CHAPTER 260 Radiosurgery for Intracranial Vascular Malformations
Intracranial vascular malformations represent a diverse group of congenital or acquired anomalies with varying risks for neurological morbidity. Included in this group are arteriovenous malformations (AVMs), dural arteriovenous fistulas (DAVFs), cavernous malformations (CMs), developmental venous anomalies (DVAs), and capillary telangiectases, each of which carries a specific chance for intracranial bleeding or other neurological sequelae.1 Because of the heterogeneous nature of intracranial vascular malformations, management of these patients must take into account the natural history of these lesions. AVMs are congenital lesions that arise from abnormal differentiation of primordial vascular channels into direct arteriovenous shunts without the appropriate intervening vascular beds, such as mature arteries, veins, and capillaries. The most common finding in patients with AVMs is intracranial hemorrhage (ICH), but patients may also have seizures, headaches, and other associated neurological deficits. DAVFs are acquired lesions thought to develop after sinus thrombosis or occlusion. The risks and associated symptoms of DAVFs are related to their location and the presence or absence of cortical venous drainage. CMs, sometimes referred to as angiographically occult vascular malformations, are thought to be acquired in the majority of cases and are composed of closely approximated endothelial-lined sinusoidal spaces without intervening neural tissue. Hemorrhages from CMs are rarely fatal, although those located in the brainstem, thalamus, or basal ganglia can cause progressive neurological disabilities from repetitive bleeding events. DVAs are congenital and more closely represent anatomic variants than true vascular malformations. Rarely symptomatic by themselves, up to a third of DVAs are associated with CMs. Capillary telangiectases are collections of dilated capillaries whose walls lack smooth muscle and elastic fibers. Capillary telangiectases may also coexist with CMs, and it has been suggested that they represent the same histopathologic entity. Capillary telangiectases are rarely symptomatic and are often discovered as incidental findings during autopsy. In this chapter the role of stereotactic radiosurgery is reviewed for patients with intracranial AVMs, DAVFs, and CMs.
Arteriovenous Malformations
Natural History
The natural history of AVMs is summarized in this section. For a comprehensive review of the natural history of AVMs, the reader is referred to Chapter 384.
Large, prospective population-based studies have determined the incidence of newly diagnosed AVM to range from 1.12 to 1.34 per 100,000 person-years.2,3 The number of incidentally discovered intracranial AVMs continues to rise as more patients undergo magnetic resonance imaging (MRI) of the head. The annual risk for ICH from AVMs has been reported to be 2% to 4%4–11; the combined annual morbidity plus mortality from intracranial AVMs is approximately 1%.9 The majority of AVMs are diagnosed at a point when patients’ life expectancy is long, so the cumulative risk for hemorrhage is often significant. The factor associated with the greatest increased risk for bleeding is previous hemorrhage.10,11 The estimated risk for rebleeding from intracranial AVMs is thought to be elevated in the first months after an ICH. Additional factors that have been correlated with AVM hemorrhage include increasing age,12 single or deep draining veins,10,11 associated arterial aneurysm,13 and diffuse AVM morphology.11 Pediatric patients,14 patients with deeply located AVMs (basal ganglia, thalamus, brainstem, cerebellum),10,11 and patients with small AVMs15 are more frequently first seen after an ICH. It remains unclear whether these patients truly have an increased annual risk for hemorrhage or whether they are just unlikely to have other symptoms that would permit the diagnosis.
Management of Arteriovenous Malformations
The definitive treatment options available for patients with intracranial AVMs are surgical resection and stereotactic radiosurgery. Patients with a large ICH require urgent surgical evacuation to eliminate the mass effect. Once the blood clot has been removed, cerebral angiography can be performed to determine a best plan for the residual nidus. Surgical resection is the preferred treatment of patients after a recent ICH if the nidus is accessible.16–22 The benefit of surgical resection versus radiosurgery is the immediate elimination of future hemorrhage risk. Embolization of AVMs is frequently performed in conjunction with either surgical resection or radiosurgery, but it is rarely curative by itself. Alternatively, fractionated radiation therapy is rarely performed because it results in a low AVM cure rate23 and the optimal dose fraction schedule has not been determined.24
In 1972, Ladislau Steiner and colleagues recognized that single-fraction, high-dose irradiation causes progressive obliteration of AVMs and subsequent cure from the risk for later hemorrhage.25 Based on the fact that AVMs could be visualized with angiography before the development of axial imaging, AVMs were a common early indication for radiosurgery: 27% (n = 204) of the first 762 patients who underwent Gamma Knife radiosurgery at the Karolinska Institute had AVMs.26 Concurrent with findings from the Karolinska Institute, Kjellberg and Fabrikant were using heavy charged particles instead of photons to irradiate AVMs.27,28 These pioneers also noted that focused radiation techniques could obliterate a high percentage of irradiated AVMs. Later studies showed that AVM radiosurgery could be performed successfully with modified linear accelerators (LINACs).29–31
If the risk associated with treatment is determined to be greater than the lifetime risk based on the natural history of untreated AVMs, observation alone is a reasonable management option. This is especially applicable for older patients with incidentally discovered AVMs. Despite technologic advances, it is recognized that the risk related to treatment in patients with Spetzler-Martin grade IV and V AVMs is substantial, so observation of large AVMs is often recommended unless the patient has bled or is suffering from a progressive neurological deficit.32 Recently, recognition of two factors has led to a re-examination of observation for patients discovered to have smaller, unruptured brain AVMs.33 First, the morbidity associated with AVM bleeding may be less than previously thought. Hartmann and colleagues reviewed 119 AVM patients and found that 47% suffered no disability related to their first ICH and an additional 37% remained independent in activities of daily living.34 Second, the incidence of neurological injury related to surgical resection of AVMs may be greater than previously described. A prospective observation study of 124 AVM patients noted that at last follow-up, 38% had new postoperative neurological deficits.35 Of these patients, 6% were disabled after surgery. Likewise, Lawton and associates found that patients with unruptured AVMs were 2.3 times more likely to experience a decline in their modified Rankin scale score than were patients with ruptured AVMs.36 Future studies on AVM management must carefully consider the risk-benefit ratio associated with the treatment of patents with unruptured AVMs.
Patient Selection
Proper patient selection is essential for successful AVM radiosurgery. In particular, a number of factors must be considered when discussing radiosurgery for AVM, including age, signs and symptoms, AVM size, and AVM location. As mentioned before, patients with a recent ICH and a surgically accessible AVM are best managed by surgical resection. However, patients with a recent hemorrhage and a surgically inaccessible AVM are generally good candidates for radiosurgery, assuming that the AVM is not too large. Moreover, patients with a distant hemorrhage should be considered for radiosurgery because they have passed the time when rehemorrhage is most likely to occur. For individual patients, a comparison of the chance of surgical resection or radiosurgery eliminating AVMs without risk for new deficits should be undertaken. Standardized scales such as the Spetzler-Martin grade16 and the Pollock-Flickinger score37 can be used to estimate the efficacy of surgical resection and radiosurgery, respectively, for individual AVM patients.
A previous history of seizures can also affect management decisions for patients with AVMs. Although between 15% and 20% of AVM patients will have a seizure,3,26,38 few patients have medically resistant epilepsy. In addition, few AVM studies have used standardized scales in reporting epilepsy outcomes. Piepgras and coworkers studied seizure outcomes after AVM surgery.39 In the low-seizure group (less than four seizures before surgery) with a follow-up longer than 2 years, 93% were seizure free, 2% improved but continued to have seizures, and 5% had worsening of their seizures. In comparison, 76% of patients with more than four seizures preoperatively were without seizures 2 or more years after surgery, 21% were improved, and 3% remained unchanged. Overall, 83% of patients remained seizure free at last follow-up in this study. Schäuble and coauthors retrospectively reviewed 65 AVM patients with one or more seizures who underwent radiosurgery between 1990 and 1998.40 Forty patients (78%) had an excellent outcome (nondisabling simple partial seizures only) at 3-year follow-up; 26 patients (51%) were seizure free. Factors associated with seizure-free outcomes included a low seizure frequency score (<4) before radiosurgery and smaller AVM size. Eleven of 18 (61%) patients with medically intractable partial epilepsy had excellent outcomes 3 years after radiosurgery. Hoh and colleagues reviewed 110 patients with seizures who underwent AVM treatment.41 Patients with a short seizure history, seizures related to an ICH, surgical resection, and complete AVM obliteration were more likely to have Engel class I outcomes. However, selection bias with regard to the different treatments prevents any meaningful conclusion whether surgical resection or radiosurgery correlates with improved seizure outcomes.
Technique of Radiosurgery for Arteriovenous Malformations
The goal of stereotactic radiosurgery is to accurately deliver a high dose of radiation to an imaging-defined target. To accomplish this goal in a single fraction, placement of a stereotactic head frame is needed to ensure rigid fixation and minimize patient movement during imaging and delivery of radiation. Head frame placement for adults is performed under local anesthesia supplemented with a low dose of a benzodiazepine. General anesthesia is typically required for patients younger than 16 years. After the head frame has been placed, patients undergo either gadolinium-enhanced MRI or contrast-enhanced computed tomography in addition to cerebral angiography. Reliance on angiography alone for radiosurgical dose planning increases the chance of treating too much adjacent normal brain tissue because of the often irregular shape of AVMs.42,43 In addition, AVMs in selected locations (posterior fossa, lateral temporal regions) are difficult to clearly visualize on angiography, so the chance of not including a portion of the nidus in the prescription isodose volume (PIV) is increased. More recently, we have become increasingly confident in excluding cerebral angiography for AVM dose planning. Ideal patients for this approach have small, compact, hemispheric AVMs with simple venous drainage. In our current practice, approximately 20% of AVM patients undergo radiosurgery based on MRI alone. However, we continue to perform complete diagnostic angiography, including appropriate external carotid injections, before any decision is made about the feasibility of radiosurgery and to determine whether the patient has any associated aneurysms.
The goal of dose planning is to completely cover the three-dimensional shape of the nidus and exclude adjacent brain parenchyma. Feeding arteries and draining veins are not included in the dose plan if possible. Inclusion of these vessels increases the PIV and may result in a lower radiation dose. Dose prescription must take into account two conflicting considerations: the chance of obliteration versus the chance of radiation-related complications. Higher radiation doses directly correlate with the chance of obliteration. Assuming that the nidus is completely covered, the chance of AVM cure is approximately 70%, 80%, and 90% for radiation doses of 16, 18, and 20 Gy, respectively.44,45 However, the likelihood of radiation-related complications after AVM radiosurgery increases at higher radiation doses and with larger AVM volumes. Dose prescription for AVM radiosurgery has traditionally followed either Kjellberg’s 3% isodose line27 or Flickinger’s integrated logistic formula46 to predict the probability of radiation-related complications. Recent studies have correlated the chance of radiation-related complications after AVM radiosurgery to some measure (10-Gy volume, 12-Gy volume) of irradiation of the surrounding brain.47–49 Patients with deeply located AVMs are more likely to exhibit neurological deficits as a result of the changes noted on MRI.47 The prescribed radiation dose is typically reduced for patients undergoing AVM radiosurgery to minimize the chance for radiation-related complications.
Patients undergo radiation therapy after the dose plan is reviewed by all members of the radiosurgical team. After radiation delivery, patients are discharged home either the day of the procedure or after an overnight observation period. Immediate complications are rare, but some patients complain of pin site discomfort, neck pain, or headache. Such symptoms are usually temporary and can be managed with over-the-counter medications. Follow-up after radiosurgery consists of clinical examination and MRI at 1, 2, and 3 years after radiosurgery. If MRI suggests that the AVM has been completely obliterated, follow-up angiography is recommended 3 or more years after radiosurgery to definitively determine the status of the AVM.50 Patients with residual AVM on follow-up angiography are evaluated for repeat radiosurgery or surgical resection based on their age, clinical condition, and response of the AVM to the first radiosurgical procedure.
Obliteration of Arteriovenous Malformations after Radiosurgery
The goal of AVM radiosurgery is complete nidus obliteration to eliminate a patient’s risk for future hemorrhage. Generally, AVM obliteration after radiosurgery requires between 1 and 5 years. Histopathologic changes after AVM radiosurgery include damage to endothelial cells, progressive thickening of the intimal layer secondary to proliferation of smooth muscle cells (which produces an extracellular matrix containing type IV collagen), and then cellular degeneration and hyaline transformation.51 Electron microscopic studies of seven AVMs resected after bleeding 10 to 52 months after radiosurgery revealed spindle cell proliferation in the connective tissue stroma and subendothelial region of irradiated vessels.52 The characteristics of the spindle cells were similar to those of myofibroblasts noted during wound healing, and these cells probably contributed to the occlusive process and obliteration of AVMs after radiosurgery.
The AVM margin dose is the most important factor associated with obliteration after radiosurgery.44,45,53–55 Several models have been developed to predict the chance for cure of AVMs after radiosurgery. Karlsson and coauthors reported the K index as a method to predict obliteration after AVM radiosurgery.45 Analysis of 945 AVM patients who underwent radiosurgery from 1970 to 1990 showed a logarithmic relationship between minimum dose and AVM obliteration: the product minimum dose (AVM volume) × 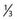 was termed the K index, and it increased to a maximum of 87%. The obliteration rate increased linearly with the K index up to a value of approximately 27, and for higher K index values, the obliteration rate had a constant value of approximately 80%. For the group of patients receiving an AVM margin dose of 25 Gy or greater, the obliteration rate at 2 years was 80%. Higher average doses also shortened the latency to AVM obliteration. Schwartz and colleagues developed the obliteration prediction index as a method to predict success or failure after AVM radiosurgery.56 By analyzing patients who underwent either Gamma Knife or LINAC-based radiosurgery, a relationship was noted between the obliteration prediction index (AVM margin dose, Gy per lesion diameter in centimeters) and AVM obliteration. A number of papers have analyzed factors associated with incomplete AVM obliteration after radiosurgery.57–60 Common reasons for incomplete obliteration of the nidus are targeting errors, recanalization of a portion of the AVM that was previously embolized, reexpansion of the nidus after hemorrhage, and low radiation dose. These studies have emphasized the need for complete nidus coverage at the time of radiosurgery. Nonetheless, part of the problem in AVM radiosurgery is defining the nidus accurately. Buis and coworkers had six independent clinicians contour the nidus of AVMs based on digital subtraction angiography.61 They noted significant interobserver variation when outlining the nidus and concluded that such variation may contribute to failure in some AVM radiosurgical cases. Yu and associates compared AVM dose plans based on a combination of angiography and MRI with those based on MRI alone.62 They concluded that AVM dose planning without angiography should be limited to patients with smaller AVMs and compact niduses. A recent study from the University of Florida suggested that AVM morphology is also an important factor associated with obliteration.63 Specifically, they noted that patients with a diffuse nidus structure and associated neovascularity were at a higher risk for incomplete nidus obliteration than were patients with compact AVMs.
was termed the K index, and it increased to a maximum of 87%. The obliteration rate increased linearly with the K index up to a value of approximately 27, and for higher K index values, the obliteration rate had a constant value of approximately 80%. For the group of patients receiving an AVM margin dose of 25 Gy or greater, the obliteration rate at 2 years was 80%. Higher average doses also shortened the latency to AVM obliteration. Schwartz and colleagues developed the obliteration prediction index as a method to predict success or failure after AVM radiosurgery.56 By analyzing patients who underwent either Gamma Knife or LINAC-based radiosurgery, a relationship was noted between the obliteration prediction index (AVM margin dose, Gy per lesion diameter in centimeters) and AVM obliteration. A number of papers have analyzed factors associated with incomplete AVM obliteration after radiosurgery.57–60 Common reasons for incomplete obliteration of the nidus are targeting errors, recanalization of a portion of the AVM that was previously embolized, reexpansion of the nidus after hemorrhage, and low radiation dose. These studies have emphasized the need for complete nidus coverage at the time of radiosurgery. Nonetheless, part of the problem in AVM radiosurgery is defining the nidus accurately. Buis and coworkers had six independent clinicians contour the nidus of AVMs based on digital subtraction angiography.61 They noted significant interobserver variation when outlining the nidus and concluded that such variation may contribute to failure in some AVM radiosurgical cases. Yu and associates compared AVM dose plans based on a combination of angiography and MRI with those based on MRI alone.62 They concluded that AVM dose planning without angiography should be limited to patients with smaller AVMs and compact niduses. A recent study from the University of Florida suggested that AVM morphology is also an important factor associated with obliteration.63 Specifically, they noted that patients with a diffuse nidus structure and associated neovascularity were at a higher risk for incomplete nidus obliteration than were patients with compact AVMs.
Bleeding of Arteriovenous Malformations after Radiosurgery
The primary drawback of AVM radiosurgery is that patients remain at risk for hemorrhage until the AVM has eventually been completely obliterated. Despite early papers on AVM radiosurgery suggesting increased risk for bleeding before documented obliteration of AVMs,30,64 later, more detailed analyses of this topic have concluded that the risk for AVM bleeding is either unchanged or reduced during this latency interval.65–69 Karlsson and colleagues analyzed the large AVM experience at the Karolinska Institute and found that some measure of protection occurred as early as 6 months after radiosurgery for patients receiving an AVM margin dose of 25 Gy.66 Maruyama and associates performed a retrospective observational study of 500 AVM patients who underwent radiosurgery.67 In comparing the risk for bleeding before and after radiosurgery, they found a 54% reduction in bleeding risk during the latency interval. The reduction in risk was greatest in patients initially seen with hemorrhage. Despite the general contention that the risk for bleeding after angiographically confirmed obliteration is zero, episodes of bleeding after AVM obliteration have been reported.70,71 Pediatric AVM patients appear to have an increased chance of this delayed complication. Consequently, follow-up angiography is indicated for these patients when they reach adulthood to ensure that they do not have any residual nidus. Moreover, if bleeding does occur after angiographically documented AVM obliteration, the clinical sequelae are generally minimal, with the hemorrhages behaving more like bleeding from CMs than AVMs.
Radiation-Related Complications after Radiosurgery for Arteriovenous Malformations
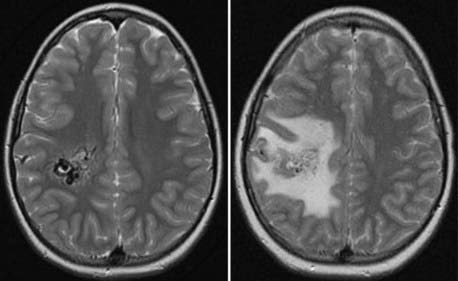
It has been demonstrated that various measures of radiation exposure correlate with the chance of areas of increased signal developing on long-TR sequences after AVM radiosurgery. The most commonly used measure is the 12-Gy volume (V12)47; other studies have shown the 10-Gy volume (V10)49 and the mean dose received by 20 cc surrounding the maximum radiation point (Dmean20)48 to also correlate with changes on MRI after AVM radiosurgery. Such imaging changes are noted in approximately 30% to 50% of AVM patients within the first year after radiosurgery, but the majority remain asymptomatic (Fig. 260-1). Comparison of AVM patients with non-AVM patients has demonstrated that the incidence of these imaging changes is greater in AVM patients. Thus, it is probable that many “radiation-associated” imaging changes relate not to radiation damage to adjacent brain but rather to alterations in regional blood flow in brain tissue adjacent to the AVM. Patients with AVMs in the thalamus, basal ganglia, and brainstem are more likely to have neurological deficits as a result of the changes noted on MRI.47 Levegrun and coworkers studied the correlation of radiation-induced imaging changes and dose distribution parameters in 73 patients who underwent AVM radiosurgery.72 They concluded that all three measures studied (V10, V12, Dmean20) yielded similar results and that no parameter was favored over the others in predicting these abnormalities on MRI. Occlusive hyperemia from early closure of draining veins before nidus obliteration may be a significant factor contributing to the development of such imaging changes.73,74
Despite the fact that most imaging changes detected on MRI after AVM radiosurgery resolve over time, radiation necrosis will develop in a small percentage of patients. MRI findings consistent with radiation necrosis are persistent enhancement at the irradiated site with associated edema and mass effect. In addition to radiation necrosis, other late complications have been noted after AVM radiosurgery, including cyst formation and diffuse white matter changes.75–78 The development of radiation-induced tumors after radiosurgery is exceedingly rare.79,80 Although the true incidence of this complication will not be known for many years, it is clear that the risk for a radiation-induced tumor after radiosurgery is significantly less than the incidence after fractionated radiation therapy.
Repeat Radiosurgery for Arteriovenous Malformations
Patients with incomplete obliteration after AVM radiosurgery remain at risk for ICH. Repeat AVM radiosurgery has proved to be a safe and effective option for the majority of patients with subtotal AVM obliteration after their initial radiosurgical procedure.81–84 Karlsson and coauthors reviewed 112 patients who underwent repeat AVM radiosurgery and compared complication rates after the first procedure with complication rates after repeat AVM radiosurgery.82 Sixty-two of 101 patients with angiographic follow-up exhibited complete obliteration; 14 patients had radiation-related complications after a second radiosurgical procedure. They concluded that the obliteration rate after repeat radiosurgery is similar to that after primary procedures but that the complication rate increases with the overall amount of radiation given. Maesawa and associates noted complete obliteration in 21 of 30 patients (70%) on angiographic follow-up after repeat AVM radiosurgery.83 Permanent radiation-related complications were noted in 2 of 41 patients (5%); the annual risk for hemorrhage after repeat AVM radiosurgery was 1.6%. Foote and colleagues analyzed 52 patients who underwent repeat LINAC-based AVM radiosurgery between 1991 and 1998.81 The mean volume of the AVMs was 66% smaller at the time of repeat radiosurgery. The cure rate after repeat radiosurgery was 60%. Schlienger and coworkers also noted complete obliteration in 19 of 32 patients (59%) who underwent repeat LINAC-based AVM radiosurgery.84
Radiosurgery for Large Intracranial Arteriovenous Malformations
Studies on the dose-volume relationship of AVM radiation-related complications have demonstrated an unacceptable rate of complications for large AVMs after radiosurgery. Miyawaki and coauthors reported that radiation necrosis developed in 22% of patients after LINAC-based radiosurgery for AVMs greater than 14 cc.85 Alternative strategies that use radiation for the management of large AVMs include embolization followed by radiosurgery, fractionated radiation techniques, and staged-volume radiosurgery. Planned embolization plus radiosurgery has been used for many years to manage patients with large AVMs.86–90 Unlike presurgical embolization, where reduction in flow is the goal, embolization before radiosurgery must achieve a permanent reduction in volume with minimal morbidity to be a useful adjunct. Gobin and colleagues published the results of 125 patients who underwent acrylate embolization followed by radiosurgery.86 Although complete AVM obliteration was noted in 53 of 90 evaluable patients (59%), 16 patients (13%) had complications from the embolization procedures, and 2 died of intracerebral hemorrhage before undergoing radiosurgery. In addition, nidus recanalization was seen in 14% of patients. Wikholm and coauthors reported the complications associated with AVM embolization as primary treatment or in preparation for either surgical resection or radiosurgery.89,90 The overall procedural complication rate was 40%, but only 7% were considered severe. The procedural mortality rate was 1.3%. Based on such studies, planned embolization before radiosurgery is rarely used to manage patients with large AVMs.
Fractionated radiation therapy has also been used to treat patients with large AVMs.23,24,91,92 Karlsson and associates reviewed 28 AVM patients who underwent fractionated radiation therapy between 1980 and 1985.23 The median volume treated was 78 cc. Only 2 patients (7%) achieved angiographically confirmed cure with a fractionation scheme of 42 Gy in 12 fractions. They concluded that conventional radiation therapy provides little protection against future bleeding. A number of centers have more recently examined the efficacy of hypofractionated radiation schedules in which higher radiation doses per fraction are used to treat patients with intracranial AVMs.24,91,92 Lindvall and coworkers used hypofractionated radiation therapy to treat 36 AVM patients.92 Two-year follow-up angiography showed that 48% of the patients were cured; angiographically corroborated obliteration rates rose to 76% at the 5-year follow-up. Veznedaroglu and colleagues managed 30 patients with large AVMs from 1995 to 1998 with a combination of preradiation embolization and hypofractionated radiation therapy.24 The radiation schedules were either 42 Gy in six fractions (n = 7) or 30 Gy in six fractions (n = 23). Obliteration was confirmed on 5-year follow-up angiography in 83% of patients in the 42-Gy group (5 of 6 patients) versus only 22% of patients in the 30-Gy group (4 of 18 patients). However, the morbidity rate in patients receiving 42 Gy was 43%, and the most effective hypofractionated dosing schedule to achieve AVM obliteration with an acceptable rate of radiation-related morbidity remains unclear.
Staged-volume radiosurgery has emerged as an option for patients with large-volume AVMs.93–96 Volume staging of large AVMs consists of performing multiple radiosurgical procedures separated by several months with each procedure covering a different portion of the nidus. This strategy permits a higher radiation dose to be delivered to the entire AVM volume while reducing radiation exposure to adjacent brain tissue. We compared the dosimetry of our first 10 patients who underwent staged-volume AVM radiosurgery with hypothetical single-session procedures covering the same volume and using the same margin and maximum radiation doses.94 Staged-volume radiosurgery decreased V12 by an average of 11.1%, and the non-AVM V12 was reduced by an average of 27.2%. Sirin and coauthors reported 28 patients who underwent staged-volume AVM radiosurgery at the University of Pittsburgh.95 The median AVM volume was 22.2 cc, and the median AVM margin dose used at each procedure was 16 Gy. Seven of 21 patients (33%) with follow-up beyond 36 months achieved AVM obliteration. Four patients (14%) had bleeding after radiosurgery, 2 patients died, and 2 had new neurological deficits. In 4 patients (14%), areas of increased signal on MRI developed and they required corticosteroid medications; no patient had a permanent new radiation-related deficit. To date, we have completed staged-volume radiosurgery in 25 patients with large AVMs. The treatment was completed in two procedures for 22 patients, three procedures for 2 patients, and four procedures for 1 patient. The median AVM volume was 16.2 cc; the median AVM margin dose was 16 Gy. Obliteration was noted in 6 of 18 patients (33%) with imaging performed 3 or more years after completion of staged-volume radiosurgery. Four patients (16%) sustained eight hemorrhages after staged-volume radiosurgery. Three patients suffered neurological deficits from the bleeding, and 1 patient died. In no patient did a radiation-related complication develop. More information is needed, however, to determine whether staged-volume AVM radiosurgery will be a useful technique in patients with large AVMs.
Radiosurgery-Based Arteriovenous Malformation Grading System
The Spetzler-Martin grading system is the most widely used system for grading intracranial AVMs.16 Consisting of three components (AVM size, location, and pattern of venous drainage), this system has been validated prospectively18 and by other cerebrovascular centers of excellence.19–22 Unfortunately, this grading scale is insensitive to factors associated with successful AVM radiosurgery, such as volume and location.97 Therefore, in collaboration with the University of Pittsburgh, our center developed a radiosurgery-based AVM grading system that accounts for these shortcomings to potentially guide management decisions for individual AVM patients.37 Initially called the Pittsburgh arteriovenous malformation (PAR) grading scale,98 it was based on multivariate analysis of factors that correlated with AVM obliteration without new neurological deficits after single-session AVM radiosurgery. Yet calculation of a patient’s PAR grade proved too cumbersome to be practical because five separate variables were required, as well as a y-intercept value. Therefore, regression analysis modeling was used to simplify the grading scale and develop the radiosurgery-based AVM grading system.37 This grading system is based on three factors (patient age, AVM volume, AVM location) and was shown to predict outcomes in patients undergoing AVM radiosurgery at the Mayo Clinic from 1990 to 1997. Of significance, the grading system does not include any treatment-related factors such as radiation dose, so it can be used to counsel individual patients on the chance of success after AVM radiosurgery. Other studies have correlated the AVM score with outcomes of patients with deep AVMs,99–101 pediatric AVM patients,102–104 and patients undergoing LINAC-based radiosurgery.99,101,104–106 Finally, although this grading system was created to predict outcomes after a single radiosurgical procedure, it also correlates with outcomes after one or more radiosurgical procedures107 and with the chance of neurological decline after AVM radiosurgery.108–110
The radiosurgery-based AVM system was recently modified by using location as a two-tiered variable (deep versus other) rather than a three-tiered variable (Table 260-1).111 This simplification did not affect the accuracy of the scale in comparison to the original version. Routine use of this system allows more accurate prediction of outcomes after radiosurgery to guide choices between surgical and radiosurgical management of individual AVM patients. To provide the best care for AVM patients, we should use every tool available to individualize their management by taking into account the patient’s age, signs and symptoms, nidus size and morphology, AVM location, and patient preference.
TABLE 260-1 Modified Radiosurgery-Based Grading System for Arteriovenous Malformations
| AVM SCORE* | EXCELLENT OUTCOMES | DECLINE IN MRS |
|---|---|---|
| ≤1.00 (n = 53) | 89% | 0% |
| 1.01-1.50 (n = 83) | 70% | 13% |
| 1.51-2.00 (n = 61) | 64% | 20% |
| >2.00 (n = 50) | 46% | 36% |
AVM, arteriovenous malformation; BG, basal ganglia; BS, brainstem; MRS, modified Rankin scale.
* AVM score = (0.1) (AVM volume [cc]) + (0.02) (Patient age [yr]) + (0.5) (AVM location [hemispheric/cerebellar = 0, BG/thalamus/BS = 1]).
Dural Arteriovenous Fistulas
Pathogenesis of Dural Arteriovenous Fistulas
DAVFs are acquired vascular malformations that are distinct from parenchymal AVMs. DAVFs are believed to account for approximately 10% to 15% of all intracranial vascular malformations. Houser and associates defined the angiographic characteristics of DAVFs and related their development to venous sinus thrombosis.112,113 DAVFs consist of a nidus of arteriovenous shunting within the dura mater. In most cases, they occur in proximity or continuity to a major dural venous sinus or cortical vein. Multiple hypotheses based on clinical observation, examination of resected pathologic specimens, and animal experiments investigating various possible provocative factors have been advanced regarding the pathogenesis of DAVFs. The primary theory on the development of DAVFs relates dural sinus thrombosis and the development of venous hypertension as the hallmark events.114,115 Normal microscopic arteriovenous shunts in the dural sinus wall are then thought to enlarge after sinus thrombosis secondary to venous hypertension. Increased blood flow through these fistulous channels leads to further venous hypertension as the patent portion of the sinus is exposed to arterial inflow pressure. This induces further thrombosis related to turbulent flow within the sinus and high pressure, as well as a spiral of events that increases the size of the lesion and the volume of arteriovenous shunting. This theory has been tested in animal models in which carotid-to–external jugular vein anastomoses, often combined with experimentally induced sinus thrombosis, resulted in venous hypertension and the development of DAVFs.116,117
However, not all DAVFs are seen in conjunction with sinus thrombosis, so it is thought that other factors also play a role in the formation of DAVFs. Another theory on DAVF formation posits that local angiogenic factors cause blood vessels to develop in response to venous hypertension. Surgical DAVF specimens have been shown to contain basic fibroblast growth factor, a potent angiogenic growth stimulator.118 Lawton and colleagues demonstrated increased angiogenesis in a rat model of venous hypertension and sinus thrombosis.119 They hypothesized that venous hypertension induces angiogenic activity as a result of local ischemia and that DAVFs may be the consequence of this induced aberrant angiogenesis. A third factor in the pathogenesis of DAVFs that has received less attention is the role of hypercoagulability.120 Patients with hypercoagulable conditions are predisposed to venous sinus thrombosis and the development of DAVFs, and this may account for the spontaneous obliteration noted in some patients. After the diagnosis of a DAVF, patients are generally evaluated with the following tests: prothrombin time, partial thromboplastin time, bleeding time, protein S and C levels, antiphospholipid and anticardiolipin antibodies, Leiden factor V mutation, and homocysteine levels.
Clinical Findings and Natural History of Dural Arteriovenous Fistulas
DAVFs can be accompanied by ICH, seizures, and focal neurological deficits, similar to pial AVMs. In addition, local symptoms such as proptosis, chemosis, or visual loss can occur in patients with a DAVF involving the cavernous sinus. Likewise, patients with DAVFs of the transverse or sigmoid sinuses frequently have pulsatile tinnitus as their primary complaint. Reviews of the literature have shown that although some locations are predisposed to hemorrhage,121–123 no location is immune from potential aggressive behavior. Awad and associates noted that although 80% to 90% of patients with DAVFs of the tentorium are initially evaluated because of ICH, only 10% to 20% of patients with DAVFs of the transverse/sigmoid region or cavernous sinus have bleeding as the initial manifestation.121 Singh and colleagues reviewed 402 patients with DAVFs evaluated from 1988 to 2004 and found five factors associated with hemorrhagic findings: cortical venous drainage (CVD), focal neurological deficits, DAVFs located in the posterior fossa, male sex, and increasing age.123
Our understanding of the natural history of DAVFs is rather limited because of their relative rarity and the tendency to treat patients with bleeding or other disabling symptoms. Generally, DAVFs are classified as high or low risk based on the pattern of venous drainage.124,125 DAVFs with retrograde drainage or CVD are typically considered to be associated more frequently with ICH, thus warranting more aggressive treatment, whereas patients without CVD are frequently observed or treatment is recommended only if their associated symptoms are significant. Brown and coauthors reported 54 untreated patients with DAVFs monitored for a mean of 6.6 years.126 Five patients experienced an ICH for an estimated annual risk of bleeding of 1.8%. Most hemorrhages occurred early, within 3 years of diagnosis. They concluded that only variceal venous drainage was a predictor of future hemorrhage. Duffau and coworkers observed 20 patients with hemorrhage from DAVFs; all the patients had CVD.127 Four patients had radiographically documented rebleeding, and another 3 had clinical suspicion of rebleeding for a recurrent hemorrhage rate of 20% to 35%. These rebleeding episodes all occurred within 2 weeks of the original hemorrhage and were associated with worsened neurological deficits. Conversely, Soderman and colleagues calculated the annual incidence of ICH based on 85 patients with DAVFs and CVD from 1978 to 2007.128 The annual ICH rate was 7.4% for patients (n = 53) who had an ICH as their initial symptom versus 1.5% in patients (n = 32) without ICH as the initial symptom. Despite these conflicting reports, most clinicians recommend treatment aimed at eliminating retrograde drainage or CVD when present.
Management of Intracranial Dural Arteriovenous Fistulas
Management of DAVFs has evolved over the past 3 decades. For minimally symptomatic or asymptomatic lesions, observation alone is often recommended because spontaneous regression of DAVFs is a well-documented phenomenon.129–131 Surgical resection of the fistulous dura, usually including the involved sinus, was often performed for symptomatic patients through the 1970s and 1980s.132,133 As the pathophysiology of DAVFs has become better understood, disconnection of the vein bridging the dural fistula and the brain has evolved to become the surgical method of choice when possible.134,135 Currently, initial treatment of most DAVFs involves endovascular techniques. Transvenous embolization has been advocated as the preferred endovascular treatment route because of higher occlusion rates, fewer complications, and a lower rate of recanalization than with transarterial embolization.136–140
Radiosurgery for Dural Arteriovenous Fistulas
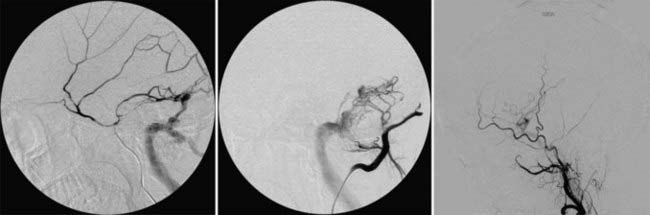
Radiosurgery has been used as a treatment option for patients with DAVFs over the past 2 decades.141–149 Similar to the experience with AVMs, radiosurgery is expected to cause obliteration of DAVFs 1 to 3 years after the procedure. At our center, radiosurgery is frequently supplemented with transarterial embolization, usually performed within 48 hours after radiosurgery, to provide early symptom relief and to reduce or eliminate CVD (Fig. 260-2).143,147,148 In most cases, the combination of radiosurgery and transarterial embolization provides both rapid symptom relief and long-term cure of DAVFs. From January 1993 to February 2008, we performed 148 radiosurgical procedures on patients with DAVF (Table 260-2). Ninety-five patients (64%) underwent embolization procedures as part of the planned treatment approach.
TABLE 260-2 Location of Dural Arteriovenous Fistulas in 148 Patients after Radiosurgery
| LOCATION | NUMBER OF PATIENTS |
|---|---|
| Transverse/sigmoid sinus | 61 |
| Cavernous sinus | 38 |
| Jugular bulb | 13 |
| Petrosal sinus | 11 |
| Vein of Galen | 9 |
| Other | 16 |
Results of Radiosurgery for Dural Arteriovenous Fistulas
Radiosurgery has proved to a safe option for the management of patients with DAVFs. In the majority of patients the initial symptoms are eliminated or improved, especially when radiosurgery is combined with embolization (Fig. 260-2). Moreover, the incidence of radiation-related complications has been exceedingly low, with most complications being related to the embolization procedures and not radiosurgery itself. We previously reviewed the outcomes of patients with DAVFs involving the cavernous sinus and transverse/sigmoid regions.143,148 Twenty patients (17 women, 3 men) with a median age of 67 years underwent radiosurgery for cavernous sinus DAVFs.148 All patients had symptoms referable to their fistula at initial evaluation; no patient had a previous ICH. Four patients had CVD. Twelve patients (60%) underwent particulate embolization after radiosurgery. At a median of 36 months after radiosurgery, 19 patients (95%) had elimination of or a significant reduction in their symptoms. Notably, 7 of 8 patients (88%) with decreased visual fields or visual acuity regained normal vision. Thirteen of 15 patients (87%) with follow-up angiography demonstrated complete obliteration of their DAVF. Friedman and associates evaluated the clinical and angiographic results of 25 patients (18 women, 7 men) with DAVFs of the transverse/sigmoid sinus region.143 The median patient age was 57 years, and the most common symptom was pulsatile tinnitus (n = 22, 88%). Two patients had a previous history of ICH. Four patients had CVD; these patients were thought to be at high risk for open surgery because of advanced age and medical comorbid conditions. The median PIV was much larger for DAVFs of the transverse/sigmoid region than for those of the cavernous sinus (9.6 versus 2.8 cc). Seventeen patients (68%) underwent transarterial embolization after radiosurgery during one (n = 13) or more (n = 4) sessions. At a mean follow-up of 50 months after radiosurgery, symptoms completely resolved in 20 patients (87%) and were significantly improved in 2 (9%). Two patients had recurrence of their tinnitus at 10 and 12 months, respectively, after combined radiosurgery and embolization. Both patients underwent repeat radiosurgery and embolization at 21 and 38 months. No patient suffered an ICH or radiation-related complication after radiosurgery. Seventeen patients had angiographic follow-up at a mean of 21 months after radiosurgery. Total angiographically documented obliteration of the DAVF was seen in 7 patients (41%). We have had 1 patient who experienced a fatal hemorrhage 17 months after radiosurgery for a superior sagittal sinus DAVF.150 There have been no clinical or radiographic hemorrhages in the other 147 patients treated to date, including patients who were initially seen with hemorrhage or had CVD (or both).
Other centers have had similar results with radiosurgery for patients with DAVFs. Patients with indirect cavernous sinus DAVFs have had the most impressive results, with nearly complete resolution of ocular symptoms and high angiographic obliteration rates. Barcia-Salorio and coauthors reported 22 patients with spontaneous, low-flow carotid-cavernous DAVFs.141 Twenty of 22 DAVFs (91%) were obliterated at a mean of 7.5 months after radiosurgery. Guo and associates noted DAVF obliteration in 12 of 15 patients (80%) after radiosurgery for their cavernous sinus DAVF.144
Cavernous Malformations
Indications for Radiosurgery for Cavernous Malformations
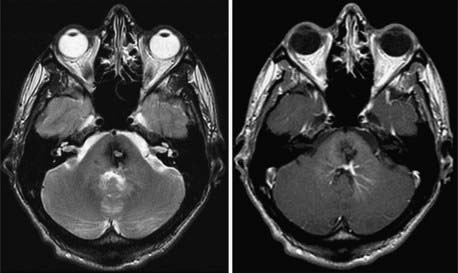
CMs are vascular lesions that account for the majority of angiographically occult vascular malformations diagnosed in the modern neuroimaging era. Although often seen on cerebral angiography, CMs have a typical appearance on MRI that has permitted better understanding of their incidence and natural history. CMs may be manifested as bleeding or seizures or be detected as incidental findings. Surgical resection of CMs is the preferred management for patients with symptomatic lesions.151,152 Resection of CMs allows clot removal, improvement of seizures, and protection against future ICH. Radiosurgery has been performed as an option for a select group of patients with CMs.153–163 The goal of performing CM radiosurgery is the same as that for AVM radiosurgery: to protect the patient from future risk for ICH.
The effect that radiosurgery has on the endothelial-lined channels of CMs is poorly understood. Gewirtz and colleagues examined 11 CMs that were resected 1 to 10 years after either radiation therapy or radiosurgery and compared them histopathologically with a group of nonirradiated controls.164 Fibrinoid necrosis was noted in the irradiated CMs. Of note, 10 of 11 patients had bleeding after radiosurgery, so they are probably not representative of successful CM radiosurgery. Karlsson and coworkers reported a patient who underwent CM radiosurgery with resection of the lesion 5 years later.157 More than 75% of the vascular channels were obliterated. Based on these few examined specimens, it appears that the response of CMs to radiosurgery is similar to what has been observed in subtotally obliterated AVMs. The current indication for CM radiosurgery is a patient with a history of multiple ICHs from a surgically inaccessible lesion (Fig. 260-3). CM radiosurgery remains controversial, although it has been performed for more than 2 decades.
Results of Radiosurgery for Cavernous Malformations
Studies examining patient outcomes after CM radiosurgery have noted higher annual hemorrhage rates for the first 1 to 3 years after the procedure and then a decline in bleeding events in the remainder of their follow-up. Hasegawa and colleagues from the University of Pittsburgh reported the result of CM radiosurgery in 82 patients from 1987 to 2000.155 The mean follow-up after radiosurgery was 5 years. The annual hemorrhage rate was 12.3% for the first 2 years after radiosurgery and then 0.8% thereafter. Kida and Hasegawa reported the outcomes of 152 patients who underwent CM radiosurgery.158 The hemorrhage rate was 8% the first year after radiosurgery, 5% the second year, and 0% by year 7. Other smaller series have documented annual hemorrhage rates from 4.5% to 9.4% for the first several years after radiosurgery and then lower bleeding rates in later years of follow-up.154,156,157,159,162
It is difficult to determine whether CM radiosurgery reduces risk for ICH for two primary reasons. First, unlike AVM radiosurgery, where obliteration is confirmed with angiography, CMs generally do not have significant changes in their MRI appearance, so it is the clinical course of the patient that is followed to determine whether radiosurgery has reduced the risk for bleeding. Second, the natural history of these lesions remains poorly understood.165–167 The statistical method most commonly used to determine the effect that radiosurgery has had on bleeding risk is to compare the hemorrhage rate in patients before and after the procedure. However, because patients tend to be treated shortly after they have bled, this methodology tends to inflate the annual bleeding risk. In addition, the observation that untreated CMs tend to bleed in “clusters” followed by a later reduction in bleeding creates doubt that radiosurgery has any true effect on hemorrhage risk in these patients.165 A prospective, randomized trial comparing radiosurgery with conservative therapy for patients with hemorrhagic, surgically high-risk CMs is needed to accurately determine the true effect that CM radiosurgery has on hemorrhage risk in these patients.
Another important concern associated with CM radiosurgery is the risk for radiation-related complications. It appears that the number of radiation-related complications noted after CM radiosurgery is greater than that in matched cohorts of AVM radiosurgical patients. The reported rate of permanent radiation-related complications after CM radiosurgery has ranged from 0% to 41%.153–163 Karlsson and coauthors reported 22 patients who underwent radiosurgery for CMs.157 Permanent radiation-related complications developed in 5 patients (23%). Amin-Hanjabi and associates found a 16% incidence of permanent radiation-associated complications in 98 patients who underwent proton beam therapy for CMs between 1977 and 1993.153 In our series, 7 of 17 patients (41%) had permanent radiation-related complications.162 The majority of patients (12 of 17, 71%) received a marginal radiation dose below the recommendations of Kondziolka and colleagues.160 Factors believed to be related to the increased complication rate observed after CM radiosurgery include the use of imaging techniques other than MRI for dose planning, targeting of the hemosiderin-stained brain rather than the nidus itself, and a radiation-sensitizing effect of iron.
Cavernous Malformation Radiosurgery: Seizure Protection
It has been noted that CM patients who undergo radiosurgery appear to have fewer seizures after the procedure. Regis and associates retrospectively reviewed the experience of five centers to evaluate the efficacy of CM radiosurgery on epilepsy control.163 Forty-nine patients with drug-resistant epilepsy were identified. At an average follow-up of 24 months, 26 patients (53%) were seizure free. Patients with CMs in the medial temporal region had the greatest risk of failure. They concluded that seizure control can be achieved when there is a clear electroclinical correlation between CM location and the epileptogenic zone. Two studies have compared seizure control in CM patients undergoing radiosurgery or surgical excision.168,169 Shih and Pan compared 46 patients with solitary supratentorial CMs who underwent craniotomy and excision (n = 16) with patients treated by radiosurgery (n = 30).169 Eleven of 14 patients (79%) in the craniotomy group remained seizure free as compared with 4 of 16 patients (25%) in the radiosurgical group. Hsu and colleagues found that 13 of 15 patients (87%) were seizure free after CM resection as opposed to 9 of 14 patients (64%) treated with LINAC-based radiosurgery.168 Thus, surgical resection should be considered the first option for CM patients with epilepsy when their lesion is surgically accessible.
Andrade-Souza YM, Zadeh G, Ramani M, et al. Testing the radiosurgery-based arteriovenous malformation score and the modified Spetzler-Martin grading system to predict radiosurgical outcome. J Neurosurg. 2005;103:642-648.
Barker FGII, Amin-Hanjabi S, Butler WE, et al. Temporal clustering of hemorrhages from untreated cavernous malformations of the central nervous system. Neurosurgery. 2001;49:15-25.
Borden JA, Wu JK, Shucart WA. A proposed classification for spinal and cranial dural arteriovenous fistulous malformations and implications for treatment. J Neurosurg. 1995;82:166-179.
Cognard C, Gobin YP, Pierot L, et al. Cerebral dural arteriovenous fistulas: Clinical and angiographic correlation with a revised classification of venous drainage. Radiology. 1995;194:671-680.
Flickinger JC, Kondziolka D, Lunsford LD, et al. Development of a model to predict permanent symptomatic post-radiosurgery injury for arteriovenous malformation patients. Int J Radiat Onol Biol Phys. 2000;46:1143-1148.
Flickinger JC, Pollock BE, Kondziolka D, et al. A dose-response analysis of arteriovenous malformation obliteration by radiosurgery. Int J Radiat Oncol Biol Phys. 1996;36:873-879.
Foote KD, Friedman WA, Ellis TL, et al. Salvage retreatment after failure of radiosurgery in patients with arteriovenous malformations. J Neurosurg. 2003;98:337-341.
Friedman WA, Blatt DL, Bova FJ, et al. The risk of hemorrhage after radiosurgery for arteriovenous malformations. J Neurosurg. 1996;84:912-919.
Friedman JA, Pollock BE, Nichols DA, et al. Results of combined stereotactic radiosurgery and transarterial embolization for dural arteriovenous fistulae of the transverse and sigmoid sinuses. J Neurosurg. 2001;94:886-891.
Hasegawa T, McInerney J, Kondziolka D, et al. Long-term results after stereotactic radiosurgery for patients with cavernous malformations. Neurosurgery. 2002;50:1190-1197.
Kida Y, Hasegawa T. Radiosurgery for cavernous malformations: results of long-term follow-up. In: Kondziolka D, editor. Radiosurgery. Basel: Karger; 2004:153-160.
Liscak R, Vladyka V, Simonova G, et al. Arteriovenous malformations after Leksell gamma knife radiosurgery: rate of obliteration and complications. Neurosurgery. 2007;60:1005-1016.
Maesawa S, Flickinger JC, Kondziolka D, et al. Repeated radiosurgery for incompletely obliterated arteriovenous malformations. J Neurosurg. 2000;92:961-970.
Maruyama K, Kawahara N, Shin M, et al. The risk of hemorrhage after radiosurgery for cerebral arteriovenous malformations. N Engl J Med. 2005;352:146-153.
Pollock BE, Flickinger JC. Modification of the radiosurgery-based arteriovenous malformation grading system. Neurosurgery. 2008;63:239-243.
Pollock BE, Gorman DA, Coffey RJ. Patient outcomes after arteriovenous malformation radiosurgical management: results based on a 5- to 14-year follow-up study. Neurosurgery. 2003;52:1291-1297.
Pollock BE, Kline RW, Stafford SL, et al. The rationale and technique of staged-volume arteriovenous malformation radiosurgery. Int J Radiat Oncol Biol Phys. 2000;48:817-824.
Pollock BE, Nichols DA, Garrity JA, et al. Stereotactic radiosurgery and particulate embolization for cavernous sinus dural arteriovenous fistulae. Neurosurgery. 1999;45:459-467.
Regis J, Bartolomei F, Kida Y, et al. Radiosurgery for epilepsy associated with cavernous malformation: retrospective study in 49 patients. Neurosurgery. 2000;47:1091-1097.
Shin M, Kawahara N, Maruyama K, et al. Risk of hemorrhage from an arteriovenous malformation confirmed to have been obliterated on angiography after stereotactic radiosurgery. J Neurosurg. 2005;102:842-846.
Singh V, Smith WS, Lawton ML, et al. Risk factors for hemorrhagic presentation in patients with dural arteriovenous fistulae. Neurosurgery. 2008;62:628-635.
Sirin S, Kondziolka D, Niranjan A, et al. Prospective staged volume radiosurgery for large arteriovenous malformations: indications and outcomes in otherwise untreatable patients. Neurosurgery. 2006;58:17-27.
Stapf C, Mast H, Sciacca JH, et al. Predictors of hemorrhage in patients with untreated brain arteriovenous malformation. Neurology. 2006;66:1350-1355.
Stapf C, Mast H, Sciacca RR, et al. The New York Islands AVM Study: design, study progress, and initial results. Stroke. 2003;34:29-33.
Zipfel GJ, Bradshaw P, Bova FJ, et al. Do the morphological characteristics of arteriovenous malformations affect the results of radiosurgery? J Neurosurg. 2004;101:393-401.
1 McCormick WE. Pathology of vascular malformations of the brain. In: Wilson CB, Stein B, editors. Intracranial Arteriovenous Malformations. Baltimore: Williams & Wilkins; 1984:44-63.
2 Al-Shahi R, Bhattacharya JJ, Currie DG, et al. Prospective, population-based detection of intracranial vascular malformations in adults: the Scottish Intracranial Vascular Malformation Study (SIVMS). Stroke. 2003;34:1163-1169.
3 Stapf C, Mast H, Sciacca RR, et al. The New York Islands AVM Study: design, study progress, and initial results. Stroke. 2003;34:29-33.
4 Brown RDJr, Wiebers DO, Forbes G, et al. The natural history of unruptured intracranial arteriovenous malformations. J Neurosurg. 1988;68:352-357.
5 Crawford PM, West CR, Park YG, et al. Arteriovenous malformations of the brain: natural history in unoperated patients. J Neurol Neurosurg Psychiatry. 1986;49:1-10.
6 Fults D, Kelly DLJr. Natural history of arteriovenous of the brain: a clinical study. Neurosurgery. 1984;15:658-662.
7 Graf CJ, Perret GB, Torner JC. Bleeding from cerebral arteriovenous malformations as part of their natural history. J Neurosurg. 1983;58:331-337.
8 Itoyama Y, Uemura S, Ushio Y, et al. Natural course of unoperated intracranial arteriovenous malformations: study of 50 cases. J Neurosurg. 1989;71:805-809.
9 Ondra SK, Troupp H, George ED, et al. The natural history of symptomatic arteriovenous malformations of the brain: a 24-year follow-up assessment. J Neurosurg. 1990;73:387-391.
10 Pollock BE, Flickinger JC, Lunsford LD, et al. Factors that predict the bleeding risk of cerebral arteriovenous malformations. Stroke. 1996;27:1-6.
11 Stapf C, Mast H, Sciacca JH, et al. Predictors of hemorrhage in patients with untreated brain arteriovenous malformation. Neurology. 2006;66:1350-1355.
12 Stapf C, Khaw AV, Sciacca RR, et al. Effect of age on clinical and morphological characteristics in patients with brain arteriovenous malformations. Stroke. 2003;34:2664-2669.
13 Stapf C, Mohr JP, Pile-Spellman J, et al. Concurrent arterial aneurysms in brain arteriovenous malformations with haemorrhagic presentation. J Neurol Neurosurg Psychiatry. 2002;73:294-298.
14 Kondziolka D, Humphreys RP, Hoffman HJ, et al. Arteriovenous malformations of the brain in children: a forty year experience. Can J Neurol Sci. 1992;19:40-45.
15 Spetzler RF, Hargraves RW, McCormick PW, et al. Relationship of perfusion pressure and size to risk of hemorrhage from arteriovenous malformations. J Neurosurg. 1992;76:918-923.
16 Spetzler RF, Martin NA. A proposed grading system for arteriovenous malformations. J Neurosurg. 1986;65:476-483.
17 de Oliveria E, Tedeschi H, Raso J. Comprehensive management of arteriovenous malformations. Neurol Res. 1998;20:673-683.
18 Hamilton MG, Spetzler RF. The prospective application of a grading system for arteriovenous malformations. Neurosurgery. 1994;34:2-7.
19 Heros RC, Korosue K, Diebold PM. Surgical excision of cerebral arteriovenous malformations. Neurosurgery. 1990;26:570-578.
20 Pik JHT, Morgan MK. Microsurgery for small arteriovenous malformations of the brain: results in 110 consecutive patients. Neurosurgery. 2000;47:571-577.
21 Pikus HJ, Beach ML, Harbaugh RE. Microsurgical treatment of arteriovenous malformations: analysis and comparison with stereotactic radiosurgery. J Neurosurg. 1998;88:641-646.
22 Schaller C, Schramm J. Microsurgical results for small arteriovenous malformations accessible for radiosurgical or embolization treatment. Neurosurgery. 1997;40:664-674.
23 Karlsson B, Lindqvist M, Blomgren H, et al. Long-term results after fractionated radiation therapy for large brain arteriovenous malformations. Neurosurgery. 2005;57:42-49.
24 Veznedaroglu E, Andrews D, Benitez R, et al. Fractionated stereotactic radiotherapy for the treatment of large arteriovenous malformations with or without previous partial embolization. Neurosurgery. 2004;55:519-530.
25 Steiner L, Leksell L, Grietz T, et al. Stereotaxic radiosurgery for cerebral arteriovenous malformations: report of a case. Acta Chir Scand. 1972;138:459-464.
26 Leksell L. Stereotactic radiosurgery. J Neurol Neurosurg Psychiatry. 1983;46:797-803.
27 Kjellberg RN, Hanamura T, Davis KR, et al. Bragg-peak proton-beam therapy for arteriovenous malformations. N Engl J Med. 1983;309:269-274.
28 Steinberg GK, Fabrikant JI, Marks MP, et al. Stereotactic heavy-charged-particle Bragg-peak radiation for intracranial arteriovenous malformations. N Engl J Med. 1990;323:96-101.
29 Betti OO, Munari C, Rosler R. Stereotactic radiosurgery with the linear accelerator: treatment of arteriovenous malformations. Neurosurgery. 1989;24:311-321.
30 Columbo F, Pozza F, Chierego G, et al. Linear accelerator radiosurgery of cerebral arteriovenous malformations: an update. Neurosurgery. 1994;34:14-21.
31 Friedman WA, Bova FJ, Mendenhall WM. Linear accelerator radiosurgery for arteriovenous malformations: the relationship of size to outcome. J Neurosurg. 1995;82:180-189.
32 Han PP, Ponce FA, Spetzler RF. Intention-to-treat analysis of Spetzler-Martin grades IV and V arteriovenous malformations: natural history and treatment paradigm. J Neurosurg. 2003;98:3-7.
33 Choi JH, Mohr JP. Brain arteriovenous malformations in adults. Lancet Neurol. 2005;4:299-308.
34 Hartmann A, Mast H, Mohr JP, et al. Morbidity of intracranial hemorrhage in patients with cerebral arteriovenous malformation. Stroke. 1998;29:931-934.
35 Hartmann A, Stapf C, Hofmeister C, et al. Determinants of neurological outcome after surgery for brain arteriovenous malformations. Stroke. 2000;31:2361-2364.
36 Lawton MT, Du R, Tran MN, et al. Effect of presenting hemorrhage on outcome after microsurgical resection of brain arteriovenous malformations. Neurosurgery. 2005;56:485-493.
37 Pollock BE, Flickinger JC. A proposed radiosurgery-based grading system for arteriovenous malformations. J Neurosurg. 2002;96:79-85.
38 Zhao J, Wang S, Li J, et al. Clinical characteristics and surgical results of patients with cerebral arteriovenous malformations. Surg Neurol. 2005;63:156-161.
39 Piepgras DG, Sundt TMJr, Ragoowansi AT, et al. Seizure outcome in patients with surgically treated cerebral arteriovenous malformations. J Neurosurg. 1993;78:5-11.
40 Schäuble B, Cascino GD, Pollock BE, et al. Seizure outcomes after stereotactic radiosurgery for cerebral arteriovenous malformations. Neurology. 2004;63:683-687.
41 Hoh BL, Chapman PH, Loeffler JS, et al. Results of multimodality treatment for 141 patients with brain arteriovenous malformations and seizures: factors associated with seizure incidence and seizure outcome. Neurosurgery. 2002;51:303-309.
42 Blatt DR, Friedman WA, Bova FJ. Modifications based on computed tomographic imaging in planning the radiosurgical treatment of arteriovenous malformations. Neurosurgery. 1993;33:588-595.
43 Kondziolka D, Lunsford LD, Kanal E, et al. Stereotactic magnetic resonance angiography for targeting in arteriovenous malformation radiosurgery. Neurosurgery. 1994;35:585-591.
44 Flickinger JC, Pollock BE, Kondziolka D, et al. A dose-response analysis of arteriovenous malformation obliteration by radiosurgery. Int J Radiat Oncol Biol Phys. 1996;36:873-879.
45 Karlsson B, Lindquist C, Steiner L. Prediction of obliteration after gamma knife surgery for cerebral arteriovenous malformations. Neurosurgery. 1997;40:425-431.
46 Flickinger JC. An integrated logistic formula for prediction of complications from radiosurgery. Int J Radiat Oncol Biol Phys. 1989;17:879-885.
47 Flickinger JC, Kondziolka D, Lunsford LD, et al. Development of a model to predict permanent symptomatic post-radiosurgery injury for arteriovenous malformation patients. Int J Radiat Oncol Biol Phys. 2000;46:1143-1148.
48 Lax I, Karlsson B. Prediction of complications in gamma knife radiosurgery of arteriovenous malformations. Acta Oncol. 1996;35:49-56.
49 Voges J, Treuer H, Lehrke R, et al. Risk analysis of LINAC radiosurgery in patients with arterio-venous malformations (AVM). Acta Neurochir. 1997;68:118-123.
50 Pollock BE, Kondziolka D, Lunsford LD, et al. Magnetic resonance imaging: an accurate method to evaluate arteriovenous malformations after stereotactic radiosurgery. J Neurosurg. 1996;85:1044-1049.
51 Schneider BF, Eberhard DA, Steiner LE. Histopathology of arteriovenous malformations after gamma knife radiosurgery. J Neurosurg. 1997;87:352-357.
52 Szeifert GT, Major O, Kemeny AA. Ultrastructural changes in arteriovenous malformations after gamma knife surgery: an electron microscopic study. J Neurosurg. 2005;102(Suppl):289-292.
53 Liscak R, Vladyka V, Simonova G, et al. Arteriovenous malformations after Leksell gamma knife radiosurgery: rate of obliteration and complications. Neurosurgery. 2007;60:1005-1016.
54 Shin M, Maruyama K, Kurita H, et al. Analysis of nidus obliteration rates after gamma knife surgery for arteriovenous malformations based on long-term follow-up data: the University of Tokyo experience. J Neurosurg. 2004;101:18-24.
55 Touboul E, Al Halabi A, Buffat L, et al. Single-fraction stereotactic radiotherapy: a dose-response analysis of arteriovenous malformation obliteration. Int J Radiat Oncol Biol Phys. 1998;41:855-861.
56 Schwartz M, Sixel K, Young C, et al. Prediction of obliteration of arteriovenous malformations: the obliteration prediction index. Can J Neurol Sci. 1997;24:106-109.
57 Ellis TL, Friedman WA, Bova FJ, et al. Analysis of treatment failure after radiosurgery for arteriovenous malformations. J Neurosurg. 1998;89:104-110.
58 Gallina P, Merienne L, Meder JF, et al. Failure in radiosurgery treatment of cerebral arteriovenous malformations. Neurosurgery. 1998;42:996-1004.
59 Kwon Y, Jeon SR, Kim JH, et al. Analysis of the causes of treatment failure in gamma knife radiosurgery for intracranial arteriovenous malformations. J Neurosurg. 2000;93(Suppl 3):104-106.
60 Pollock BE, Kondziolka D, Lunsford LD, et al. Repeat stereotactic radiosurgery of arteriovenous malformations: factors associated with incomplete obliteration. Neurosurgery. 1996;38:318-324.
61 Buis DR, Lagerwaard FJ, Barkhof F, et al. Stereotactic radiosurgery for brain AVMs: role of interobserver variation in target definition on digital subtraction angiography. Int J Radiat Oncol Biol Phys. 2005;62:246-252.
62 Yu C, Petrovich Z, Apuzzo ML, et al. Study of magnetic resonance imaging–based arteriovenous malformation delineation without conventional angiography. Neurosurgery. 2004;54:1104-1107.
63 Zipfel GJ, Bradshaw P, Bova FJ, et al. Do the morphological characteristics of arteriovenous malformations affect the results of radiosurgery? J Neurosurg. 2004;101:393-401.
64 Pollock BE, Lunsford LD, Kondziolka D, et al. Patient outcomes after stereotactic radiosurgery for “operable” arteriovenous malformations. Neurosurgery. 1994;35:1-8.
65 Friedman WA, Blatt DL, Bova FJ, et al. The risk of hemorrhage after radiosurgery for arteriovenous malformations. J Neurosurg. 1996;84:912-919.
66 Karlsson B, Lax I, Söderman M. Risk of hemorrhage during the 2-year latency period following gamma knife radiosurgery for arteriovenous malformations. Int J Radiat Oncol Biol Phys. 2001;49:1045-1051.
67 Maruyama K, Kawahara N, Shin M, et al. The risk of hemorrhage after radiosurgery for cerebral arteriovenous malformations. N Engl J Med. 2005;352:146-153.
68 Nataf F, Ghossoub M, Schlienger M, et al. Bleeding after radiosurgery for cerebral arteriovenous malformations. Neurosurgery. 2004;55:298-305.
69 Pollock BE, Flickinger JC, Lunsford LD, et al. Hemorrhage risk after stereotactic radiosurgery of cerebral arteriovenous malformations. Neurosurgery. 1996;38:652-661.
70 Lindqvist M, Karlsson B, Guo W, et al. Angiographic long-term follow-up data for arteriovenous malformations previously proven to be obliterated after gamma knife radiosurgery. Neurosurgery. 2000;46:803-810.
71 Shin M, Kawahara N, Maruyama K, et al. Risk of hemorrhage from an arteriovenous malformation confirmed to have been obliterated on angiography after stereotactic radiosurgery. J Neurosurg. 2005;102:842-846.
72 Levegrun S, Hof H, Essig M, et al. Radiation-induced changes of brain tissue after radiosurgery in patients with arteriovenous malformations: correlation with dose distribution parameters. Int J Radiat Oncol Biol Phys. 2004;59:796-808.
73 Chapman PH, Ogilvy CS, Loeffler JS. The relationship between occlusive hyperemia and complications associated with the radiosurgical treatment of arteriovenous malformations: report of two cases. Neurosurgery. 2004;55:228-234.
74 Pollock BE. Occlusive hyperemia. A radiosurgical phenomenon? Neurosurgery. 2000;47:1178-1184.
75 Kihlström L, Guo W, Karlsson B, et al. Magnetic resonance imaging of obliterated arteriovenous malformations up to 23 years after radiosurgery. J Neurosurg. 1997;86:589-593.
76 Pollock BE, Brown RDJr. Management of cysts arising after radiosurgery of intracranial arteriovenous malformations. Neurosurgery. 2001;49:259-265.
77 Yamamoto M, Jimbo M, Hara M, et al. Gamma knife radiosurgery for arteriovenous malformations: long-term follow-up results focusing on complications occurring more than 5 years after irradiation. Neurosurgery. 1996;38:906-914.
78 Yamamoto M, Ban S, Ide M, et al. A diffuse white matter ischemic lesion appearing 7 years after stereotactic radiosurgery for cerebral arteriovenous malformations: case report. Neurosurgery. 1997;41:1405-1409.
79 Kaido T, Hoshida T, Uranishi R, et al. Radiosurgery-induced brain tumor: case report. J Neurosurg. 2001;95:710-713.
80 Loeffler JS, Niemierko A, Chapman PH. Second tumors after radiosurgery: tip of the iceberg or a bump in the road? Neurosurgery. 2003;52:1436-1442.
81 Foote KD, Friedman WA, Ellis TL, et al. Salvage retreatment after failure of radiosurgery in patients with arteriovenous malformations. J Neurosurg. 2003;98:337-341.
82 Karlsson B, Kihlstrom L, Lindquist C, et al. Gamma knife surgery for previously irradiated arteriovenous malformations. Neurosurgery. 1998;42:1-5.
83 Maesawa S, Flickinger JC, Kondziolka D, et al. Repeated radiosurgery for incompletely obliterated arteriovenous malformations. J Neurosurg. 2000;92:961-970.
84 Schlienger M, Nataf F, Lefkopoulos D, et al. Repeat linear accelerator radiosurgery for cerebral arteriovenous malformations. Int J Radiat Oncol Biol Phys. 2003;56:529-536.
85 Miyawaki L, Dowd C, Wara W, et al. Five year results of LINAC radiosurgery for arteriovenous malformations: outcome for large AVMs. Int J Radiat Oncol Biol Phys. 1999;44:1089-1096.
86 Gobin YP, Laurent A, Merienne L, et al. Treatment of brain arteriovenous malformations by embolization and radiosurgery. J Neurosurg. 1996;85:19-28.
87 Mathis JA, Barr JD, Horton JA, et al. The efficacy of particulate embolization combined with stereotactic radiosurgery for treatment of large arteriovenous malformations of the brain. AJNR Am J Neuroradiol. 1995;16:299-306.
88 Paulsen RD, Steinberg GK, Norbash AM, et al. Embolization of basal ganglia and thalamic arteriovenous malformations. Neurosurgery. 1999;44:991-997.
89 Wikholm G, Lundqvist C, Svendsen P. Embolization of cerebral arteriovenous malformations: Part I—Technique, morphology, and complications. Neurosurgery. 1996;39:448-459.
90 Wikholm G, Lundqvist C, Svendsen P. Embolization of cerebral arteriovenous malformations: Part II—Aspects of complications and late outcome. Neurosurgery. 1996;39:460-469.
91 Chang T, Shirato H, Aoyama H, et al. Stereotactic irradiation for intracranial arteriovenous malformations using stereotactic radiosurgery or hypofractionated stereotactic radiotherapy. Int J Radiat Oncol Biol Phys. 2004;60:861-870.
92 Lindvall P, Bergström P, Löfroth P, et al. Hypofractionated conformal stereotactic radiotherapy for arteriovenous malformations. Neurosurgery. 2003;53:1036-1042.
93 Pendl G, Unger F, Papaefthymiou G, et al. Staged radiosurgical treatment for large benign cerebral lesions. J Neurosurg. 2000;93(Suppl 3):107-112.
94 Pollock BE, Kline RW, Stafford SL, et al. The rationale and technique of staged-volume arteriovenous malformation radiosurgery. Int J Radiat Oncol Biol Phys. 2000;48:817-824.
95 Sirin S, Kondziolka D, Niranjan A, et al. Prospective staged volume radiosurgery for large arteriovenous malformations: indications and outcomes in otherwise untreatable patients. Neurosurgery. 2006;58:17-27.
96 Petti PL, Coleman J, McDermott M, et al. Anatomic landmarks versus fiducials for volume-staged gamma knife radiosurgery for large arteriovenous malformations. Int J Radiat Oncol Biol Phys. 2007;67:1578-1585.
97 Pollock BE, Flickinger JC, Lunsford LD, et al. Factors associated with successful arteriovenous malformation radiosurgery. Neurosurgery. 1998;42:1239-1247.
98 Pollock BE, Flickinger JC, Lunsford LD, et al. The Pittsburgh arteriovenous malformation radiosurgery (PAR) grading scale. In: Kondziolka D, editor. Radiosurgery. Basel: Karger; 1997:137-146.
99 Andrade-Souza Y, Zadeh G, Scora D, et al. Radiosurgery for basal ganglia, internal capsule, and thalamus arteriovenous malformations: clinical outcomes. Neurosurgery. 2005;56:56-64.
100 Pollock BE, Gorman DA, Brown PD. Radiosurgery for arteriovenous malformations of the basal ganglia, thalamus, and brainstem. J Neurosurg. 2004;100:210-214.
101 Zabel-du Bois A, Milker-Zabel S, Huber P, et al. Stereotactic LINAC-based radiosurgery in the treatment of cerebral arteriovenous malformations located deep, involving corpus callosum, motor cortex, or brainstem. Int J Radiat Oncol Biol Phys. 2006;64:1044-1048.
102 Buis DR, Dirven CM, Lagerwaard FJ, et al. Radiosurgery of brain arteriovenous malformations in children. J Neurol. 2008;255:551-560.
103 Cohen-Gadol AA, Pollock BE. Radiosurgery for pediatric arteriovenous malformations. J Neurosurg. 2006;104(6 Suppl):388-391.
104 Zabel-du Bois A, Milker-Zabel S, Huber P, et al. Pediatric cerebral arteriovenous malformations: the role of stereotactic LINAC-based radiosurgery. Int J Radiat Oncol Biol Phys. 2006;65:1206-1211.
105 Andrade-Souza YM, Zadeh G, Ramani M, et al. Testing the radiosurgery-based arteriovenous malformation score and the modified Spetzler-Martin grading system to predict radiosurgical outcome. J Neurosurg. 2005;103:642-648.
106 Lee JS, Girvigian MR, Miller MJ, et al. Validation of a radiosurgery-based grading system for arteriovenous malformations. In: Kondziolka D, editor. Radiosurgery. Basel: Karger; 2006:221-228.
107 Pollock BE, Gorman DA, Coffey RJ. Patient outcomes after arteriovenous malformation radiosurgical management: results based on a 5- to 14-year follow-up study. Neurosurgery. 2003;52:1291-1297.
108 Maruyama K, Shin M, Tago M, et al. Gamma knife surgery for arteriovenous malformations involving the corpus callosum. J Neurosurg. 2005;102(Suppl):49-52.
109 Maruyama K, Kondziolka D, Niranjan A, et al. Stereotactic radiosurgery for brainstem arteriovenous malformations: factors affecting outcome. J Neurosurg. 2004;100:407-413.
110 Pollock BE, Brown RDJr. Use of the modified Rankin scale to assess outcome after arteriovenous malformation radiosurgery. Neurology. 2006;67:1630-1634.
111 Pollock BE, Flickinger JC. Modification of the radiosurgery-based arteriovenous malformation grading system. Neurosurgery. 2008;63:239-243.
112 Houser OW, Baker HLJr, Rhoton ALJr, et al. Intracranial dural arteriovenous malformations. Radiology. 1972;105:55-64.
113 Houser OW, Campbell JK, Campbell RJ, et al. Arteriovenous malformation affecting the transverse dural venous sinus—An acquired lesion. Mayo Clin Proc. 1979;54:651-661.
114 Chaudhary MY, Sachdev VP, Cho SH, et al. Dural arteriovenous malformation of the major venous sinuses: An acquired lesion. AJNR Am J Neuroradiol. 1982;3:13-19.
115 Kutluk K, Schumacher M, Mironov A. The role of sinus thrombosis in occipital dural arteriovenous malformations—development and spontaneous closure. Neurochirurgica. 1991;34:144-147.
116 Terada T, Higashida RT, Halbach VV, et al. Development of acquired arteriovenous fistulas in rats due to venous hypertension. J Neurosurg. 1994;80:884-889.
117 Herman JM, Spetzler RF, Bederson JB, et al. Genesis of a dural arteriovenous malformation in a rat model. J Neurosurg. 1995;83:539-545.
118 Terada T, Tsuura M, Komai N, et al. The role of angiogenic factor bFGF in the development of dural AVFs. Acta Neurochir. 1996;138:877-883.
119 Lawton MT, Jacobowitz R, Spetzler RF. Redefined role of angiogenesis in the pathogenesis of dural arteriovenous malformations. J Neurosurg. 1997;87:267-274.
120 Kraus JA, Stuper BK, Berlit P. Association of resistance to activated protein C and dural arteriovenous fistulas. J Neurol. 1998;245:731-733.
121 Awad IA, Little JR, Akarawi WP, et al. Intracranial dural arteriovenous malformations: factors predisposing to an aggressive neurological course. J Neurosurg. 1990;72:839-850.
122 Lasjaunias P, Chiu M, Ter Brugge K, et al. Neurological manifestations of intracranial dural arteriovenous malformations. J Neurosurg. 1986;64:724-730.
123 Singh V, Smith WS, Lawton ML, et al. Risk factors for hemorrhagic presentation in patients with dural arteriovenous fistulae. Neurosurgery. 2008;62:628-635.
124 Borden JA, Wu JK, Shucart WA. A proposed classification for spinal and cranial dural arteriovenous fistulous malformations and implications for treatment. J Neurosurg. 1995;82:166-179.
125 Cognard C, Gobin YP, Pierot L, et al. Cerebral dural arteriovenous fistulas: Clinical and angiographic correlation with a revised classification of venous drainage. Radiology. 1995;194:671-680.
126 Brown RDJr, Wiebers DO, Nichols DA. Intracranial dural arteriovenous fistulae: angiographic predictors of intracranial hemorrhage and clinical outcome in nonsurgical patients. J Neurosurg. 1994;81:531-538.
127 Duffau H, Lopes M, Janosevic V, et al. Early rebleeding from intracranial dural arteriovenous fistulas: report of 20 cases and review of the literature. J Neurosurg. 1999;90:78-84.
128 Soderman M, Pavic L, Edner G, et al. Natural history of dural arteriovenous shunts. Stroke. 2008;39:1735-1739.
129 Bitoh S, Sakaki S. Spontaneous cure of dural arteriovenous malformation in the posterior fossa. Surg Neurol. 1979;12:111-114.
130 Kupersmith MJ, Berenstein A, Choi IS, et al. Management of nontraumatic vascular shunts involving the cavernous sinus. Ophthalmology. 1988;95:121-130.
131 Vinuela F, Fox AJ, Debrun GM, et al. Spontaneous carotid-cavernous fistulas: clinical, radiological, and therapeutic considerations. Experience with 20 cases. J Neurosurg. 1984;60:976-984.
132 Barnwell SL, Halbach VV, Higashida RT, et al. Complex dural arteriovenous fistulas. Results of combined endovascular and neurosurgical treatment in 16 patients. J Neurosurg. 1989;71:352-358.
133 Sundt TMJr, Piepgras DG. The surgical approach to arteriovenous malformations of the lateral and sigmoid dural sinuses. J Neurosurg. 1983;59:32-39.
134 Thompson BG, Doppman JL, Oldfield EH. Treatment of cranial dural arteriovenous fistulae by interruption of leptomeningeal venous drainage. J Neurosurg. 1994;80:617-623.
135 Hoh BL, Choudhri TF, Connolly ESJr, et al. Surgical management of high-grade intracranial dural arteriovenous fistulas: Leptomeningeal venous disruption without nidus excision. Neurosurgery. 1998;42:796-805.
136 Dawson RCIII, Joseph GJ, Owens DS, et al. Transvenous embolization as the primary therapy for arteriovenous fistulas of the lateral and sigmoid sinuses. AJNR Am J Neuroradiol. 1998;19:571-576.
137 Halbach VV, Higashida RT, Hieshima GB, et al. Transvenous embolization of dural fistulas involving the cavernous sinus. AJNR Am J Neuroradiol. 1989;10:377-383.
138 Halbach VV, Higashida RT, Hieshima GB, et al. Transvenous embolization of dural fistulas involving the transverse and sigmoid sinuses. AJNR Am J Neuroradiol. 1989;10:385-392.
139 Nakamura M, Tamaki N, Kawaguchi T, et al. Selective transvenous embolization of dural carotid-cavernous sinus fistulas with preservation of sylvian venous outflow. Report of three cases. J Neurosurg. 1998;89:825-829.
140 Roy D, Raymond J. The role of transvenous embolization in the treatment of intracranial dural arteriovenous fistulas. Neurosurgery. 1997;40:1133-1144.
141 Barcia-Salorio JL, Soler F, Barcia JA, et al. Stereotactic radiosurgery for the treatment of low-flow carotid-cavernous fistulae: Results in a series of 25 cases. Stereotact Funct Neurosurg. 1994;63:266-270.
142 Chandler HCJr, Friedman WA. Successful radiosurgical treatment of a dural arteriovenous malformation: case report. Neurosurgery. 1993;33:139-142.
143 Friedman JA, Pollock BE, Nichols DA, et al. Results of combined stereotactic radiosurgery and transarterial embolization for dural arteriovenous fistulae of the transverse and sigmoid sinuses. J Neurosurg. 2001;94:886-891.
144 Guo W, Pan DHC, Wu H, et al. Radiosurgery as a treatment alternative for dural arteriovenous fistulas of the cavernous sinus. AJNR Am J Neuroradiol. 1998;19:1081-1087.
145 Koebbe CJ, Singhal D, Sheehan J, et al. Radiosurgery for dural arteriovenous fistulas. Surg Neurol. 2005;64:392-398.
146 Lewis AI, Tomsick TA, Tew JMJr. Management of tentorial arteriovenous malformations: transarterial embolization combined with stereotactic radiation or surgery. J Neurosurg. 1994;81:851-859.
147 Link MJ, Coffey RJ, Nichols DA, et al. The role of radiosurgery and particulate embolization in the treatment of dural arteriovenous fistulas. J Neurosurg. 1996;84:804-809.
148 Pollock BE, Nichols DA, Garrity JA, et al. Stereotactic radiosurgery and particulate embolization for cavernous sinus dural arteriovenous fistulae. Neurosurgery. 1999;45:459-467.
149 Shin M, Kurita H, Tago M, et al. Stereotactic radiosurgery for tentorial dural arteriovenous fistulae draining into the vein of Galen: Report of two cases. Neurosurgery. 2000;46:730-734.
150 Freidman JA, Meyer FB, Nichols DA, et al. Fatal progression of posttraumatic dural arteriovenous fistulae refractory to multimodality therapy. J Neurosurg. 2001;94:831-835.
151 Porter RW, Detwiler PW, Spetzler RF, et al. Cavernous malformations of the brainstem: experience with 100 patients. J Neurosurg. 1999;90:50-58.
152 Steinberg GK, Chang SD, Gewirtz RJ, et al. Microsurgical resection of brainstem, thalamic, and basal ganglion angiographically occult vascular malformations. Neurosurgery. 2000;46:260-271.
153 Amin-Hanjabi S, Ogilvy CS, Candia GJ, et al. Stereotactic radiosurgery for cavernous malformations: Kjellberg’s experience with proton beam therapy in 98 cases at the Harvard cyclotron. Neurosurgery. 1998;42:1229-1238.
154 Chang SD, Levy RP, Adler JRJr, et al. Stereotactic radiosurgery of angiographically occult vascular malformations: 14-year experience. Neurosurgery. 1998;43:213-221.
155 Hasegawa T, McInerney J, Kondziolka D, et al. Long-term results after stereotactic radiosurgery for patients with cavernous malformations. Neurosurgery. 2002;50:1190-1197.
156 Huang Y, Tseng C, Chang C, et al. LINAC radiosurgery for intracranial cavernous malformation: 10-year experience. Clin Neurol Neurosurg. 2006;108:750-756.
157 Karlsson B, Kihlström L, Lindquist C, et al. Radiosurgery for cavernous malformations. J Neurosurg. 1998;88:293-297.
158 Kida Y, Hasegawa T. Radiosurgery for cavernous malformations: results of long-term follow-up. In: Kondziolka D, editor. Radiosurgery. Basel: Karger; 2004:153-160.
159 Kim DG, Choe WJ, Paek SH, et al. Radiosurgery of intracranial cavernous malformations. Acta Neurochir. 2002;144:869-878.
160 Kondziolka D, Lunsford D, Coffey R, et al. Stereotactic radiosurgery of angiographically occult vascular malformations: Indications and preliminary experience. Neurosurgery. 1990;27:892-900.
161 Kondziolka D, Lunsford D, Flickinger J, et al. Reduction of hemorrhage risk after stereotactic radiosurgery for cavernous malformations. J Neurosurg. 1995;83:825-831.
162 Pollock BE, Garces YI, Stafford SL, et al. Stereotactic radiosurgery of cavernous malformations. J Neurosurg. 2000;93:987-991.
163 Regis J, Bartolomei F, Kida Y, et al. Radiosurgery for epilepsy associated with cavernous malformation: retrospective study in 49 patients. Neurosurgery. 2000;47:1091-1097.
164 Gewirtz RJ, Steinberg GK, Crowley R, et al. Pathological changes in surgically resected angiographically occult vascular malformations after radiation. Neurosurgery. 1998;42:738-743.
165 Barker FGII, Amin-Hanjabi S, Butler WE, et al. Temporal clustering of hemorrhages from untreated cavernous malformations of the central nervous system. Neurosurgery. 2001;49:15-25.
166 Kondziolka D, Lunsford D, Kestle J. The prospective natural history of cerebral cavernous malformations. J Neurosurg. 1995;83:820-824.
167 Porter PJ, Willinsky RA, Harper W, et al. Cerebral cavernous malformations: natural history and prognosis after clinical deterioration with or without hemorrhage. J Neurosurg. 1997;87:190-197.
168 Hsu P, Chang C, Tseng C, et al. Treatment of epileptic cavernomas: surgery versus radiosurgery. Cerebrovasc Dis. 2007;24:116-120.
169 Shih Y, Pan DH. Management of supratentorial cavernous malformations: craniotomy versus Gamma Knife surgery. Clin Neurol Neurosurg. 2005;107:108-112.