CHAPTER 75 Tracheobronchial Endoscopy
Airway Anatomy and Nomenclature
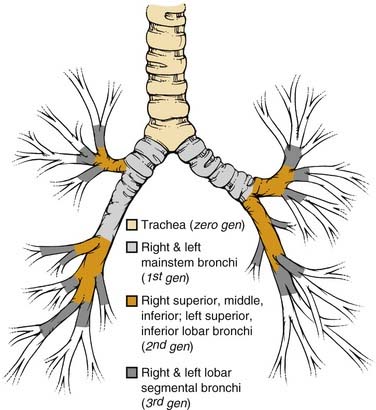
The basic pulmonary function of gas exchange occurs at the level of the alveolus, located in the distal acinus beyond respiratory bronchioles, but for the purpose of the bronchoscopist, it is more important to recognize the more proximal airway divisions. Distal to the trachea and the mainstem bronchi, the lobar bronchus defines the division of the lobes, and the segmental bronchi the pulmonary lobules (Fig. 75-1). There are thus three lobes in the right lung, with normally ten segmental lobules, although there may be some anatomic variants. In the left lung, the left upper lobar segments and the lingular subsegments come off the same left upper lobe bronchial division, which also explains why resection of the left upper lobe often requires inclusion of the lingular, and vice versa. Because of the anatomic divisions, the left lung is often divided into nine segmental lobules, although here again there are anomalies, and the bronchoscopist should be familiar with the variations. Because of the location of the heart, the right lung usually accounts for 55% to 60% of the total lung parenchyma, and the left lung the smaller remainder. This information becomes important in estimations of residual pulmonary function after a planned lobar resection or pneumonectomy.
An additional source of frequent confusion in the naming of the segmental and subsegmental bronchi has to do with the frequent but incorrect interchange of the terms generation and order of the bronchial segments. Standard nomenclature of the human airways denotes the trachea as the zero-generation airway, with each of the right and left mainstem bronchi being the first generation, lobar bronchi the second generation, lobar segmental bronchi the third generation, and so forth. Conversely, order of airway segment refers to the retrograde counting from the “lobular bronchiole,” which is the first airway segment with a diameter of less than 0.7 mm. Hence, more central airways of a larger dimension actually have a higher order.1,2 However, given the uneven segmentation and subsequent diminution of airway calibers, there is far less uniformity of division of airway in the different lobes, and lobules narrow down to 0.7 mm. Hence, the standard textbook description of the 0.7-mm lobular bronchiole located in the 10th- to 14th-generation bronchi does not match the bronchoscopic findings of airways navigable by a bronchoscope significantly wider than 0.7 mm beyond the number of branchings described previously.2–4 In summary, it is best to use the term generation in the description of sequential airway branching, starting from the trachea. Dr. Shigeto Ikeda,5 regarded as the founder of modern fiberoptic bronchoscopy, also developed a detailed system of naming segmental and subsegmental airways branchings, but that topic is beyond the scope of this chapter.
Indications and Preparations for Tracheobronchoscopy
Indications
Indications for tracheobronchoscopy include the evaluation of acute or chronic respiratory symptoms such as hemoptysis, nonresolving and worsening cough, and acute or worsening subacute dyspnea that may be accompanied by wheezing or stridor, pleurisy, chest pain, fever, or other symptoms suggestive of a pulmonary process (Box 75-1). Symptoms may be accompanied by radiographic abnormalities suggesting an endobronchial lesion, extrinsic compression of the airways by a mediastinal or lung mass, or presence of an infiltrate. Patients are often also referred for evaluation of asymptomatic lung or mediastinal masses and nonresolving parenchymal lung infiltrates. Pleural effusions usually lead to evaluation of the pleural fluid; however, effusions most often have an underlying parenchymal pulmonary cause, and a nonresolving effusion without an established cause after thoracentesis or thoracoscopy should prompt an examination for endobronchial obstruction. Bronchoscopy is helpful in assessing the placement of endotracheal tubes, especially in difficult intubation cases or when double-lumen tubes are required. A flexible fiberoptic bronchoscope (FOB) can be used to guide placement of an endotracheal (ET) tube in a difficult airway; in such cases, the ET tube is prepositioned and advanced over the bronchoscope. An FOB can also be used to guide percutaneous tracheostomy. Indications for tracheobronchoscopy in children include suspected foreign body, respiratory distress secondary to tracheomalacia or bronchomalacia, and evaluation of other congenital anomalies. Depending on the findings on radiography or during a diagnostic bronchoscopy, therapeutic interventions may be performed with an FOB to maintain airway patency and improve gas exchange.6,7
Box 75-1 Indications for Tracheobronchoscopy
Diagnostic Evaluation
Sedation and Airway Management
For patients undergoing bronchoscopy under conscious sedation, topical anesthetics are commonly used to reduce the amount of systemic sedative-narcotics needed to suppress cough, and also to diminish local discomfort in the nasopharynx during the passage of the bronchoscope. Lidocaine and its derivatives are most commonly used and applied topically as a gel to the nasal passage or as a spray to the posterior oropharynx. Solutions with concentrations between 1% and 4% are available, but the operator must pay attention to the total dosage applied because systemic lidocaine toxicities, including inadvertent deaths in healthy research subjects, have been reported when more than 500 to 1000 mg is delivered topically in a single session.8 Therefore, except for spraying to the posterior oropharynx, we limit the use of topical lidocaine injected via the operating channel of the bronchoscope onto the vocal cords and airway mucosa to less than 3 to 4 mg/kg total dosage (1% lidocaine has 10 mg of lidocaine per l mL solution). Cocaine is also a very effective topical anesthetic that has the advantage of vasoconstricting the nasal mucosa membrane; it is, however, not generally available for use in the average endoscopy suite.
Rigid Bronchoscopy
Before insertion of the rigid bronchoscope, either alone or with a telescope lens through it to provide a better distal view, the patient must be examined for neck stability and for any loose teeth or dentures. A shoulder roll may give room for added extension of the neck. With or without a tooth guard to protect the upper teeth, the operator’s left thumb is placed over the upper teeth and the index finger scissors open to lift up the lower teeth of the relaxed jaw. The bronchoscope with the bevel up is then directed midline and almost perpendicularly toward the hypopharynx until the uvula is passed. The operator thereafter slowly levels the bronchoscope angle toward the horizontal, seeking out the epiglottis while lifting the base of the tongue. When the vocal cords are clearly visualized, the bronchoscope is rotated 90 degrees such that the beveled edge can enter the trachea along the length of the vocal cord, thereby limiting trauma to the cords. Once the tip of the rigid bronchoscope is clearly in the trachea, it is rotated back to its starting position with the bevel up. The operator’s left hand and finger positions are maintained to protect the teeth, although it is also necessary to occasionally use the left hand to provide a better seal around the cuffless rigid bronchoscope at the level of the cricoid and thyroid cartilages when ventilation is applied. Because larger operative rigid bronchoscopes with diameters of 11 to 12 mm are now available for insertion of silicone stents, upper airway and vocal cord edema may be a more severe problem at the end of a prolonged procedure; hence careful examination of the cords and hypopharynx is important to avoid postprocedural stridor and upper airway obstruction. With the advent of flexible fiberoptic bronchoscopy, the number of pulmonary teaching programs routinely including rigid bronchoscopy in their training curriculum is falling. Therefore, even though rigid bronchoscopy should be a skill acquired by all bronchoscopists, generally only those being trained in otolaryngology–head and neck surgery, thoracic surgery, or interventional pulmonology generally receive adequate exposure to and practice with this technique.9
Flexible Fiberoptic Bronchoscopy
Range of Flexible Bronchoscopes and Special Features
Although there remains the tendency to subdivide bronchoscopes into “adult” and “pediatric” categories depending on their outer diameters, this distinction is arbitrary. Because bronchoscopists venture more peripherally with FOBs to sample focal lesions, and more interventional procedures via a flexible FOB are performed in the pediatric population, it makes more sense to describe the bronchoscopes and leave their judicious application to the bronchoscopist according to experience and the situational need.3,4
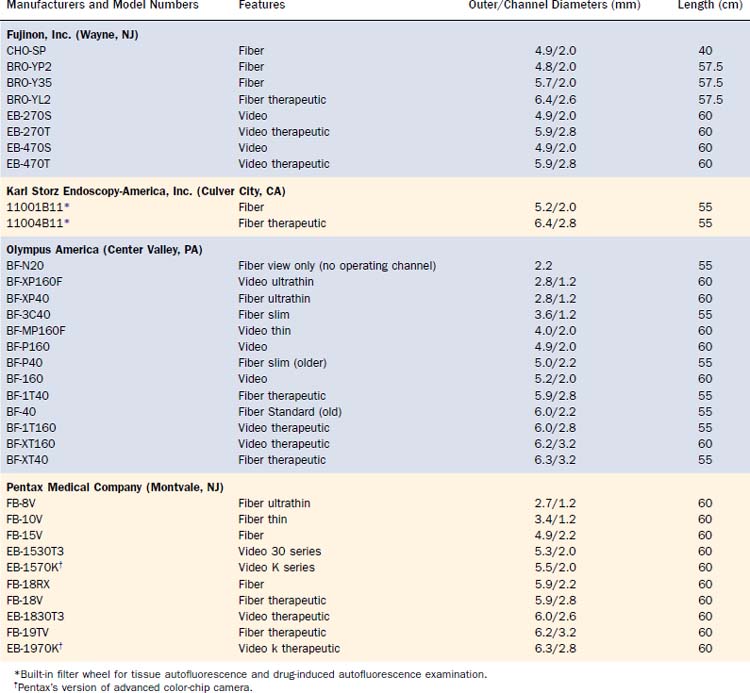
All FOBs have an illumination fiberoptic bundle and imaging fiberoptics or a camera. With the exception of the very few “ultrathin” bronchoscopes, there is also a channel for suction of secretions and blood, for the passage of topical medication and fluid for washing, and for the passage of various instruments for diagnostic retrieval of tissues or for therapeutic procedures (Table 75-1). The “average” diagnostic bronchoscope has an outer diameter of 5.0 to 5.5 mm and an operating channel of 2.0 to 2.2 mm. This caliber channel admits most cytology brushes, bronchial biopsy forceps, and transbronchial aspiration needles with sheathed outer diameters between 1.8 and 2.0 mm. Smaller bronchoscopes, in the range of 3.0 to 4.0 mm at the outer diameter and correspondingly smaller channels, are usually given a “P” designation (for pediatrics), but they can, of course, be used in the adult airways when narrowing is present because of benign strictures or malignant stenosis. Newer generations of “slim” video and fiberoptic bronchoscopes have a 2.0-mm operating channel with a 4.0-mm outer diameter. The one disadvantage of these bronchoscopes is the sacrifice of a smaller image area because of fewer optical bundles.
Larger “therapeutic” bronchoscopes (often designated with a “T” in the model number) can, of course, also be used for diagnostic purposes, but the larger outer diameter can cause greater discomfort and mucosal trauma in a conscious patient and can be harder to pass through an ET or tracheostomy tube, and thus can also impair gas exchange to a greater degree. Such therapeutic bronchoscopes have outer diameters between 6.0 and 6.3 mm, with operating channel lumens between 2.6 and 3.2 mm. Certain therapeutic instruments, including larger laser fibers, larger electrocautery forceps designed for gastrointestinal endoscopes, cryotherapy probes, and expandable balloons for bronchoplasty, require these larger diameters for their use through FOBs. Prototype bronchoscopes with a 9-mm outer diameter and a 5-mm operating channel have been made, the main application of which is to provide access for therapeutic instruments to airway segments that cannot normally be reached by rigid open-tube bronchoscopes and telescopes (Fig. 75-2).
At the other extreme are the ultrathin bronchoscopes, with outer diameters smaller than 3 mm. The Olympus production models fiber BF-XP40 and video BF-XP160F (Olympus America, Center Valley, PA) have outer diameters of 2.8 mm and operating channels of 1.2 mm. Special instruments (e.g., reusable cytology brush and forceps) of the proper caliber are available for tissue sampling (Fig. 75-3). Handling of these ultrathin bronchoscopes is often more challenging because their very floppy tips make steering more difficult. Suction via the narrow channels is also much more limited. Nevertheless, with practice and the use of small amounts of saline to flush open the distal airways in guiding the bronchoscope forward, the ultrathin bronchoscopes can traverse 12 to 16 generations of airways and, under fluoroscopic or CT guidance, are seen to approach the periphery of the lungs to sample focal lesions.3,4 The passage of a 2.8-mm instrument beyond the mid-teens division of the lobular bronchiole (measured at 0.7 mm in fixed tissue) calls into question some of the accepted measurements of the adult human airways. To permit the greater distance traversed, such ultrathin bronchoscopes usually have an operating length of 60 cm, 5 cm longer than the older bronchoscopes, although the current generation of videobronchoscopes are all built with a 60-cm working length.
New bronchoscopes manufactured today usually have a white ceramic insulated tip; this feature permits the safe use of electrocautery instruments with reduced risk of retrograde electric shock to the bronchoscopist and damage to the bronchoscope (see Figure 75-2).
Diagnostic Fiberoptic Bronchoscopy
General Approaches
Although there is usually one primary indication for bronchoscopy, an orderly and uniform approach should be taken for airway examination so that important pathology will not be missed because of impatience to attend to the primary focus. Starting at the upper trachea, mucosal integrity should be examined. Even when there are no gross endobronchial lesions, the presence of extrinsic tracheal deviation and compression due to paratracheal masses should be noted. TBNA can often successfully provide a tissue diagnosis of extrinsic lesions.10,11 Still under development is an array of ultrasound devices, balloon probes, and actual dedicated EBUS bronchoscopes that should help in the localization and characterization of such lesions. The posterior membranous portion of the trachea is sometimes the site of airway compromise caused by tracheomalacia or tumor invasion from esophageal cancers, and it is also the site of tracheoesophageal fistula. However, because of inflammation and redundant tissue, a tracheoesophageal fistula may be difficult to locate. Having the patient swallow 15 mL of methylene blue dye or food coloring immediately preceding bronchoscopy can aid in the localization of a tracheoesophageal tear or fistula. The distal trachea and main carina are important sites for examination because malignant diseases often metastasize to the surrounding mediastinal lymph nodes. The subcarinal, anterior carinal, and low paratracheal lymph nodes bilaterally are particularly suitable for TBNA.10,12
Endobronchial and Transbronchial Biopsy
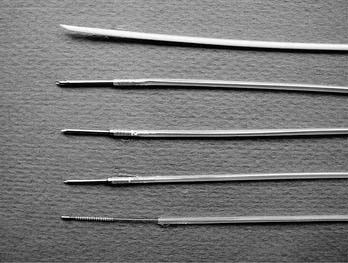
A number of biopsy forceps are available for biopsy of bronchial mucosa or endobronchial lesions, and for transbronchial biopsy of airway and parenchyma beyond direct bronchoscopic vision (Fig. 75-4). The catheters are of various diameters and lengths, with larger “therapeutic” forceps fitted with larger forceps cups. The edges of the cups are either smooth-cutting or jagged “alligator” type. A number of the larger forceps are designed primarily as forceps for use in upper and lower gastrointestinal endoscopes, and hence their cables are much longer than the 55 to 60 cm of the bronchoscope working channel. There are additional forceps with a needle or “spike” in the center of the opened jaw near the fulcrum of the opened forceps; this spike aids in the anchoring of the forceps on mucosa, scar tissue, or tumor. These needle forceps are most useful for endobronchial biopsy, especially when the target may be along an airway wall tangential to the bronchoscope and thus cannot be easily grasped by forceps opening at a very shallow angle to the lesion. Some forceps also have an attachment for electrocautery. These may be useful for sampling or removing friable tissue that bleeds, because electrocautery can effectively provide thermal hemostasis.
Transbronchial biopsy uses the same instruments, although there is much less reason to use the needle forceps. This procedure is usually directed toward a focal mass lesion most often suspicious for lung carcinoma, or toward focal or diffuse infiltrates suggestive of infection, inflammatory lung processes, fibrotic lung parenchyma, or metastatic carcinoma with lymphangitic or diffuse hematogenous spread. Although it is possible to perform successful transbronchial biopsy without radiographic guidance, the yield is lower and the risk of complications increased; therefore, we prefer the availability of fluoroscopy to guide transbronchial biopsy, especially of smaller, more focal, and more peripheral lesions (Fig. 75-5).11,13
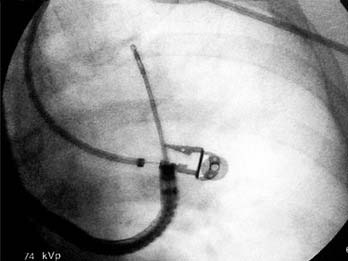
Figure 75-5. Transbronchial biopsy of a peripheral (left upper lobe) lung mass under fluoroscopic guidance.
Complications of TBBX are bleeding and pneumothorax. As aforementioned, performing fluoroscopy and querying for symptoms of pleurisy reduce the risk of pneumothorax to less than 10%, with overall rates of 3% to 20% reported.11 Significant bleeding is not increased in patients taking aspirin and cannot be predicted by routinely ordering measurements of platelet counts and coagulation parameters. Nevertheless, in critically ill or pancytopenic patients, an attempt is made to keep the platelet counts above 50,000/µL, to correct coagulopathy if present, and to temporarily suspend anticoagulation. Bleeding, when brisk, is managed by tamponading of the bleeding subsegment with the bronchoscope tip. Depending on operator preference, suction can be applied to collapse the segmental airways to prevent further retrograde bleeding into the central airways, and lavage with room-temperature or cooled saline can be performed to slow the bleeding. There are no randomized trials to suggest efficacy of one approach over another. Needless to say, life-threatening hemorrhage from transbronchial biopsy is rare but reported.
Transbronchial Needle Aspiration
The introduction and refinement of TBNA needles attached to a flexible catheter has permitted sampling, with a flexible FOB, of a larger number of lymph node regions, endobronchial lesions in the segmental and subsegmental bronchi, and peripheral, endoscopically occult lesions. The majority of the needles are made of stainless steel with calibers ranging from 22 to 19 gauge (Fig. 75-6). There remains one plastic needle with a flexible tip (Microvasive Sofcor, Boston Scientific, Natick, MA) that may be bent to facilitate passage into subsegments of the upper lobes and certain lower-lobe subsegments that are otherwise often not reachable with metal transbronchial needles because the 13- to 15-mm lengths of these rigid needles reduce the tip angulations of the flexible FOBs.
Although the flexible needles have been divided into smaller gauge (20, 21, and 22 gauge) “cytology” needles and larger gauge (19-gauge metal and 18-gauge Sofcor) “histology” needles (see Figure 75-6), the choice of the needle depends on operator experience, because the larger “histology” needles still provide tissue for cytologic examination by slide smear, and samples aspirated with smaller “cytology” needles can be spun down into a cell block and cut for histologic examination. The longer needle lengths (15 mm) of the 21-gauge and larger needles offer the advantage of greater depth penetration. However, a 19-gauge needle is more unwieldy to maneuver, takes more practice for penetration of the bronchial mucosa, and can potentially cause more trauma. It is often reserved for the sampling of larger lymph nodes suspected of harboring lymphoproliferative tissue (e.g., lymphoma, Castleman’s disease) or granulomatous diseases (e.g., sarcoid, mycobacterial, and fungal adenitis) because, with practice, a 21-gauge core sample can be retrieved.
Mediastinoscopy remains the “gold standard” for examination and sampling of mediastinal lymph nodes; however, access to the subcarina, posterior tracheal, subaortic left-paratracheal/aortopulmonary window and hilar lymph nodes is limited. TBNA via FOB has the advantage of access to a larger number of lymph node stations and of being a less invasive procedure that can be combined with the endoscopic examination of the airways plus as-needed sampling of peripheral lung disease (see Figure 75-6). Therefore, TBNA may be one of multiple procedures performed in a sequence of “one-stop” diagnosis and staging of thoracic malignancies. Certain lymph node stations are also inaccessible to TBNA via FOB; these include the paraesophageal and lateral aortopulmonary-window lymph nodes, which can be sampled by real-time endoscopic ultrasonography–guided fine-needle aspiration (FNA) via the esophagus and by anterior median sternotomy (Chamberlain procedure), respectively.
Technical Aspects
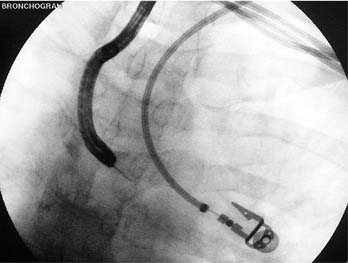
The actual mucosal penetration and spearing of the mediastinal target can be performed by one of two techniques, the “stab” or the “push-jab.”10 The stabbing approach entails holding the bronchoscope in place and pushing the deployed needle catheter forcefully forward, thus traversing the bronchial mucosal into a lymph node or tumor mass. The limitation of this technique is that it is suited only for lesions that are situated directly in the line of sight of the bronchoscope; the needle will more than likely take a somewhat more oblique pathway across the bronchial mucosa, thus leaving less needle length to penetrate the lesion. In a spontaneously breathing patient, subtle airway motion can translate to missing the lesion or having the needle hang up on bronchial cartilage instead of passing through the membranous portion of the mucosa. Finally, if the bronchoscope-needle setup is positioned too far away from the bronchial mucosa point of entry, pushing the flexible TBNA catheter outside the bronchoscope channel will allow the catheter to bend and kink, further limiting its effectiveness.
For these reasons, the piggy-back “push-jab” technique is preferred. In this approach, the deployed and locked needle is kept partially withdrawn into the distal bronchoscope and the sharp needle tip is carefully directed toward a selected entry point; the membranous mucosa between cartilage rings is chosen. The bronchoscope tip can be partially flexed such that the bronchoscope and needle catheter behave as a curved needle set, with the needle anchored at about a 45-degree angle. Control of both the catheter and bronchoscope is obtained, either by the bronchoscopist alone or with an assistant holding the proximal end of the catheter and being ready to advance it on command, and the bronchoscope is pushed forward firmly as the piggy-backed needle-catheter is jabbed forward. With practice, the TBNA needle can be made to traverse the mucosa at a nearly 90-degree angle, thus affording sampling of lesions in the distal left and right paratracheal angles, anterior precarinal space, and right bronchial lymph nodes (Fig. 75-7). Once the locked needle-catheter set is advanced until the entire length of the needle is buried up to the hub, the clear catheter is pushed from the proximal end until a bit of it is visibly protruding from the distal bronchoscope tip. At this point, inadvertent penetration of one of the major vascular structures, such as the aorta, pulmonary artery branches, superior vena cava, or azygous vein would be obvious from the complete backfill of the distal catheter with blood even without any suction applied. If there is no unexpected return of blood, a cytology or histology sample can be obtained with the forward-and-backward movement of the needle. Although suction has most often been applied by syringe attached to the proximal end of the TBNA catheters, this manipulation is not always necessary because it is really the capillary action plus the regular agitation motion that draws tissue into the lumen of the needle. This principle is especially applicable for looser, less organized malignant tissue, whereas suction may be required for acquisition of an adequate sample of a denser granulomatous or reactive lymph node.
The same TBNA needles can be used for the transbronchial sampling of peripheral lung masses, although as mentioned earlier, the acute bending of certain lobar segments may preclude successful passage of the TBNA needle into the desired airway segments.11,13 Unlike in sampling of central mediastinal lesions or endobronchial pathology, the needle is not deployed until the catheter hub with the protected needle tip is advanced into position as indicated by fluoroscopy or CT.
The retrieved samples can be prepared and preserved in a number of ways. Generally, more than a single pass is made to ensure adequate sampling, with the maximum yield leveling off after between four and seven passes at a particular site. The presence of rapid on-site evaluation by a pathologist or an experienced cytopathology technician is a helpful adjunct that improves the diagnostic yield of TBNA.14 The immediate feedback will direct the need for additional TBNA passes. Even when disease is noted, such as carcinoma or abnormal lymphocytes, extra passes for additional material to be spun down for immunostaining or preservation in media for flow analysis may aid in making the identification of a tumor source or cell type, with important implications for prognosis and as a guide for therapy. In the absence of cytopathology assistance, samples can also be injected into an aliquot of preservative solution such as Saccomanno’s fixative and spun down in the laboratory for further analysis. Flushing out the needle with 1 to 2 mL of saline after each pass, as reported earlier, is no longer practiced because this dilutes out small quantities of sample, and the needle loses some of its capillary effect when flushed with liquid. We therefore eject the sample from the needle with an air-filled syringe.
Published series quote a diagnostic yield rate of 75% to 90% for TBNA sampling of mediastinal lymph nodes, with lower rates for peripheral pulmonary lesions, once again depending on the location and size of the targets.10,11,13 TBNA, however, remains an underused minimally invasive sampling technique because of uneven teaching of the technique and the average low yield for the occasional practitioner of this technique.9 A number of radiographic techniques have been used or are being developed as adjuncts to help in localization and to guide TBNA sampling of lung pathology. Fluoroscopy can help visualize the proper penetration of a lesion in the lung parenchyma but is generally less useful in accurately localizing mediastinal adenopathy and central masses that may be obscured by the cardiac silhouette and other normal mediastinal structures. Spiral CT, either with stopped frames or fluoroscopic, can be used to help localize and prove TBNA penetration of target lesions, but it is cumbersome to perform these bronchoscopic procedures in the CT scanner, and the patient and staff are exposed to a much higher levels of radiation.
The advent of rapid multislice detector CT scanners and the development of software to improve three-dimensional rendering may soon make available a number of high-fidelity “virtual bronchoscopy” programs, including the capability of rendering the airway wall “transparent” and highlighting the adjacent mediastinal structures of interest. The purpose of these programs is not to obviate the need for tissue diagnosis but to guide the approach for procedures such as TBNA and transbronchial biopsy. Another real-time guidance technique is the use of EBUS to localize lymph nodes and other mediastinal structures of interest for tissue sampling.15,16 Most EBUS procedures use a radial balloon probe introduced via the working bronchoscope channel. After EBUS imaging is completed, the probe is withdrawn and TBNA is performed. Report of simultaneous imaging and sampling via a double-lumen bronchoscope is not generally applicable owing to the rarity of these bronchoscopes, which are largely being phased out, and by the imminent introduction of a dedicated EBUS bronchoscope with an angled side-port for TBNA. EBUS is currently available for clinical use, but in only at a limited number of centers because of the lack of experienced practitioners and the equipment cost.
Bronchoscopic Brush
The use of a cytology brush for the sampling of an endobronchial lesion or its use under fluoroscopic guidance for the sampling of a peripheral lesion appears a simple enough procedure, but a review of the yield and complications is warranted. Because the stiff metallic bristles of the cytology brush can be traumatic over a larger area, bleeding from bronchoscopic brushing can be severe. In spite of a vigorous effort to retrieve sample, the yield can be disappointingly low for carcinoma and generally ranks below directed-forceps biopsy and endobronchial needle aspiration.11,13 There are multiple explanations for this apparent paradox. Much as with TBNA, in which active negative-pressure aspiration can cause more trauma and bleeding than agitation without suction, the nonspecific trauma induced by the bristles leads to a usually bloody sample that can obscure the malignant cells behind a field of red blood cells, fibrin, and other debris. The superficial endobronchial layer of a central tumor can often consist of a biofilm of mucus, necrotic cell debris, and recruited inflammatory cells, thus sampling along the surface with a cytology brush may pick up only these nondiagnostic contaminants and miss the underlying true pathology. The yield of a cytology brush for the sampling of peripheral lesions is equally disappointing, also because of the sampling issue of picking up mostly normal or inflamed bronchial or bronchiolar epithelial cells. In a number of instances, unless an airway leads directly up into a tumor mass, the catheter of the cytology brush is directed away from the tumor that pushes away adjacent bronchi.17 In a similar circumstance, the needle of a TBNA can be extended directly toward a tumor, penetrating the smaller airways as necessary. Therefore, except as a poor substitute for directed-forceps biopsy and TBNA of central endobronchial and peripheral lesions, bronchoscopic brushing for cytology has relatively low merit and rarely adds to the other sampling procedures in cancer diagnosis.
Interventional Bronchoscopic Procedures
General Principles
Virtual bronchoscopy is becoming more popular in simulating diagnostic bronchoscopy to diagnose airway pathology as a prequel to therapy, and minimally invasive therapies are now more and more favored over aggressive operations because of improved technology. Therefore the paradigm of bronchoscopy is shifting slightly from diagnostic implications to treatment with bronchoscopic interventional procedures.18–20
Preparation of the Patient and Selection of Instruments
Careful history-taking should include any use of anticoagulants and antiplatelet agents. Physical examination, review of radiographs (preferably including a recent contrast CT scan), and measurement of platelet counts and other relevant bleeding parameters are routine. Because many of the patients undergoing interventional bronchoscopic procedures often have more advanced pulmonary diseases and possible comorbidities, a baseline electrocardiogram is often requested. Because the purpose of the procedures is often palliative in nature and the procedures are accompanied by higher risk than a standard diagnostic bronchoscopy, a detailed discussion of possible complications and the realistic likelihood of achieving subjective relief should be discussed with the patients and their families. In an airway interventional series of laser débridement in 1585 patients by Cavaliere and associates,21 the overall procedure-related mortality was 0.3% and the rate of major complications (including significant hemorrhage, pneumothorax, and pneumomediastinum) was 1.5%.
Most interventional procedures can be performed via an FOB, a rigid bronchoscope, or an FOB introduced via a rigid bronchoscope or via direct suspension laryngoscope. There are pros and cons to both approaches, but the operator should be capable of converting a procedure begun with an FOB into one using a rigid bronchoscope when the need arises.9,18 The larger channel of the rigid bronchoscope facilitates the use of larger-caliber instruments, and certain rigid silicone stents require rigid instrumentation for their deployment. The bronchoscope itself can be used to core out tumor tissue or to tamponade hemorrhage from the side walls. It is also easier to remove noncancerous foreign bodies and to dilate benign stenotic segments via a larger rigid tube. Conversely, the larger and rigid instruments are more liable to cause trauma to the vocal cords and potentially cause a fistula when used to débride necrotic tissue in distorted airways. The constrained angulation provided by manipulation of the patient’s head and neck positions can limit the bronchial segments reachable for intervention. The rigid instruments also mandate the use of general anesthesia. When a flexible FOB is chosen for interventional procedures, larger-channel “therapeutic” bronchoscopes that can accommodate flexible interventional instruments are selected, but it is often helpful to also have available thinner pediatric bronchoscopes that can be used to bypass critical narrowings.
Foreign Body Removal
Although foreign body aspiration (FBA) is classically a pediatric problem, with the highest incidence occurring in children younger than 5 years, bronchial foreign body can occur in adults as well, albeit often with less acute symptoms.22 The classic signs—witnessed acute choking, wheezing, loss of unilateral breath sounds with corresponding volume loss, or hyperinflation due to air-trapping on the radiographs—have a high sensitivity (about 70%) but variable specificity for FBA. Other findings, such as chronic cough, recurrent pneumonias in the same chest region, atelectasis, and even pneumothoraces or pneumomediastinum, are more common in adults, who may or may not recall an episode of possible aspiration.18
Rigid bronchoscopy has been the standard procedure for removal of airway foreign bodies and remains so in the pediatric population. Conversely, in adults, a trial of foreign body removal with instruments compatible with a flexible FOB would be reasonable, although there should always be the backup plan for rigid bronchoscopy should the aspirated foreign body prove too stubborn for removal by the smaller flexible bronchoscopy instruments. Flexible bronchoscopy is indicated for patients with cervical spine instability and skull and jaw fractures, and such trauma patients may be particularly at risk for the aspiration of broken teeth or dentures, or a preceding aspiration leading to near asphyxiation and loss of consciousness may even be the cause of the subsequent head and neck trauma from a fall or a vehicular accident. Helpful adjuncts in directing the foreign body centrally include repositioning the patient in a Trendelenburg or decubitus position, movements that would not interfere with flexible FOB but may prove more challenging with a rigid bronchoscope. A number of “nonpulmonary” instruments can be adapted to assist in the retrieval of a foreign body; these include a Fogarty catheter pushed distal to the foreign body, then inflated and slowly pulled back, simulating the retrograde clot removal from a vascular lumen. Snares and baskets, such as the Roth basket developed for gastrointestinal procedures, can also be used to retrieve smooth or rounded items that are difficult to grasp, such as peanuts and marbles that are akin in texture and size to biliary stones. Cryotherapy probes used primarily for tumor and granulation tissue ablation can also be used to retrieve a foreign body that has a layer of aqueous condensation or secretions around it by virtue of the process of cryoadhesion.23 (Recall the unfortunate child testing this hypothesis on a cold winter’s day and getting his tongue stuck to the metal pole.)
Tissue Débridement
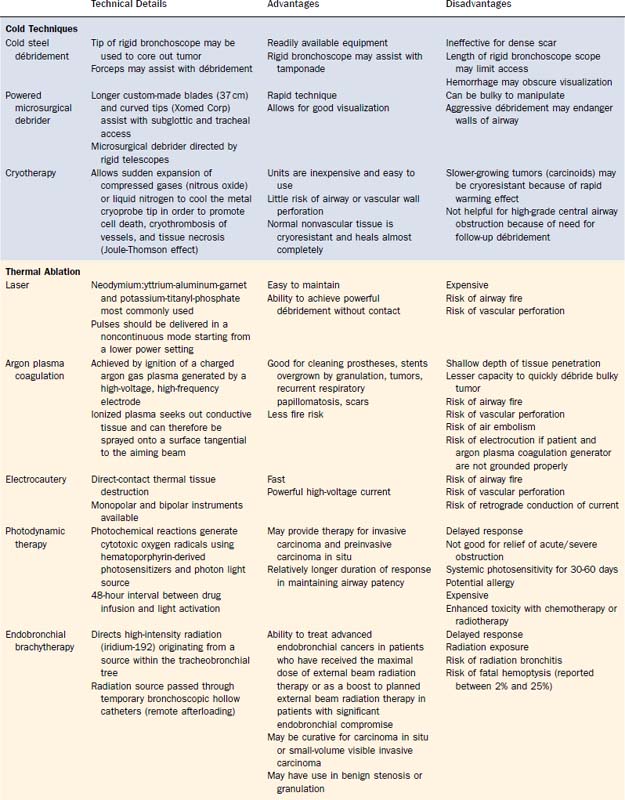
The discussion of interventional bronchoscopic approaches in the débridement of malignant or scar tissue causing airway obstruction often becomes a catalog of technology and devices to deliver these tissue-destructive techniques. However, it is fair to say that the underlying common denominator is mechanical resection assisted by freezing, heating, or biochemical methods of causing cell death. Cold techniques include use of forceps, powered microdebrider, or cryotherapy. Laser, argon plasma coagulation (APC), and electrocautery are three methods of heat-assisted mechanical resection. Finally, photodynamic therapy (PDT) and endobronchial brachytherapy (EBBT) are biochemical destructive methods. Methods for tissue débridement are detailed in Table 75-2.
Endobronchial Stent Placement
Placement of airway stents may be the definitive treatment or may maintain airway patency long enough for adjunctive treatment to control the underlying disease in a number of obstructive conditions. A large number of endobronchial stents have been developed or adapted for airway use over the past 20 years.9,18,20,24 A detailed discussion of all of the individual types and their variants goes beyond the scope of this chapter. The stents can be grouped into metallic and silicone types. Most but not all of the metallic stents are self-expanding, whereas most of the silicone stents are fairly rigid and require rigid bronchoscopy or direct suspension laryngoscopy for placement.
Complications of stent placement include trauma to a compromised airway during stent insertion, such as inadvertent tears to the tracheobronchial mucosa already compromised by tumor invasion. An improperly sized covered stent can occlude functioning airway segments because it is too large or too long; if it is too small, it can subsequently migrate. All stents, covered or otherwise, interfere with normal mucociliary clearance and, as foreign bodies, will promote biofilm formation. Stents can therefore become quickly overgrown with a variety of microorganisms. The problems of granulation and tumor ingrowth have been mentioned. The metallic stents can suffer metal fatigue and undergo fracture and, with the single strand weave of nitinol, can unravel.25 This problem is especially prominent with tracheal stents in which the constant change in curvature of the posterior tracheal membrane with breathing in and out results in metal fatigue. We have therefore avoided placing self-expandable metal stents in the trachea if we anticipate long-term need for the stent in benign conditions such as tracheobronchomalacia or if the patient with a malignant condition has an anticipated survival beyond several months.
Innovations and Future Developments
Autofluorescence Bronchoscopy
Autofluorescence bronchoscopy is used to detect early, preinvasive lung cancers and can also help thoracic surgeons in planning lung cancer resection. Since the early 1990s, trials have been ongoing to test and improve systems to detect alteration of tissue autofluorescence in dysplastic and cancerous bronchial tissues. Reduction of tissue autofluorescence in precancerous and cancerous tissue results in a change in the green autofluorescent hue of normal bronchial mucosa toward red-brown to blue-black tints. U.S. trials of several systems, including the LIFE (Laser Imaging Fluorescence Endoscope, Xillix Technologies, Richmond, BC, Canada) and D-Light (Karl Storz Endoscopy-America, Inc., Culver City, CA), validate a higher yield in the detection and localization of severely dysplastic and carcinoma in situ lesions in comparison with conventional white-light broncoscopy.26–28
Endobronchial Ultrasonography
Endoscopic application of ultrasonography for characterization and localization of extraluminal structures such as lymph nodes, masses, and blood vessels has been mostly developed in the gastrointestinal field. The adaptation of a balloon-tipped ultrasound probe for use within the airway lumen allows for a more precise localization of regional lymph nodes for tumor staging and for diagnosis of lymphoma and other lymphoproliferative disorders. Ultrasound probes introduced through the working channel of the bronchoscope and directed peripherally have also been used to characterize pulmonary consolidation and may distinguish malignant from nonmalignant causes.15,16,18 Higher-frequency high-resolution EBUS can also provide additional information about extrabronchial invasion of the serosal surface or submucosal infiltration by cancer, again with prognostic and therapeutic impact. Currently, sampling with EBUS-directed TBNA requires imaging followed by the removal of the probe and substitution of a sampling needle into the working channel. The next adaptation of ultrasound technology into the airways will be the introduction of a dedicated EBUS bronchoscope with a side-directed radial probe and aspiration port that will allow real-time aspiration.
Endoscopic/Bronchoscopic Lung Volume Reduction
The National Emphysema Treatment Trial established the benefit of surgical lung volume reduction surgery as an alternative to lung transplantation in selected patients with COPD.29 Parallel studies have been undertaken to examine the possibility of affecting lung volume reduction through selective blockage of segmental bronchi leading to regional atelectasis. A number of such endoscopic/bronchoscopic lung volume reduction studies using biologic or synthetic adhesives, such as fibrin glue and removable valves, are currently ongoing. These procedures hold the promise of either being effective and less invasive methods of lung volume reduction surgery or of offering a less invasive and reversible trial of lung volume reduction before patients are subjected to the rigors of thoracic surgery.30,31
Bergler W, Hönig M, Götte K, et al. Treatment of recurrent respiratory papillomatosis with argon plasma coagulation. J Laryngol Otol. 1997;111:381.
Boiselle PM, Ernst A. State-of-the-art imaging of the central airways. Respiration. 2003;70:383.
British Thoracic Society Bronchoscopy Guidelines Committee. British Thoracic Society guidelines on diagnostic flexible bronchoscopy. Thorax. 2001;56(Suppl 1):11-21.
Burgers JA, Herth F, Becker HD. Endobronchial ultrasound. Lung Cancer. 2001;34(Suppl 2):S109.
Cavaliere S, Foccoli P, Toninelli C, et al. Nd:YAG laser therapy in lung cancer: an 11-year experience with 2,253 applications in 1,585 patients. J Bronchol. 1994;1:105.
Chan AL, Yoneda KY, Allen RP, et al. Advances in the management of endobronchial lung malignancies. Curr Opin Pulm Med. 2003;9:301.
Diette G, White PJr, Terry P, et al. Utility of on-site cytopathology assessment for bronchoscopic evaluation of lung masses and adenopathy. Chest. 2000;117:1186.
Ernst A, Silvestri GA, Johnstone D, American College of Chest Physicians. Interventional pulmonary procedures: guidelines from the American College of Chest Physicians. Chest. 2003;123:1693.
Feller-Kopman D, Lunn W, Ernst A. Autofluorescence bronchoscopy and endobronchial ultrasound: a practical review. Ann Thorac Surg. 2005;80:2395.
Flint PW. Powered surgical instruments for laryngeal surgery. Otolaryngol Head Neck Surg. 2000;122:263.
Gasparini S, Ferretti M, Secchi EB, et al. Integration of transbronchial and percutaneous approach in the diagnosis of peripheral pulmonary nodules or masses: experience with 1,027 consecutive cases. Chest. 1995;108(1):131.
, 1987 Guidelines for fiberoptic bronchoscopy in adults. American Thoracic Society. Medical Section of the American Lung Association. Am Rev Respir Dis. 1987;136:1066.
Hayata Y, Kato H, Konaka C, Okunaka T. Photodynamic therapy (PDT) in early stage lung cancer. Lung Cancer. 1993;9:287.
Herth F, Becker HD, Ernst A. Conventional vs endobronchial ultrasound-guided transbronchial needle aspiration: a randomized trial. Chest. 2004;125:322.
Homasson JP. Bronchoscopic cryotherapy. J Bronchology. 1995;2:145.
Jones LM, Mair EA, Fitzpatrick TM, et al. Multidisciplinary airway stent team: a comprehensive approach and protocol for tracheobronchial stent treatment. Ann Otol Rhinol Laryngol. 2000;109:889.
Kennedy TC, Lam S, Hirsch FR. Review of recent advances in fluorescence bronchoscopy in early localization of central airway lung cancer. Oncologist. 2001;6:257.
Martinot A, Closset M, Marquette CH, et al. Indications for flexible versus rigid bronchoscopy in children with suspected foreign body aspiration. Am J Respir Crit Care Med. 1997;155:1676.
Mathisen DJ, Grillo HC. Endoscopic relief of malignant airway obstruction. Ann Thorac Surg. 1989;48:469.
Mountain CF, Dresler CM. Regional lymph node classification for lung cancer staging. Chest. 1997;111:1718.
Saad CP, Murthy S, Krizmanich G, et al. Self-expandable metallic airway stents and flexible bronchoscopy: long-term outcomes analysis. Chest. 2003;124:1993.
Speiser BL, Spratling L. Remote afterloading brachytherapy for the local control of endobronchial carcinoma. Int J Radiat Oncol Biol Phys. 1993;25:579.
Toma TP, Hopkinson NS, Hillier J, et al. Bronchoscopic volume reduction with valve implants in patients with severe emphysema. Lancet. 2003;361:931.
Wahidi MM, Herth FJ, Ernst A. State of the art: interventional pulmonology. Chest. 2007;131:261.
Yung RC. Tissue diagnosis of suspected lung cancer: selecting between sputum cytology, transthoracic needle aspiration, bronchoscopy, and resectional biopsy. Respir Care Clin. 2003;9:51.
1. Thurlbeck WM, Haines JR. Bronchial dimensions and stature. Annu Rev Respir Dis. 1975;112:142.
2. Weibel ER. Morphometry of the Human Lung. New York: Academic; 1963.
3. Kikawada M, Ichinose Y, Miyamoto D, et al. Peripheral airway findings in chronic obstructive pulmonary disease using an ultrathin bronchoscope. Eur Respir J. 2000;15:105.
4. Rooney C, Wolf K, McLennan G. Ultrathin bronchoscopy as an adjunct to standard bronchoscopy in the diagnosis of peripheral lung lesions. Respiration. 2002;69:63.
5. Ikeda S. Flexible bronchofiberscope. Ann Otol Rhinol Laryngol. 1970;79:916.
6. British Thoracic Society Bronchoscopy Guidelines Committee. British Thoracic Society guidelines on diagnostic flexible bronchoscopy. Thorax. 2001;56(Suppl 1):11-21.
7. Guidelines for fiberoptic bronchoscopy in adults. American Thoracic Society. Medical Section of the American Lung Association. Am Rev Respir Dis. 1987;136:1066.
8. Pereira W, Kovnat DM, Snider GL. A prospective cooperative study of complications following flexible fiberoptic bronchoscopy. Chest. 1978;73:813.
9. Ernst A, Silvestri GA, Johnstone D, American College of Chest Physicians. Interventional pulmonary procedures: guidelines from the American College of Chest Physicians. Chest. 2003;123:1693.
10. Wang K, Brower R, Haponik EF, et al. Flexible transbronchial needle aspiration for staging of bronchogenic carcinoma. Chest. 1983;84:571.
11. Yung RC. Tissue diagnosis of suspected lung cancer: selecting between sputum cytology, transthoracic needle aspiration, bronchoscopy, and resectional biopsy. Respir Care Clin. 2003;9:51.
12. Mountain CF, Dresler CM. Regional lymph node classification for lung cancer staging. Chest. 1997;111:1718.
13. Gasparini S, Ferretti M, Secchi EB, et al. Integration of transbronchial and percutaneous approach in the diagnosis of peripheral pulmonary nodules or masses: experience with 1,027 consecutive cases. Chest. 1995;108:131.
14. Diette G, White PJr, Terry P, et al. Utility of on-site cytopathology assessment for bronchoscopic evaluation of lung masses and adenopathy. Chest. 2000;117:1186.
15. Burgers JA, Herth F, Becker HD. Endobronchial ultrasound. Lung Cancer. 2001;34(Suppl 2):S109.
16. Herth F, Becker HD, Ernst A. Conventional vs endobronchial ultrasound-guided transbronchial needle aspiration: a randomized trial. Chest. 2004;125:322.
17. Tsuboi E, Ikeda S, Tajima M, et al. Transbronchial biopsy smears for diagnosis of peripheral pulmonary carcinoma. Cancer. 1967;20:687.
18. CT. Bolliger, PN. Mathur. Basel: S. Karger AG. 2000.
19. Mathisen DJ, Grillo HC. Endoscopic relief malignant airway obstruction. Ann Thorac Surg. 1989;48:469.
20. Seijo LM, Sterman DH. Interventional pulmonology. N Engl J Med. 2001;344:740.
21. Cavaliere S, Foccoli P, Toninelli C, et al. Nd:YAG laser therapy in lung cancer: an 11-year experience with 2,253 applications in 1,585 patients. J Bronchol. 1994;1:105.
22. Martinot A, Closset M, Marquette CH, et al. Indications for flexible versus rigid bronchoscopy in children with suspected foreign body aspiration. Am J Respir Crit Care Med. 1997;155:1676.
23. Homasson JP. Bronchoscopic cryotherapy. J Bronchology. 1995;2:145.
24. Jones LM, Mair EA, Fitzpatrick TM, et al. Multidisciplinary airway stent team: a comprehensive approach and protocol for tracheobronchial stent treatment. Ann Otol Rhinol Laryngol. 2000;109:889.
25. Saad CP, Murthy S, Krizmanich G, et al. Self-expandable metallic airway stents and flexible bronchoscopy: long-term outcomes analysis. Chest. 2003;124:1993.
26. Haussinger K, Stanzel F, Huber RM, et al. Autofluorescence detection of bronchial tumors with the D-Light/AF. Diag Ther Endosc. 1999;5:105.
27. Kennedy TC, Lam S, Hirsch FR. Review of recent advances in fluorescence bronchoscopy in early localization of central airway lung cancer. Oncologist. 2001;6:257.
28. Lam S, Kennedy T, Unger M, et al. Localization of bronchial intraepithelial neoplastic lesions by fluorescence bronchoscopy. Chest. 1998;113:696.
29. Naunheim KS, Wood DE, Mohsenifar Z, et al. Long-term follow-up of patients receiving lung-volume-reduction surgery versus medical therapy for severe emphysema by the National Emphysema Treatment Trial Research Group. Ann Thorac Surg. 2006;82:431-443.
30. Ingenito EP, Berger RL, Henderson AC, et al. Bronchoscopic lung volume reduction using tissue engineering principles. Am J Respir Crit Care Med. 2003;167:771.
31. Toma TP, Hopkinson NS, Hillier J, et al. Bronchoscopic volume reduction with valve implants in patients with severe emphysema. Lancet. 2003;361:931.