CHAPTER 22 Radiopharmaceutical Single Photon Emission Computed Tomography Imaging Agents
* The views expressed in this chapter are those of the author and do not necessarily reflect the official policy or position of the Department of the Navy, Army, Department of Defense, or the U.S. government.
We certify that all individuals who qualify as authors have been listed; each has participated in the conception and design of this work, the analysis of data (when applicable), the writing of the document, and the approval of the submission of this version; that the document represents valid work; that if we used information derived from another source, we obtained all necessary approvals to use it and made appropriate acknowledgments in the document; and that each takes public responsibility for it.
Myocardial perfusion imaging agents have become critical to diagnosis, prognosis, and clinical decision-making in the setting of coronary artery disease. Radiopharmaceuticals have been used to determine regional perfusion since the 1950s, although the imaging techniques have been refined and improved over time. In general, myocardial perfusion radiotracers share many of the following common characteristics:
DESCRIPTION OF TECHNICAL REQUIREMENTS
Thallium 201
The Saperstein principle states that a given type of radiopharmaceutical will be distributed in proportion to regional perfusion if its extraction by the organ of interest is high and if its clearance from the blood is rapid.1 This principle led to the development of the potassium analogues, one of the first radiopharmaceuticals used to image myocardial perfusion. These agents are monovalent cations of potassium, cesium, rubidium, and thallium. They enter the myocardium by active channels through the Na+, K+-ATPase pump. Thallium 201 (201Tl) has many of the best physical and biologic characteristics for imaging in humans, making it one of the most popular myocardial perfusion agents used since the 1970s.1 Thallium continues to find significant utility in the diagnosis of coronary artery disease and in the evaluation of prognosis and revascularization in those with ischemic heart disease.
Extraction and Biodistribution
The flow dependence of myocardial tracer uptake limits the sensitivity of a given tracer for the detection of coronary artery disease. In Figure 22-1, the curves of tracer myocardial uptake versus myocardial blood flow flatten as coronary blood flow increases. This implies that at a given level of coronary blood flow, the tracer imaging defect will be much less than the actual coronary blood flow disparity. In Figure 22-1, there is a linear relation of uptake over a wide range of coronary blood flow using a standard of radiolabeled microspheres. At high flow rates (~2.5 mL/min/g), there is a diffusion limitation and a fall in tracer extraction with increased cellular tracer washout, leading to a plateau of myocardial uptake. This phenomenon leads to an underestimation of coronary blood flow at high flow rates and overestimation at very low flow rates when there is increased myocardial extraction relative to blood flow.
In general, 201Tl has an extraction fraction of 87% at normal flow rates. Only viable myocardial cells that maintain a transmembrane potassium gradient will retain 201Tl. In general, a maximum of 4% of the injected dose is taken up in the myocardium (occurring within 10 to 15 minutes of injection), with high concentrations also found in the liver and kidney. 201Tl disappearance from the blood compartment is rapid; more than 90% of blood activity clears with a half-life of 5 minutes, resulting in a decreased blood pool background.2
Redistribution
Myocardial redistribution is one of the most clinically important characteristics of 201Tl. On a practical level, redistribution is manifested as a “filling in” of a myocardial perfusion defect occurring 3 to 5 hours after the injection of 201Tl at peak stress. The myocardial uptake of thallium is not static over time, and redistribution is related to the rate of influx of 201Tl into the myocardium from the blood pool and the rate of clearance or washout of thallium from the myocardium. As described in the previous section, no more than 3% to 5% of tracer is delivered to the myocardium. The amount of washout from the myocardium depends not only on the amount of tracer leaving through blood flow but also on how much is being continuously accumulated by exchange from other compartments.3 Figure 22-2 further illustrates this concept for various levels of myocardial blood flow. All the curves show some level of rapid early myocardial uptake, roughly proportional to myocardial perfusion. Blood levels of tracer fall rapidly as tracer is extracted by the heart and other systemic compartments. After this initial extraction, tracer molecules are slowly released back into the blood from body compartments, maintaining a constant low-level blood concentration of tracer. There is a subsequent exchange of tracer between the blood and myocardial cells until an equilibrium point is reached. This exchange equilibrium is not dependent on blood flow but only on the relative concentration of intravascular and extravascular tracer molecules and the subsequent concentration gradient supported by the membrane potentials or by active membrane transport.4 All tracers must redistribute to some extent, although thallium displays this property to a greater extent because of the ease with which its molecules are transported in and out of the myocardium through active channels. With thallium, a reversible myocardial perfusion defect related to ischemic heart disease will show normalization of 201Tl uptake at 3 to 4 hours after injection at stress because of delayed accumulation into the ischemic segment and a more rapid washout from normal myocardial segments than from hypoperfused segments.1
Clinical Considerations
Viability
In many clinical scenarios, patients with known coronary artery disease and history of myocardial infarction are considered for revascularization. However, exposure of patients to the risks of possible complex percutaneous or surgical revascularization options in the absence of true long-term benefit, either from a mortality or on a symptomatic basis, may preclude a more aggressive approach. Truly infarcted myocardium will not improve in contractile function regardless of coronary blood flow to the region of interest. Myocardium that is chronically ischemic or hibernating but not truly infarcted can achieve significant benefit from revascularization. These myocardial cells have downregulated their metabolic processes as a protective mechanism to counteract a lack of blood flow until future oxygen delivery capabilities return. Optimization of blood flow to hibernating myocardium indeed does improve long-term outcomes and can improve symptoms in carefully selected patients.5 This concept has led to an enormous growth in the use of nuclear perfusion imaging to assess for viability to guide clinical decision-making with regard to revascularization.
Thallium has proved to be a useful agent in the assessment of viability. Its redistribution properties lend themselves to a sensitive physiologic assessment of tissue integrity, even in the absence of adequate blood flow to a region of myocardium.6 In general, cationic tracers that are retained by membrane potentials will be “viability” agents. Significant tracer uptake into the myocardium requires delivery, implying perfusion, and retention, implying enough cellular integrity to generate membrane potentials to fuel active transport channels. Even in the absence of a delivery mechanism, thallium can redistribute over time to myocardial cells that have intact cell membranes. Practically, this would be manifested as a lack of perfusion (or counts) on rest imaging with a “filling in” of any defects with delayed imaging. Infarcted tissue would not display similar count statistics owing to an inability of thallium tracer molecules to enter dead myocardial cells (Fig. 22-3). As a result, patients with symptoms of ischemic heart disease but history of prior infarction (and, often, systolic left ventricular dysfunction) may benefit from revascularization in the setting of viability in the region subtended by obstructive epicardial coronary lesions. In general, redistribution imaging is performed at least 4 to 6 hours after rest imaging to allow maximal thallium uptake into hibernating but viable myocardium. Some studies have shown that 24-hour late thallium imaging enhances the detection of myocardial viability after myocardial infarction.7 From a practical perspective, 24-hour thallium redistribution imaging is often preferred to early (4- to 6-hour) redistribution studies for the assessment of viability.
Radiation Exposure
In the 21st century, the medical use of radiation is the largest man-made source of radiation. More than 5 billion imaging examinations are performed worldwide each year, with a majority employing ionizing radiation with radiology or nuclear medicine.8 In modern medicine, attributable cancer risk increases significantly with increasing lifetime radiation exposure. Cardiac studies account for 57% of all nuclear medicine examinations and 85% of the effective dose.9 Radiologic dose estimates can be expressed as multiples of a single posterior-anterior chest radiograph (equivalent to 0.02 mSv). As can be seen in Table 22-1, a stress technetium sestamibi study is the radiation dose equivalent of 600 chest radiographs, and a similar thallium study is the equivalent of 1500 chest radiographs. For thallium, this corresponds to an extra lifetime cancer risk of about 1 in 1000 patients (Fig. 22-4). Indeed, as our technology improves and the demand grows for noninvasive cardiac studies, radiation exposure will continue to be a strong consideration in choosing the most appropriate imaging modality with consideration of balancing lifetime cancer risk with the optimal, cost-efficient diagnostic approach.
Dose and Image Quality
The major driving force behind the increased radiation exposure risk for thallium is its long half-life, especially compared with technetium. As a result, dosing is limited to 2 to 4 mCi, a fraction of that feasible for technetium-based agents (25 to 30 mCi), for a typical myocardial perfusion study. Count statistics for thallium therefore prove to be less robust, often making imaging artifacts more prominent and actual scan interpretation more of a problem. Studies have consistently shown that image quality is better and interobserver reader variability is lower with technetium-based agents compared with thallium.10 In addition, the ability to inject much higher doses of technetium and the improved counts therein allow ECG-based gating of technetium images, adding left ventricular wall motion to the perfusion data, which can improve image interpretation sensitivity and specificity.
Technetium Tc 99m Labeled Myocardial Perfusion Agents
Despite the clinical value of 201Tl as a myocardial perfusion agent, its physical characteristics are suboptimal for gamma camera imaging. Since its introduction to clinical use in the 1970s, intense research subsequently led to the discovery of other cardiac nuclear tracers, particularly technetium Tc 99m labeled agents. These agents have many physical properties that are superior to 201Tl as listed in Table 22-1, but the two of most interest are its ideal photon energy peak (140 keV) and short half-life (6 hours) that allows a 10-fold dose increase in comparison with 201Tl. These two attributes result in image quality superior to that of 201Tl-based cardiac imaging, particularly in obese patients.10,11,12 The two Tc 99m labeled perfusion agents of most clinical use today are sestamibi and tetrofosmin. Two other tracers that have been developed are N-NOET and teboroxime.
As opposed to 201Tl, which is cyclotron produced and thus must be obtained from commercial suppliers, Tc 99m is eluted from a small molybdenum 99 (99Mo) generator, allowing possible 24-hour use. Tc 99m is produced in the form of Tc 99m pertechnetate, which can be eluted from the generator with sterile normal saline. After an elution of the 99Mo generator, maximal activity is achieved after 24 hours, but clinical doses of Tc 99m are available as soon as 3 to 6 hours later. The free Tc 99m pertechnetate can then be reconstituted with the aforementioned perfusion agents to be used for myocardial perfusion imaging.13
Tc 99m Sestamibi
Extraction and Biodistribution
Tc 99m sestamibi has an in vivo first-pass myocardial extraction of 55% to 68%, which is significantly less than that of 201Tl. Related to a reduced extraction rate is a nonlinear cellular uptake particularly above coronary flow rates of 2 to 2.5 mL/min/g, which theoretically reduces its sensitivity to detect less critical stenosis. Cellular uptake of Tc 99m sestamibi, like that of 201Tl, is more linear at basal flow rates and actually increases relative to absolute blood flow at lower rates. Approximately 1% of the dose is taken up by the myocardium and remains in the blood pool 1 hour after injection.14
Tc 99m Tetrofosmin
Extraction and Biodistribution
The first-pass myocardial extraction rate for Tc 99m tetrofosmin is 54%, which is somewhat lower than that of Tc 99m sestamibi, with a slow rate of myocardial clearance and redistribution. As a result of the lower extraction rate, uptake of the tracer plateaus at higher coronary flow rates, particularly above 2 mL/min/g, potentially underestimates more modest coronary stenosis.14
Tc 99m Teboroxime
Extraction and Biodistribution
The neutral lipophilic characteristics of Tc 99m teboroxime result in an extraction rate that exceeds 90%. Tc 99m teboroxime approximates a freely diffusible radiotracer with a concomitant rapid myocardial washout that is independent of metabolic activity. Myocardial uptake is linear with coronary blood flow even at high flow rates and thus does not experience a “roll-off” phenomenon as does Tc 99m sestamibi or even 201Tl. However, as a result of the rapid myocardial washout of Tc 99m teboroxime, subsequent gamma imaging needs to occur rapidly to avoid underestimation of a potential defect. In animal models, defect normalization can occur as rapidly as 8 minutes by use of adenosine stress in dogs with and without coronary occlusions. This has led to the limited use of this agent for clinical applications.10
Tc 99m N-NOET
Extraction and Biodistribution
The myocardial extraction fraction of Tc 99m N-NOET is similar to that of 201Tl at 82% to 87%, with an initial cardiac retention higher than that of 201Tl. This linear uptake with flow in the hyperemic range has the potential to increase sensitivity for detection of subtle ischemic changes in relation to the other Tc 99m based tracers. Cardiac transport of Tc 99m N-NOET appears to be less sensitive than 201Tl to ischemic injury. Myocardial washout of Tc 99m N-NOET is rapid, resulting in reductions in defect contrast soon after injection, which is related to the rapid redistribution of the tracer. This is similar to Tc 99m teboroxime, but to a lesser extent with Tc 99m N-NOET.10
1 Taillefer R. Kinetics of myocardial perfusion imaging radiotracers. In: Iskandrian AE, Verani MS, editors. Nuclear Cardiac Imaging: Principles and Applications. 3rd ed. New York: Oxford University Press; 2003:51.
2 Krahwinkel W, Herzog H, Feinendegen LE. Pharmacokinetics of thallium-201 in normal individuals after routine myocardial scintigraphy. J Nucl Med. 1988;29:1582-1587.
3 Grunwald AM, Watson DD, Hoszgrete HH, et al. Thallium-201 kinetics in normal and ischemic myocardium. Circulation. 1981;64:610-618.
4 Watson D, Glover D. Overview of Kinetics and Modeling. In: Zaret BL, Beller GA, editors. Clinical Nuclear Cardiology: State of the Art and Future Directions. 3rd ed. Philadelphia: Mosby; 2005:6-7. pp
5 Sansoy V, Glover DK, Watson DD, et al. Comparison of thallium-201 resting redistribution with technetium-99m–sestamibi uptake and functional response to dobutamine for assessment of myocardial viability. Circulation. 1995;92:994-1004.
6 Dilsizian V, Rocco TP, Nanette MD, et al. Enhanced detection of ischemic but viable myocardium by the reinjection of thallium after stress-redistribution imaging. N Engl J Med. 1990;323:141-146.
7 Allman KC, Shaw LJ, Hachamovitch R, Udelson JE. Myocardial viability testing and impact of revascularization on prognosis in patients with coronary artery disease and left ventricular dysfunction. J Am Coll Cardiol. 2002;39:1151-1158.
8 Picano E, Vano E, Semelka R, Regulla D. The American College of Radiology white paper on radiation dose in medicine: deep impact on the practice of cardiovascular imaging. Cardiovasc Ultrasound. 2007;5:37-43.
9 Thompson R, Cullon S. Issues regarding radiation dosage of cardiac nuclear and radiography procedures. J Nucl Cardiol. 2006;13:19-23.
10 Beller GA, Bergmann SR. Myocardial perfusion imaging agents: SPECT and PET. J Nucl Cardiol. 2004;11:71-86.
11 Kailasnath P, Sinusas AJ. Technetium-99m–labeled myocardial perfusion agents: are they better than thallium-201? Cardiol Rev. 2001;9:160-172.
12 Kailasnath P, Sinusas AJ. Comparison of Tl-201 with Tc-99m–labeled myocardial perfusion agents: technical, physiologic, and clinical issues. J Nucl Cardiol. 2001;8:482-498.
13 Baggish AL, Boucher CA. Radiopharmaceutical agents for myocardial perfusion imaging. Circulation. 2008;118:1668-1674.
14 Hendel R, Parker M, Zaret B, et al. Reduced variability of interpretation and improved image quality with a technetium 99m myocardial perfusion agent: comparison of thallium-201 and technetium 99m–labeled tetrofosmin. J Nucl Cardiol. 1994:509-514.

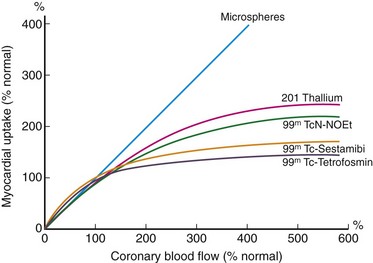
 FIGURE 22-1
FIGURE 22-1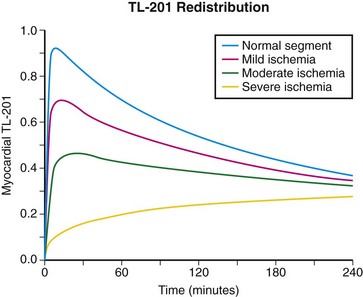
 FIGURE 22-2
FIGURE 22-2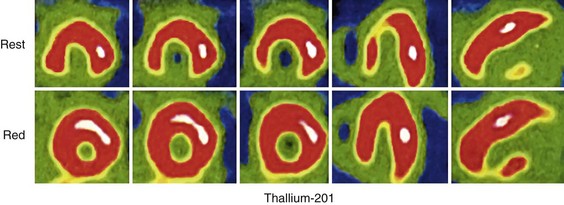
 FIGURE 22-3
FIGURE 22-3
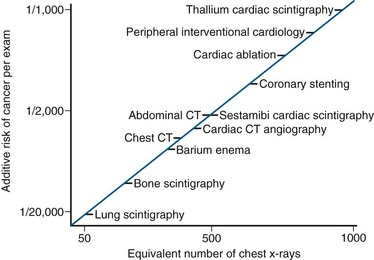
 FIGURE 22-4
FIGURE 22-4


