Chapter 62 Rare Coagulation Factor Deficiencies
Table 62-2 Treatment Considerations for Rare Coagulation Factor Deficiencies
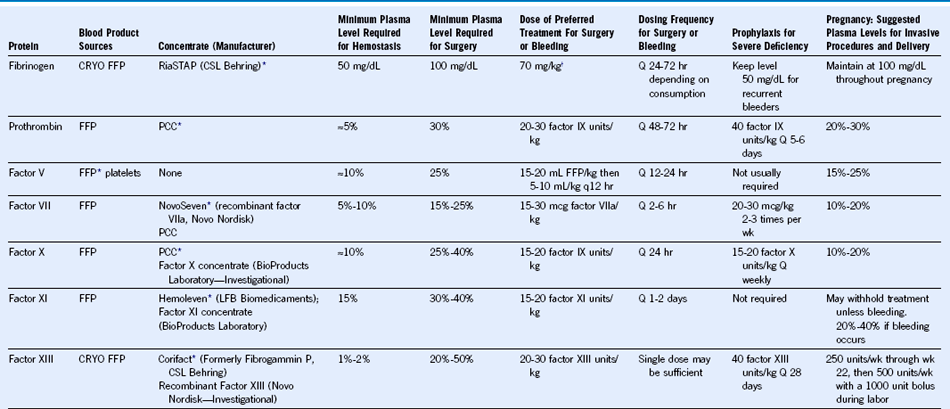
FFP, Fresh frozen plasma; CRYO, cryoprecipitate; PCC, prothrombin complex concentrate.
Chapter 62 Rare Coagulation Factor Deficiencies
Table 62-2 Treatment Considerations for Rare Coagulation Factor Deficiencies

FFP, Fresh frozen plasma; CRYO, cryoprecipitate; PCC, prothrombin complex concentrate.
