CHAPTER 263 Radiosurgery for Benign Spine Tumors and Vascular Malformations
Stereotactic radiosurgery has become a widely accepted primary and adjuvant treatment of both malignant and benign primary brain tumors, intracranial metastases, and intracranial arteriovenous malformations (AVMs), with large studies demonstrating effective tumor control and low morbidity.1–6 Technologic advances have more recently allowed radiosurgery to transcend the confines of the stereotactic head frame such that extracranial targets are now feasible.7 The current body of evidence supporting the use of extracranial radiosurgery for malignant spine tumors is considerable,8–27 and radiosurgery has only recently become part of the multimodality management of benign spinal tumors and spinal vascular malformations.
Benign spinal tumors represent a group of intradural extramedullary neoplasms that include meningiomas, schwannomas, and neurofibromas. The primary treatment option for most benign spinal neoplasms is microsurgical resection. The safety and effectiveness of such surgery have clearly been documented.7,28–33 The majority of spinal meningiomas, schwannomas, and neurofibromas are not infiltrative and can be completely and safely resected with microsurgical techniques.31,34–36 When complete tumor removal is achieved, recurrence is unlikely.28,31,37,38
In certain circumstances, however, some patients are less than ideal candidates for open standard surgical resection because of age, medical comorbid conditions, and the recurrent nature of their tumor.7 Multiple benign spinal tumors, as commonly seen in familial neurocutaneous disorders, may be a pattern of spinal pathology better suited for the less invasive radiosurgical option. In addition, tumors that have recurred after open surgical resection may make safe surgical resection challenging or impossible. It is in such clinical circumstances that radiosurgery might be an important clinical option for these patients.
Stereotactic radiosurgery for the treatment of a wide variety of benign intracranial lesions has become widely accepted and has excellent long-term outcomes and minimal toxicity.1,3,4,39 Radiation therapy has been used for the treatment of numerous benign diseases since discovery of the therapeutic potential of ionizing radiation.40–44 Although similar benign tumor histologies can be found in and around the spine, the initial radiosurgical instruments were frame based and unable to treat such extracranial targets.7 The recent emergence of frameless image-guided radiosurgery allows the treatment of such tumors throughout the body.23,45,46 There is now a substantial body of literature that supports the use of extracranial radiosurgery for the treatment of a variety of malignant spinal tumors,8–2747 but clinical experience with radiosurgery for the management of benign spine tumors is more limited.7,48 Because patients with benign lesions have prolonged life expectancies in comparison to their malignant counterparts, the potential for delayed radiation-induced myelopathy is a special concern. In addition, benign spine tumors have their own unique clinical manifestations, relationship to the spinal cord, and radiobiologic response to radiosurgery, any of which could represent unique challenges to safe and effective application of radiosurgical ablation.7
Besides treating poor surgical candidates and recurrent or residual tumor after surgery, spinal radiosurgery is most appropriate for well-circumscribed lesions associated with minimal spinal cord compromise and no biomechanical instability.49 Physicians at the Memorial Sloan-Kettering Cancer Center have proposed a four-tiered grading system for spinal tumors based on the degree of epidural involvement and spinal cord compromise; patients with severe grade 2 and 3 spinal cord compromise are deemed more appropriate candidates for surgery than for radiotherapy. Relative contraindications to spinal radiosurgery include evidence of overt spinal instability, neurological deficit resulting from bony compression, or previous radiation treatment to the spinal cord tolerance dose. A theoretical advantage of using spinal radiosurgery as frontline management of spinal tumors is the possibility that such treatment may act as prophylaxis against future spinal instability or compression of neural elements and thus obviate the need for extensive spinal surgery and instrumentation. Moreover, early radiosurgery may obviate the need for large-field external beam radiation, which is known to suppress bone marrow function. Tumor shrinkage and complete obliteration aside, the minimally invasive technique of spinal radiosurgery may develop into an effective palliative strategy solely through local tumor control. Finally, the ability to perform spinal radiosurgery in the outpatient setting is an advantage that may spare patients with spinal tumors both time and the morbidity of hospitalization.
Special Considerations for Benign Tumors
Radiosurgery has been documented to be an effective treatment modality for a variety of malignant tumors of the spine and spinal cord.8–27 Because the life expectancy of most of these patients is measured in months and radiation injury can take years to become evident,7 there is more controversy regarding radiosurgery for the management of benign tumors of the spine. The low radiation tolerance of the spinal cord is the primary limiting factor in the dose of therapeutic ionizing radiation that can be used for spinal tumors. Conventional external beam radiotherapy lacks the precision to deliver large single-fraction doses to benign spinal tumors in close proximity to the spinal cord. In fact, the radiosensitivity of the spinal cord has often required that the treatment dose be far below the optimal therapeutic dose.23,50,51 Spinal radiosurgery aims to deliver a highly conformal radiation dose to the treatment volume, which should increase the likelihood of successful tumor control and minimize the risk for spinal cord injury.8,9,23,25 Especially in patients with greater survival and a longer life span because of benign spinal tumor histology, the potential for delayed radiation-induced myelopathy must be entertained. The intradural nature of benign spinal tumors engenders a close proximity between the tumor and spinal cord or cauda equina, an anatomic relationship that may influence the risk for neural toxicity. Moreover, because benign spinal tumors are prone to late recurrence and the late toxicity of radiation delivered to the spinal cord may take years to develop, evaluation of radiosurgical treatment in terms of efficacy, safety, and durability will necessitate longer follow-up than what has been granted to patients with metastatic spine tumors.52 Finally, because benign spinal tumors are rarely life-threatening or destabilizing to the spinal column, treatment with high-dose ionizing radiation or by any other means creates more controversy than such treatment in the context of malignant spinal tumors; morbidity from any intervention, whether immediate or delayed, becomes less acceptable in this younger, healthier patient population.
Technical and Dose Considerations
Accurate geometric visualization of the tumor target on imaging is essential for radiosurgical contouring and treatment planning. The diagnostic imaging modality of choice for benign spinal tumors is magnetic resonance imaging (MRI). However, optimal radiosurgical treatment planning often rests on the modality of computed tomography (CT). Modern commercial radiosurgery systems use CT-based imaging for planning and delivery, and benign extramedullary spinal tumors are typically well visualized with contrast-enhanced CT. Most systems today also permit MRI-CT image fusion. Such fusion may improve the target definition for spinal tumors, especially when the neoplasms exhibit heterogeneous contrast enhancement. MRI-CT image fusion in the spine is usually more challenging than that required for intracranial radiosurgery because it is more dependent on the technical aspects of image acquisition. Quality spinal image fusion often requires that the patient’s imaging position closely match the intended treatment position. Issues of spatial distortion need to be considered if MRI is used directly for planning. Because signal intensities in MRI do not reflect a direct relationship with electron densities, unless attenuation coefficients are manually assigned to the region of interest, spatial distortion limits the accuracy of using MRI directly for radiosurgical planning.53 Virtually all extramedullary spinal tumors show some degree of contrast enhancement, so contrast-enhanced CT is sometimes used to directly define the target. CT myelography is an alternative imaging strategy that can provide superior visualization of the tumor and spinal cord. For spinal AVMs, the use of three-dimensional angiography has improved the ability to visualize these vascular lesions.54
The goal of spinal radiosurgery for benign spinal tumors is to deliver a clinically significant radiation dose to the tumor via a plan that respects the radiation dose tolerance limits of the nearby spinal cord, cauda equina, and surrounding organs such as the intestines, esophagus, kidneys, larynx, and liver. By virtue of their origin along the dura and spinal nerve roots, extramedullary spinal tumors can significantly impinge on the spinal cord or cauda equina and thus make contouring difficult. The single-fraction doses prescribed for spinal radiosurgery have varied from 12 to 20 Gy (Table 263-1).7,8,14,55–57 Although spine radiosurgery is often performed with a single–radiation fraction technique, the formal definition includes hypofractionated dosing plans with a maximum of five treatment sessions. Doses as high as 30 Gy have been reported in the setting of hypofractionation, and even higher total cumulative doses have been administered when one considers patients who have undergone conventional radiotherapy before spinal radiosurgery. The linear quadratic equation remains the most accepted mathematical model of cell kill secondary to ionizing radiation.47 The equation has been used to compare various dose fractionation schedules.
The spinal cord is one of the most radiosensitive structures considered in radiosurgical treatment plans. Radiation-induced myelopathy may occur in delayed fashion, and the spinal cord tolerance of single-fraction radiation has not been carefully determined with long-term follow-up studies. Nevertheless, some data point to less than a 5% probability of myelopathy at 5 years when the cord receives a 60-Gy dose via standard fractionation.47 Most centers have tailored plans to ensure that the spinal cord is exposed to no more than 20 Gy during fractionated therapy.58 Borrowing again from the intracranial radiosurgical experience, some physicians have fashioned a cord avoidance strategy based on dosage tolerance data for the optic nerves and chiasm. In contrast to patients with metastatic cancer, patients undergoing radiosurgery for benign paraspinal neoplasms are expected to survive longer with higher functional status. Consequently, when planning treatment of benign tumors, it is prudent to err on underestimating spinal cord tolerance in the event that an unrecognized form of radiation-induced myelopathy may become manifested over a period of decades. Whether previous radiosurgery will sensitize the spinal cord and cauda equina to degenerative insults in aging patients is also unknown.
Clinical Results
There is a growing amount of experience to date with clinical outcomes after spine radiosurgery for benign tumors.7,8,14,53,59,60 Similar to the application of radiosurgery for intracranial pathology, spine radiosurgery has primarily been embraced as a adjunctive treatment technique for tumor recurrence or residual tumor after open surgery, for progression of tumor after failure of conventional radiation treatment, in patients who have significant medical comorbid conditions that preclude open surgery, or for “salvage” therapy when further conventional irradiation or surgery is not appropriate. Spinal radiosurgery will probably with time be explored as the initial treatment of both malignant and benign spinal tumors.
Given their pathologic similarities, it has been speculated that benign spinal lesions would be equally responsive to radiosurgery as their intracranial counterparts.7 It is for this reason that centers experienced in radiosurgery explored this developing technology to treat benign spine tumors. The extramedullary intradural spinal neoplasms treated with radiosurgery have primarily included meningiomas, schwannomas, and neurofibromas. However, the first reported benign spinal tumor treated with radiosurgery was actually a hemangioblastoma.7 Ryu and coauthors published the first description of radiosurgery used to treat such lesions.23 In the initial experience from Stanford using the CyberKnife for 15 benign spinal tumors, no tumor progression was reported with a follow-up period of 12 months.
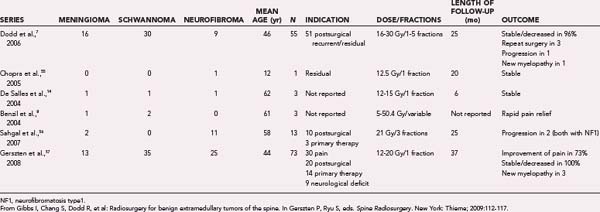
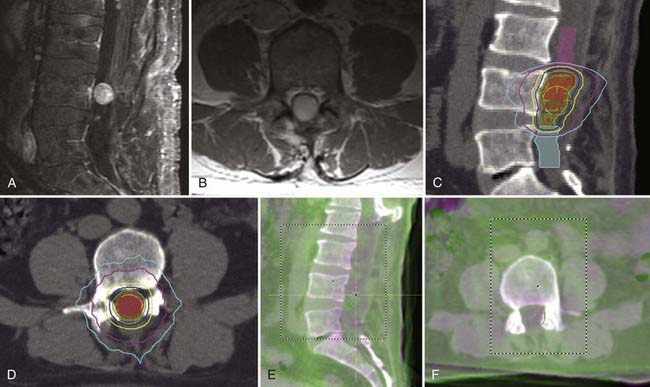
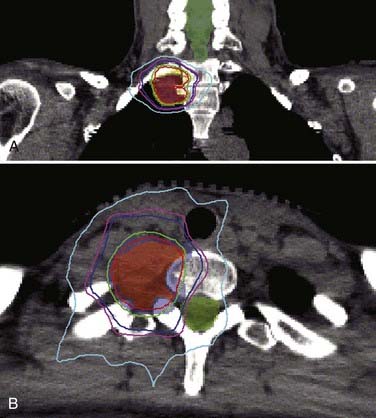
Dodd and associates, in the largest published series to date for benign intradural extramedullary spinal tumors, reported the results of radiosurgical treatment of 55 such tumors (30 schwannomas, 9 neurofibromas, and 16 meningiomas).7 Total treatment doses ranged from 16 to 30 Gy delivered in one to five fractions to tumor volumes that varied from 0.136 to 24.6 cm3. Less than 1 year after radiosurgery, three patients (one with meningioma, one with schwannoma, and one with neurofibroma) required open surgical resection of their tumor because of persistent or worsening symptoms. Only 1 of these 3 lesions was larger radiographically. Twenty-eight of the 55 lesions had greater than 24 months’ follow-up. The mean follow-up in this group was 36 months. All lesions in this group were either stable (61%) or smaller (39%), with no tumor increasing in size. The following sections, subdivided by histopathology, summarize the available clinical outcome data for benign spinal tumor radiosurgery (Figs. 263-1 and 263-2).
Meningiomas
Spinal meningiomas are arachnoid cap cell–derived tumors that develop in individuals in the fifth to seventh decade, have a female preponderance, and occur mainly in the thoracic region. They arise from cells of the meningeal coverings of the central nervous system and occur more frequently within the brain than the spine at a 5 : 1 ratio. Gross total surgical resection optimizes outcome.61 Radiosurgery has become a well-accepted treatment option for the management of intracranial meningiomas and schwannomas.3,11 Kondziolka and coauthors reported that the long-term tumor control rate in a series of patients with benign intracranial tumors treated by radiosurgery was 93%, with 53% of the lesions decreasing in size.3 A more recent publication of more than 1000 meningiomas revealed a 96% tumor control rate with a follow-up of up to 18 years.61 Other studies have shown similar efficacy and safety of radiosurgery for meningiomas.3,62,63 The long-term growth control rate after radiosurgery for vestibular schwannomas has been documented to be 95% to 98%.2,64
Dodd and colleagues reported 16 patients who underwent radiosurgery for the treatment of spinal meningiomas (mean dose, 20 Gy; mean tumor volume, 2.4 cm3; mean follow-up, 27 months) and demonstrated radiologic stabilization in 67% and radiologic decrease in tumor size in 33% of the 15 who had radiographic follow-up. Only 1 patient required open surgery, and another sustained the only complication reported in the series. Seventy percent of the meningiomas treated in this series were symptomatically stable or improved. Most patients experienced an improvement in pain and strength with radiosurgery.7
From our institution’s published series, 13 spinal meningiomas were treated with a single-fraction technique (mean dose, 21 Gy; mean tumor volume, 4.9 cm3). Eleven of 13 patients underwent radiosurgery as an adjunctive treatment of residual or recurrent tumor after open surgical resection. Radiographic tumor control was demonstrated in all cases with a median follow-up of 17 months.57
In a smaller series, Sahgal and associates reported using radiosurgery to treat two spinal meningiomas (mean dose, 23 Gy delivered in two fractions; mean tumor volume, 1.6 cm3), with no evidence of radiographic tumor progression.56 Benzil and coworkers and De Salles and coauthors have also reported good results in treating spine meningiomas with long-term radiographic follow-up.8,14
Schwannomas
Nerve sheath tumors consist of schwannomas and neurofibromas. Schwannoma is the most common spinal tumor and has no proclivity for a particular spinal region or gender.33 Spinal schwannomas typically arise from the posterolaterally placed dorsal nerve root. Given their posterior position relative to the spinal cord or cauda equina, removal via a laminectomy approach is usually straightforward. From the Stanford series, Dodd and colleagues reported on 30 tumors (mean dose, 19 Gy; mean tumor volume, 5.7 cm3; mean follow-up, 26 months), and all but one patient exhibited radiographic tumor control after radiosurgery. A third of the patients reported improvement in pain, weakness, or sensation, but 18% experienced a clinical decline after treatment.7 Forty percent of patients in this series had spinal schwannomas in the setting of neurofibromatosis type 2 (NF2).
From our institution, 35 schwannomas were treated with spine radiosurgery (mean dose, 22 Gy; mean tumor volume, 11.0 cm3). Fourteen of 17 patients (82%) for whom pain was the primary indication for radiosurgery reported significant improvement of pain. Radiographic tumor control was demonstrated in 6 of 7 patients (86%) in whom radiosurgery was used as the primary treatment. Three patients eventually underwent surgical resection for new or persistent neurological deficits.57 Benzil, De Salles, and colleagues have also reported their experience in treating spine schwannomas, with good results on long-term radiographic follow-up and rapid pain relief being achieved.8,14
Neurofibromas
Sahgal and coauthors reported a series of 11 radiosurgically treated neurofibromas (mean dose, 21 Gy delivered in three fractions; mean tumor volume, 6.0 cm3).56 Radiographic control was documented in nine patients. Three patients had NF1, with progression developing in two of them. In the published series from Stanford, 9 neurofibromas in seven patients with NF1 (mean dose, 10.6 Gy; mean tumor volume, 4.31 cm3) were treated with radiosurgery, and tumor stabilization was documented on imaging in six of the seven (86%) patients. After a mean follow-up of 20 months, half of the patients reported an improvement in symptoms after radiosurgery, and half reported worsening of pain, weakness, or numbness at their last follow-up.7 However, the tumors were radiographically stable in all patients. The authors cautioned that the role of radiosurgery for neurofibromas remains unclear, particularly since a significant number of the NF1 patients were myelopathic at initial evaluation. They further stated that the most realistic and attainable goal of treatment of neurofibroma in myelopathic patients is tumor control without significant expectations for improvement in symptoms.62
Our institution has published its experience in treating 25 neurofibromas (mean dose, 21.3 Gy; mean tumor volume, 12.6 cm3). Similar to the Stanford experience, no patient has had evidence of radiographic tumor progression on follow-up. Twenty-one of these patients had NF1 and 9 had NF2. Radiosurgery ameliorated the discomfort in 8 of the 13 patients (61.5%) treated for pain. All patients with no improvement in pain had NF1. These findings echo outcomes from the Stanford series, in which it was found that pain in patients with NF1-associated spinal neurofibromas responds less well to radiosurgery.7 Poorer microsurgical results for neurofibromas have also been observed in patients with NF1.33 The multiplicity of neurofibromas in patients with NF1 may be partially to blame because this factor makes identifying symptomatic neurofibromas in need of treatment more difficult. Furthermore, given that many of the patients with neurofibromas have multiple lesions along their spine, it can often be difficult to determine whether symptom progression is due to the treated lesion or any of the other neurofibroma lesions within the spine.62 Moreover, the infiltrative nature of neurofibromas, in contrast to the other benign extramedullary intradural spinal tumors, may engender more irreversible neural damage and increase the susceptibility of the native nerve root to injury from both microsurgical and radiosurgical treatment. Finally, future genomic investigations may reveal that the intrinsic genetic differences in NF1-associated neurofibromas predispose to a weaker radiobiologic response.
Arteriovenous Malformations
Stereotactic radiosurgery has emerged as a successful alternative to microsurgical resection and embolization of cerebral AVMs. Since this technique was first described by Steiner and colleagues in 1972,65 more than 5000 patients have been treated successfully with this modality. Radiosurgery causes gradual hyperplasia of the endothelial tissue within the arteries of the nidus of the AVM, which leads to occlusion of the vessel.66 Numerous reports have documented that the rate of obliteration of cerebral AVMs is 80% to 85% when smaller than 2.5 cm.12,67–72 With the success of radiosurgery in the management of brain AVMs, treatment of spinal cord AVMs with radiosurgery would be a logical next step.73
Spinal AVMs have been successfully treated with radiosurgery.74 Much of radiosurgery for spinal AVMs was based on previous experience of radiosurgery for other benign spinal tumors.75 Given the various subtypes of spinal cord AVMs (see Chapter 395 for classification of spinal cord AVMs), those with a relatively compact nidus would represent the optimal targets.73 Type I and type IV spinal cord AVMs are dural and perimedullary arteriovenous fistulas and are often optimally treated by endovascular embolization or microsurgical resection (or both). Type III spinal cord AVMs, also called juvenile-type spinal AVMs, are characterized by a large and diffuse intramedullary nidus that can also extend into the extramedullary space. They are less well defined spinal cord AVMs and also do not represent optimal radiosurgical targets. Type II spinal cord AVMs, also called glomus AVMs, have a compact vascular nidus and are often suitable radiosurgical targets. These type II spinal cord AVMs are also difficult if not impossible to treat with embolization and microsurgery alone and frequently were not treated before the development of spinal radiosurgery.
The largest experience to date has been in patients with type II spinal cord AVMs who are not candidates for either microsurgical removal or endovascular embolization. In addition, a small number of type III spinal cord AVMs are considered candidates for radiosurgery if the vascular nidus is a reasonable target based on size. Type I and type IV spinal cord AVMs are not optimally treated with radiosurgery. Low-flow spinal cord and vascular malformations, such as arteriovenous fistulas and cavernous malformations, are currently not considered candidates for spine radiosurgery.73
The largest experience with spinal AVM radiosurgery comes from the Stanford group.73 They reported a series of 23 patients with spinal cord AVMs treated with the CyberKnife system. Twenty-two of these patients had type II or glomus AVMs, and 1 patient had a type III juvenile spinal cord AVM. Spinal AVM locations included the cervical spine (12 patients), thoracic spine (8 patients), and conus region (3 patients). Thirteen of these 23 patients (57%) initially had hemorrhage, which was multiple in 6 of these patients. In the remaining 10 patients, progressive neurological dysfunction was the initial symptom. Ten of the 23 patients (43%) previously underwent embolization of their AVM. A median of three treatment sessions was used. The mean target volume was 2.8 cm3 with a range of 0.26 to 15 cm3. The mean marginal dose used was 20.3 Gy with a range of 16 to 21 Gy. A median of two sessions with a range of one to four treatments was used in this cohort. The mean maximal internidal dose was 25.8 Gy with a range of 22.5 to 30 Gy.
Clinical follow-up ranged from 4 to 83 months with a mean of 35 months.73 Radiographic follow-up averaged 25 months. Although postoperative MRI demonstrated a noticeable reduction in the volume of all AVMs over the course of follow-up, only 8 of the 23 patients underwent formal spine angiography. Of these 8 patients, 3 exhibited complete angiographic obliteration. To date, no patient has suffered rebleeding after spine radiosurgery, and the clinical outcome was improved or unchanged in 96% of patients. A single patient who was neurologically severely impaired before radiosurgery deteriorated further at 9 months after radiosurgery. MRI demonstrated a significant decrease in flow void, as well as high signal in the adjacent spinal cord, on T2-weighted imaging, consistent with radiation-induced edema. One other patient experienced a significant onset of radiation-induced edema (for a conus AVM), but this AVM obliterated rapidly over a period of 9 months, and the patient was 1 of the 3 documented to have complete obliteration of the AVM. The future of radiosurgery for the treatment of spinal AVMs has still not been determined.
Radiation Toxicity
Complications from the radiosurgical treatment of benign spinal tumors are rare. Symptomatic radiation-induced myelopathy confirmed by MRI was reported in 3 of 73 (4.1%) patients treated at our institution, and all tumors were located in the cervical spinal column. All of these patients were treated with a single-fraction technique. Dodd and colleagues used multiple fractions much more commonly and reported a new-onset neurological deficit (posterior column dysfunction) in a single patient that was presumed to be due to radiation-induced myelopathy (1.8%).7 In a series of 19 benign spine tumors from the University of California, San Francisco, no late toxicity occurred, including myelopathy.56 Previous studies have suggested that the risk for radiation-induced myelopathy is a function of the total dose, fraction size, length of spinal cord exposed, and duration of treatment.76,77 The benign spinal neoplasms that have thus far been treated with radiosurgery represent a heterogeneous group of neoplasms, some of which have had no previous treatment and others that have been treated with surgery, external beam radiation, or both. Although already small, the risk for radiation-induced myelopathy with radiosurgical treatment of benign spinal tumors may be further minimized once physicians understand how the radiation tolerance of the spinal cord is influenced by previous treatments such as microsurgery.
Benzil DL, Saboori M, Mogilner AY, et al. Safety and efficacy of stereotactic radiosurgery for tumors of the spine. J Neurosurg. 2004;101:413.
Betti O, Munari C, Rosler R. Stereotactic radiosurgery with the linear accelerator: treatment of arteriovenous malformations. Neurosurgery. 1989;24:311.
Bhatnagar AK, Gerszten PC, Ozhasoglu C, et al. CyberKnife frameless radiosurgery for the treatment of extracranial benign tumors. Technol Cancer Res Treat. 2005;4:1.
Bilsky MH, Yamada Y, Yenice KM, et al. Intensity-modulated stereotactic radiotherapy of paraspinal tumors: a preliminary report. Neurosurgery. 2004;54:823.
Chang EL, Shiu AS, Lii M-F, et al. Phase I clinical evaluation of near-simultaneous computed tomographic image–guided stereotactic body radiotherapy for spinal metastases. Int J Radiat Oncol Biol Phys. 2004;59:1288.
Chang et al Chang S, Hancock S, Gibbs I, et al. Spinal cord arteriovenous malformation radiosurgery. In: Gerszten PC, Ryu SI, eds. Spine Radiosurgery. New York: Thieme; 2009:123-127.
Chopra R, Morris C, Friedman W, et al. Radiotherapy and radiosurgery for benign neurofibromas. Am J Clin Oncol. 2005;28:317.
Conti P, Pansini G, Mouchaty H, et al. Spinal neurinomas: retrospective analysis and long-term outcome of 179 consecutively operated cases and review of the literature. Surg Neurol. 2004;61:34.
Dodd RL, Ryu MR, Kammerdsupaphon P, et al. CyberKnife radiosurgery for benign intradural extramedullary spinal tumors. Neurosurgery. 2006;58:674.
Gerszten PC, Burton SA, Ozhasoglu C, et al. Radiosurgery for benign intradural spinal tumors. Neurosurgery. 2008;62:887.
Gibbs et al Gibbs I, Chang S, Dodd R, et al. Radiosurgery for beningn extramedullary tumors of the spine. In: Gerszten PC, Ryu SI, eds. Spine Radiosurgery. New York: Thieme; 2009:112-117.
Isaacson S. Radiation therapy and the management of intramedullary spinal cord tumors. J Neurooncol. 2000;47:231.
Lukacs S, Braun-Falco O, Goldschmidt H. Radiotherapy of benign dermatoses: indications, practice and results. J Dermatol Surg Oncol. 1978;4:620.
Peker S, Cerci A, Ozgen S, et al. Spinal meningiomas: evaluation of 41 patients. J Neurosurg Sci. 2005;49:7.
Ryu SI, Kim DH, Chang SD. Stereotactic radiosurgery for hemangiomas and ependymomas of the spinal cord. Neurosurg Focus. 2003;15(5):E10.
Sahgal A, Chou D, Ames C, et al. Image-guided robotic stereotactic body radiotherapy for benign spinal tumors: the University of California San Francisco preliminary experience. Technol Cancer Res Treat. 2007;6:595.
Sinclair J, Chang SD, Gibbs IC, et al. Multisession CyberKnife radiosurgery for intramedullary spinal cord arteriovenous malformations. Neurosurgery. 2006;58:1081.
Sperduto P, Scott C, Andrews D. Stereotactic radiosurgery with whole brain radiation therapy improves survival in patients with brain metastases: report of radiation therapy oncology group phase III study 95-08. Int J Radiat Oncol Biol Phys. 2002;54:3a.
Stancanello J, Cavedon C, Francescon P. Development and validation of a CT–3D rotational angiography registration method for AVM radiosurgery. Med Phys. 2004;31:1363.
Steinberg G, Fabrikant J, Marks M, et al. Stereotactic heavy-charged-particle Bragg-peak radiation for intracranial arteriovenous malformations. N Engl J Med. 1990;323:96.
Steiner L, Lesksell L, Greitz T, et al. Stereotaxic radiosurgery for cerebral arteriovenous malformations. Report of a case. Acta Chir Scand. 1972;138:459.
Yamada Y, Lovelock D, Bilsky M. A review of image-guided intensity-modulated radiotherapy for spinal tumors. Neurosurgery. 2007;61:226.
Yin FF, Ryu S, Ajlouni M, et al. Image-guided procedures for intensity-modulated spinal radiosurgery. J Neurosurgery. 2004;101:419.
1 Flickinger JC, Pollock BE, Kondziolka D. A dose-response analysis of arteriovenous malformation obliteration after radiosurgery. Int J Radiat Oncol Biol Phys. 1996;36:873.
2 Kondziolka D, Lunsford L, McLaughlin M. Long-term outcomes after radiosurgery for acoustic neuromas. N Engl J Med. 1998;339:1426.
3 Kondziolka D, Nathoo N, Flickinger JC. Long term results after radiosurgery for benign intracranial tumors. Neurosurgery. 2003;53:815.
4 Lunsford LD, Kondziolka D, Flickinger JC. Stereotactic radiosurgery for arteriovenous malformations of the brain. J Neurosurgery. 1991;75:512.
5 Chang SD, Lee E, Sakamoto GT, et al. Stereotactic radiosurgery in patients with multiple brain metastases. Neurosurg Focus. 2000;9(2):e3.
6 Bhatnagar A, Flickinger J, Kondziolka D, et al. Stereotactic radiosurgery for four or more intracranial metastases. Int J Radiat Oncol Biol Phys. 2006;64:898.
7 Dodd RL, Ryu MR, Kammerdsupaphon P, et al. CyberKnife radiosurgery for benign intradural extramedullary spinal tumors. Neurosurgery. 2006;58:674.
8 Benzil DL, Saboori M, Mogilner AY, et al. Safety and efficacy of stereotactic radiosurgery for tumors of the spine. J Neurosurg. 2004;101:413.
9 Bilsky MH, Yamada Y, Yenice KM, et al. Intensity-modulated stereotactic radiotherapy of paraspinal tumors: a preliminary report. Neurosurgery. 2004;54:823.
10 Chang EL, Shiu AS, Lii M-F, et al. Phase I clinical evaluation of near-simultaneous computed tomographic image–guided stereotactic body radiotherapy for spinal metastases. Int J Radiat Oncol Biol Phys. 2004;59:1288.
11 Chang S, Adler J. Current status and optimal use of radiosurgery. Oncology. 2001;15:209.
12 Colombo F, Pozza F, Chierego G. Linear accelerator radiosurgery of cerebral arteriovenous malformations: an update. Neurosurgery. 1994;34:14.
13 Degen JW, Gagnon GJ, Voyadzis J-M, et al. CyberKnife stereotactic radiosurgical treatment of spinal tumors for pain control and quality of life. J Neurosurg Spine. 2005;2:540.
14 De Salles AA, Pedroso A, Medin P, et al. Spinal lesions treated with Novalis shaped beam intensity modulated radiosurgery and stereotactic radiotherapy. J Neurosurg. 2004;101:435.
15 Gerszten PC, Welch WC. CyberKnife radiosurgery for metastatic spine tumors. Neurosurg Clin N Am. 2004;15:491.
16 Gerszten PC, Welch WC. Combined percutaneous transpedicular tumor debulking and kyphoplasty for pathological compression fractures. J Neurosurg Spine. 2007;6:92.
17 Hamilton A, Lulu B, Fosmire H, et al. Preliminary clinical experience with linear accelerator–based spinal stereotactic radiosurgery. Neurosurgery. 1995;36:311.
18 Hitchcock E, Kitchen G, Dalton E, et al. Stereotactic LINAC radiosurgery. Br J Neurosurg. 1989;3:305.
19 Medin P, Solberg T, De Salles A. Investigations of a minimally invasive method for treatment of spinal malignancies with LINAC stereotactic radiation therapy: accuracy and animal studies. Int J Radiat Oncol Biol Phys. 2002;52:1111.
20 Milker-Zabel S, Zabel A, Thilmann C, et al. Clinical results of retreatment of vertebral bone metastases by stereotactic conformal radiotherapy and intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2003;55:162.
21 Pirzkall A, Lohr F, Rhein B, et al. Conformal radiotherapy of challenging paraspinal tumors using a multiple arc segment technique. Int J Radiat Oncol Biol Phys. 2000;48:1197.
22 Rock JP, Ryu S, Yin FF. Novalis radiosurgery for metastatic spine tumors. Neurosurg Clin N Am. 2004;15:503.
23 Ryu S, Chang S, Kim D, et al. Image-guided hypo-fractionated stereotactic radiosurgery to spinal lesions. Neurosurgery. 2001;49:838.
24 Ryu S, Rock J, Rosenblum M, et al. Patterns of failure after single-dose radiosurgery for spinal metastasis. J Neurosurg. 2004;101:402.
25 Ryu S, Yin FF, Rock J, et al. Image-guided and intensity-modulated radiosurgery for patients with spinal metastasis. Cancer. 2003;97:2013.
26 Sperduto P, Scott C, Andrews D. Stereotactic radiosurgery with whole brain radiation therapy improves survival in patients with brain metastases: report of Radiation Therapy Oncology Group phase III study 95-08. Int J Radiat Oncol Biol Phys. 2002;54:3a.
27 Yin FF, Ryu S, Ajlouni M, et al. Image-guided procedures for intensity-modulated spinal radiosurgery. J Neurosurg. 2004;101:419.
28 Conti P, Pansini G, Mouchaty H, et al. Spinal neurinomas: retrospective analysis and long-term outcome of 179 consecutively operated cases and review of the literature. Surg Neurol. 2004;61:34.
29 Gezen F, Kahraman S, Canakci Z, et al. Review of 36 cases of spinal cord meningioma. Spine. 2000;27:727.
30 Klekamp J, Samii M. Surgery of spinal nerve sheath tumors with special reference to neurofibromatosis. Neurosurgery. 1998;42:279.
31 Parsa A, Lee J, Parney I, et al. Spinal cord and intradural-extraparenchymal spinal tumors: current best care practices and strategies. J Neurooncol. 2004;69:291.
32 Seppala M, Haltia M, Sankila R, et al. Long-term outcome after removal of spinal neurofibroma. J Neurosurg. 1995;82:572.
33 Seppala M, Haltia M, Sankila R, et al. Long term outcome after removal of spinal schwannoma: a clinicopathological study of 187 cases. J Neurosurg. 1995;83:621.
34 Asazuma T, Toyama Y, Maruiwa H, et al. Surgical strategy for cervical dumbbell tumors based on a three-dimensional classification. Spine. 2004;29:E10.
35 McCormick P. Surgical management of dumbbell and paraspinal tumors of the thoracic and lumbar spine. Neurosurgery. 1996;38:67.
36 McCormick P. Surgical management of dumbbell tumors of the cervical spine. Neurosurgery. 1996;38:294.
37 Cohen-Gadol A, Zikel O, Koch C, et al. Spinal meningiomas in patients younger than 50 years of age: a 21-year experience. J Neurosurg Spine. 2003;98:258.
38 Roux F, Nataf F, Pinaudeau M, et al. Intraspinal meningiomas: review of 54 cases with discussion of poor prognosis factors and modern therapeutic management. Surg Neurol. 1996;46:458.
39 Steiner L, Leksell L, Forster D. Stereotactic radiosurgery in intracranial arterio-venous malformations. Acta Neurochir Suppl. 1974;21:195.
40 Lukacs S, Braun-Falco O, Goldschmidt H. Radiotherapy of benign dermatoses: indications, practice and results. J Dermatol Surg Oncol. 1978;4:620.
41 Solan M, Kramer. The role of radiation therapy in the management of intracranial meningiomas. Int J Radiat Oncol Biol Phys. 1985;11:675.
42 Klumpar D, Murray J, Anscher M. Keloids treated with excision followed by radiation therapy. J Am Acad Dermatol. 1994;31:225.
43 Keilholz L, Seegenschmiedt M, Sauer R. Radiotherapy for prevention of disease progression in early-stage Dupuytren’s contracture: initial and long-term results. Int J Radiat Oncol Biol Phys. 1996;36:891.
44 Seegenschmiedt M, Martus P, Goldmann A. Preoperative versus postoperative radiotherapy for prevention of heterotopic ossification (HO): first results of a randomized trial in high-risk patients. Int J Radiat Oncol Biol Phys. 1994;30:63.
45 Adler JR, Chang SD, Murphy MJ, et al. The CyberKnife: a frameless robotic system for radiosurgery. Stereotact Funct Neurosurg. 1997;69:124.
46 Gerszten PC, Bilsky MH. Spine radiosurgery. Contemp Neurosurg. 2006;28:1.
47 Yamada Y, Lovelock D, Bilsky M. A review of image-guided intensity-modulated radiotherapy for spinal tumors. Neurosurgery. 2007;61:226.
48 Bhatnagar AK, Gerszten PC, Ozhasoglu C, et al. CyberKnife frameless radiosurgery for the treatment of extracranial benign tumors. Technol Cancer Res Treat. 2005;4:1.
49 Gerszten P. The role of minimally invasive techniques in the management of spine tumors: percutaneous bone cement augmentation, radiosurgery and microendoscopic approaches. Orthop Clin North Am. 2007;38:441.
50 Faul CM, Flickinger JC. The use of radiation in the management of spinal metastases. J Neurooncol. 1995;23:149.
51 Loblaw DA, Laperriere NJ. Emergency treatment of malignant extradural spinal cord compression: an evidence-based guideline. J Clin Oncol. 1998;16:1613.
52 Gerszten P, Ozhasoglu C, Burton S, et al. Feasibility of frameless single-fraction stereotactic radiosurgery for spinal lesions. Neurosurg Focus. 2002;13(4):e2.
53 Gibbs I, Chang S, Dodd R, et al. Radiosurgery for benign extramedullary tumors of the spine. In: Gerszten PC, Ryu SI, eds. Spine Radiosurgery. New York: Thieme; 2009:112-117.
54 Stancanello J, Cavedon C, Francescon P. Development and validation of a CT–3D rotational angiography registration method for AVM radiosurgery. Med Phys. 2004;31:1363.
55 Chopra R, Morris C, Friedman W, et al. Radiotherapy and radiosurgery for benign neurofibromas. Am J Clin Oncol. 2005;28:317.
56 Sahgal A, Chou D, Ames C, et al. Image-guided robotic stereotactic body radiotherapy for benign spinal tumors: the University of California San Francisco preliminary experience. Technol Cancer Res Treat. 2007;6:595.
57 Gerszten PC, Burton SA, Ozhasoglu C, et al. Radiosurgery for benign intradural spinal tumors. Neurosurgery. 2008;62:887.
58 Gerszten PC, Burton SA, Ozhasoglu C, et al. Radiosurgery for the management of spinal metastases. In: Kondziolka D, editor. Radiosurgery, Vol 6. Basel: Karger; 2006:199.
59 Gerszten PC, Ozhasoglu C, Burton S, et al. CyberKnife frameless single-fraction stereotactic radiosurgery for benign tumors of the spine. Neurosurg Focus. 2003;14(5):e16.
60 Peker S, Cerci A, Ozgen S, et al. Spinal meningiomas: evaluation of 41 patients. J Neurosurg Sci. 2005;49:7.
61 Kondziolka D, Mathieu D, Martin JJ, et al: The long-term perspective on meningioma radiosurgery: experience from over 1,000 tumors. Paper presented at the 75th Annual Meeting of the American Academy of Neurological Surgeons, Washington, DC.
62 Chang S, Adler JJr. Treatment of cranial base meningiomas with linear accelerator radiosurgery. Neurosurgery. 1997;41:1019.
63 Lee J, Niranjan A, McInerney J, et al. Stereotactic radiosurgery providing long-term tumor control of cavernous sinus meningiomas. J Neurosurg. 2002;97:65.
64 Prasad D, Steiner M, Steiner L. Gamma surgery for vestibular schwannoma. J Neurosurg. 2000;92:745.
65 Steiner L, Lesksell L, Greitz T, et al. Stereotaxic radiosurgery for cerebral arteriovenous malformations. Report of a case. Acta Chir Scand. 1972;138:459.
66 Chang S, Shuster D, Steinberg G, et al. Stereotactic radiosurgery of arteriovenous malformations: pathologic changes in resected tissue. Clin Neuropathol. 1997;16:111.
67 Betti O, Munari C, Rosler R. Stereotactic radiosurgery with the linear accelerator: treatment of arteriovenous malformations. Neurosurgery. 1989;24:311.
68 Coffey R, Lunsford L, Bissonette D, et al. Stereotactic gamma radiosurgery for intracranial vascular malformations and tumors: report of the initial North American experience in 331 patients. Stereotact Funct Neurosurg. 1990;54:535.
69 Colombo F, Benedetti A, Pozza F, et al. Linear accelerator radiosurgery of cerebral arteriovenous malformations. Neurosurgery. 1989;24:833-840.
70 Friedman W, Bova F. Linear accelerator radiosurgery for arteriovenous malformations. J Neurosurg. 1992;77:832.
71 Steinberg G, Fabrikant J, Marks M, et al. Stereotactic heavy-charged-particle Bragg-peak radiation for intracranial arteriovenous malformations. N Engl J Med. 1990;323:96.
72 Steiner L. Radiosurgery in cerebral arteriovenous malformations. In: Fein JM, Flamm ES, editors. Cerebrovascular Surgery, Vol 4. New York: Springer-Verlag; 1985:1161.
73 Chang S, Hancock S, Gibbs I, et al. Spinal cord arteriovenous malformation radiosurgery In: Gerszten PC, Ryu SI, eds. Spine Radiosurgery. New York: Thieme; 2009:123-127.
74 Sinclair J, Chang SD, Gibbs IC, et al. Multisession CyberKnife radiosurgery for intramedullary spinal cord arteriovenous malformations. Neurosurgery. 2006;58:1081.
75 Ryu SI, Kim DH, Chang SD. Stereotactic radiosurgery for hemangiomas and ependymomas of the spinal cord. Neurosurg Focus. 2003;15(5):E10.
76 Isaacson S. Radiation therapy and the management of intramedullary spinal cord tumors. J Neurooncol. 2000;47:231.
77 Rampling R, Symonds P. Radiation myelopathy. Curr Opin Neurol. 1998;11:627.