CHAPTER 25 Radiopharmaceuticals and Radiation Dose Considerations in Cardiac Positron Emission Tomography and PET/CT
PET RADIOPHARMACEUTICALS
Perfusion Agents
There are currently three PET perfusion agents that are approved for clinical use (13N-ammonia, 15O-water, and 82Rb), although several other PET perfusion agents are under preclinical and clinical evaluation. We focus on the clinically available agents (Table 25-1).
13N-Ammonia
13N-Ammonia is a cyclotron produced PET perfusion agent with a physical half-life of 10 minutes requiring an on-site cyclotron and radiochemistry synthesis capability. In the blood, 13N-ammonia exists in equilibrium between two states, neutral ammonia (NH3) and charged ammonium (NH4+). The neutral ammonia rapidly crosses cell membranes. Once it is inside the cell, glutamine synthase takes glutamate, ammonia, and ATP and produces glutamine, which remains intracellular, thereby fixing the 13N within the cell with very little diffusion back into the blood pool. 13N-Ammonia has a high extraction rate, with a significant percentage of the agent entering the myocyte.1 Animal models demonstrate, however, a nonlinear extraction of 13N-ammonia compared with the gold standard of microsphere flow measurements. At flows in the normal resting physiologic range of about 50 to 150 mL/min/100 g, 13N-ammonia uptake is nearly linear. However, at higher flows of more than 200 mL/min/100 g, such as those produced with vasodilator stress, there is a plateau effect, and higher flow rates are not associated with a further linear increase of 13N-ammonia uptake and retention.2 The nonlinear uptake at higher flow rates does not reflect a limitation of the diffusion of 13N-ammonia; rather, it is a limitation of metabolic trapping through the glutamine synthase pathway. This roll-off phenomenon may lead to underestimation of the true myocardial perfusion at higher flows, a statement true for all extracted radiotracers (Fig. 25-1). 13N-Ammonia image quality is generally excellent, with rapid clearance of the tracer from the blood pool and lung, although lung uptake can be an issue in patients with chronic lung disease or depressed left ventricular function.3
15O-Water
15O-Water is also a cyclotron-produced agent with an ultrashort half-life of 2 minutes. Unlike 13N-ammonia and 82Rb, 15O-water is a diffusible agent that is not extracted by or trapped within the myocytes but rather freely diffuses across membranes. The kinetics of 15O-water are dependent only on myocardial perfusion and are not affected by the metabolic rate-limiting steps that affect extracted tracers, eliminating the issues with tracer roll-off. However, PET 15O-water imaging generally is performed only as a dynamic study to determine uptake kinetics. Application of a single-compartment model to the dynamic image data allows a highly accurate estimation of absolute myocardial flow across a wide range of blood flow rates (0.29 to 5.04 mL/min/g).4 15O-water can be administered as an intravenous bolus, as a slow infusion, or by inhalation of 15O-CO2, which is converted to 15O-water in the lungs by carbonic anhydrase.
The advantage of 15O-water as an “ideal” flow tracer is counterbalanced by the complex acquisition protocols and analyses required for use in clinical practice. Because the 15O-water is not extracted from the blood pool, the images are contaminated by the high level of residual activity within the blood pool, which must be subtracted from the final image to properly evaluate myocardial perfusion. Two techniques are used to accomplish this goal. The blood pool can be labeled with 15O-carbon monoxide (CO). Inhalation of 15O-CO leads to irreversible binding of the CO to hemoglobin, thereby labeling the red blood cells and the blood pool. Blood pool data obtained during this acquisition can be subtracted from the 15O-water perfusion, eliminating the contribution of the blood pool from the final 15O-water data set. Alternatively, very early acquisitions (20 to 40 seconds after tracer infusion) provide an image of the blood pool before tracer flow has reached the myocardium; these are then used to remove background blood pool signal from the myocardium.5 The difficulties in image processing as well as the increased radiation exposure to the lung with use of the inhaled 15O-CO technique limit the clinical application of 15O-water.
Rubidium 82
82Rb is a radioactive monovalent cationic potassium analogue. Unlike the other perfusion agents used in cardiac PET, 82Rb is produced in a commercially available generator, alleviating the need for an on-site cyclotron, which makes it a more practical perfusion tracer for widespread clinical application. 82Rb is obtained by elution of 25 to 50 mL of normal saline with use of a computerized pump through a strontium 82 (82Sr) generator. The generator is rapidly replenished, with 90% of the maximal activity available after 5 minutes, and full replenishment occurs by 10 minutes. 82Rb has an ultrashort half-life of 75 seconds, which allows rapid acquisition of sequential stress and rest images (Fig. 25-2). The half-life of 82Sr is 25.5 days, and therefore the generator needs to be replaced every 4 weeks.
82Rb is extracted from the blood pool by the Na+,K+-ATPase pump. Extraction of 82Rb is similar to that of thallium 201 (201Tl), another potassium analogue,6 but it is less than that of 13N-ammonia7; therefore, the issues associated with roll-off may be present in 82Rb as they are in all other extractable tracers. Extraction of 82Rb is affected by myocardial perfusion as well as by the metabolic milieu. Severe acidosis and hypoxemia can affect 82Rb extraction.6 In addition, 82Rb is a less ideal imaging agent because the 82Rb positron has a high kinetic energy, which allows the positron to travel a relatively long distance before an annihilation event occurs. The increased positron range of the parent molecule results in a reduced spatial resolution of 82Rb imaging compared with that possible with other PET agents. Despite these issues, 82Rb has become one of the most commonly used PET perfusion agents because of the availability, ease of use, and acceptable image quality.
Summary of PET Perfusion Imaging
PET perfusion has become a routine tool in the management of cardiac patients. Multiple studies have evaluated the accuracy of perfusion PET for the detection of coronary artery disease. PET has consistently been found to have a high sensitivity and specificity for the detection of coronary artery disease. The accuracy of this technique makes the use of PET perfusion an attractive option in patients with chest pain or suspected coronary artery disease despite the increased cost and relative technical complexity (Table 25-2).8–15
Metabolic Agents
18F-Fluorodeoxyglucose (18F-FDG)
Fluorodeoxyglucose (2-fluoro-2-deoxy-d-glucose, FDG) is a glucose analogue that enters the cell through facilitated diffusion. Once inside cells, it is phosphorylated by hexokinase into FDG 6-phosphate, which is metabolically inert and is trapped within the myocyte. Therefore, retention of FDG is an energy-requiring process and is dependent on intact metabolic pathways (Fig. 25-3). FDG can be labeled with fluorine18 (18F), which decays by positron emission with a half-life of 110 minutes. The prolonged half-life of 18F allows local distribution of the agent from a central cyclotron site to regional imaging centers. Whereas it is the most commonly used agent in oncologic PET imaging, 18F-FDG is used primarily for the evaluation of myocardial viability in cardiac imaging.
Preparation of the patient is important in the proper acquisition of 18F-FDG images. In the fasting state, glucose uptake is low and inhomogeneous. To maximize glucose use by the myocardium, the patient is asked to fast for 6 to 12 hours. An oral glucose load (25 to 100 g) or an intravenous glucose load is administered and followed by insulin as needed before imaging. In diabetic patients, a more rigorous methodology is necessary to produce high-quality scans, and a euglycemic hyperinsulinemic clamp is often used. A dose of 5 to 15 mCi is injected, and imaging begins at least 45 minutes after tracer infusion. Viability studies with 18F-FDG are performed in conjunction with a perfusion study. Perfusion can be acquired with PET perfusion agents as described before or with SPECT perfusion agents such as 201Tl or technetium Tc 99m. Preserved FDG uptake in the setting of reduced perfusion suggests the presence of viable myocardium. These segments are likely to have improved function after revascularization. Segments that have both diminished perfusion and diminished FDG uptake are unlikely to recover function with revascularization and consist mostly of infarcted tissue (Fig. 25-4).
11C-Palmitate and 11C-Acetate
As noted previously, free fatty acids are the primary source of energy in normal myocardium, accounting for 60% to 80% of ATP produced in nonischemic myocytes. Free fatty acids are activated as acyl coenzyme A. Acyl coenzyme A enters the mitochondria through the acyl carnitine transport system and becomes part of the beta-oxidation pathway. Whereas this pathway is a rich source of ATP, it is highly oxygen dependent. During periods of ischemia, there is a rapid shift away from fatty acid metabolism to increased glucose use (see Fig. 25-3). Free fatty acids account for the preponderance of myocardial energy formation, and these pathways are dramatically altered by ischemia. Therefore, free fatty acid imaging is an attractive target for noninvasive imaging of ischemia and the associated alterations in oxidative metabolism. PET imaging of fatty acid metabolism generally involves imaging with carbon 11 (11C)–labeled medium-sized straight-chain fatty acids like palmitate, which undergo beta-oxidation. 11C has also been used to label acetate as an alternative approach for imaging of oxidative metabolism. 11C is cyclotron produced and has a relatively short half-life of 20.4 minutes. Initially produced in 1934 and first studied in humans in 1945, it has the advantage of being an organic molecule and therefore can potentially be used to target a wide variety of metabolic processes.16 Unfortunately, 11C-labeled metabolic agents are more difficult to employ in clinical practice and have been primarily used for research purposes. However, both 11C-palmitate and 11C-acetate have been effectively employed for the evaluation of myocardial oxidative metabolism.
Several studies have evaluated fatty acid metabolism in normal volunteers and patients. Walsh and colleagues17 demonstrated decreased mitochondrial metabolism in infarcted myocardium. In addition, 11C-palmitate infusion during dobutamine stress testing demonstrated the expected rise in fatty acid metabolism in the normal myocardial segments, whereas areas supplied by stenosed vessels did not show a rise in fatty acid use.18 Last, free fatty acid metabolism imaging has been used to demonstrate differences in myocardial metabolism in the elderly19 as well as in inherited nonischemic cardiomyopathies.20 In both cases, fatty acid metabolism was decreased compared with normal controls. Although the clinical application of some of these findings is not fully elucidated, free fatty acid PET offers a powerful method for the in vivo evaluation of myocardial metabolism and therefore provides an important tool for gaining insight into disease processes associated with altered myocardial metabolism.
Neurohormonal Cardiac PET
Multiple tracers have been developed for the evaluation of both the sympathetic and parasympathetic pathways (Table 25-3). One PET agent that has been extensively used in the evaluation of neurohormonal activation in heart failure and other cardiac disease states is m-hydroxyephedrine (HED). HED, a derivative of the amine metaraminol, is an analogue of norepinephrine that is resistant to metabolism through the typical monoamine oxidase pathway. Therefore, sympathetic activation of the heart can be effectively evaluated with 11C-labeled HED ([11C]HED) PET imaging. This radiopharmaceutical has been studied in multiple conditions including cardiac transplantation, cardiomyopathies, diabetes, and myocardial infarction. Evaluation of neuroactivation can infer disease outcome; for example, lower levels of [11C]HED are associated with a poor prognosis in heart failure.21 Whereas neurohormonal PET imaging remains in an early stage of development, many potential future avenues of investigation are present, including the prediction of sudden cardiac death.
| Tracer | PET/SPECT Tracer | Type |
|---|---|---|
| 11C-m-hydroxyephedrine (11C-HED) | PET | Analogue |
| 123I-MIBG (metaiodobenzylguanidine) | SPECT | Analogue |
| 18F-6-fluorodopamine | PET | Catecholamine |
| 11C-epinephrine | PET | Catecholamine |
| 18F-6-fluoronorepinephrine | PET | Catecholamine |
| 11C-phenylephrine | PET | Analogue |
| 18F-fluorometaraminol | PET | Analogue |
| 18F-p-fluorobenzylguanidine | PET | Analogue |
| 18F-fluoroiodobenzylguanidine | PET | Analogue |
| 18F-6-fluorometaraminol | PET | Analogue |
123I-MIBG has been the most studied SPECT agent. The PET agents are divided into those that are radiolabeled catecholamines and those that are catecholamine analogues.
Modified from Langer O, Halldin C. PET and SPECT tracers for mapping the cardiac nervous system. Eur J Nucl Med Mol Imaging 2002; 29:416-434.
IONIZING RADIATION IN PET/CT
The true risk to the patient from exposure to diagnostic medical radiation is difficult to estimate. The relatively low dose of radiation delivered with each exposure and the long latency time before the development of adverse events make it difficult to establish a causal link as large studies with long follow-up times are necessary to evaluate such effects. Therefore, much of the data available is derived from the study of nuclear plant workers involving occupational or accidental exposures or from the atom bomb experience at Hiroshima and Nagasaki. There are increased rates of leukemia and breast, thyroid, colorectal, and lung cancer in the exposed population.22 Excessive radiation exposure also has an adverse effect on mortality.23 Radiation exposure to young people carries a greater risk than to the elderly as the longer time horizon allows the clinical appearance of malignant change. In addition, the variable sensitivity of different tissues places women at higher risk from exposure to the thorax as breast tissue is sensitive to the effects of radiation (Fig. 25-5). Einstein and colleagues24 estimated a lifetime risk of 1 in 143 for a 20-year-old woman exposed to a single coronary CT study as opposed to a lifetime risk of 1 in 3261 for an 80-year-old man. It is therefore reasonable to exercise greater caution in scanning younger patients, especially a pediatric population.
In 2006, nuclear medicine scans and CT scans accounted for 22% of all radiologic studies, excluding dental studies. These procedures accounted for 75% of the effective dose administered with diagnostic procedures, with CT making up 46% and nuclear medicine accounting for 26% of the total exposure. The number of CT scans has increased by 10% annually since 1993, with an estimated 67 million CT scans being performed in the United States in 2006. In 2005, it was estimated that 19.7 million nuclear medicine procedures were performed in the United States. Nuclear cardiology accounted for 57% of all nuclear studies; most of these studies were myocardial perfusion scans.25 Therefore, it is important to take into account the radiation issues associated with PET/CT. PET/CT poses a unique challenge in diagnostic imaging as two separate systems with significant associated radiation exposure are employed (Fig. 25-6 and Table 25-4).
PET/CT
An alternative technique for attenuation correction that was employed routinely in the previous generation PET scanners involves the use of a rotating external radionuclide source, such as germanium 68 (68Ge), for creation of a transmission attenuation scan. The effective radiation dose from the 68Ge transmission scan is negligible compared with a CT scan. This represents a significant advantage over PET/CT.26 Whereas an external source may be adequate for attenuation correction, CT provides important anatomic data that can be used to localize and to quantify radiotracer activity as well as to provide complementary diagnostic information. Even a low-dose nongated CT scan, which is inadequate for quantification of coronary calcifications, or coronary CTA provides diagnostic anatomic information of the thorax and heart that is unavailable with an external radionuclide dose.
Radiation Exposure Associated with CT
One of two principal scanning methods can be used in cardiac CTA (Fig. 25-7). Traditional CTA uses a retrospective ECG gating technique. In this scan, a helical acquisition necessitates continuous radiation exposure throughout the cardiac cycle, increasing the overall ionizing radiation exposure. Several acquisition strategies can be employed to reduce exposure during retrospective ECG-gated cardiac CT. One strategy involves dose modulation, which allows lowering of the tube current during preselected portions of the cardiac cycle that are unlikely to provide diagnostic information. The coronary arteries move relatively rapidly through the imaging field during the cardiac cycle, while the temporal resolution of cardiac CT is relatively low. Therefore, data obtained during much of the cardiac cycle are unnecessary for the evaluation of the coronary arteries because the coronaries are in motion and cannot be imaged optimally secondary to through-plane motion. The coronaries are relatively still during end-systole and diastasis, and therefore only information acquired during those two selected phases of the cardiac cycle is generally clinically useful in the evaluation of epicardial coronary arteries. By maximizing the x-ray tube output during those two phases and, importantly, lowering tube output during the rest of the cardiac cycle, dose savings of 30% to 40% can be achieved.
The second CTA technique, which is increasingly used, is prospective ECG-gated image acquisition. This technique allows greater dose savings than those available with helical techniques. This scan uses an axial acquisition as opposed to the helical acquisition in the retrospective technique. The CT scanner is turned on only during the phase of the cardiac cycle in which coronary motion is minimized, avoiding radiation exposure at other portions of the cardiac cycle. This scanning approach requires longer scan times and therefore longer breath-hold times as well as a stable cardiac rhythm. However, as the number of CT detector rows has increased from 4 to 64 and beyond, breath-hold times are sufficiently short, making this technique more clinically viable in practice. Whereas the main advantage of prospective gating is decreased radiation dose, the primary disadvantage is the loss of functional information as the heart is imaged for only a short period of the entire cardiac cycle. In the context of PET/CT imaging, this limitation is partially overcome as ECG-gated PET scans can provide information about regional and global left ventricular function. Therefore, in the context of PET/CT imaging, prospective CTA is an especially appealing technologic advancement. Exposure from cardiac CT ranges from 7 to 21.4 mSv with use of 64–detector row scanners employing the retrospective acquisition techniques27; however, the newer prospective CT acquisition techniques offer much lower effective doses, potentially less than 3 mSv.28 This represents a substantial radiation dose savings to the patient.
In addition to the choice between helical and axial scanning, there are other CT parameters that can be used to minimize exposure. Tube current and tube voltage can and should be adjusted to the lowest levels that produce diagnostic-quality images. These adjustments are especially applicable in smaller and thinner patients because less x-ray attenuation occurs in these patients. Last, the width of the scan field in the z direction should be minimized to include only the heart and coronary arteries to avoid unnecessary radiation to adjacent structures (Table 25-5).
TABLE 25-5 Dose Reduction Strategies for the CT Portion of the PET/CT Examination
| CT Dose Reduction Strategy | Disadvantages |
|---|---|
| Low-dose transmission scan |
Radiation Exposure Associated with PET
Radiation dosimetry of nuclear radiopharmaceuticals is calculated by a mathematical biokinetic model that incorporates the tissue biodistribution of the agent as well as biologic and physical decay. Different PET agents have different target organs, dictating which tissues receive the maximal radiation exposure. The bladder is the organ of maximal dose for 13N-ammonia and 18F-FDG. The heart is the target organ for 15O-water, and the lungs are the target organ for inhaled 15O-CO gas. Total effective dose is then calculated from the sum of the organ doses. Effective radiation dose to the patient associated with PET imaging varies with the type of agent used, from 2.4 mSv for 13N-ammonia perfusion studies to 13.5 mSv for 82Rb perfusion studies (Fig. 25-8). Note that these radiation doses compare favorably with standard SPECT agents, for which doses can range from 6.6 mSv for stress-only 99mTc-tetrofosmin studies to 29.2 mSv for dual-isotope stress-rest studies using Tc 99m and 201Tl because those agents have significantly longer half-lives.27
Last, in addition to consideration of the radiation exposure to the patient, occupational exposure related to PET radiopharmaceuticals is also important. As positron annihilation events produce high-energy photons, these photons can travel long distances and affect bystanders. This is of particular importance to the staff of a PET facility. Technologists working with PET agents tend to have higher exposure to radiation compared with those working with SPECT agents.29 Mitigation strategies for occupational exposure center on the three principles of radiation safety, maximizing distance and shielding while minimizing exposure time. Proper shielding of syringes and vials reduces hand doses. Maintaining an appropriate distance from a recently injected patient reduces occupational exposure. Finally, proper setup of the patient before injection of the tracer and an efficient system of removal of intravenous lines and equipment and handling after the procedure can reduce the time of exposure for the PET laboratory staff. Used in concert, these techniques reduce radiation exposure and increase safety for PET personnel.
Dose Reduction in Cardiac PET/CT
Because PET/CT may potentially involve significant radiation exposure to the patient, care must be taken to maximize safety of the patient while maintaining image quality. Each technique should be optimized individually, allowing the best results in each portion of the study. Several considerations are taken into account in reducing radiation exposure from PET/CT studies. The first consideration is whether additional information is gained from combining both modalities. In some cases, a normal or abnormal test result from one of the two imaging techniques may be sufficient to make the diagnosis and to define future management of the patient. In such cases, the second study may be avoided entirely, saving the patient unnecessary cost and exposure. If just the PET study is necessary, an attenuation map can be constructed from either an external radionuclide transmission scan or a low-dose transmission CT scan for attenuation correction, minimizing the radiation exposure. Conversely, a normal coronary CTA study may obviate the need for perfusion imaging, avoiding the additional radiation associated with PET imaging. Considering which population of patients will benefit most from the combination of physiologic and anatomic information allows the greatest dose savings overall (Figs. 25-9 and 25-10).
When both studies are performed, efforts should be made to reduce exposure with each modality. The appropriate radionuclide dose should be selected according to the patient’s size. Exposure to radiation with 18F-FDG can be mitigated by encouraging hydration and frequent bladder emptying after the scan; radioisotope in the bladder is a major source of radiation exposure to the bladder and abdominal organs.30
A large proportion of the effective radiation dose in PET/CT is related directly to the CT scan. In a study involving four centers using diagnostic-quality contrast-enhanced CT as part of a whole-body 18F-FDG-PET/CT protocol (Fig. 25-11), an average of 16.1 mSv of the total 24.8 mSv for the entire procedure was accounted for by the diagnostic-quality CT (65%).30 By comparison, low-dose (not “diagnostic quality”) CT has an effective dose of 4.5 mSv. The logical conclusion is that low-dose CT for attenuation correction alone should be preferentially performed in instances in which a high-dose diagnostic CT examination is unnecessary.
KEY POINTS
 Increasing prevalence of hybrid PET/CT imaging systems permits the evaluation of anatomy and physiology in a single test.
Increasing prevalence of hybrid PET/CT imaging systems permits the evaluation of anatomy and physiology in a single test. PET perfusion agents are divided into extractable agents (13N-ammonia and 82Rb) and diffusible agents (15O-water).
PET perfusion agents are divided into extractable agents (13N-ammonia and 82Rb) and diffusible agents (15O-water). 82Rb provides high-quality myocardial perfusion images and has become more widely used since the isotope can be produced by a small, on-site, fully shielded, portable generator and infusion system.
82Rb provides high-quality myocardial perfusion images and has become more widely used since the isotope can be produced by a small, on-site, fully shielded, portable generator and infusion system. Myocytes use fatty acids as their main energy source. However, in ischemia, the heart quickly switches to glucose metabolism. PET tracers can be used to image these metabolic processes.
Myocytes use fatty acids as their main energy source. However, in ischemia, the heart quickly switches to glucose metabolism. PET tracers can be used to image these metabolic processes. 18F-FDG, a cyclotron-produced tracer with a relatively long half-life, identifies viable tissues by identifying intact glucose metabolism within myocytes.
18F-FDG, a cyclotron-produced tracer with a relatively long half-life, identifies viable tissues by identifying intact glucose metabolism within myocytes. CT and nuclear medicine individually account for a substantial proportion of medical radiation exposure in the population.
CT and nuclear medicine individually account for a substantial proportion of medical radiation exposure in the population.Beller GA, Bergmann SR. Myocardial perfusion imaging agents: SPECT and PET. J Nucl Cardiol. 2004;11:71-86.
Bergmann SR. Imaging of myocardial fatty acid metabolism with PET. J Nucl Cardiol. 2007;14:S118-S124.
Brix G, Beyer T. PET/CT: dose-escalated image fusion? Nuklearmedizin. 2005;44(Suppl 1):S51-S57.
Di Carli MF. Advances in positron emission tomography. J Nucl Cardiol. 2004;11:719-732.
Einstein AJ, Moser KW, Thompson RC, et al. Radiation dose to patients from cardiac diagnostic imaging. Circulation. 2007;116:1290-1305.
Hall EJ, Brenner DJ. Cancer risks from diagnostic radiology. Br J Radiol. 2008;81:362-378.
Machac J. Cardiac positron emission tomography imaging. Semin Nucl Med. 2005;35:17-36.
Townsend DW. Combined positron emission tomography–computed tomography: the historical perspective. Semin Ultrasound CT MR. 2008;29:232-235.
Townsend DW. Positron emission tomography/computed tomography. Semin Nucl Med. 2008;38:152-166.
1 Schelbert HR, Phelps ME, Huang SC, et al. N-13 ammonia as an indicator of myocardial blood flow. Circulation. 1981;63:1259-1272.
2 Shah A, Schelbert HR, Schwaiger M, et al. Measurement of regional myocardial blood flow with N-13 ammonia and positron-emission tomography in intact dogs. J Am Coll Cardiol. 1985;5:92-100.
3 Wahl RL, Buchanan JW. Principles and Practice of Positron Emission Tomography. Philadelphia: Lippincott Williams & Wilkins; 2002.
4 Bergmann SR, Herrero P, Markham J, et al. Noninvasive quantitation of myocardial blood flow in human subjects with oxygen-15–labeled water and positron emission tomography. J Am Coll Cardiol. 1989;14:639-652.
5 Beller GA, Bergmann SR. Myocardial perfusion imaging agents: SPECT and PET. J Nucl Cardiol. 2004;11:71-86.
6 Goldstein RA, Mullani NA, Marani SK, et al. Myocardial perfusion with rubidium-82. II. Effects of metabolic and pharmacologic interventions. J Nucl Med. 1983;24:907-915.
7 Becker L, Ferreira R, Thomas M. Comparison of Rb-86 and microsphere estimates of left ventricular blood flow distribution. J Nucl Med. 1977;15:969-973.
8 Gould KL, Goldstein RA, Mullani NA, et al. Noninvasive assessment of coronary stenoses by myocardial perfusion imaging during pharmacologic coronary vasodilation. VIII. Clinical feasibility of positron cardiac imaging without a cyclotron using generator-produced rubidium-82. J Am Coll Cardiol. 1986;7:775-789.
9 Demer LL, Gould KL, Goldstein RA, et al. Assessment of coronary artery disease severity by positron emission tomography. Comparison with quantitative arteriography in 193 patients. Circulation. 1989;79:825-835.
10 Go RT, Marwick TH, MacIntyre WJ, et al. A prospective comparison of rubidium-82 PET and thallium-201 SPECT myocardial perfusion imaging utilizing a single dipyridamole stress in the diagnosis of coronary artery disease. J Nucl Med. 1990;31:1899-1905.
11 Schelbert HR, Wisenberg G, Phelps ME, et al. Noninvasive assessment of coronary stenoses by myocardial imaging during pharmacologic coronary vasodilation. VI. Detection of coronary artery disease in human beings with intravenous N-13 ammonia and positron computed tomography. Am J Cardiol. 1982;49:1197-1207.
12 Yonekura Y, Tamaki N, Senda M, et al. Detection of coronary artery disease with 13N-ammonia and high-resolution positron-emission computed tomography. Am Heart J. 1987;113:645-654.
13 Williams BR, Jansen DE, Wong LF, et al. Positron emission tomography for the diagnosis of coronary artery disease: a non-university experience and correlation with coronary angiography. J Nucl Med. 1989;30:845.
14 Stewart RE, Schwaiger M, Molina E, et al. Comparison of rubidium-82 positron emission tomography and thallium-201 SPECT imaging for detection of coronary artery disease. Am J Cardiol. 1991;67:1303-1310.
15 Tamaki N, Yonekura Y, Senda M, et al. Value and limitation of stress thallium-201 single photon emission computed tomography: comparison with nitrogen-13 ammonia positron tomography. J Nucl Med. 1988;29:1181-1188.
16 Allard M, Fouquet E, James D, Szlosek-Pinaud M. State of art in 11C labelled radiotracers synthesis. Curr Med Chem. 2008;15:235-277.
17 Walsh MN, Geltman EM, Brown MA, et al. Noninvasive estimation of regional myocardial oxygen consumption by positron emission tomography with carbon-11 acetate in patients with myocardial infarction. J Nucl Med. 1989;30:1798-1808.
18 Tamaki N, Kawamoto M, Takahashi N, et al. Assessment of myocardial fatty acid metabolism with positron emission tomography at rest and during dobutamine infusion in patients with coronary artery disease. Am Heart J. 1993;125:702-710.
19 Kates AM, Herrero P, Dence C, et al. Impact of aging on substrate metabolism by the human heart. J Am Coll Cardiol. 2003;41:293-299.
20 Davila-Roman VG, Vedala G, Herrero P, et al. Altered myocardial fatty acid and glucose metabolism in idiopathic dilated cardiomyopathy. J Am Coll Cardiol. 2002;40:271-277.
21 Pietila M, Malminiemi K, Ukkonen H, et al. Reduced myocardial carbon-11 hydroxyephedrine retention is associated with poor prognosis in chronic heart failure. Eur J Nucl Med. 2001;28:373-376.
22 Pierce DA, Shimizu Y, Preston DL, et al. Studies of the mortality of atomic bomb survivors. Report 12, Part I. Cancer: 1950-1990. Radiat Res. 1996;146:1-27.
23 Cologne JB, Preston DL. Longevity of atomic-bomb survivors. Lancet. 2000;356:303-307.
24 Einstein AJ, Henzlova MJ, Rajagopalan S. Estimating risk of cancer associated with radiation exposure from 64-slice computed tomography coronary angiography. JAMA. 2007;298:317-323.
25 Mettler FAJ, Thomadsen BR, Bhargavan M, et al. Medical radiation exposure in the U.S. in 2006: preliminary results. Health Phys. 2008;95:502-507.
26 Wu TH, Huang YH, Lee JJ, et al. Radiation exposure during transmission measurements: comparison between CT- and germanium-based techniques with a current PET scanner. Eur J Nucl Med Mol Imaging. 2004;31:38-43.
27 Einstein AJ, Moser KW, Thompson RC, et al. Radiation dose to patients from cardiac diagnostic imaging. Circulation. 2007;116:1290-1305.
28 Abada HT, Larchez C, Daoud B, et al. MDCT of the coronary arteries: feasibility of low-dose CT with ECG-pulsed tube current modulation to reduce radiation dose. AJR Am J Roentgenol. 2006;186:S387-S390.
29 Chiesa C, De Sanctis V, Crippa F, et al. Radiation dose to technicians per nuclear medicine procedure: comparison between technetium-99m, gallium-67, and iodine-131 radiotracers and fluorine-18 fluorodeoxyglucose. Eur J Nucl Med. 1997;24:1380-1389.
30 Brix G, Lechel U, Glatting G, et al. Radiation exposure of patients undergoing whole-body dual-modality 18F-FDG PET/CT examinations. J Nucl Med. 2005;46:608-613.


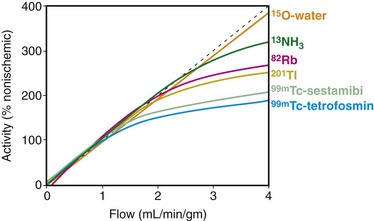
 FIGURE 25-1
FIGURE 25-1
 FIGURE 25-2
FIGURE 25-2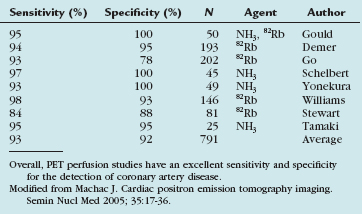
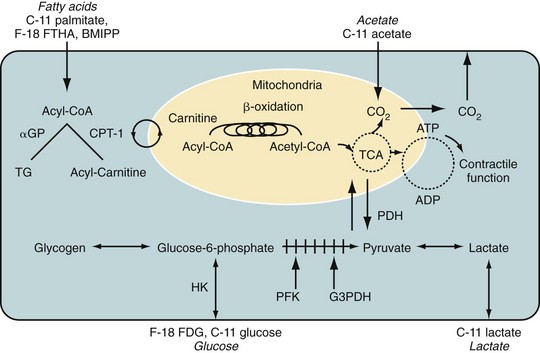
 FIGURE 25-3
FIGURE 25-3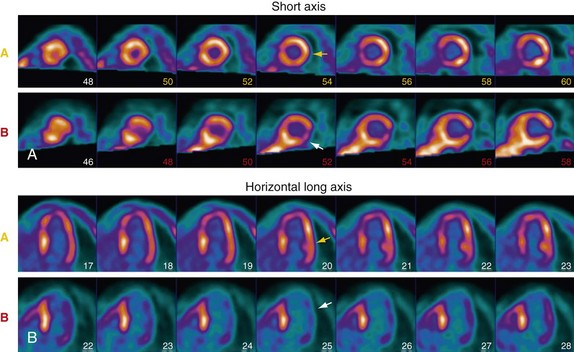
 FIGURE 25-4
FIGURE 25-4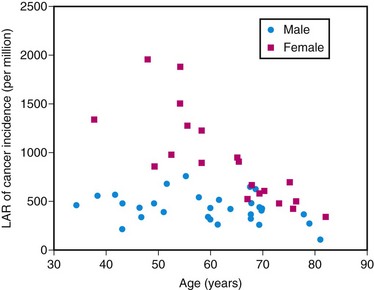
 FIGURE 25-5
FIGURE 25-5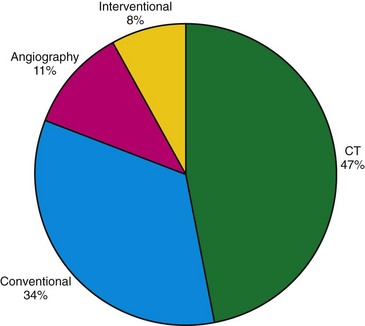
 FIGURE 25-6
FIGURE 25-6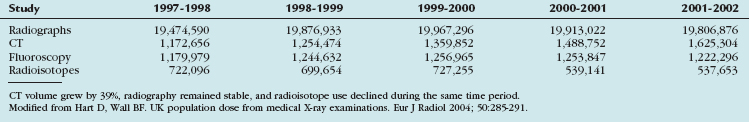
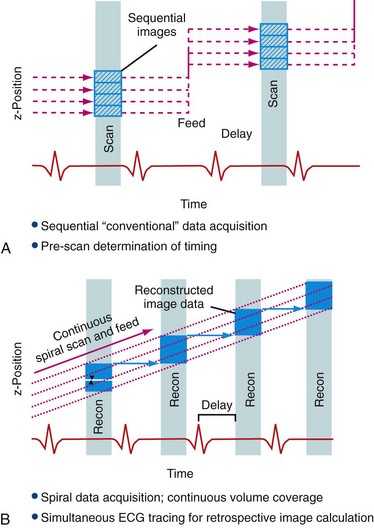
 FIGURE 25-7
FIGURE 25-7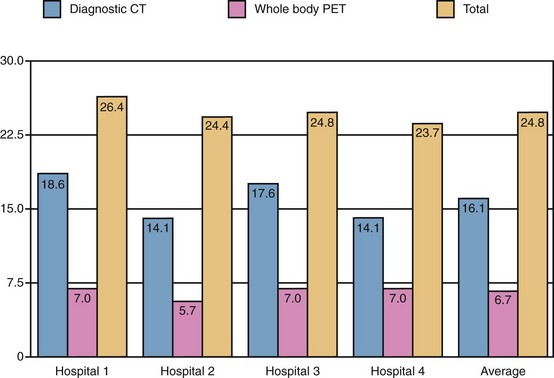
 FIGURE 25-8
FIGURE 25-8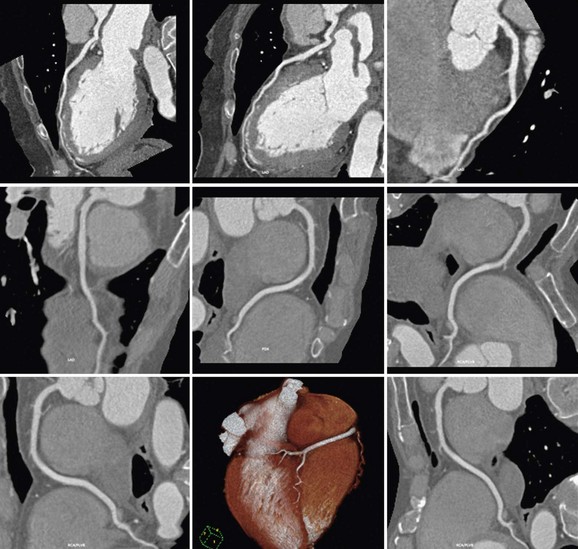
 FIGURE 25-9
FIGURE 25-9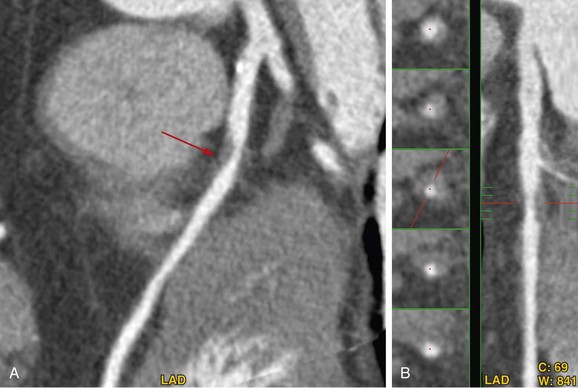
 FIGURE 25-10
FIGURE 25-10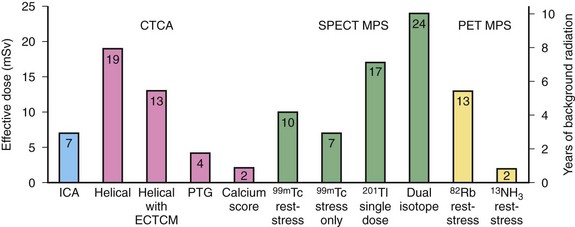
 FIGURE 25-11
FIGURE 25-11


