Chapter 46 Radiosurgery of Central Nervous System Tumors
• Randomized clinical trials confirm the survival benefit of stereotactic radiosurgery for patients with single brain metastases and the local control benefit for patients with multiple (two to four) tumors.
• Four matched cohort studies (class II evidence) that compare Gamma Knife radiosurgery to resection for patients with acoustic neuromas less than 2.5 cm in diameter show improved outcomes after radiosurgery with lower morbidity rate. Indications for microsurgery at institutions with radiosurgery expertise include disabling symptomatic brainstem compression, severe headache, hydrocephalus, trigeminal neuralgia, and patient choice.
• Long-term data are available over the past 10 years from numerous institutions on the value of stereotactic radiosurgery for different benign intracranial tumors.
Stereotactic radiosurgery (SRS) has become one of the most important concepts in the management of patients with central nervous system tumors. The neurosurgeon uses SRS to destroy a tumor using precise, image-guided ionizing irradiation in a single procedure.1 Over the past two decades, radiosurgery has supplanted fractionated radiation therapy in the care of many tumors, and has forced an evolution of thinking regarding the roles of resection or observation. At a biological level, radiosurgery can halt cell division, cause vascular occlusion, induce apoptosis or necrosis, and affect blood-brain barrier integrity.2–6
Radiosurgical devices include the cobalt-60 Gamma Knife, modified linear accelerator systems, and charged particle generators.7–10 Although initially designed for functional neurosurgery, the use of brain tumor radiosurgery took off in the era of higher-resolution parenchymal brain imaging with magnetic resonance imaging (MRI). Now robotic devices such as the Perfexion or model 4C gamma units or the Cyberknife facilitate dose planning and delivery.11,12 For use in the brain, accuracy and reliability are crucial. At present no pharmacological agents are used to modify the target response to radiosurgery.13,14 A listing of indications is shown in Table 46.1.
TABLE 46.1 Applications for Gamma Knife Radiosurgery for Brain Tumors∗
| Diagnosis | Number of Procedures |
|---|---|
| Vestibular schwannoma | 1439 |
| Trigeminal schwannoma | 45 |
| Other schwannomas | 65 |
| Meningioma | 1377 |
| Pituitary tumor | 300 |
| Craniopharyngioma | 76 |
| Hemangioblastoma | 50 |
| Hemangiopericytoma | 41 |
| Glomus tumor | 24 |
| Pineocytoma | 16 |
| Malignant pineal tumor | 13 |
| Chordoma | 31 |
| Chondrosarcoma | 25 |
| Choroid plexus papilloma | 12 |
| Hemangioma | 7 |
| Glioblastoma multiforme | 349 |
| Anaplastic astrocytoma | 134 |
| Fibrillary astrocytoma | 43 |
| Mixed glioma | 78 |
| Pilocytic astrocytoma | 89 |
| Ependymoma | 74 |
| Medulloblastoma | 24 |
| CNS lymphoma | 12 |
| Hypothalamic hamartoma | 6 |
| Brain metastasis | 3264 |
| Invasive skull base tumor | 32 |
| Other tumors | 66 |
| Total | 7692 |
CNS, central nervous system.
∗ Data from clinical series at the University of Pittsburgh: total procedures = 10,158; total brain tumors = 7692.
Not all radiosurgery systems are the same and they differ in both hardware and software. One should seek a system that allows the surgeon to efficiently create highly conformal and selective volumetric dose plans for irregular lesion volumes, because tumors are rarely “spherical.”15 The steep falloff of radiation into the surrounding structures (selectivity) helps to achieve safety. The location of many tumors either within or adjacent to critical brain or nerve makes the requirement for conformal and selective radiosurgery paramount.
Radiosurgery or radiotherapy?
Fractionated stereotactic irradiation has been used by some centers to treat benign tumors,16,17 and of course, large-field conventional or whole-brain radiotherapy has been used for decades. Any advantage for the use of fractionated radiotherapy becomes important when large volumes of sensitive surrounding normal tissue need to be included in the treatment volume, such as with the standard 2-cm margin around a malignant glioma. If the volume of normal tissue irradiated inside or outside the target volume is small, then fractionated radiation may be of no additional value. Certain tumors appear to be more resistant to traditional radiotherapy and include meningiomas, schwannomas, and some metastatic tumors such as melanoma, sarcoma, and renal cell carcinoma. The radiobiological response can be addressed with the α/β ratio. A lower number refers to later responding tissue.18–20
Vestibular Schwannomas
The goals of vestibular schwannoma radiosurgery are to prevent tumor growth, preserve cochlear and other cranial nerve function, maintain general function and quality of life, and avoid the risks associated with open surgical resection. Available long-term data have established radiosurgery as an important alternative to resection. Radiosurgery was first offered to patients who were elderly or medically infirm, but with experience, was found ideal for patients of all ages.21–24 We have found consistent results across age groups.21,25
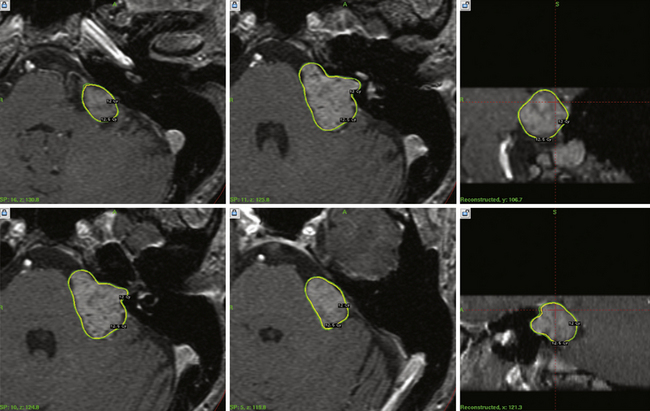
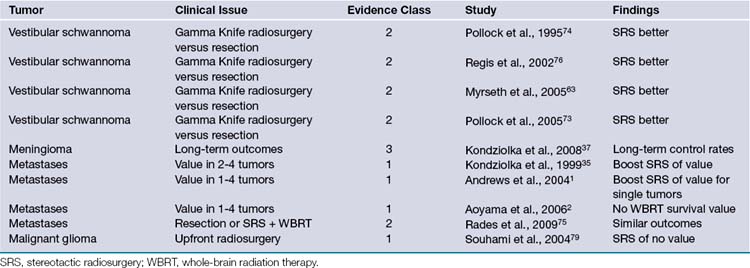
To date we have managed 1397 patients with vestibular schwannomas using Gamma Knife radiosurgery (Fig. 46.1). The mean patient age in our series was 57 years (range, 12 to 95). Eight percent had neurofibromatosis (93 patients). Symptoms before radiosurgery included hearing loss (92%), balance symptoms or ataxia (51%), tinnitus (43%), or other neurological deficit (19.5%). Thirty-four percent of our patients had useful hearing, Gardner-Robertson grade I (speech discrimination score ≥70%; pure tone average ≤30 dB) or grade II (speech discrimination score ≥50%; pure tone average ≤50 dB). Since 1992, the average dose prescribed to the tumor margin was 13 Gy. The 50% isodose line was used in 90% of patients.
Our long-term study documented a 98% clinical tumor control rate (no requirement for surgical intervention) at 5 to 10 years.21,23 Since the institution of MRI-guided dose planning, there has been a significant reduction in morbidity.26,27 Currently, the risk for any grade delayed facial nerve dysfunction is below 1%.26,27 Patients with useful hearing before radiosurgery continue to report an approximate 75% overall rate for maintenance of useful hearing, depending on tumor size, with even better results for intracanalicular tumors.27–29 In comparison studies, radiosurgery has been shown to be a cost-effective alternative to microsurgery for these patients.
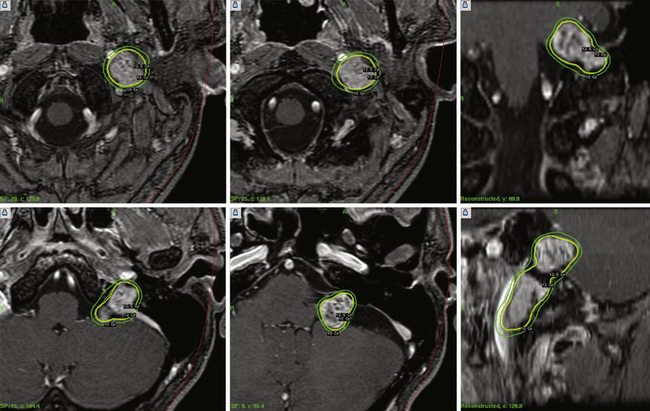
For smaller tumors, it is likely that more patients now receive radiosurgery as primary care. No randomized clinical trials have been conducted, but there are now four matched cohort studies (class II evidence). These studies evaluated patients with similar sized tumors, and evaluated clinical, imaging, and quality of life outcomes. All showed better results after radiosurgery for most clinical measures, similar results for the symptoms of tinnitus and imbalance, and similar freedom from tumor progression rates.30–33 It is important to tell patients that there can sometimes be a transient expansion of the tumor capsule after radiosurgery and that it usually can be observed without further treatment.34,35 The value of radiosurgery in neurofibromatosis type 2 has been studied.36 Based on these data, we believe that there are few remaining indications for surgical resection in a patient with a small to moderate size tumor. These indications include disabling symptomatic brainstem compression, severe headache, hydrocephalus, trigeminal neuralgia, and patient choice. Radiosurgery has also been performed and evaluated for patients with other cranial nerve schwannomas (Fig. 46.2).
Meningiomas
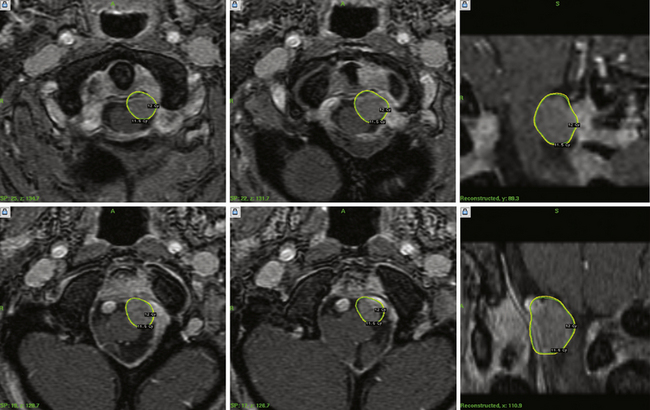
Radiosurgery has transformed the management of intracranial meningiomas, particularly for tumors of the skull base.37 As for most indications, radiosurgery was first considered for residual or recurrent tumors after prior resection.38 The steep radiation falloff can be directly conformed to the well-defined tumor margin. The role of aggressive skull base surgery has waned as reports showing excellent clinical outcomes for small basal tumors have been published.39,40 Larger tumors with mass effect benefit from subtotal resection followed by radiosurgery if complete resection is not feasible. However, because of their more aggressive nature, atypical or anaplastic/malignant meningiomas (WHO [World Health Organization] grade II or III) are best managed with complete resection followed by fractionated radiotherapy because of their tendency to extend beyond the borders seen on imaging.41 Radiosurgery also is of value in the setting of incomplete resection.
Our 22-year experience includes 1302 intracranial meningiomas. Recently, we published data from 972 patients with 1045 meningiomas (Fig. 46.3).42 Half of the patients had undergone a prior resection and the average age was 57 years. Tumor locations included middle fossa (351 patients), posterior fossa (307), convexity (126), anterior fossa (88), parasagittal region (113), or other sites (115). Follow-up over the past 5, 7, 10, and 12 years was obtained in 327, 190, 90, and 41 patients, respectively.
The control rate for patients who had radiosurgery for known WHO grade I (benign) meningiomas (after prior resection) was 93%. Primary radiosurgery patients (no prior histological confirmation; n = 482), had a tumor control rate of 97%. Adjuvant radiosurgery for patients with WHO grades II and III tumors had tumor control rates of 50% and 17%, respectively. Delayed resection after radiosurgery was performed in 51 patients (5%) at a mean period of 3 years. Additional radiosurgery was performed in 41 patients, usually for new tumors. At 10 years or more, adjuvant grade I tumors were controlled in 91% (n = 53), and primary tumors in 95% (n = 22). There was no case of a subsequent radiation-induced tumor. The morbidity rate was 7.7%. Most centers restrict the dose received by the optic nerve or chiasm to 8 to 9 Gy or less, which should keep the risk for delayed radiation-related optic neuropathy very low.
We believe that SRS has changed meningioma management significantly. Rather than performing a subtotal resection and “following the patient,” we now advocate postoperative radiosurgery to reduce the risk of delayed progression.43–45 We believe this strategy is particularly valuable for younger patients (<75 years). Several longitudinal studies have shown that untreated meningiomas under observation continue to grow over time. We also believe that radiosurgery is the preferred option for a young patient with a critically located small meningioma. No randomized trials comparing radiosurgery to other options have been performed, and all reports represent level 3 evidence.
Pituitary Adenomas
The goal of pituitary adenoma radiosurgery is to arrest the tumor, maintain pituitary and neurological function, and normalize hormonal secretion in case of functional adenomas.46 We have managed 290 patients with pituitary adenomas. For functional tumor, acromegaly patients typically respond best,47 with normalization of growth hormone hypersecretion in over 70% of patients, and in approximately half of those with Cushing disease. Follow-up varied from 6 to 102 months (mean 29.5 months); 14 patients had follow-up in excess of 40 months. The mean radiation dose to the tumor margin was 16 Gy.
We found that all patients with microadenomas and 97% of patients with macroadenomas had tumor control after radiosurgery. Gamma Knife radiosurgery was essentially equally effective for control of adenomas with cavernous sinus invasion and suprasellar extension. Endocrine deficits are less common after radiosurgery, although some recent reports with detailed testing show some hormone deficiencies over time. One patient demonstrated tumor growth that resulted in a decline in visual function. A single patient had visual deterioration despite a decrease in tumor size. Advances in dose planning and dose selection facilitated tumor management even when the adenoma was adjacent to the optic apparatus or invaded the cavernous sinus. As for meningiomas, there are no class I or II studies. All published reports represent clinical series with class III evidence.
Malignant Cranial Base Tumors
Malignant tumors of the skull base cause formidable challenges. Such tumors include chordomas, chondrosarcomas, nasopharyngeal carcinomas, or other adenocarcinomas or squamous cell carcinomas from regional structures. To date we have used radiosurgery in 30 patients with chordoma and 22 with chondrosarcoma.48 In our analysis, the actuarial local tumor control for chondrosarcomas at 5 years was 84.4% ± 10.2%. The overall survival was 84% at a mean of 6.2 years. The actuarial tumor control and survival for chordomas at 10 years was 63.2% ± 10.9%. New techniques in cranial base microsurgery or endoscopic surgery coupled with radiosurgery have improved outcomes.
Brain Metastases
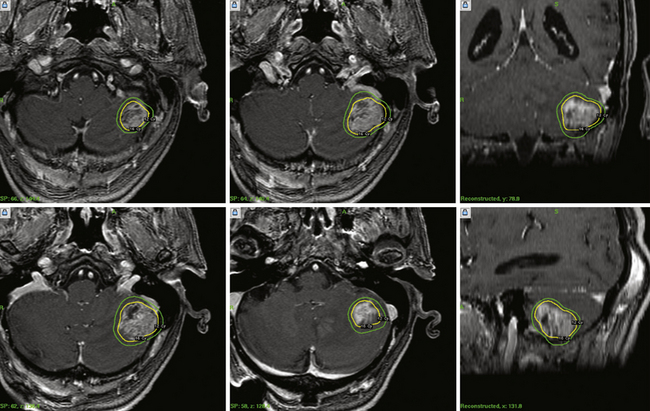
Radiosurgery as the sole approach or as a boost before or after fractionated whole-brain radiotherapy (WBRT) has become a widely practiced treatment for brain metastases (Fig. 46.4). The value of radiosurgery for brain metastases is well reported, including its evaluation in class I studies.1,49–63 When performed as sole management without WBRT, the goal is tumor control without the potential longer term neurotoxic or cognitive side effects of WBRT.64 The rationale for radiosurgery used in addition to WBRT is to achieve improved tumor control. For decades, most patients had been managed with WBRT with the goal of simple palliation.52 Hair loss, fatigue, and delayed cognitive deficits (in longer-term survivors) are noted after WBRT and can adversely impact the patient’s quality of life.65 To achieve better clinical results, neurosurgeons and radiation oncologists have partnered in the evaluation of radiosurgery alone for solitary tumors, or radiosurgery plus WBRT for multiple tumors. In addition, radiosurgery is an attractive concept to medical oncologists because it is effective, is performed as an outpatient, provides excellent local control, palliates symptoms, avoids craniotomy, and can be used in any brain location. To date, the results after radiosurgery plus WBRT appear to be as good as those after surgical resection plus WBRT for “resectable” tumors.53 Radiosurgery has shown to be more cost effective than any other option. As for other tumor types, patients with large tumors causing mass effect and disabling symptoms should be considered for resection. However, over 50% of brain metastases are now identified in asymptomatic patients, and most are of smaller volume.
We advocate radiosurgery for patients with multiple brain metastases, whether with active or inactive systemic disease, who remain in good neurological condition. Initially, this paradigm was considered controversial because conventional teaching was that the recognition of more than one metastasis heralded widespread subclinical micrometastases. This concept is no longer valid in the era of double-dose, high-definition MRIs. We conducted a randomized trial that compared radiosurgery plus WBRT to WBRT alone for patients with two to four brain metastases. Improved tumor control was observed when patients received both SRS and WBRT.65,66 A similar and larger study was conducted by the Radiation Therapy Oncology Group (RTOG).49 This study and others found that the presence of multiple metastases did not automatically herald the onset of more and more tumors. Thus, patients should continue to be managed aggressively if effective therapies remain for their extracranial cancer.56 In a recent study, we found that the survival expectation for patients with five to eight tumors was not significantly different from that for patients with two to four tumors, as long as the total tumor burden was less than 7 mL.50 Recently, guidelines for the management of brain metastases, including the role of radiosurgery, were published in the Journal of Neuro-Oncology.67 This provides an excellent current summary of the literature regarding radiosurgery, radiotherapy, and resection.
Glial Neoplasms
For decades, neurosurgeons have tried to maximize the benefits of radiation on glial tumors of the brain.18,68 Stereotactic brachytherapy with temporary or permanent radioactive isotopes, intracavitary irradiation with colloidal isotopes, and balloon placement of radioactive isotopes have been used. Radiosurgery is a minimally invasive method to boost the radiation effect of fractionated radiotherapy for patients with such tumors.44,69,70 Radiosurgery has been used mainly for carefully selected patients with residual or deep-seated malignant glial tumors less than 3.5 cm in diameter as part of a multimodality approach. To date, we have performed radiosurgery on 327 patients with glioblastomas and 96 patients with anaplastic astrocytomas. In comparison to patients who received radiotherapy alone, we found that glioblastoma patients had significant prolongation of survival.66 However, no prospective randomized trial has been completed to study the benefit of boost radiosurgery after radiation therapy for glioblastoma multiforme. There is level one evidence that evaluated the role of upfront radiosurgery, a concept that was uncommonly practiced. This randomized trial showed no benefit from upfront radiosurgery plus radiotherapy and carmustine, compared to radiotherapy and carmustine alone.71 We think that radiosurgery is a useful concept for residual or recurrent smaller-volume malignant gliomas after radiation therapy and chemotherapy. It may also be of value at the time of a small volume late recurrence.
The role of radiosurgery for low-grade astrocytomas has been evaluated.72,73 We have managed 86 patients with pilocytic astrocytomas and 42 with fibrillary astrocytomas. In addition, we cared for 69 patients with mixed gliomas and 19 patients with oligodendrogliomas.74 Neurocytomas appear to regress impressively after radiosurgery, but the clinical experience is small.75
Perhaps the greatest value of glioma radiosurgery so far has been in the management of deep-seated small pilocytic astrocytomas.76,77 We used SRS in 86 patients whose mean age was 17 years (range, 4-52). Many of these tumors were in the brainstem. Radiosurgery is also used as an alternative to fractionated radiation therapy in the management of patients with residual or recurrent ependymomas, or as additional treatment after tumor recurrence following radiation therapy. To date we have treated 71 patients with ependymomas78 and 24 patients with medulloblastomas.79 For all of these tumors, the available literature provides only class III evidence. Table 46.2 summarizes different tumor clinical trials in radiosurgery.
Chan A., Black P., Ojemann R., et al. Stereotactic radiotherapy for vestibular schwannomas: favorable outcome with minimal toxicity. Neurosurgery. 2005;57:60-70.
Flickinger J., Kondziolka D. Radiosurgery instead of resection for solitary brain metastases: redefining the gold standard. Int J Radiat Oncol Biol Phys. 1996;35:185-186.
Kondziolka D., Mathieu D., Lunsford L.D., et al. Radiosurgery as definitive management of meningiomas. Neurosurgery. 2008;62:53-60.
Kondziolka D., Patel A., Lunsford L.D., et al. Stereotactic radiosurgery plus whole brain radiotherapy versus radiotherapy alone for patients with multiple brain metastases. Int J Radiat Oncol Biol Phys. 1999;45:427-434.
Linskey M., Andrews D., Asher A., et al. The role of stereotactic radiosurgery in the management of newly diagnosed brain metastases: a systematic review and evidence-based clinical practice guideline. J Neurooncol. 2010;96:45-68.
Please go to expertconsult.com to view the complete list of references.
1. Atteberry D., Szeifert G., Kondziolka D., et al. Radiosurgical pathology observations on cerebral metastases after gamma knife radiosurgery. Radiosurgery. 2006;6:173-185.
2. Kondziolka D., Lunsford L., Claassen D., et al. Radiobiology of radiosurgery. Part 2: the rat C6 glioma model. Neurosurgery. 1992;31:280-288.
3. Kondziolka D., Lunsford L., Flickinger J. The radiobiology of radiosurgery. Neurosurg Clin North Am. 1999;10:157-166.
4. Kondziolka D., Lunsford L., Witt T., Flickinger J. The future of radiosurgery: radiobiology, technology, and applications. Surg Neurol. 2000;54:406-414.
5. Niranjan A., Gobbel G., Kondziolka D., et al. Experimental radiobiological investigations into radiosurgery: present understanding and future directions. Neurosurgery. 2004;55:495-504.
6. Witham T., Okada H., Fellows W., et al. The characterization of tumor apoptosis after experimental radiosurgery. Stereotactic Funct Neurosurg. 2005;83:17-24.
7. Chang S., Adler J. Treatment of cranial base meningiomas with linear accelerator radiosurgery. Neurosurgery. 1997;41:1019-1027.
8. Chang S., Gibbs I., Sakamoto G., et al. Staged stereotactic irradiation for acoustic neuroma. Neurosurgery. 2005;56:1254-1263.
9. Friedman W., Murad G., Bradshaw P., Amdur R., et al. Linear accelerator radiosurgery for meningiomas. J Neurosurg. 2005;103:206-209.
10. Hakim R., Alexander E., Loeffler J. Results of linear accelerator-based radiosurgery for intracranial meningiomas. Neurosurgery. 1998;42:446-454.
11. Niranjan A., Novotny J., Bhatnagar J., et al. Efficiency and dose-planning comparisons between the Perfexion and 4C Leksell Gamma Knife units. Stereotact Funct Neurosurg. 2009;87:191-198.
12. Novotny J., Bhatnagar J., Niranjan A., et al. Dosimetric comparison of the Leksell Gamma Knife Perfexion and 4C. J Neurosurg. 2008;109:8-14.
13. Kondziolka D., Mori Y., Martinez A., et al. Beneficial effects of the radioprotectant 21-aminosteroid U-74389G in a radiosurgery rat malignant glioma model. Int J Radiat Oncol Biol Phys. 1999;44:179-184.
14. Kondziolka D., Somaza S., Martinez A., et al. Radioprotective effects of the 21-aminosteroid U74389G for stereotactic radiosurgery. Neurosurgery. 1997;41:203-208.
15. Lunsford L., Kondziolka D., Niranjan A., et al. Concepts of conformality and selectivity in acoustic tumor radiosurgery. Radiosurgery. 2006;6:98-107.
16. Chan A., Black P., Ojemann R., et al. Stereotactic radiotherapy for vestibular schwannomas: favorable outcome with minimal toxicity. Neurosurgery. 2005;57:60-70.
17. Combs S., Volk S., Schulz-Ertner D., et al. Management of acoustic neuromas with fractionated stereotactic radiotherapy (FSRT): long-term results in 106 patients treated in a single institution. Int J Radiat Oncol Biol Phys. 2005;63:75-81.
18. Bentzen S.M., Overgaard J., Thames H.D., et al. Clinical radiobiology of malignant melanoma. Radiother Oncol. 1989;16:169-182.
19. Dasu A. Is the alpha/beta value for prostate tumours low enough to be safely used in clinical trials? Clin Oncol (R Coll Radiol). 2007;19:289-301.
20. Garcia-Barros M., Paris F., Cordon-Cardo C., et al. Tumor response to radiotherapy regulated by endothelial cell apoptosis. Science. 2003;300:1155-1159.
21. Flickinger J., Kondziolka D., Niranjan A., Lunsford L. Results of acoustic neuroma radiosurgery: an analysis of 5 years’ experience using current methods. J Neurosurg. 2001;94:1-6.
22. Flickinger J., Kondziolka D., Pollock B., Lunsford L. Evolution of technique for vestibular schwannoma radiosurgery and effect on outcome. Int J Radiat Oncol Biol Phys. 1996;36:275-280.
23. Kondziolka D., Lunsford L., McLaughlin M., Flickinger J. Long-term outcomes after radiosurgery for acoustic neuromas. N Engl J Med. 1998;339:1426-1433.
24. Subach B., Kondziolka D., Lunsford L., et al. Stereotactic radiosurgery in the management of acoustic neuromas associated with neurofibromatosis—type II. J Neurosurg. 1999;90:815-822.
25. Flickinger J., Kondziolka D., Niranjan A., et al. Acoustic neuroma radiosurgery with marginal tumor doses of 12 to 13 Gy. Int J Radiat Oncol Biol Phys. 2004;60:225-230.
26. Kano H., Kondziolka D., Flickinger J., Lunsford L. Predictors of hearing preservation after stereotactic radiosurgery for acoustic neuromas. J Neurosurg. 2009;111:863-873.
27. Niranjan A., Mathieu D., Flickinger J., et al. Hearing preservation after intracanalicular vestibular schwannoma radiosurgery. Neurosurgery. 2008;63:1054-1063.
28. Lunsford L., Niranjan A., Flickinger J., et al. Radiosurgery of vestibular schwannomas: summary of experience in 829 cases. J Neurosurg. 2005;102(Suppl):195-199.
29. Niranjan A., Lunsford L., Flickinger J., et al. Dose reduction improves hearing preservation rates after intracanalicular acoustic tumor radiosurgery. Neurosurgery. 1999;45:753-765.
30. Myrseth E., Moller P., Pedersen P., et al. Vestibular schwannomas: clinical results and quality of life after microsurgery or gamma knife radiosurgery. Neurosurgery. 2005;56:927-935.
31. Pollock B., Driscoll C., Foote R., et al. Patient outcomes after vestibular schwannoma management: a prospective comparison of microsurgical resection and stereotactic radiosurgery. Neurosurgery. 2006;59:77-85.
32. Pollock B., Lunsford L., Kondziolka D., et al. Outcome analysis of acoustic neuroma management: a comparison of microsurgery and stereotactic radiosurgery. Neurosurgery. 1995;36:215-229.
33. Regis J., Pellet W., Delsant C. Functional outcome after gamma knife surgery or microsurgery for vestibular schwannomas. J Neurosurg. 2002;97:1091-1100.
34. Hasegawa T., Kida Y., Yoshimoto M., et al. Evaluation of tumor expansion after stereotactic radiosurgery in patients harboring vestibular schwannomas. Neurosurgery. 2006;58:1119-1128.
35. Pollock B. Management of vestibular schwannomas that enlarge after stereotactic radiosurgery: treatment recommendations based on a 15 year experience. Neurosurgery. 2006;58:241-248.
36. Mathieu D., Kondziolka D., Flickinger J.C., et al. Stereotactic radiosurgery for vestibular schwannomas in patients with neurofibromatosis type 2: an analysis of tumor control, complications and hearing preservation rates. Neurosurgery. 2007;60:460-470.
37. Flickinger J., Kondziolka D., Maitz A., Lunsford L. Gamma knife radiosurgery of imaging-defined intracranial meningioma. Int J Radiat Oncol Biol Phys. 2003;56:801-806.
38. Kondziolka D., Lunsford L., Coffey R., Flickinger J. Gamma knife radiosurgery of meningiomas. Stereotact Funct Neurosurg. 1991;57:11-21.
39. Kondziolka D., Levy E., Niranjan A., et al. Long-term outcomes after meningioma radiosurgery: physicians and patients perspective. J Neurosurg. 1999;91:44-50.
40. Kondziolka D., Nathoo N., Flickinger J., et al. Long-term results after radiosurgery for benign intracranial tumors. Neurosurgery. 2003;53:815-822.
41. Condra K., Buatt I.J., Mendenhall W., et al. Benign meningiomas: primary treatment selection affects survival. Int J Radiat Oncol Biol Phys. 1997;39:427-436.
42. Kondziolka D., Mathieu D., Lunsford L.D., et al. Radiosurgery as definitive management of meningiomas. Neurosurgery. 2008;62:53-60.
43. Lee J., Niranjan A., McInerney J., et al. Stereotactic radiosurgery provides long term tumor control of cavernous sinus meningiomas. J Neurosurg. 2002;97(1):65-72.
44. Mahajan A., McCutcheon I., Suki D., et al. Case-control study of stereotactic radiosurgery for recurrent glioblastoma multiforme. J Neurosurg. 2005;103:210-217.
45. Muthukumar N., Kondziolka D., Lunsford L., Flickinger J. Stereotactic radiosurgery for anterior foramen magnum meningiomas. Surg Neurol. 1999;51:268-273.
46. Sheehan J., Niranjan A., Sheehan J., et al. Stereotactic radiosurgery for pituitary adenomas: an intermediate review of its safety, efficacy, and role in the neurosurgical treatment armamentarium. J Neurosurg. 2005;102:678-691.
47. Niranjan A., Szeifert G., Lunsford L., et al. Gamma knife radiosurgery for the treatment of growth hormone secreting pituitary adenomas. Radiosurgery. 2001;4:93-101.
48. Martin J., Niranjan A., Kondziolka D., et al. Radiosurgery for chordomas and chondrosarcomas of the skull base. J Neurosurg. 2007;107:758-764.
49. Andrews D., Scott C., Sperduto P. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomized trial. Lancet. 2004;363:1665-1672.
50. Bhatnagar A., Flickinger J., Kondziolka D., Lunsford L. Stereotactic radiosurgery for four or more intracranial metastases. Int J Radiat Oncol Biol Phys. 2006;64:898-903.
51. Firlik K., Kondziolka D., Flickinger J., Lunsford L. Stereotactic radiosurgery for brain metastases from breast cancer. Ann Surg Oncol. 2000;7:333-338.
52. Flickinger J., Kondziolka D. Radiosurgery instead of resection for solitary brain metastases: redefining the gold standard. Int J Radiat Oncol Biol Phys. 1996;35:185-186.
53. Hasegawa T., Kondziolka D., Flickinger J., et al. Brain metastases treated with radiosurgery alone: an alternative to whole brain radiotherapy? Neurosurgery. 2003;52:1318-1326.
54. Hasegawa T., Kondziolka D., Flickinger J., Lunsford L. Stereotactic radiosurgery for brain metastases from gastrointestinal tract cancer. Surg Neurol. 2003;60:506-514.
55. Kondziolka D., Patel A., Lunsford L.D., et al. Stereotactic radiosurgery plus whole brain radiotherapy versus radiotherapy alone for patients with multiple brain metastases. Int J Radiat Oncol Biol Phys. 1999;45:427-434.
56. Kondziolka D., Martin J., Flickinger J., et al. Long-term survivors after gamma knife radiosurgery for brain metastases. Cancer. 2005;104:2784-2791.
57. Maesawa S., Kondziolka D., Thompson T., et al. Brain metastases in patients with no known primary tumor: the role of stereotactic radiosurgery. Cancer. 2000;89:1095-1101.
58. Mathieu D., Kondziolka D., Cooper P., et al. Gamma knife radiosurgery in the management of malignant melanoma brain metastases. Neurosurgery. 2007;60:471-482.
59. Mori Y., Kondziolka D., Flickinger J., et al. Stereotactic radiosurgery for cerebral metastatic melanoma: factors affecting local disease control and survival. Int J Radiat Oncol Biol Phys. 1998;42:581-589.
60. Mori Y., Kondziolka D., Lunsford L., et al. Stereotactic radiosurgery for brain metastases from renal cell carcinoma. Cancer. 1998;83:344-353.
61. Peterson A., Meltzer C., Evanson E., et al. MR imaging response of brain metastases after gamma knife stereotactic radiosurgery. Radiology. 1999;211:807-814.
62. Rades D., Kueter J.D., Veninga T., et al. Whole brain radiotherapy plus stereotactic radiosurgery versus surgery plus whole brain radiotherapy for 1-3 brain metastases: results of a matched pair analysis. Eur J Cancer. 2009;45:400-404.
63. Sheehan J., Sun M., Kondziolka D., et al. Radiosurgery for patients with renal cell carcinoma metastatic to the brain: long-term outcomes and prognostic factors influencing survival and local tumor control. J Neurosurg. 2003;98:342-349.
64. Chang E.L., Wefel J.S., Hess K., et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole brain irradiation: a randomized controlled trial. Lancet Oncol. 2009;10(11):1037-1044.
65. Aoyama H., Shirato H., Tago M., et al. Stereotactic radiosurgery plus whole brain radiation therapy versus stereotactic radiosurgery alone for treatment of brain metastases. JAMA. 2006;295:2483-2491.
66. Reference deleted in proof.
67. Linskey M., Andrews D., Asher A., et al. The role of stereotactic radiosurgery in the management of newly diagnosed brain metastases: a systematic review and evidence-based clinical practice guideline. J Neurooncol. 2010;96:45-68.
68. Kondziolka D., Somaza S., Comey C., et al. Radiosurgery and fractionated radiation therapy: comparison of different techniques in an in vivo rat glioma model. J Neurosurg. 1996;84:1033-1038.
69. Larson D., Gutin P., McDermott M., et al. Gamma knife radiosurgery for gliomas: selection factors and results. Int J Radiat Oncol Biol Phys. 1996;36:1045-1053.
70. Ulm A., Friedman W., Bradshaw P., et al. Radiosurgery in the treatment of malignant gliomas: the University of Florida experience. Neurosurgery. 2005;57:512-517.
71. Souhami L., Seiferheld W., Brachman D. Randomized comparison of stereotactic radiosurgery followed by conventional radiotherapy plus carmustine to conventional radiotherapy with carmustine for patients with glioblastoma multiforme: report of Radiation Therapy Oncology Group 93-05 protocol. Int J Radiat Oncol Biol Phys. 2004;60:853-860.
72. Hadjipanayis C., Kondziolka D., Flickinger J., Lunsford L. The role of stereotactic radiosurgery for low grade astrocytomas. Neurosurg Focus. 2003;14:1-7.
73. Hadjipanayis C., Niranjan A., Tyler-Kabara E., et al. Stereotactic radiosurgery for well circumscribed fibrillary grade II astrocytomas and initial experience. Stereotact Funct Neurosurg. 2002;79:13-24.
74. Kano H., Niranjan A., Khan A., et al. Does radiosurgery have a role in the management of oligodendrogliomas? J Neurosurg. 2009;110:564-571.
75. Tyler-Kabara E., Kondziolka D., Lunsford L., Flickinger J. Stereotactic radiosurgery for residual neurocytoma. J Neurosurg. 2001;95:879-882.
76. Kano H., Kondziolka D., Niranjan A., et al. Stereotactic radiosurgery for pilocytic astrocytomas part 1: outcomes in adult patients. J Neurooncol. 2009;95;(2):211-218. (Epub 2009 May 26)
77. Kano H., Niranjan A., Kondziolka D., et al. Adjuvant stereotactic radiosurgery for pilocytic astrocytomas. Part 2: outcomes in pediatric patients. J Neurooncol. 2009;95:219-229.
78. Kano H., Niranjan A., Kondziolka D., et al. Outcome predictors for intracranial ependymomas after stereotactic radiosurgery. Neurosurgery. 2009;64:279-288.
79. Germanwala A., Mai J., Tomycz N., et al. Boost gamma knife radiosurgery during multimodality management of adult medulloblastoma. J Neurosurg. 2008;108(2):204-209.