CHAPTER 52 Radiosurgery for Meningiomas (With Special Emphasis on Skull-Base Meningiomas)
INTRODUCTION
Meningiomas account for approximately 20% of all intracranial tumors in males and 38% in females.1 Although the primary treatment for intracranial meningioma is surgical removal when feasible, not all tumors are managed best by attempted resection. Increasingly, small, even asymptomatic meningiomas are detected incidentally in critical areas of the brain during an imaging evaluation. Observation with follow-up imaging may be an appropriate initial management for these patients. If tumor growth is documented by serial imaging or symptoms develop, many meningiomas can be treated effectively by radiosurgery, a minimally invasive option. In our experience, tumor growth can be documented in more than 80% of patients by the time ten years have passed in an observation strategy.
Surgical resection is the preferred treatment whenever total removal can be proposed with acceptable morbidity.2 The ideal resection (Simpson grade 1) includes the entire tumor and all involved and hyperostotic bone. The remaining dural rim must be free of tumor. Large meningioma surgery series document that gross total resection was possible in 38% to 80% of patients, depending primarily on tumor location.3–8 However, the intimate relationship between some meningiomas and critical neurovascular structures makes complete resection (with acceptable risk) impossible.9,10 The challenges of operating on intracranial meningiomas include tumor location (critical sites), close association with important neurovascular structures, and infiltration of adjacent cranial nerves.
Progression or recurrence after initial resection is a concern with all types of intracranial meningiomas regardless of extent of resection. Even after gross total resection, tumor recurrence rates at 5 and 10 years have varied from 4% to 14% and from 18% to 25%, respectively.3–8 The recurrence rate after total resection varies with histologic grade (12% for benign grade I,11–14 52% for atypical grade II,15 and 84% for malignant grade III meningiomas15,16). Studies have identified numerous factors associated with meningioma recurrence: tumor location, incomplete surgical resection, dissemination of tumor fragments, histologic classification as atypical or malignant, prominent nucleoli, more than two mitotic figures per 10 high-power fields, and heterogeneous contrast enhancement on computed tomography (CT).8,17
The extent of surgical removal is very important. In 1957, Simpson2 published a landmark paper that documented the direct correlation between the extent of meningioma resection and later clinical tumor recurrence (in an era without CT or magnetic resonance imaging [MRI]). With a follow-up interval extending more than 20 years, the rate of symptomatic clinical tumor recurrence was 9%, 19%, and 29% for patients with grade 1, 2, and 3 resection, respectively. Radical extirpation (Simpson grade 1) was strongly linked with prolonged survival. For patients with atypical or malignant meningiomas that are incompletely excised (Simpson grades 2–3), the prognosis is poor.15 In addition to factors related to tumor location, the molecular genetics, pathology, and cell kinetics of meningiomas may also influence recurrence. Cytogenetic studies have suggested that deletions of chromosomes 10, 14, 18, and 22 are associated with more atypical features and more aggressive clinical behavior in meningiomas.8 In addition to cytogenetic investigation, quantitative MIB-1 proliferation index labeling is useful for routine diagnostic assessment of meningiomas. This testing also provides information about prognosis, and therefore, helps in planning the most suitable treatment. High levels of MIB-1 (an indicator of Ki-67 expression) are thought to be strongly associated with tumor recurrence.18–20
STEREOTACTIC RADIOSURGERY
Stereotactic radiosurgery (SRS) is now performed for an increasing number of patients with small to moderate-size meningiomas as an alternative to surgical excision.21–32 Gamma Knife® radiosurgery is used as a first-line management or for postoperative adjuvant management. By 2006, 49,558 meningioma patients worldwide had undergone Gamma Knife radiosurgery management. Frequent indications include patients with skull-base meningiomas, especially those in the posterior fossa or cavernous sinus. Such tumors are not eligible for Simpson grade 1 resections.33 The combination of microsurgery and SRS extends the therapeutic spectrum for meningioma patients. Small, sharply demarcated tumors are the best candidates for radiosurgery. SRS can be performed even after surgery and radiation therapy have failed.34 Gamma Knife radiosurgery (GKRS) has proven an effective strategy for many patients with recurrent meningiomas.35
In general, the biological nature of the meningioma is the main factor that determines how effectively radiosurgery will control tumor growth. Studies have shown that cases of atypical or malignant meningioma exhibit high rates of recurrence despite surgery, radiation therapy, and radiosurgery.31,36 Younger age and smaller tumor size are associated with better tumor control after SRS.37,38 In addition to being an attractive adjuvant treatment for some meningiomas, high tumor control rates and low morbidity indicated that SRS may provide better outcome than microsurgery.39 To reduce morbidity, some surgeons recommend the combination of subtotal resection and subsequent SRS.38,40
UNIVERSITY OF PITTSBURGH EXPERIENCE
Over a 20-year period, 972 patients with 1045 intracranial meningioma underwent Gamma Knife SRS at our institution. Six-hundred and ninety-one patients underwent radiosurgery for skull base meningiomas (Table 52-1). Detailed outcome analysis was performed for 982 available tumors. The mean patient age was 57 years. Five hundred and forty patients had no prior treatment. Patients who underwent prior surgeries usually had subtotal tumor removals. Multiple tumors were present in 161 patients and solitary tumors in 818 patients. Neurofibromatosis type 2 was diagnosed in 26 patients. There were 299 men (30%) and 683 women (70%). Men had a higher rate of atypical and malignant meningiomas. At the time of initial craniotomy 49 of 317 tumors in male patients (15.5%) were found to have World Health Organization (WHO) grade II or III. The incidence of atypical and malignant meningiomas in women was 5.2%. Other prior treatments included radiation therapy (54 patients), radiosurgery at another institution (2 patients), and chemotherapy (8 patients).
TABLE 52-1 Locations of 1045 intracranial meningiomas managed with Gamma Knife radiosurgery at the University of Pittsburgh
| Location | Number of tumors | |
|---|---|---|
| Posterior fossa | ||
| Petroclival | 122 | |
| Petrous ridge | 66 | |
| Foramen magnum | 22 | |
| Other | 42 | |
| Middle fossa | ||
| Cavernous sinus | 306 | |
| Sphenoid wing | 32 | |
| Other | 13 | |
| Anterior fossa | ||
| Olfactory groove | 29 | |
| Planum sphenoidale | 29 | |
| Anterior clinoid | 17 | |
| Parasellar | 13 | |
| Convexity | 126 | |
| Others | ||
| Parasagittal | 113 | |
| Tentorial notch | 40 | |
| Torcular | 6 | |
| Falcine | 47 | |
| Intraorbital | 13 | |
| Intraventricular | 9 | |
| Total | 1045 | |
DOSE PLANNING
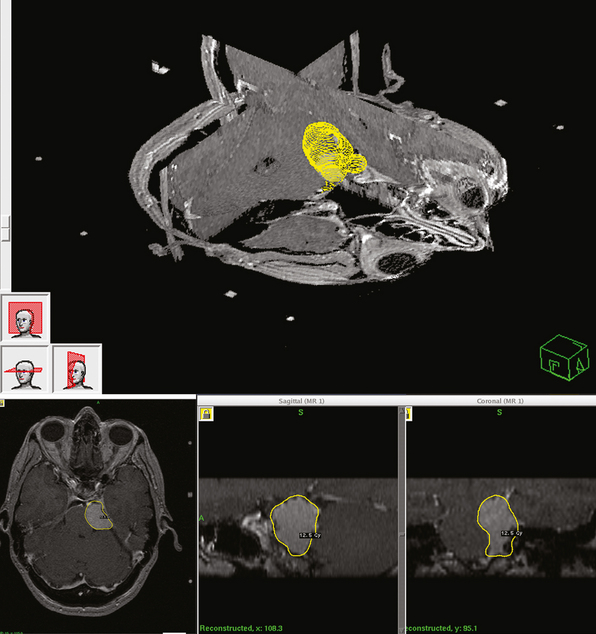
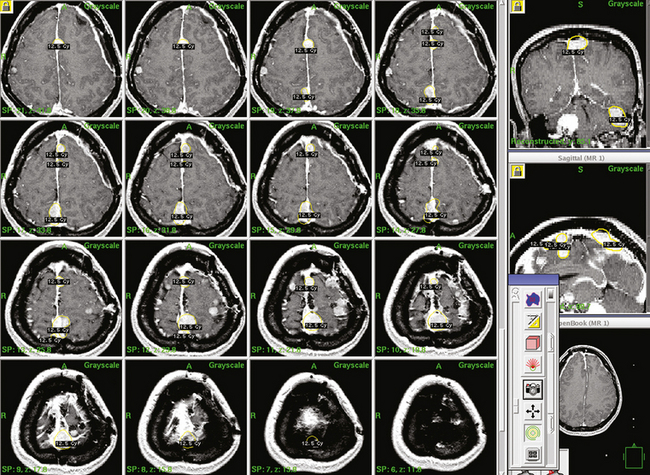
Dose planning is one of the most critical aspects of radiosurgery. A 3-D conformal radiosurgery plan is necessary to reduce radiation fall off in adjacent critical structures (selectivity). Highlights of Gamma Knife meningioma radiosurgery planning include accurate 3-D delineation of tumor volume, use of multiple isocenters, beam weighting, and use of beam blocking patterns when appropriate to achieve selectivity (Figs. 52-1 and 52-2). Precise three-dimensional conformality between planned isodose volume and tumor volumes is needed to avoid radiation related complications.41 This degree of conformality can be achieved through complex multiisocenter planning. Meningioma dose planning at our center was usually performed using a combination of small beam diameters (4- and 8-mm) collimators. For large tumors, 14-mm collimators were also used. A series of 4 mm isocenters were used to create a tapered isodose plan to conform to the dural tail portion of the tumor. The mean tumor volume in our series was 7.4 cc. A mean of 7 isocenters were used to provide a conformal and selective radiosurgery.
RESULTS
Primary Radiosurgery
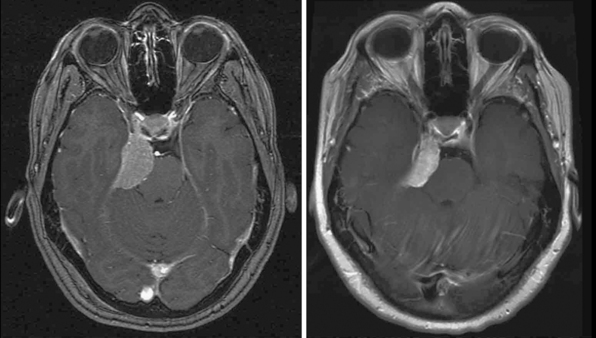
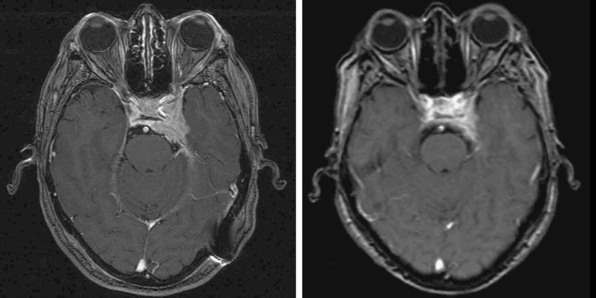
Radiosurgery was performed as primary management in 536 tumors diagnosed by neuroimaging characteristics. Follow-up imaging showed regression in 215, stabilization in 256, and delayed enlargement in 19 tumors. The tumor control rate was 97% at a median of 4 years. Ninety-three percent of patients either improved or remained stable clinically (Figs. 52-3 and 52-4). Subsequent surgical resection was performed in 51 patients (5.2%) due to tumor growth or increased symptoms at an average of 35 months. Additional fractionated radiation therapy was used in 2.9% patients at an average of 29 months. A second stage SRS procedure was performed at an average of 49 months in 41 (4%) patients, usually for new tumors. Chemotherapy was performed in eight patients (0.8%).42
Adjuvant Radiosurgery
All patients in this series had prior surgery and histologic diagnosis. SRS was performed because of confirmed tumor growth despite resection. Thus all patients had failed resection as their primary management. Of 424 WHO grade I tumors, 384 were evaluated in this study. Imaging showed regression in 172, stabilization in 186, and enlargement in 26 tumors. The tumor control rate was 93% at a median of 4 years. For grade I meningioma 5-, 10-, and 15-year actuarial control rates were 97%, 87%, and 87% respectively. Disease-specific survival was 99%, 96%, and 96% respectively. Of 56 WHO grade 2 tumors, 54 were available for analysis. Follow-up imaging showed regression in 16, stabilization in 11, and subsequent enlargement in 27 tumors. The tumor control rate was 50% at a median of 2 years. Of 31 grade III tumors, 29 were available for review. Imaging showed regression in 4, stabilization in 1, and enlargement in 24 tumors with a tumor control rate of 17% at a median of 15 months.42 Our results confirm that histologically aggressive meningiomas (grade II) or malignant meningiomas (grade III) require aggressive surgery followed by early radiosurgery and potentially fractionated radiation therapy if outcomes are to be improved.
Potential Long-Term Radiation-Related Complications
Potential long-term radiation-related complications after radiosurgery must be considered when discussing this option with patients. Radiation-related neoplasms have been reported after radiosurgery of meningiomas.43 Nonetheless, the relative incidence of radiation-related tumors will probably be at least a log factor less than the 1% to 3% typically quoted after fractionated radiotherapy.44 Another rare late complication is delayed occlusion of major intracranial arteries. Asymptomatic occlusion of the internal carotid artery has been reported after radiosurgery of cavernous sinus meningiomas.29,31
Comparing Resection vs. Radiosurgery
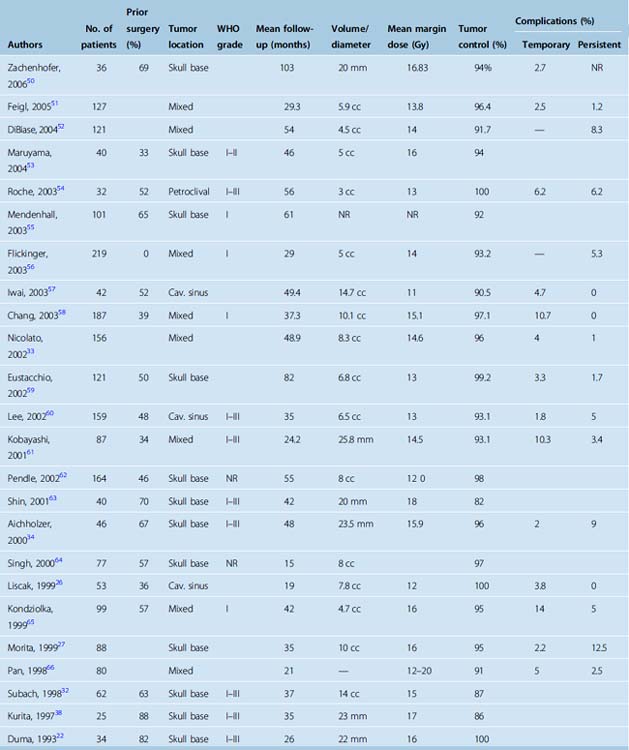
Table 52-2 shows published report of radiosurgery for skull-base meningiomas. Park and colleagues analyzed outcomes after surgery, radiosurgery, and radiation therapy for petroclival meningiomas to compare therapeutic strategies. These authors managed 75 petroclival meningioma patients with microsurgery (n = 49), radiosurgery (n = 12), radiation therapy (n = 5), or observation (n = 9). In the microsurgery group, the tumor was completely resected in 10 patients (20%). Eleven of the 39 patients with incomplete resections sequentially underwent adjuvant radiation therapy or radiosurgery. In the microsurgery group, 11 (22.4%) patients showed tumor progression. However, there was only one clinical recurrence if adjuvant therapy was used after incomplete removal. The likelihood of a favorable outcome for cranial neuropathies was better in the incomplete resection group (69.2%) than for patients who had “complete resection” (20%). More patients had a favorable functional outcome after an incomplete resection (76.9%) compared with complete resection (30%, P = .049). Tumor growth, functional status, and cranial nerve function was maintained in both the radiation therapy and the radiosurgery groups during the follow-up period. These authors recommended radiosurgery as the primary treatment in patients with small tumors. Because the growth rate of most petroclival meningiomas is slow and good functional status can be guaranteed, incomplete resection followed by adjuvant treatment was recommended as an acceptable treatment option for patients who have symptomatic mass effect. In a comparative study of tumor control rates after surgical resection or stereotactic radiosurgery for patients with small to medium-size intracranial meningiomas, Pollock and colleagues noted that the progression-free survival (PFS) rate after radiosurgery was equivalent to that after resection of a Simpson grade 1 tumor and was superior to grade 2 and 3–4 resections.39 Moreover, Pollock and colleagues reported that radiosurgery patients required less additional tumor management during the follow-up period. As expected, the ability to achieve a Simpson grade 1 resection strongly correlated with tumor location in the surgical resection group. Fewer than one third of patients with tumors involving the skull base had grade 1 resections. Thus, in a relatively large series of patients with a mean follow-up more than 5 years, radiosurgery provided better tumor control than surgical resection for patients with small- to moderate-size intracranial meningiomas. This study noted 5-year PFS rates of 96%, 82%, and 34% for Simpson grade 1, 2, and 3–4 resections, respectively. In the original paper by Simpson2 absence of clinically recognizable tumor progression was noted in 91%, 81%, and 71% of patients with grade 1, 2, and 3 resection, respectively.
More recently, Adegbite and colleagues3 had a 5-year PFS rate of 86% for grade 1 and 82% for grade 2 resection. In a series restricted to patients with cranial base meningiomas, Mathiesen and colleagues6 reported a 5-year recurrence rate of 3.5% for patients undergoing grade 1 resection, 4% for grades 2, 25% for grade 3, and 45% for grade 4 resection. As the follow-up interval exceeded 5 years 16% of grade 1 and 20% of grade 2 patients had symptomatic recurrences. The majority of grade 4 and 5 patients showed symptomatic progression. It should be noted that in the study by Pollock and colleagues, PFS rate was calculated on the basis of radiographic progression, whereas earlier studies relied primarily on symptomatic progression to calculate the recurrence rates.
Other centers have reported tumor control rates between 84% and 98% after meningioma radiosurgery with a range of follow-up intervals.21,23–26,29,30 Significantly, most prior studies included patients with atypical or malignant tumors who had lower control rates after radiosurgery.28,31 Long-term follow-up (up to 20 years) now confirms the high tumor control rate and low morbidity of radiosurgery. At many centers, radiosurgery has become the preferred treatment for patients with small to moderate-size meningiomas without symptomatic mass effect.
Our own analysis supports the concept that radiosurgery should be considered as primary management option for patients with tumors involving the skull base,42 where a Simpson grade 1 resection cannot be accomplished with acceptable risk.45–47 The morbidity of radiosurgery for meningiomas of the cavernous sinus and petroclival regions has been specifically analyzed and is considered to be less than 10%.22,26,27,29,32 Unless complete tumor removal is performed many patients with extended life expectancies eventually will recur.2,6,8,48 On the basis of the present data, radiosurgery can be recommended as primary treatment for suitable patients with small- to moderate-size meningiomas.42 Patients with symptomatic mass effect should undergo enough tumor removal to decompress the brain. After such surgery, the tumor remnant should undergo radiosurgery for benign meningiomas (for WHO grade I and II tumors) or fractionated radiotherapy and radiosurgery for malignant meningiomas (WHO grade III).49 Patients with extended life expectancies and subtotal resection of a meningioma should have radiosurgery if the remaining tumor is a discrete mass. In the absence of an imaging defined target after surgery, early stereotactic fractionated radiotherapy may reduce recurrent rates of aggressive meningiomas.
[1] Bondy M., Ligon B.L. Epidemiology and etiology of intracranial meningiomas: a review. J Neurooncol. 1996;29:197-205.
[2] Simpson D. The recurrence of intracranial meningiomas after surgical treatment. J Neurol Neurosurg Psychiatr. 1957;20:22-39.
[3] Adegbite A.B., Khan M.I., Paine K.W., Tan L.K. The recurrence of intracranial meningiomas after surgical treatment. J Neurosurg. 1983;58:51-56.
[4] Barbaro N.M., Gutin P.H., Wilson C.B., et al. Radiation therapy in the treatment of partially resected meningiomas. Neurosurgery. 1987;20:525-528.
[5] Condra K.S., Buatti J.M., Mendenhall W.M., et al. Benign meningiomas: primary treatment selection affects survival. Int J Radiat Oncol Biol Phys. 1997;39:427-436.
[6] Mathiesen T., Lindquist C., Kihlstrom L., Karlsson B. Recurrence of cranial base meningiomas. Neurosurgery. 1996;39:2-7. discussion 8–9
[7] Mirimanoff R.O., Dosoretz D.E., Linggood R.M., et al. Meningioma: analysis of recurrence and progression following neurosurgical resection. J Neurosurg. 1985;62:18-24.
[8] Stafford S.L., Perry A., Suman V.J., et al. Primarily resected meningiomas: outcome and prognostic factors in 581 Mayo Clinic patients, 1978 through 1988. Mayo Clin Proc. 1998;73:936-942.
[9] Kotapka M.J., Kalia K.K., Martinez A.J., Sekhar L.N. Infiltration of the carotid artery by cavernous sinus meningioma. J Neurosurg. 1994;81:252-255.
[10] Larson J.J., van Loveren H.R., Balko M.G., Tew J.M.Jr. Evidence of meningioma infiltration into cranial nerves: clinical implications for cavernous sinus meningiomas. J Neurosurg. 1995;83:596-599.
[11] Jaaskelainen J. Seemingly complete removal of histologically benign intracranial meningioma: late recurrence rate and factors predicting recurrence in 657 patients. A multivariate analysis. Surg Neurol. 1986;26:461-469.
[12] Mahmood A., Caccamo D.V., Tomecek F.J., Malik G.M. Atypical and malignant meningiomas: a clinicopathological review. Neurosurgery. 1993;33:955-963.
[13] Maier H., Ofner D., Hittmair A., et al. Classic, atypical, and anaplastic meningioma: three histopathological subtypes of clinical relevance. J Neurosurg. 1992;77:616-623.
[14] Perry A., Stafford S.L., Scheithauer B.W., et al. Meningioma grading: an analysis of histologic parameters. Am J Surg Pathol. 1997;21:1455-1465.
[15] Palma L., Celli P., Franco C., et al. Long-term prognosis for atypical and malignant meningiomas: a study of 71 surgical cases. J Neurosurg. 1997;86:793-800.
[16] Younis G.A., Sawaya R., DeMonte F., et al. Aggressive meningeal tumors: review of a series. J Neurosurg. 1995;82:17-27.
[17] Ayerbe J., Lobato R.D., de la Cruz J., et al. Risk factors predicting recurrence in patients operated on for intracranial meningioma. A multivariate analysis. Acta Neurochir (Wien). 1999;141:921-932.
[18] Karamitopoulou E., Perentes E., Tolnay M., Probst A. Prognostic significance of MIB-1, p53, and bcl-2 immunoreactivity in meningiomas. Hum Pathol. 1998;;29:140-145.
[19] Matsuno A., Nagashima T., Matsuura R., et al. Correlation between MIB-1 staining index and the immunoreactivity of p53 protein in recurrent and non-recurrent meningiomas. Am J Clin Pathol. 1996;106:776-781.
[20] Ohkoudo M., Sawa H., Hara M., et al. Expression of p53, MDM2 protein and Ki-67 antigen in recurrent meningiomas. J Neurooncol. 1998;38:41-49.
[21] Chang S.D., Adler J.R.Jr. Treatment of cranial base meningiomas with linear accelerator radiosurgery. Neurosurgery. 1997;41:1019-1025. discussion 1025–7
[22] Duma C.M., Lunsford L.D., Kondziolka D., et al. Stereotactic radiosurgery of cavernous sinus meningiomas as an addition or alternative to microsurgery. Neurosurgery. 1993;32:699-704. discussion 704–5
[23] Hakim R., Alexander E.3rd, Loeffler J.S., et al. Results of linear accelerator-based radiosurgery for intracranial meningiomas. Neurosurgery. 1998;42:446-453. discussion 453–4
[24] Kondziolka D., Flickinger J.C., Perez B. Judicious resection and/or radiosurgery for parasagittal meningiomas: outcomes from a multicenter review. Gamma Knife Meningioma Study Group. Neurosurgery. 1998;43:405-413. discussion 413–4
[25] Kondziolka D., Levy E.I., Niranjan A., et al. Long-term outcomes after meningioma radiosurgery: physician and patient perspectives. J Neurosurg. 1999;91:44-50.
[26] Liscak R., Simonova G., Vymazal J., et al. Gamma knife radiosurgery of meningiomas in the cavernous sinus region. Acta Neurochir (Wien). 1999;141:473-480.
[27] Morita A., Coffey R.J., Foote R.L., et al. Risk of injury to cranial nerves after gamma knife radiosurgery for skull base meningiomas: experience in 88 patients. J Neurosurg. 1999;90:42-49.
[28] Ojemann R.G. Management of cranial and spinal meningiomas (honored guest presentation). Clin Neurosurg. 1993;40:321-383.
[29] Roche P.H., Regis J., Dufour H., et al. Gamma knife radiosurgery in the management of cavernous sinus meningiomas. J Neurosurg. 2000;93(Suppl):68-73. 3
[30] Shafron D.H., Friedman W.A., Buatti J.M., et al. Linac radiosurgery for benign meningiomas. Int J Radiat Oncol Biol Phys. 1999;43:321-327.
[31] Stafford S.L., Pollock B.E., Foote R.L., et al. Meningioma radiosurgery: tumor control, outcomes, and complications among 190 consecutive patients. Neurosurgery. 2001;49:1029-1037. discussion 1037–8
[32] Subach B.R., Lunsford L.D., Kondziolka D., et al. Management of petroclival meningiomas by stereotactic radiosurgery. Neurosurgery. 1998;42:437-443. discussion 443–5
[33] Nicolato A., Foroni R., Alessandrini F., et al. Radiosurgical treatment of cavernous sinus meningiomas: experience with 122 treated patients. Neurosurgery. 2002;51:1153-1159. discussion 1159–61
[34] Aichholzer M., Bertalanffy A., Dietrich W., et al. Gamma knife radiosurgery of skull base meningiomas. Acta Neurochir (Wien). 2000;142:647-652. discussion 652–3
[35] Pollock B.E., Stafford S.L., Link M.J. Gamma knife radiosurgery for skull base meningiomas. Neurosurg Clin N Am. 2000;11:659-666.
[36] Kobayashi T., Kida Y., Mori Y. Long-term results of stereotactic gamma radiosurgery of meningiomas. Surg Neurol. 2001;55:325-331.
[37] Iwai Y., Yamanaka K., Ishiguro T. Gamma knife radiosurgery for the treatment of cavernous sinus meningiomas. Neurosurgery. 2003;52:517-524. discussion 523–4
[38] Kurita H., Sasaki T., Kawamoto S., et al. Role of radiosurgery in the management of cavernous sinus meningiomas. Acta Neurol Scand. 1997;96:297-304.
[39] Pollock B.E., Stafford S.L., Utter A., et al. Stereotactic radiosurgery provides equivalent tumor control to Simpson Grade 1 resection for patients with small- to medium-size meningiomas. Int J Radiat Oncol Biol Phys. 2003;55:1000-1005.
[40] Villavicencio A.T., Black P.M., Shrieve D.C., et al. Linac radiosurgery for skull base meningiomas. Acta Neurochir (Wien). 2001;143:1141-1152.
[41] Linskey M.E. Stereotactic radiosurgery versus stereotactic radiotherapy for patients with vestibular schwannoma: a Leksell Gamma Knife Society 2000 debate. J Neurosurg. 2000;93(Suppl 3):90-95.
[42] Kondziolka D., Mathieu D., Lunsford L.D., et al. Radiosurgery as definitive management of meningiomas. Neurosurgery. 2008;62:53-58.
[43] Yu J.S., Yong W.H., Wilson D., Black K.L. Glioblastoma induction after radiosurgery for meningioma. Lancet. 2000;356:1576-1577.
[44] al-Mefty O., Kersh J.E., Routh A., Smith R.R. The long-term side effects of radiation therapy for benign brain tumors in adults. J Neurosurg. 1990;73:502-512.
[45] Couldwell W.T., Fukushima T., Giannotta S.L., Weiss M.H. Petroclival meningiomas: surgical experience in 109 cases. J Neurosurg. 1996;84:20-28.
[46] De Jesus O., Sekhar L.N., Parikh H.K., et al. Long-term follow-up of patients with meningiomas involving the cavernous sinus: recurrence, progression, and quality of life. Neurosurgery. 1996;39:915-919. discussion 919–20
[47] DeMonte F., Smith H.K., al-Mefty O. Outcome of aggressive removal of cavernous sinus meningiomas. J Neurosurg. 1994;81:245-251.
[48] Kallio M., Sankila R., Hakulinen T., Jaaskelainen J. Factors affecting operative and excess long-term mortality in 935 patients with intracranial meningioma. Neurosurgery. 1992;31:2-12.
[49] Goldsmith B.J., Wara W.M., Wilson C.B., Larson D.A. Postoperative irradiation for subtotally resected meningiomas. A retrospective analysis of 140 patients treated from 1967 to 1990. J Neurosurg. 1994;80:195-201.
[50] Zachenhofer I., Wolfsberger S., Aichholzer M., et al. Gamma-knife radiosurgery for cranial base meningiomas: experience of tumor control, clinical course, and morbidity in a follow-up of more than 8 years. Neurosurgery. 2006;58:28-36.
[51] Feigl G.C., Bundschuh O., Gharabaghi A., et al. Volume reduction in meningiomas after gamma knife surgery. J Neurosurg. 2005;102:189-194.
[52] DiBiase S.J., Kwok Y., Yovino S., et al. Factors predicting local tumor control after gamma knife stereotactic radiosurgery for benign intracranial meningiomas. Int J Radiat Oncol Biol Phys. 2004;60:1515-1519.
[53] Maruyama K., Shin M., Kurita H., et al. Proposed treatment strategy for cavernous sinus meningiomas: a prospective study. Neurosurgery. 2004;55:1068-1075.
[54] Roche P.H., Pellet W., Fuentes S., et al. Gamma knife radiosurgical management of petroclival meningiomas results and indications. Acta Neurochir (Wien). 2003;145:883-888.
[55] Mendenhall W.M., Morris C.G., Amdur R.J., et al. Radiotherapy alone or after subtotal resection for benign skull base meningiomas. Cancer. 2003;98:1473-1482.
[56] Flickinger J.C., Kondziolka D., Maitz A.H., Lunsford L.D. Gamma knife radiosurgery of imaging-diagnosed intracranial meningioma. Int J Radiat Oncol Biol Phys. 2003;56:801-806.
[57] Iwai Y., Yamanaka K., Ishiguro T. Gamma knife radiosurgery for the treatment of cavernous sinus meningiomas. Neurosurgery. 2003;52:517-524.
[58] Chang J.H., Chang J.W., Choi J.Y., et al. Complications after gamma knife radiosurgery for benign meningiomas. J Neurol Neurosurg Psychiatry. 2003;74:226-230.
[59] Eustacchio S., Trummer M., Fuchs I., et al. Preservation of cranial nerve function following Gamma Knife radiosurgery for benign skull base meningiomas: experience in 121 patients with follow-up of 5 to 9.8 years. Acta Neurochir Suppl. 2002;84:71-76.
[60] Lee J.Y., Niranjan A., McInerney J., et al. Stereotactic radiosurgery providing long-term tumor control of cavernous sinus meningiomas. J Neurosurg. 2002;97:65-72.
[61] Kobayashi T., Kida Y., Mori Y. Long-term results of stereotactic gamma radiosurgery of meningiomas. Surg Neurol. 2001;55:325-331.
[62] Pendl G., Eustacchio S., Unger F. Radiosurgery as alternative treatment for skull base meningiomas. J Clin Neurosci. 2001;8(Suppl 1):12-14.
[63] Shin M., Kurita H., Sasaki T., et al. Analysis of treatment outcome after stereotactic radiosurgery for cavernous sinus meningiomas. J Neurosurg. 2001;95:435-439.
[64] Singh V.P., Kansai S., Vaishya S., et al. Early complications following gamma knife radiosurgery for intracranial meningiomas. J Neurosurg. 2000;93(Suppl 3):57-61.
[65] Kondziolka D., Niranjan A., Lunsford L.D., Flickinger J.C. Stereotactic radiosurgery for meningiomas. Neurosurg Clin N Am. 1999;10:317-325.
[66] Pan D.H., Guo W.Y., Chang Y.C., et al. The effectiveness and factors related to treatment results of gamma knife radiosurgery for meningiomas. Stereotact Funct Neurosurg. 1998;70(Suppl 1):19-32.