CHAPTER 258 Radiosurgery of Malignant Tumors
The management of patients diagnosed with malignant brain tumors is wide ranging. Depending on the primary pathology of the tumor, a combination of surgical resection, therapeutic radiation, and chemotherapy may be used for treatment.1–10 With the advent of stereotactic radiosurgery (SRS), neuro-oncologists have added another tool to their armamentarium. This technology was initially developed in the 1950s by Lars Leksell, and has since been modified to treat multiple types of intracranial pathology.11,12 Unlike conventional radiotherapy, SRS allows for very high doses of radiation to be delivered in a single treatment to a small volume of tissue with very little dose to surrounding tissue.13 This feature makes SRS ideal for any lesion that is readily discernible from normal structures on imaging, and thus the majority of experience has been with extra-axial lesions, such as meningiomas,14 acoustic neuromas,15 and pituitary tumors,16 or with intra-axial lesions that have readily discernible structures, such as arteriovenous malformations.17 The most extensive experience with SRS for the treatment of intra-axial tumors involves brain metastases. Metastases differ from primary brain tumors in that they are usually distinct contrast-enhancing lesions that are much less likely to infiltrate surrounding brain tissue.18 SRS has also been used to treat multiple other types of malignant brain tumors. This chapter focuses on the use of SRS for the treatment of these tumors, including brain metastases, malignant astrocytomas, ependymomas, brainstem gliomas, and medulloblastomas.
Metastatic Brain Tumors
Conservative estimates indicate that there are about 170,000 new cases of metastatic brain disease each year in the United States,19 and it has been suggested that there may be as many as 500,000.20 This means that brain metastases are the most common intracranial tumors, outnumbering primary brain tumors by more than 10 : 1.21 Twenty percent to 40% of patients with systemic cancer will develop metastatic brain disease; 30% to 40% of these patients will present with a single brain metastasis, and the remaining 60% to 70% will present with multiple brain metastases.22 It has also been suggested that the incidence of brain metastases is increasing, owing in part to earlier diagnosis and more effective treatment of primary neoplastic disease, often with chemotherapeutic agents that do not effectively cross the blood-brain barrier, and to the rising incidence of lung cancer, the most common primary source of brain metastases.23
The primary goal in the management of metastatic brain disease is palliation of debilitating neurological symptoms to attain an optimal combination of duration and quality of life. The median survival of patients with metastatic brain disease is a dismal 1 month without intervention. Corticosteroid therapy generally produces rapid palliation of symptoms, which are usually primarily attributable to peritumoral vasogenic edema, but extends median survival to only 2 months.24 Whole-brain fractionated external-beam radiotherapy (WBRT), generally administered to a total dose of 30 Gy in ten 3-Gy fractions,25 further extends median survival to 4 to 6 months,26–28 but at least half of all patients treated with this modality ultimately die from progression of their metastatic brain disease.8 Two landmark studies in the early 1990s proved that for patients with a single resectable brain metastasis and good neurological performance status, the addition of microsurgical resection to WBRT significantly extends survival,7,29 and a recent prospective randomized trial demonstrated comparable outcome with the addition of SRS to WBRT.22
The use of SRS for metastatic brain disease was first described in the late 1980s.30,31 Since that time, tens of thousands of patients with metastatic brain tumors have been treated with both linear accelerator (LINAC)-based and Gamma Knife (GK) SRS, and both platforms have proved safe and effective.22,32 SRS is well suited for the treatment of brain metastases. It is minimally invasive and does not require general anesthesia, making it a viable option for patients who cannot tolerate surgery, and it is generally performed as an outpatient procedure. Metastatic tumors are typically spherical, clearly defined lesions on neuroimaging because of contrast uptake, facilitating treatment planning, and are frequently small and histologically well defined, justifying the rapid fall-off of radiation dose at the periphery of the target volume generated by SRS.33 SRS is readily applicable to lesions located nearly anywhere in the brain, including eloquent locations and deep brain locations34,35 that would be difficult or impossible to access surgically with acceptable morbidity, and can be used to treat multiple lesions in a single treatment session.36–43 It has proved to be an effective treatment even for melanoma, sarcoma, and renal cell carcinoma metastases—lesions traditionally considered radioresistant.38,44–51 SRS may also be used in combination with WBRT, surgery, or both, and may be repeated when necessary to treat recurrent disease.38,42,52–54 Additionally, SRS has been shown to be more cost-effective than surgery.55,56
Like all treatment modalities, SRS has disadvantages as well. It is incapable of rapidly resolving symptoms due to tumor mass effect, and surgical resection is generally a more appropriate treatment for patients with symptomatic mass effect. SRS also has limited applicability for large metastatic lesions (≥3 cm in diameter), owing to increasing radiation exposure to normal brain with increasing target volume,54 and for lesions located within close proximity of structures with low radiation tolerance, such as the optic nerves and chiasm.57
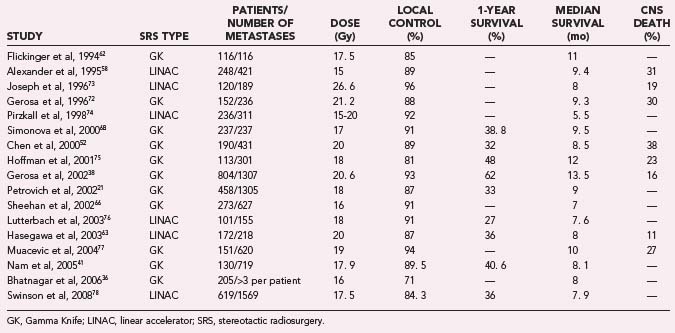
Multiple large radiosurgical series report median survival in a range from about 7 to 13. 5 months,21,36,38,40,43,45,52,58–78 and 1- and 2-year survival probabilities typically range from 31% to 53% and 13% to 30%, respectively.21,41,45,59,65,67,71,79,80 Many of these series were limited to solitary brain metastasis,59,62,68,70 excluded patients with large tumor volumes,38,45,67 or excluded patients with unfavorable characteristics.38,58,59,63 Local control values have also been reported in large series, and rates typically range from 82% to 96%,* and 1- and 2-year local control probabilities typically range from 64% to 91% and 46% to 77%, respectively.† Treatment paradigms vary from center to center, and care is generally individualized based on known prognostic factors. Numerous studies have found younger age (usually <65 years),36,58,85 higher Karnofsky performance score (KPS, usually ≥70)21,42,64,66,68,73,85–87 controlled primary tumor,40,60,86,88,89 absence of systemic metastatic disease,‡ and asynchronous presentation of brain metastases66,90 to be significantly associated with improved survival. Many have also found Radiation Therapy Oncology Group (RTOG) Recursive Partitioning Analysis (RPA) class,91 a composite of age, KPS, primary tumor status, and status of systemic metastatic disease, to be highly correlated with survival (Table 258-1).36,40,41,44,53,65
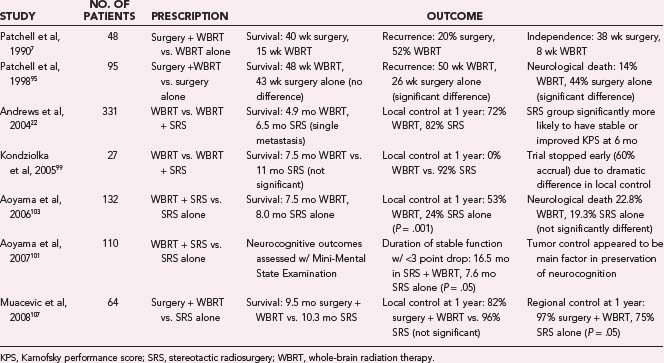
Currently, the optimal role of SRS in the management of metastatic brain disease is not well defined. Results of the first prospective randomized trials of WBRT with or without SRS have only recently been reported,22,92 and although they have shown that SRS offers a significant survival benefit for patients with a single brain metastasis, they have not corroborated a large body of retrospective literature suggesting that SRS similarly improves survival for patients with multiple brain metastases.36,40,43 Although large SRS series consistently report survival durations comparable to those reported in surgical series, no randomized prospective comparison of these two treatment modalities has been conducted.* The available class I data for surgery, WBRT, and SRS in treatment of brain metastases are summarized in Table 258-2 and are discussed in some detail here.
Patients treated with SRS for metastatic brain disease often also receive WBRT. The rationale behind this treatment strategy is the assumption that WBRT will effectively treat micrometastatic foci outside the radiosurgical field, hopefully preventing them from progressing to become detectable, symptomatic brain lesions that may threaten duration and quality of life.97 However, there is evidence that the addition of WBRT to SRS is associated with an increased incidence of mild to moderate treatment-related morbidity, and there is concern that WBRT may also cause serious neurological deficit in some long-term survivors.32,98
Kondziolka and colleagues99 reported the results of 104 surveys completed by patients (or their families) treated with SRS with or without WBRT. They found that patients treated with SRS plus WBRT were significantly more likely to report fatigue, concentration problems, short- and long-term memory problems, and disorders of mood compared with patients treated with SRS alone. DeAngelis and associates100 reported 12 patients cured of brain metastases who developed progressive dementia, ataxia, and urinary incontinence a median of 14 months (range, 5 to 36 months) after treatment with WBRT (total dose of 25 to 39 Gy delivered in 3- to 6-Gy fractions) with or without surgery. They found a 1.9% to 5.1% incidence of WBRT-induced dementia in their study population and estimated that the true incidence is probably higher.
Aoyama and coworkers101 reported the results of the Mini-Mental State Examination (MMSE) in 110 patients who were evaluated as part of a prospective randomized study of patients with one to four brain metastases, who were treated with either SRS alone or with SRS and WBRT. The average baseline MMSE did not differ significantly between treatment groups, but there was a significantly lower score in patients with tumor volume larger than 3 cm3, more edema, and lower KPS (70 to 80 versus 90 to 100). Ninety-two patients underwent follow-up MMSE, and of these, 39 had initial scores of less than 27. Of these patients, 9 of the 17 in the SRS plus WBRT group and 11 of the 22 in the SRS alone group improved by 3 points or more. Deterioration of the MMSE by 3 points or more occurred in 26 patients, and mean time to deterioration was significantly longer for the SRS plus WBRT group (13.6 months) compared with the SRS alone group (6.8 months). The authors attributed this to tumor recurrence in the SRS group and concluded that neurocognitive function most closely correlated with tumor burden and tumor control. They could not, however, comment on long-term neurocognitive effects of the WBRT and stated that they may not be negligible.
Chang and colleagues102 reported a prospective series of 15 patients treated with SRS alone for one to three brain metastases, and followed them with baseline neurocognitive evaluations that were repeated every 20 to 40 days for 1 year. Nine of the patients had impairment at baseline, and of the five who survived for more than 200 days, four had improved or stable learning and memory, three had improved executive function, and three had improved dexterity. The authors concluded that SRS alone was a safe therapy but that randomized studies with more patients were indicated. As primary treatment of metastatic brain disease continues to become more and more effective, the same patients who will benefit most from freedom from new brain metastases will be at the greatest risk for developing long-term treatment-related morbidities. Accordingly, many studies have sought to better define the effect of WBRT on survival, local control, and regional control following SRS.
Andrews and coworkers22 recently reported the phase III results of RTOG protocol 9508, the first prospective, randomized trial of WBRT with or without SRS for the treatment of metastatic brain disease to reach full accrual. Three hundred thirty-one patients with one to three newly diagnosed brain metastases, KPS score of 70 or higher, and no history of WBRT were randomly allocated to either WBRT alone (167 patients) or WBRT followed by an SRS boost (164 patients). Analysis revealed a significant survival advantage for patients with a single brain metastasis treated with WBRT plus SRS rather than WBRT alone (median survival, 6.5 months versus 4.9 months) but failed to reveal a similar advantage for patients with two to three brain metastases. However, all patients in the WBRT plus SRS group were significantly more likely to have a stable or improved KPS 6 months after treatment and were less likely to be dependent on corticosteroids than those treated with WBRT alone. Multivariate analysis revealed RPA class I and non–small cell lung primary tumor to be significantly associated with improved survival. Analysis also revealed significantly better 1-year local control of lesions treated with WBRT plus SRS compared with those treated with WBRT alone (82% versus 71%). The authors concluded that the addition of SRS to WBRT should be standard treatment for patients with a single brain metastasis and considered for patients with two to three brain metastases.
Kondziolka and colleagues92 had previously conducted a similar randomized prospective study, in which patients with two to four brain metastases and KPS of 70 or greater were randomly allocated to either WBRT alone or WBRT plus SRS; however, this study was stopped at an interim evaluation (60% accrual: 14 patients in the WBRT alone group and 13 in the WBRT plus SRS group) because detection of significantly better local control following WBRT plus SRS compared with WBRT alone. Median time to local failure was 6 months after WBRT alone compared with 36 months after WBRT plus SRS, and 1 year after treatment, no patient in the WBRT group had maintained local control, compared with 92% of patients in the WBRT plus SRS group. Although interim analysis revealed a trend toward improved survival after WBRT plus SRS compared with WBRT alone (median survival durations of 11 months and 7.5 months, respectively), no conclusions could be made owing to the small number of patients in the study.
Aoyama and associates103 recently reported results of the first prospective, multi-institutional, randomized trial of SRS with or without WBRT for the treatment of metastatic brain disease. One hundred thirty-two patients with one to four brain metastases 3 cm or less in diameter and KPS of 70 or greater were randomly allocated to either SRS plus WBRT (65 patients) or SRS alone (67 patients). Adjuvant WBRT did not affect survival; patients who received both SRS and WBRT had a median survival of 7.5 months and a 1-year actuarial survival rate of 38.5%, compared with 8.0 months and 28.4% for those treated with SRS alone. However, patients who did not initially receive WBRT underwent significantly more salvage procedures. Multivariate analysis revealed age younger than 65 years, controlled primary tumor, stable systemic disease, and KPS of 90 or greater to be significantly associated with improved survival. Analysis revealed significantly better local, regional, and total brain control after treatment with SRS plus WBRT compared with SRS alone. Patients treated with SRS plus WBRT had 1-year actuarial local control, regional control, and total brain control rates of 88.7%, 58.5%, and 53.2%, respectively, compared with 72.5%, 36.3%, and 23.6%, respectively, for patients treated with SRS alone. Multivariate analysis also revealed stable systemic disease and KPS of 80 or greater to be significantly associated with improved regional control, and a single brain metastasis approached significance (P = . 06). Analysis revealed no difference in posttreatment neurological performance; patients treated with SRS plus WBRT had a 1-year actuarial neurological preservation rate of 72.1%, compared with 70.3% for patients treated with SRS alone. These findings are consistent with the only prospective, randomized trial to date on the role of postoperative WBRT in the management of metastatic brain disease, in which Patchell and colleagues95 randomized 95 patients with a single resectable brain metastasis and KPS of 70 or greater to surgery plus WBRT or surgery alone and determined that patients who received postoperative WBRT did not have a survival advantage but did have significantly better local, regional, and total brain control.
Two similar randomized prospective trials of SRS with or without WBRT for the treatment of metastatic brain disease are underway. North Central Cancer Treatment Group (NCCTG) protocol N0574 will randomize 528 patients with one to three newly diagnosed brain metastases to treatment with SRS plus WBRT or SRS alone. Primary outcome will be duration of survival; secondary outcomes will be time to central nervous system failure, quality of life, duration of functional independence, long-term neurocognitive status, and posttreatment toxicity.104 This trial is a continuation of American College of Surgeons Oncology Group (ACOSOG) protocol Z0300, which was inactive following suspension of enrollment in December 2004.105 European Organisation for Research and Treatment of Cancer (EORTC) protocol 22952-26001 will accumulate 340 patients with one to three newly diagnosed brain metastases. One hundred seventy of these patients will have undergone complete surgical resection and will then be randomized to postoperative WBRT or no postoperative WBRT. The other 170 patients will be randomized to SRS plus WBRT or SRS alone. Primary outcome will be duration of survival with a World Health Organization performance status of 2 or greater; secondary outcomes will be duration of survival, duration of progression-free survival, time to neurological progression, acute toxicity, late toxicity, and quality of life.106
Another study that falls into this category for patients with a single brain metastasis was recently published by Muacevic and associates107 comparing surgery followed by WBRT with SRS alone. This was a prospective randomized trial consisting of 64 patients: 33 in the surgery group and 31 in the SRS group. Median survival for the two groups was not significantly different, with 9.5 months for the surgery group and 10.3 months for the SRS alone patients. Local control at 1 year was 82% for surgery and 96. 8% for SRS, again a nonsignificant difference. Regional control was significantly better in the surgery group, with 97% of patients free from distant metastases at 1 year, compared with 74.2% of the SRS group. These authors concluded that SRS and surgery provided similar local control, but WBRT significantly improved control of distant metastases. Rades and colleagues79 reported on a retrospective analysis of patients with one or two brain metastases treated with SRS alone or with surgical resection plus WBRT. There were 94 patients in the SRS group and 112 in the surgical group. These authors found no significant differences between the groups in terms of overall survival, local control, and regional control. There was no evaluation of cognitive function or quality of life.
The role of repeat SRS compared with WBRT as an upfront therapy remains an unanswered question. Because of the current paucity of class I data, several groups have conducted matched nonrandomized prospective and retrospective studies of SRS with or without WBRT for metastatic brain disease. They have consistently determined that the addition of WBRT to SRS does not affect survival but may affect local and regional control.32,74,98,108 Results of this nature have led some authors to conclude that it may be best to initially employ SRS as a monotherapy and reserve WBRT for possible salvage treatment.61,64,82,99,102,109 Many centers often employ repeat SRS in lieu of WBRT for salvage treatment,52 and newer reports exist of radiosurgical boost to the resection cavity after surgery alone, also in lieu of WBRT.110
Anaplastic Astrocytoma and Glioblastoma
Most of the reported experience with SRS to treat primary intrinsic brain tumors has been for patients with malignant astrocytomas. Primary brain tumors are by nature more infiltrative and are more difficult to target as separate from normal brain, and thus SRS for these lesions would not seem optimal. However, in the case of malignant gliomas, radiation therapy (RT) is the most effective management tool, and overall survival appears to be correlated with the total dose delivered, up to a total dose of 60 Gy.111,112 Also, most tumor recurrences occur within 2 cm of the enhancing margin of the original tumor, leading to the postulate that increasing dose to this area may improve survival and progression-free survival.113,114 This realization led to the initial interest in and use of interstitial brachytherapy for the treatment of malignant gliomas.115 In this technique, radio-isotopes, usually iodine-125, are implanted directly into the tumor cavity after resection to provide maximal radiation dose to the surrounding tissue. Early evidence in the 1990s appeared to show that brachytherapy increased survival in brain tumor patients.115 However, since that time, three separate clinical trials, most recently in 2007, have shown no evidence of a survival benefit, and an increased risk for radiation necrosis, toxicity, and reoperations,116–118 and it became clear that selection bias led to the erroneous conclusion that brachytherapy had a positive treatment effect.
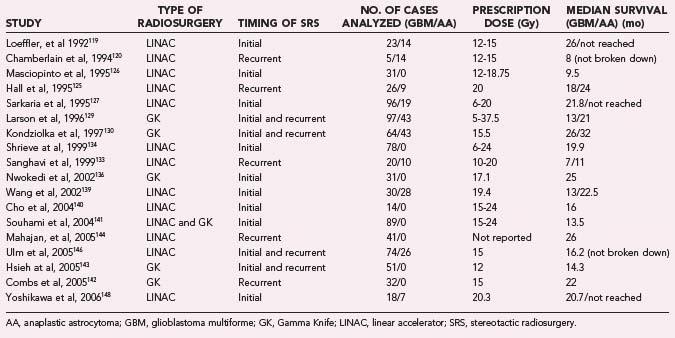
Likewise, in the 1990s, reports appeared to show good efficacy and increased survival benefit with the use of SRS at the time of surgical resection in conjunction with conventional radiotherapy and chemotherapy (Table 258-3).119–148 However, this survival benefit may have been more related to patient selection than actual treatment effect.149 Similar to the experience with brachytherapy, selection bias is likely to have been the driving force behind the apparent efficacy of SRS in these early reviews.
To eliminate the problem of selection bias, the RTOG 93-05 protocol was established for a randomized comparison of SRS followed by conventional RT and carmustine (BCNU) chemotherapy compared with conventional RT and BCNU chemotherapy alone.141 A total of 203 patients with supratentorial glioblastoma (GBM) smaller than 40 mm were randomized to receive SRS plus RT (60 Gy) and BCNU or RT and BCNU alone. SRS dose ranged from 15 to 24 Gy depending on tumor size. At a median follow-up of 61 months, patients had a median survival of 13.5 months in the SRS group compared with 13.6 months in the conventional treatment group. The data were also analyzed to determine whether there was a tail effect, leading to more long-term survivors, but 2- and 3-year survival rates were not significantly different. Recurrence patterns, cognitive decline, quality of life, and type of SRS used (GK or LINAC) were also analyzed and did not show significant differences. The results of this trial proved that upfront SRS is not of benefit for patient survival or quality of life, and prior reports of efficacy were likely due to selection bias.
Subsequently, in 2005, the American Society for Therapeutic Radiology and Oncology (ASTRO) published an evidence-based review of the role of SRS in malignant glioma.145 In this review, the previously mentioned randomized trial was reviewed, along with five prospective119,121,122,124,131 and seven retrospective studies.123,127,129,132,134,136,137 These studies did not show significant improvement in survival, quality of life, or tumor progression. Toxicity did not appear to be increased as a result of the therapy. Overall, there was no evidence that SRS was of benefit as an upfront treatment. A second portion of the review concentrated on SRS as salvage therapy for recurrent or progressive malignant gliomas. There have been no randomized trials to answer this question. There were three prospective120,125,135 and four retrospective128,129,132,138 studies that tried to assess this question. These studies did not find a survival benefit for SRS, and none of them addressed quality-of-life issues. Recurrence patterns in these patients also did not change. As a result, SRS could not be recommended as a salvage treatment either.
Ependymoma
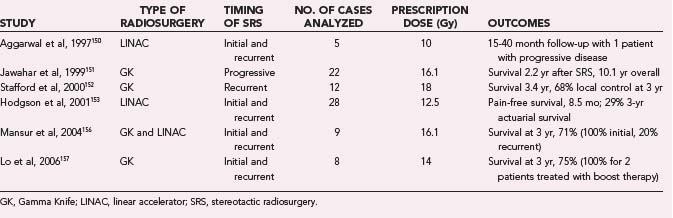
Ependymomas represent a small percentage of intracranial glial tumors. These tumors are more common in children and often occur in the posterior fossa. Currently, therapeutic options for ependymoma include surgical resection followed by external-beam RT (EBRT) for localized disease. Residual tumor remaining after surgery or distant central nervous system spread of disease have been shown to be poor prognostic factors.2 For these lesions, there are no randomized trials on the use of SRS. However, there do exist some small case series of patients treated with SRS boost at the time of initial RT or for recurrent disease (Table 258-4).150–158 Jawahar and coworkers151 reported on 22 patients with progressive anaplastic ependymomas treated with GK SRS. At a median follow-up of 21 months, 16 of the patients had stable or decreased disease volume. The median survival after SRS was 2.2 years, and median overall survival from diagnosis was 10.1 years. There were no complications. Stafford and colleagues152 reported on 12 patients with a total of 17 recurrent ependymomas. Eleven of the patients had undergone surgery followed by EBRT, and 1 had undergone surgery alone. After a median imaging follow-up of 22.5 months, in-field local control was maintained in 14 of 17 patients, and median survival was 3.4 years. One patient developed radiation-induced complications. Hodgson and colleagues153 reported on 90 patients treated with SRS for multiple different tumors, of which 28 were ependymoma. Median follow-up for all patients was 24 months. Progression-free survival for the ependymoma group was 8.5 months, and the 3-year actuarial control rate was 29%. There were 19 complications, but these were not broken down by histology. Mansur and associates156 reported on 9 ependymomas treated with GK and LINAC SRS. Two patients had grade III lesions, and 7 patients had grade II lesions. Eight of the patients received prior RT. Median follow-up was 28 months, and median age at treatment was 35 years. Four patients (44%) developed progressive disease, and 2 patients died from progressive disease. Four patients had SRS as an initial treatment. All these patients were progression free. Of the 5 remaining patients, 4 were treated at recurrence and subsequently had radiographic disease progression. Lo and coworkers157 reported on 8 patients with a total of 13 lesions. Of these, 5 were treated for recurrence, 2 were treated for gross residual disease after surgery and EBRT, and 1 (age 1.3 years) was treated with SRS alone. Of the lesions, the 1-year actuarial in-field control rate was 76.2%, with a 3-year control rate of 61%. Overall survival was 75% at a median of 30 months, with a 50% progression-free survival rate. The 2 patients treated with radiosurgical boost were both alive without disease progression at 39 and 65 months.
Brainstem Gliomas
Brainstem gliomas are a heterogeneous population of tumors. These lesions range from highly aggressive and infiltrative to focal and indolent. Their location by definition makes them difficult to access surgically in a safe manner. Focal lesions are often treated with a combination of open surgical resection (although most often subtotal), biopsy, or imaging follow-up with possible radiotherapy.4 SRS has been investigated as an alternative for primary treatment as well as an adjunct for residual disease after surgery. There have been no controlled trials of this technology for these lesions, but multiple small case series do appear to show that SRS is safe and at least fairly effective for brainstem lesions (Table 258-5).159–163 Unfortunately, some of these series are contaminated by the fact that pathologic tissue is not available for all of the tumors, making generalization of their treatment outcomes impossible.
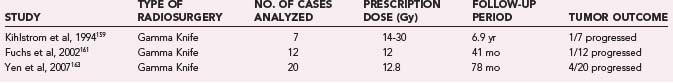
Kihlstrom and coworkers159 reported on 7 patients with tectal tumors treated with GK SRS. These tumors were all shown on biopsy to be low-grade astrocytomas. After a median follow-up of 6.9 years, 4 tumors disappeared, 2 tumors remained the same, and 1 tumor progressed. Clinically, 4 patients improved, and 1 patient worsened. Fuchs and associates161 reported on 21 patients with tumors of the midbrain, pons, and medulla who were treated with SRS. All tumors were histologically verified, with 12 low-grade gliomas and 9 high-grade tumors. After a mean follow-up of 41 months for the low-grade tumors, 6 tumors had decreased in size, 5 were unchanged, and 1 was larger. Clinically, 4 patients improved, 2 patients worsened, and 3 died during the follow-up period. In the high-grade group, 8 patients had follow-up. Three patients with glioblastoma died after a median of 5.5 months, 3 with anaplastic astrocytoma died after a median of 28 months, and the remaining 3 patients with anaplastic pathology were alive after a median of 74 months. Yen and coworkers163 reported on 20 patients with tumors of the midbrain, pons, and medulla. Ten of these patients had histologic diagnosis, 5 had pilocytic tumors, and 5 had low-grade astrocytoma. After a median follow-up of 78 months, 4 tumors disappeared, 12 regressed, and 4 increased in size. Clinically, 8 patients improved, 3 patients worsened, and 1 patient died over the follow-up period. One patient had transient radiation-induced complications.
Medulloblastoma
Medulloblastoma is the most common pediatric brain tumor and is highly malignant. Most of these tumors are located in the posterior fossa, and there is a 2 : 1 male predominance. Newer treatment methods with radiotherapy, high-dose chemotherapy, and stem cell rescue have led to 5-year survival rates as high as 80%.164 Adult medulloblastoma appears to be a somewhat different pathologic entity, although most treatment is in keeping with pediatric tumor protocols. Newer data for adult patients show 5-year survival rates of up to 80%, although 10-year survival rates drop off to 40% to 50%.165,166
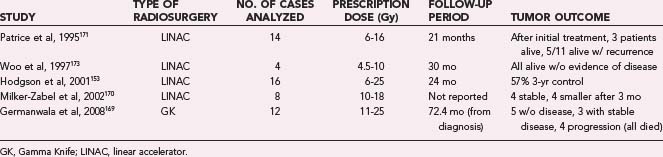
Medulloblastomas do have a higher propensity to metastasize throughout the central nervous system than do other glial tumors. Focal recurrences outside the radiation treatment field are common. SRS was originally suggested as a treatment therapy in 1993167 in the hope of lowering total radiation dose and subsequent radiation-induced complications, and has since been reported as an adjunct for nodular residual, recurrent, or metastatic disease (Table 258-6).168–173 Patrice and associates171 reported on 14 pediatric patients with medulloblastoma treated with SRS. Three patients underwent treatment with boost SRS for residual tumor after initial treatment, and all were alive at a median of 27 months after treatment. Eleven patients had treatment of recurrent disease. Six of these patients died, and overall survival from the time of treatment was a median of 10 months. Progressive disease occurred outside of the treatment volume in all 6 of the patients who died, and there were 2 marginal recurrences. One patient developed acute complications, 2 patients developed transient complications, and 1 patient had a cerebellar hemorrhage in the treatment field. Hodgson and colleagues153 reported on 16 pediatric patients treated with SRS. Median progression-free survival in these patients was 11 months, with a 57% 3-year local control rate. Six patients were treated with upfront SRS, and of these, 3 were alive without progression at a mean of 47 months. More detailed information about all 16 patients was not reported, nor were specific complication rates. Woo and coworkers173 reported on 4 patients (2 pediatric, 2 adult) treated with boost SRS after initial resection and radiotherapy. These patients were alive without progression of disease at 12 to 48 months. There were no complications aside from one episode of nausea and vomiting. One patient developed panhypopituitarism, although this was likely related to his prior conventional radiotherapy. Germanwala and colleagues169 reported on 12 adult patients treated with SRS. Six of the patients had posttreatment residual tumors, and 6 patients had recurrent disease, including distant metastases. Three patients had two SRS treatments, and 3 patients had three treatments. At a mean follow-up of 72.4 months, 5 patients were alive without disease, 3 patients were alive with stable disease, and 4 patients had progressive disease, all of whom died. There were no acute radiation complications or delayed radionecrosis.
Andrews DW, Scott CB, Sperduto PW, et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet. 2004;363:1665.
Aoyama H, Shirato H, Tago M, et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA. 2006;295:2483.
Bhatnagar AK, Flickinger JC, Kondziolka D, et al. Stereotactic radiosurgery for four or more intracranial metastases. Int J Radiat Oncol Biol Phys. 2006;64:898.
Germanwala AV, Mai JC, Tomycz ND, et al. Boost Gamma Knife surgery during multimodality management of adult medulloblastoma. J Neurosurg. 2008;108:204.
Gerosa M, Nicolato A, Foroni R, et al. Gamma Knife radiosurgery for brain metastases: a primary therapeutic option. J Neurosurg. 2002;97:515.
Jawahar A, Kondziolka D, Flickinger JC, et al. Adjuvant stereotactic radiosurgery for anaplastic ependymoma. Stereotact Funct Neurosurg. 1999;73:23.
Kondziolka D, Niranjan A, Flickinger JC, et al. Radiosurgery with or without whole-brain radiotherapy for brain metastases: the patients’ perspective regarding complications. Am J Clin Oncol. 2005;28:173.
Lo SS, Abdulrahman R, Desrosiers PM, et al. The role of Gamma Knife Radiosurgery in the management of unresectable gross disease or gross residual disease after surgery in ependymoma. J Neurooncol. 2006;79:51.
Mansur DB, Drzymala RE, Rich KM, et al. The efficacy of stereotactic radiosurgery in the management of intracranial ependymoma. J Neurooncol. 2004;66:187.
McDermott MW, Sneed PK. Radiosurgery in metastatic brain cancer. Neurosurgery. 2005;57:45.
Muacevic A, Wowra B, Siefert A, et al. Microsurgery plus whole brain irradiation versus Gamma Knife surgery alone for treatment of single metastases to the brain: a randomized controlled multicentre phase III trial. J Neurooncol. 2008;87:299.
Nam TK, Lee JI, Jung YJ, et al. Gamma Knife surgery for brain metastases in patients harboring four or more lesions: survival and prognostic factors. J Neurosurg. 2005;102(suppl):147.
Patchell RA, Tibbs PA, Regine WF, et al. Postoperative radiotherapy in the treatment of single metastases to the brain: a randomized trial. JAMA. 1998;280:1485.
Patchell RA, Tibbs PA, Walsh JW, et al. A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med. 1990;322:494.
Petrovich Z, Yu C, Giannotta SL, et al. Survival and pattern of failure in brain metastasis treated with stereotactic Gamma Knife radiosurgery. J Neurosurg. 2002;97:499.
Souhami L, Seiferheld W, Brachman D, et al. Randomized comparison of stereotactic radiosurgery followed by conventional radiotherapy with carmustine to conventional radiotherapy with carmustine for patients with glioblastoma multiforme: report of Radiation Therapy Oncology Group 93-05 protocol. Int J Radiat Oncol Biol Phys. 2004;60:853.
Swinson BM, Friedman WA. Linear accelerator stereotactic radiosurgery for metastatic brain tumors: 17 years of experience at the University of Florida. Neurosurgery. 2008;62:1021.
Tsao MN, Mehta MP, Whelan TJ, et al. The American Society for Therapeutic Radiology and Oncology (ASTRO) evidence-based review of the role of radiosurgery for malignant glioma. Int J Radiat Oncol Biol Phys. 2005;63:47.
Woo C, Stea B, Lulu B, et al. The use of stereotactic radiosurgical boost in the treatment of medulloblastomas. Int J Radiat Oncol Biol Phys. 1997;37:761.
Yen CP, Sheehan J, Steiner M, et al. Gamma Knife surgery for focal brainstem gliomas. J Neurosurg. 2007;106:8.
1 Grier JT, Batchelor T. Low-grade gliomas in adults. Oncologist. 2006;11:681.
2 Jaing TH, Wang HS, Tsay PK, et al. Multivariate analysis of clinical prognostic factors in children with intracranial ependymomas. J Neurooncol. 2004;68:255.
3 Koeller KK, Rushing EJ. From the archives of the AFIP. Pilocytic astrocytoma: radiologic-pathologic correlation. Radiographics. 2004;24:1693.
4 Mauffrey C. Paediatric brainstem gliomas: prognostic factors and management. J Clin Neurosci. 2006;13:431.
5 Norden AD, Wen PY. Glioma therapy in adults. Neurologist. 2006;12:279.
6 Stupp R, Hegi ME, Gilbert MR, et al. Chemoradiotherapy in malignant glioma: standard of care and future directions. J Clin Oncol. 2007;25:4127.
7 Patchell RA, Tibbs PA, Walsh JW, et al. A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med. 1990;322:494.
8 Shaw EG. Radiotherapeutic management of multiple brain metastases: “3000 in 10” whole brain radiation is no longer a “no brainer.”. Int J Radiat Oncol Biol Phys. 1999;45:253.
9 Mintz A, Perry J, Spithoff K, et al. Management of single brain metastasis: a practice guideline. Curr Oncol. 2007;14:131.
10 Smith ML, Lee JY. Stereotactic radiosurgery in the management of brain metastasis. Neurosurg Focus. 2007;22:E5.
11 Leksell L. The stereotaxic method and radiosurgery of the brain. Acta Chir Scand. 1951;102:316.
12 Leksell L. Sterotaxic radiosurgery in trigeminal neuralgia. Acta Chir Scand. 1971;137:311.
13 Kondziolka D, Lunsford LD, Loeffler JS, et al. Radiosurgery and radiotherapy: observations and clarifications. J Neurosurg. 2004;101:585.
14 Friedman WA, Murad GJ, Bradshaw P, et al. Linear accelerator surgery for meningiomas. J Neurosurg. 2005;103:206.
15 Chopra R, Kondziolka D, Niranjan A, et al. Long-term follow-up of acoustic schwannoma radiosurgery with marginal tumor doses of 12 to 13 Gy. Int J Radiat Oncol Biol Phys. 2007;68:845.
16 Sheehan JP, Niranjan A, Sheehan JM, et al. Stereotactic radiosurgery for pituitary adenomas: an intermediate review of its safety, efficacy, and role in the neurosurgical treatment armamentarium. J Neurosurg. 2005;102:678.
17 Zipfel GJ, Bradshaw P, Bova FJ, et al. Do the morphological characteristics of arteriovenous malformations affect the results of radiosurgery? J Neurosurg. 2004;101:393.
18 McDermott MW, Sneed PK. Radiosurgery in metastatic brain cancer. Neurosurgery. 2005;57:S45.
19 Landis SH, Murray T, Bolden S, et al. Cancer statistics, 1999. CA Cancer J Clin. 1999;49:8.
20 Sheehan J, Niranjan A, Flickinger JC, et al. The expanding role of neurosurgeons in the management of brain metastases. Surg Neurol. 2004;62:32.
21 Petrovich Z, Yu C, Giannotta SL, et al. Survival and pattern of failure in brain metastasis treated with stereotactic Gamma Knife radiosurgery. J Neurosurg. 2002;97:499.
22 Andrews DW, Scott CB, Sperduto PW, et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet. 2004;363:1665.
23 Tosoni A, Ermani M, Brandes AA. The pathogenesis and treatment of brain metastases: a comprehensive review. Crit Rev Oncol Hematol. 2004;52:199.
24 Ruderman NB, Hall TC. Use of glucocorticoids in the palliative treatment of metastatic brain tumors. Cancer. 1965;18:298.
25 Borgelt B, Gelber R, Kramer S, et al. The palliation of brain metastases: final results of the first two studies by the Radiation Therapy Oncology Group. Int J Radiat Oncol Biol Phys. 1980;6:1.
26 Komarnicky LT, Phillips TL, Martz K, et al. A randomized phase III protocol for the evaluation of misonidazole combined with radiation in the treatment of patients with brain metastases (RTOG-7916). Int J Radiat Oncol Biol Phys. 1991;20:53.
27 Phillips TL, Scott CB, Leibel SA, et al. Results of a randomized comparison of radiotherapy and bromodeoxyuridine with radiotherapy alone for brain metastases: report of RTOG trial 89-05. Int J Radiat Oncol Biol Phys. 1995;33:339.
28 Sause WT, Scott C, Krisch R, et al. Phase I/II trial of accelerated fractionation in brain metastases RTOG 85-28. Int J Radiat Oncol Biol Phys. 1993;26:653.
29 Noordijk EM, Vecht CJ, Haaxma-Reiche H, et al. The choice of treatment of single brain metastasis should be based on extracranial tumor activity and age. Int J Radiat Oncol Biol Phys. 1994;29:711.
30 Lindquist C. Gamma Knife surgery for recurrent solitary metastasis of a cerebral hypernephroma: case report. Neurosurgery. 1989;25:802.
31 Sturm V, Kober B, Hover KH, et al. Stereotactic percutaneous single dose irradiation of brain metastases with a linear accelerator. Int J Radiat Oncol Biol Phys. 1987;13:279.
32 Sneed PK, Suh JH, Goetsch SJ, et al. A multi-institutional review of radiosurgery alone vs. radiosurgery with whole brain radiotherapy as the initial management of brain metastases. Int J Radiat Oncol Biol Phys. 2002;53:519.
33 Maor MH, Dubey P, Tucker SL, et al. Stereotactic radiosurgery for brain metastases: results and prognostic factors. Int J Cancer. 2000;90:157.
34 Kased N, Huang K, Nakamura JL, et al. Gamma Knife radiosurgery for brainstem metastases: the UCSF experience. J Neurooncol. 2008;86:195.
35 Yen CP, Sheehan J, Patterson G, et al. Gamma Knife surgery for metastatic brainstem tumors. J Neurosurg. 2006;105:213.
36 Bhatnagar AK, Flickinger JC, Kondziolka D, et al. Stereotactic radiosurgery for four or more intracranial metastases. Int J Radiat Oncol Biol Phys. 2006;64:898.
37 Fuentes S, Delsanti C, Metellus P, et al. Brainstem metastases: management using Gamma Knife radiosurgery. Neurosurgery. 2006;58:37.
38 Gerosa M, Nicolato A, Foroni R, et al. Gamma Knife radiosurgery for brain metastases: a primary therapeutic option. J Neurosurg. 2002;97:515.
39 Huang CF, Kondziolka D, Flickinger JC, et al. Stereotactic radiosurgery for brainstem metastases. J Neurosurg. 1999;91:563.
40 Jawahar A, Shaya M, Campbell P, et al. Role of stereotactic radiosurgery as a primary treatment option in the management of newly diagnosed multiple (3-6) intracranial metastases. Surg Neurol. 2005;64:207.
41 Nam TK, Lee JI, Jung YJ, et al. Gamma Knife surgery for brain metastases in patients harboring four or more lesions: survival and prognostic factors. J Neurosurg. 2005;102(suppl):147.
42 Serizawa T, Iuchi T, Ono J, et al. Gamma Knife treatment for multiple metastatic brain tumors compared with whole-brain radiation therapy. J Neurosurg. 2000;93(suppl 3):32.
43 Seung SK, Sneed PK, McDermott MW, et al. Gamma Knife radiosurgery for malignant melanoma brain metastases. Cancer J Sci Am. 1998;4:103.
44 Brown PD, Brown CA, Pollock BE, et al. Stereotactic radiosurgery for patients with “radioresistant” brain metastases. Neurosurgery. 2002;51:656.
45 Chang EL, Hassenbusch SJ3rd, Shiu AS, et al. The role of tumor size in the radiosurgical management of patients with ambiguous brain metastases. Neurosurgery. 2003;53:272.
46 Lavine SD, Petrovich Z, Cohen-Gadol AA, et al. Gamma Knife radiosurgery for metastatic melanoma: an analysis of survival, outcome, and complications. Neurosurgery. 1999;44:59.
47 Mingione V, Oliveira M, Prasad D, et al. Gamma surgery for melanoma metastases in the brain. J Neurosurg. 2002;96:544.
48 Mori Y, Kondziolka D, Flickinger JC, et al. Stereotactic radiosurgery for cerebral metastatic melanoma: factors affecting local disease control and survival. Int J Radiat Oncol Biol Phys. 1998;42:581.
49 Mori Y, Kondziolka D, Flickinger JC, et al. Stereotactic radiosurgery for brain metastasis from renal cell carcinoma. Cancer. 1998;83:344.
50 Noel G, Valery CA, Boisserie G, et al. LINAC radiosurgery for brain metastasis of renal cell carcinoma. Urol Oncol. 2004;22:25.
51 Gaudy-Marqueste C, Regis JM, Muracciole X, et al. Gamma-Knife radiosurgery in the management of melanoma patients with brain metastases: a series of 106 patients without whole-brain radiotherapy. Int J Radiat Oncol Biol Phys. 2006;65:809.
52 Chen JC, Petrovich Z, Giannotta SL, et al. Radiosurgical salvage therapy for patients presenting with recurrence of metastatic disease to the brain. Neurosurgery. 2000;46:860.
53 Noel G, Proudhom MA, Valery CA, et al. Radiosurgery for re-irradiation of brain metastasis: results in 54 patients. Radiother Oncol. 2001;60:61.
54 Shaw E, Scott C, Souhami L, et al. Single dose radiosurgical treatment of recurrent previously irradiated primary brain tumors and brain metastases: final report of RTOG protocol. Int J Radiat Oncol Biol Phys. 2000;47:90-105.
55 Mehta M, Noyes W, Craig B, et al. A cost-effectiveness and cost-utility analysis of radiosurgery vs. resection for single-brain metastases. Int J Radiat Oncol Biol Phys. 1997;39:445.
56 Rutigliano MJ, Lunsford LD, Kondziolka D, et al. The cost effectiveness of stereotactic radiosurgery versus surgical resection in the treatment of solitary metastatic brain tumors. Neurosurgery. 1995;37:445.
57 Stafford SL, Pollock BE, Leavitt JA, et al. A study on the radiation tolerance of the optic nerves and chiasm after stereotactic radiosurgery. Int J Radiat Oncol Biol Phys. 2003;55:1177.
58 Alexander E3rd, Moriarty TM, Davis RB, et al. Stereotactic radiosurgery for the definitive, noninvasive treatment of brain metastases. J Natl Cancer Inst. 1995;87:34.
59 Auchter RM, Lamond JP, Alexander E, et al. A multiinstitutional outcome and prognostic factor analysis of radiosurgery for resectable single brain metastasis. Int J Radiat Oncol Biol Phys. 1996;35:27.
60 Breneman JC, Warnick RE, Albright REJr, et al. Stereotactic radiosurgery for the treatment of brain metastases. Results of a single institution series. Cancer. 1997;79:551.
61 Deinsberger R, Tidstrand J. LINAC radiosurgery as single treatment in cerebral metastases. J Neurooncol. 2006;76:77.
62 Flickinger JC, Kondziolka D, Lunsford LD, et al. A multi-institutional experience with stereotactic radiosurgery for solitary brain metastasis. Int J Radiat Oncol Biol Phys. 1994;28:797.
63 Hasegawa T, Kondziolka D, Flickinger JC, et al. Brain metastases treated with radiosurgery alone: an alternative to whole brain radiotherapy? Neurosurgery. 2003;52:1318.
64 Kondziolka D, Martin JJ, Flickinger JC, et al. Long-term survivors after Gamma Knife radiosurgery for brain metastases. Cancer. 2005;104:2784.
65 Noel G, Bollet MA, Noel S, et al. Linac stereotactic radiosurgery: an effective and safe treatment for elderly patients with brain metastases. Int J Radiat Oncol Biol Phys. 2005;63:1555.
66 Sheehan JP, Sun MH, Kondziolka D, et al. Radiosurgery for non-small cell lung carcinoma metastatic to the brain: long-term outcomes and prognostic factors influencing patient survival time and local tumor control. J Neurosurg. 2002;97:1276.
67 Shehata MK, Young B, Reid B, et al. Stereotactic radiosurgery of 468 brain metastases < or = 2 cm: implications for SRS dose and whole brain radiation therapy. Int J Radiat Oncol Biol Phys. 2004;59:87.
68 Simonova G, Liscak R, Novotny JJr, et al. Solitary brain metastases treated with the Leksell Gamma Knife: prognostic factors for patients. Radiother Oncol. 2000;57:207.
69 Ulm AJ, Friedman WA, Bova FJ, et al. Linear accelerator radiosurgery in the treatment of brain metastases. Neurosurgery. 2004;55:1076.
70 Valentino V. The results of radiosurgical management of 139 single cerebral metastases. Acta Neurochir Suppl. 1995;63:95.
71 Yu CP, Cheung JY, Chan JF, et al. Prolonged survival in a subgroup of patients with brain metastases treated by Gamma Knife surgery. J Neurosurg. 2005;102(suppl):262.
72 Gerosa M, Nicolato A, Severi F, et al. Gamma Knife radiosurgery for intracranial metastases: from local tumor control to increased survival. Stereotact Funct Neurosurg. 1996;66(suppl 1):184.
73 Joseph J, Adler JR, Cox RS, et al. Linear accelerator-based stereotaxic radiosurgery for brain metastases: the influence of number of lesions on survival. J Clin Oncol. 1996;14:1085.
74 Pirzkall A, Debus J, Lohr F, et al. Radiosurgery alone or in combination with whole-brain radiotherapy for brain metastases. J Clin Oncol. 1998;16:3563.
75 Hoffman R, Sneed PK, McDermott MW, et al. Radiosurgery for brain metastases from primary lung carcinoma. Cancer J. 2001;7:121.
76 Lutterbach J, Cyron D, Henne K, et al. Radiosurgery followed by planned observation in patients with one to three brain metastases. Neurosurgery. 2003;52:1066.
77 Muacevic A, Kreth FW, Tonn JC, et al. Stereotactic radiosurgery for multiple brain metastases from breast carcinoma. Cancer. 2004;100:1705.
78 Swinson BM, Friedman WA. Linear accelerator stereotactic radiosurgery for metastatic brain tumors: 17 years of experience at the University of Florida. Neurosurgery. 2008;62:1021.
79 Rades D, Bohlen G, Pluemer A, et al. Stereotactic radiosurgery alone versus resection plus whole-brain radiotherapy for 1 or 2 brain metastases in recursive partitioning analysis class 1 and 2 patients. Cancer. 2007;109:2515.
80 Rades D, Pluemer A, Veninga T, et al. Whole-brain radiotherapy versus stereotactic radiosurgery for patients in recursive partitioning analysis classes 1 and 2 with 1 to 3 brain metastases. Cancer. 2007;110:2285.
81 Black PM. Solitary brain metastases. Radiation, resection, or radiosurgery? Chest. 1993;103:367S.
82 Kihlstrom L, Karlsson B, Lindquist C. Gamma Knife surgery for cerebral metastases. Implications for survival based on 16 years experience. Stereotact Funct Neurosurg. 1993;61(suppl 1):45.
83 Selek U, Chang EL, Hassenbusch SJ3rd, et al. Stereotactic radiosurgical treatment in 103 patients for 153 cerebral melanoma metastases. Int J Radiat Oncol Biol Phys. 2004;59:1097.
84 Somaza S, Kondziolka D, Lunsford LD, et al. Stereotactic radiosurgery for cerebral metastatic melanoma. J Neurosurg. 1993;79:661.
85 Fernandez-Vicioso E, Suh JH, Kupelian PA, et al. Analysis of prognostic factors for patients with single brain metastasis treated with stereotactic radiosurgery. Radiat Oncol Investig. 1997;5:31.
86 Cho KH, Hall WA, Gerbi BJ, et al. Patient selection criteria for the treatment of brain metastases with stereotactic radiosurgery. J Neurooncol. 1998;40:73.
87 Hoshi S, Jokura H, Nakamura H, et al. Gamma-knife radiosurgery for brain metastasis of renal cell carcinoma: results in 42 patients. Int J Urol. 2002;9:618.
88 Kim YS, Kondziolka D, Flickinger JC, et al. Stereotactic radiosurgery for patients with nonsmall cell lung carcinoma metastatic to the brain. Cancer. 1997;80:2075.
89 Vogelbaum MA, Angelov L, Lee SY, et al. Local control of brain metastases by stereotactic radiosurgery in relation to dose to the tumor margin. J Neurosurg. 2006;104:907.
90 Flannery TW, Suntharalingam M, Kwok Y, et al. Gamma Knife stereotactic radiosurgery for synchronous versus metachronous solitary brain metastases from non-small cell lung cancer. Lung Cancer. 2003;42:327.
91 Gaspar L, Scott C, Rotman M, et al. Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys. 1997;37:745.
92 Kondziolka D, Patel A, Lunsford LD, et al. Stereotactic radiosurgery plus whole brain radiotherapy versus radiotherapy alone for patients with multiple brain metastases. Int J Radiat Oncol Biol Phys. 1999;45:427.
93 Bindal RK, Sawaya R, Leavens ME, et al. Surgical treatment of multiple brain metastases. J Neurosurg. 1993;79:210.
94 Paek SH, Audu PB, Sperling MR, et al. Reevaluation of surgery for the treatment of brain metastases: review of 208 patients with single or multiple brain metastases treated at one institution with modern neurosurgical techniques. Neurosurgery. 2005;56:1021.
95 Patchell RA, Tibbs PA, Regine WF, et al. Postoperative radiotherapy in the treatment of single metastases to the brain: a randomized trial. JAMA. 1998;280:1485.
96 Wronski M, Arbit E, McCormick B. Surgical treatment of 70 patients with brain metastases from breast carcinoma. Cancer. 1997;80:1746.
97 Lunsford LD, Flickinger JC. Radiosurgery plus or minus whole brain radiation therapy for the treatment of brain metastases. An editorial comment. Surg Neurol. 2006;66:461.
98 Sneed PK, Lamborn KR, Forstner JM, et al. Radiosurgery for brain metastases: is whole brain radiotherapy necessary? Int J Radiat Oncol Biol Phys. 1999;43:549.
99 Kondziolka D, Niranjan A, Flickinger JC, et al. Radiosurgery with or without whole-brain radiotherapy for brain metastases: the patients’ perspective regarding complications. Am J Clin Oncol. 2005;28:173.
100 DeAngelis LM, Delattre JY, Posner JB. Radiation-induced dementia in patients cured of brain metastases. Neurology. 1989;39:789.
101 Aoyama H, Tago M, Kato N, et al. Neurocognitive function of patients with brain metastasis who received either whole brain radiotherapy plus stereotactic radiosurgery or radiosurgery alone. Int J Radiat Oncol Biol Phys. 2007;68:1388.
102 Chang EL, Wefel JS, Maor MH, et al. A pilot study of neurocognitive function in patients with one to three new brain metastases initially treated with stereotactic radiosurgery alone. Neurosurgery. 2007;60:277.
103 Aoyama H, Shirato H, Tago M, et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA. 2006;295:2483.
104 http://www.cancer.gov/clinicaltrials/NCCTG-N0574. Accessed 6/01/2008
105 http://www.acosog.org/studies/synopses/z0300_synopsis.pdf. Accessed 6/01/08
106 http://www.eortc.be/protoc/details.asp?protocol=22952. Accessed 6/01/08
107 Muacevic A, Wowra B, Siefert A, et al. Microsurgery plus whole brain irradiation versus Gamma Knife surgery alone for treatment of single metastases to the brain: a randomized controlled multicentre phase III trial. J Neurooncol. 2008;87:299.
108 Li B, Yu J, Suntharalingam M, et al. Comparison of three treatment options for single brain metastasis from lung cancer. Int J Cancer. 2000;90:37.
109 Muacevic A, Kreth FW, Horstmann GA, et al. Surgery and radiotherapy compared with Gamma Knife radiosurgery in the treatment of solitary cerebral metastases of small diameter. J Neurosurg. 1999;91:35.
110 Soltys SG, Adler JR, Lipani JD, et al. Stereotactic radiosurgery of the postoperative resection cavity for brain metastases. Int J Radiat Oncol Biol Phys. 2008;70:187.
111 Bleehen NM, Stenning SP. A Medical Research Council trial of two radiotherapy doses in the treatment of grades 3 and 4 astrocytoma. The Medical Research Council Brain Tumour Working Party. Br J Cancer. 1991;64:769.
112 Walker MD, Strike TA, Sheline GE. An analysis of dose-effect relationship in the radiotherapy of malignant gliomas. Int J Radiat Oncol Biol Phys. 1979;5:1725.
113 Hochberg FH, Pruitt A. Assumptions in the radiotherapy of glioblastoma. Neurology. 1980;30:907.
114 Wallner KE, Galicich JH, Krol G, et al. Patterns of failure following treatment for glioblastoma multiforme and anaplastic astrocytoma. Int J Radiat Oncol Biol Phys. 1989;16:1405.
115 Vitaz TW, Warnke PC, Tabar V, et al. Brachytherapy for brain tumors. J Neurooncol. 2005;73:71.
116 Chen AM, Chang S, Pouliot J, et al. Phase I trial of gross total resection, permanent iodine-125 brachytherapy, and hyperfractionated radiotherapy for newly diagnosed glioblastoma multiforme. Int J Radiat Oncol Biol Phys. 2007;69:825.
117 Laperriere NJ, Leung PM, McKenzie S, et al. Randomized study of brachytherapy in the initial management of patients with malignant astrocytoma. Int J Radiat Oncol Biol Phys. 1998;41:1005.
118 Selker RG, Shapiro WR, Burger P, et al. The Brain Tumor Cooperative Group NIH Trial 87-01: a randomized comparison of surgery, external radiotherapy, and carmustine versus surgery, interstitial radiotherapy boost, external radiation therapy, and carmustine. Neurosurgery. 2002;51:343.
119 Loeffler JS, Alexander E3rd, Shea WM, et al. Radiosurgery as part of the initial management of patients with malignant gliomas. J Clin Oncol. 1992;10:1379.
120 Chamberlain MC, Barba D, Kormanik P, et al. Stereotactic radiosurgery for recurrent gliomas. Cancer. 1994;74:1342.
121 Mehta MP, Masciopinto J, Rozental J, et al. Stereotactic radiosurgery for glioblastoma multiforme: report of a prospective study evaluating prognostic factors and analyzing long-term survival advantage. Int J Radiat Oncol Biol Phys. 1994;30:541.
122 Stea B, Rossman K, Kittelson J, et al. A comparison of survival between radiosurgery and stereotactic implants for malignant astrocytomas. Acta Neurochir Suppl. 1994;62:47.
123 Buatti JM, Friedman WA, Bova FJ, et al. LINAC radiosurgery for high-grade gliomas: the University of Florida experience. Int J Radiat Oncol Biol Phys. 1995;32:205.
124 Gannett D, Stea B, Lulu B, et al. Stereotactic radiosurgery as an adjunct to surgery and external beam radiotherapy in the treatment of patients with malignant gliomas. Int J Radiat Oncol Biol Phys. 1995;33:461.
125 Hall WA, Djalilian HR, Sperduto PW, et al. Stereotactic radiosurgery for recurrent malignant gliomas. J Clin Oncol. 1995;13:1642.
126 Masciopinto JE, Levin AB, Mehta MP, et al. Stereotactic radiosurgery for glioblastoma: a final report of 31 patients. J Neurosurg. 1995;82:530.
127 Sarkaria JN, Mehta MP, Loeffler JS, et al. Radiosurgery in the initial management of malignant gliomas: survival comparison with the RTOG recursive partitioning analysis. Radiation Therapy Oncology Group. Int J Radiat Oncol Biol Phys. 1995;32:931.
128 Shrieve DC, Alexander E3rd, Wen PY, et al. Comparison of stereotactic radiosurgery and brachytherapy in the treatment of recurrent glioblastoma multiforme. Neurosurgery. 1995;36:275.
129 Larson DA, Gutin PH, McDermott M, et al. Gamma Knife for glioma: selection factors and survival. Int J Radiat Oncol Biol Phys. 1996;36:1045.
130 Kondziolka D, Flickinger JC, Bissonette DJ, et al. Survival benefit of stereotactic radiosurgery for patients with malignant glial neoplasms. Neurosurgery. 1997;41:776.
131 Shenouda G, Souhami L, Podgorsak EB, et al. Radiosurgery and accelerated radiotherapy for patients with glioblastoma. Can J Neurol Sci. 1997;24:110.
132 Selch MT DA, Goetsch SJ, Holly FE, et al. Single-fraction radiosurgery for primary and recurrent malignant glioma. J Radiosurg. 1998;1:155.
133 Sanghavi S, Badie B, Skrupky R, et al. Recurrent malignant gliomas treated with radiosurgery. J Radiosurg. 1999;2:119.
134 Shrieve DC, Alexander E3rd, Black PM, et al. Treatment of patients with primary glioblastoma multiforme with standard postoperative radiotherapy and radiosurgical boost: prognostic factors and long-term outcome. J Neurosurg. 1999;90:72.
135 Larson DA, Prados M, Lamborn KR, et al. Phase II study of high central dose Gamma Knife radiosurgery and marimastat in patients with recurrent malignant glioma. Int J Radiat Oncol Biol Phys. 2002;54:1397.
136 Nwokedi EC, DiBiase SJ, Jabbour S, et al. Gamma Knife stereotactic radiosurgery for patients with glioblastoma multiforme. Neurosurgery. 2002;50:41.
137 Prisco FE, Weltman E, de Hanriot RM, et al. Radiosurgical boost for primary high-grade gliomas. J Neurooncol. 2002;57:151.
138 Sarkar A, Pollock BE, Brown PD, et al. Evaluation of Gamma Knife radiosurgery in the treatment of oligodendrogliomas and mixed oligodendroastrocytomas. J Neurosurg. 2002;97:653.
139 Wang YY, Bao XF, Li SY, et al. [Prognostic factors for deep situated malignant gliomas treated with LINAC radiosurgery]. Ai Zheng. 2002;21:1149.
140 Cho KH, Hall WA, Lo SS, et al. Stereotactic radiosurgery versus fractionated stereotactic radiotherapy boost for patients with glioblastoma multiforme. Technol Cancer Res Treat. 2004;3:41.
141 Souhami L, Seiferheld W, Brachman D, et al. Randomized comparison of stereotactic radiosurgery followed by conventional radiotherapy with carmustine to conventional radiotherapy with carmustine for patients with glioblastoma multiforme: report of Radiation Therapy Oncology Group 93-05 protocol. Int J Radiat Oncol Biol Phys. 2004;60:853.
142 Combs SE, Widmer V, Thilmann C, et al. Stereotactic radiosurgery (SRS): treatment option for recurrent glioblastoma multiforme (GBM). Cancer. 2005;104:2168.
143 Hsieh PC, Chandler JP, Bhangoo S, et al. Adjuvant Gamma Knife stereotactic radiosurgery at the time of tumor progression potentially improves survival for patients with glioblastoma multiforme. Neurosurgery. 2005;57:684.
144 Mahajan A, McCutcheon IE, Suki D, et al. Case-control study of stereotactic radiosurgery for recurrent glioblastoma multiforme. J Neurosurg. 2005;103:210.
145 Tsao MN, Mehta MP, Whelan TJ, et al. The American Society for Therapeutic Radiology and Oncology (ASTRO) evidence-based review of the role of radiosurgery for malignant glioma. Int J Radiat Oncol Biol Phys. 2005;63:47.
146 Ulm AJ3rd, Friedman WA, Bradshaw P, et al. Radiosurgery in the treatment of malignant gliomas: the University of Florida experience. Neurosurgery. 2005;57:512.
147 Crowley RW, Pouratian N, Sheehan JP. Gamma Knife surgery for glioblastoma multiforme. Neurosurg Focus. 2006;20:E17.
148 Yoshikawa K, Saito K, Kajiwara K, et al. CyberKnife stereotactic radiotherapy for patients with malignant glioma. Minim Invasive Neurosurg. 2006;49:110.
149 Irish WD, Macdonald DR, Cairncross JG. Measuring bias in uncontrolled brain tumor trials—to randomize or not to randomize? Can J Neurol Sci. 1997;24:307.
150 Aggarwal R, Yeung D, Kumar P, et al. Efficacy and feasibility of stereotactic radiosurgery in the primary management of unfavorable pediatric ependymoma. Radiother Oncol. 1997;43:269.
151 Jawahar A, Kondziolka D, Flickinger JC, et al. Adjuvant stereotactic radiosurgery for anaplastic ependymoma. Stereotact Funct Neurosurg. 1999;73:23.
152 Stafford SL, Pollock BE, Foote RL, et al. Stereotactic radiosurgery for recurrent ependymoma. Cancer. 2000;88:870.
153 Hodgson DC, Goumnerova LC, Loeffler JS, et al. Radiosurgery in the management of pediatric brain tumors. Int J Radiat Oncol Biol Phys. 2001;50:929.
154 Endo H, Kumabe T, Jokura H, et al. Stereotactic radiosurgery for nodular dissemination of anaplastic ependymoma. Acta Neurochir (Wien). 2004;146:291.
155 Kinoshita M, Izumoto S, Kagawa N, et al. Long-term control of recurrent anaplastic ependymoma with extracranial metastasis: importance of multiple surgery and stereotactic radiosurgery procedures—case report. Neurol Med Chir (Tokyo). 2004;44:669.
156 Mansur DB, Drzymala RE, Rich KM, et al. The efficacy of stereotactic radiosurgery in the management of intracranial ependymoma. J Neurooncol. 2004;66:187.
157 Lo SS, Abdulrahman R, Desrosiers PM, et al. The role of Gamma Knife radiosurgery in the management of unresectable gross disease or gross residual disease after surgery in ependymoma. J Neurooncol. 2006;79:51.
158 Lo SS, Chang EL, Sloan AE. Role of stereotactic radiosurgery and fractionated stereotactic radiotherapy in the management of intracranial ependymoma. Expert Rev Neurother. 2006;6:501.
159 Kihlstrom L, Lindquist C, Lindquist M, et al. Stereotactic radiosurgery for tectal low-grade gliomas. Acta Neurochir Suppl. 1994;62:55.
160 Hirato M, Nakamura M, Inoue HK, et al. Gamma Knife radiosurgery for the treatment of brainstem tumors. Stereotact Funct Neurosurg. 1995;64(suppl 1):32.
161 Fuchs I, Kreil W, Sutter B, et al. Gamma Knife radiosurgery of brainstem gliomas. Acta Neurochir Suppl. 2002;84:85.
162 Pollock BE. Gamma Knife surgery for focal brainstem gliomas. J Neurosurg. 2007;106:6.
163 Yen CP, Sheehan J, Steiner M, et al. Gamma Knife surgery for focal brainstem gliomas. J Neurosurg. 2007;106:8.
164 Gajjar A, Chintagumpala M, Ashley D, et al. Risk-adapted craniospinal radiotherapy followed by high-dose chemotherapy and stem-cell rescue in children with newly diagnosed medulloblastoma (St Jude Medulloblastoma-96): long-term results from a prospective, multicentre trial. Lancet Oncol. 2006;7:813.
165 Brandes AA, Ermani M, Amista P, et al. The treatment of adults with medulloblastoma: a prospective study. Int J Radiat Oncol Biol Phys. 2003;57:755.
166 Spreafico F, Massimino M, Gandola L, et al. Survival of adults treated for medulloblastoma using paediatric protocols. Eur J Cancer. 2005;41:1304.
167 Inoue HK, Nakamura M, Ono N, et al. Long-term clinical effects of radiation therapy for primitive gliomas and medulloblastomas: a role for radiosurgery. Stereotact Funct Neurosurg. 1993;61(suppl 1):51.
168 Abe M, Tokumaru S, Tabuchi K, et al. Stereotactic radiation therapy with chemotherapy in the management of recurrent medulloblastomas. Pediatr Neurosurg. 2006;42:81.
169 Germanwala AV, Mai JC, Tomycz ND, et al. Boost Gamma Knife surgery during multimodality management of adult medulloblastoma. J Neurosurg. 2008;108:204.
170 Milker-Zabel S, Zabel A, Thilmann C, et al. Results of three-dimensional stereotactically-guided radiotherapy in recurrent medulloblastoma. J Neurooncol. 2002;60:227.
171 Patrice SJ, Tarbell NJ, Goumnerova LC, et al. Results of radiosurgery in the management of recurrent and residual medulloblastoma. Pediatr Neurosurg. 1995;22:197.
172 Suh JH, Barnett GH. Stereotactic radiosurgery for brain tumors in pediatric patients. Technol Cancer Res Treat. 2003;2:141.
173 Woo C, Stea B, Lulu B, et al. The use of stereotactic radiosurgical boost in the treatment of medulloblastomas. Int J Radiat Oncol Biol Phys. 1997;37:761.