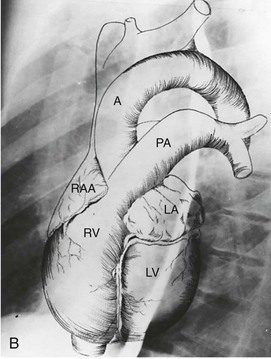
 B, Superimposed drawing of the cardiac chambers and the major vessels.
B, Superimposed drawing of the cardiac chambers and the major vessels.
(From Van Houten FX, Adams DF, Abrams HL: Radiology of valvular heart disease. In Sonnenblick E, Lesch M [eds]: Valvular Heart Disease. New York, Grune & Stratton, 1974.)
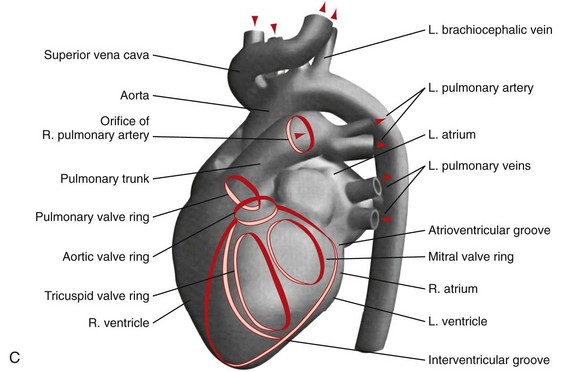 C, A computer-generated model in the lateral projection demonstrates the valves, chambers, and sulci.
C, A computer-generated model in the lateral projection demonstrates the valves, chambers, and sulci.
(From Steiner RM: Radiology of the heart and great vessels. In Braunwald E, Zipes PD, Libby P [eds]: Heart Disease: A Textbook of Cardiovascular Medicine, 6th ed. Philadelphia, Saunders, 2001.)
TABLE 5-1 Cardiovascular and Pulmonary Devices and Other Appliances in the ICU Patient
CHAPTER 5 Radiology of the Heart
Plain Film Imaging and Diagnosis
INTRODUCTION
All of these newer modalities yield anatomically detailed images of the heart and great vessels not possible with plain film radiography alone. However, the chest radiograph continues to provide valuable and often unique information concerning the structure and function of the cardiovascular system. The chest radiograph presents an opportunity to recognize subtle and/or overlooked abnormalities such as cardiac chamber enlargement, clinically important calcifications, and evidence of right and left heart dysfunction. Because the pulmonary arteries and veins are visualized in exquisite detail on a well-performed posterior-anterior (PA) and lateral chest radiograph, over and under pulmonary circulation as well as the findings of pulmonary arterial and venous hypertension can be appreciated. Adult onset congenital heart disease, often misdiagnosed or overlooked clinically, can often be identified on a plain film chest radiograph.1,2
The portable chest radiograph, usually performed in the anterior-posterior (AP) supine or erect position is the imaging modality of choice for the evaluation and monitoring of patients with cardiopulmonary disease in the intensive care unit (ICU) including postoperative cardiac patients and in those with implanted cardiovascular devices.3,4
NORMAL AND ABNORMAL CARDIAC BORDERS
Chest Radiograph
In a well-positioned PA or frontal chest radiograph, the cardiac silhouette and other vascular structures are predictably outlined against the lung as a series of bulges and indentations along the right and left mediastinal borders (Fig. 5-1).
Left Mediastinal Border
In the normal patient, the most superior border-forming structure along the left side of the mediastinum is the left subclavian artery (LSCA). Positioned just above the aortic arch and below the left clavicle, it usually forms a concave border with the lung and lies medial to the aortic arch (see Fig. 5-1). When blood flow through the LSCA is increased, as in a postductal coarctation of the aorta, or when the vessel is tortuous because of atherosclerosis, it will lie at or lateral to the aortic knob or arch—an important clue to the diagnosis of disease. A vertical, straight, or convex, double shadow parallel to the LSCA border is found when a left superior vena cava is present (Fig. 5-2). A cervical aortic arch or the elongated aortic arch of a pseudocoarctation of the aorta may also obscure the normal LSCA border.
Aortic Arch
The aortic arch or “knob” forms a convex segment along the left mediastinal border below the LSCA border and above the main pulmonary artery border. The aortic convexity is the distal posterior portion of the arch measuring 2.0 ± 1 cm in the young adult and 2.5 to 3.5 cm in the normal middle-aged individual. A clue to the location of the medial border of the aortic arch is the right-sided displacement of the tracheal airway at the same level as the arch. Although the aortic arch border usually has smooth margins, a rounded 2 to 3 mm extension or “nipple” may be present. This structure, seen in up to 10% of normal individuals, is the left superior intercostal vein (LSIV) (Fig. 5-3). An enlarged LSIV will suggest obstruction to blood flow in the deep mediastinal venous system such as superior vena caval obstruction. A dilated LSIV is similar in significance to a dilated azygos vein.
The left side of the descending thoracic aorta forms a clearly visualized interface with the left lung behind the left heart border on a well-penetrated frontal chest film (see Fig. 5-4). Pleural effusion and pulmonary masses, left lower lobe pneumonia, and other soft tissue opacities may obliterate the descending aortic border.
When the aortic arch border on the left side of the mediastinum is not visible and when the trachea is displaced to the left, the presence of an aortic arch on the right side of the mediastinum will suggest the diagnosis of right aortic arch (RAA) (Fig. 5-4). It is associated in most cases with an aberrant left subclavian artery; however, RAA may occur without an associated aberrant subclavian artery in mirror image RAA with or without associated cyanotic heart disease.
Main Pulmonary Artery Border (see Fig. 5-1)
The normally convex main left pulmonary artery (LPA) is situated immediately below the aorticopulmonary window and extends posteriorly as it arches over the left bronchus. It will enlarge because of increased pulmonary blood flow due to a left-to-right shunt, increased pulmonary pressure in patients with obliterative lung disease or Eisenmenger physiology, pulmonary valve stenosis, or weakness of the pulmonary arterial wall in an arteritis (Fig. 5-5). The main pulmonary artery (MPA) and LPA will bulge to the left and the pulmonary artery branches will converge at the lateral border of the enlarged vessel. Alternatively, if the pulmonary artery border is flat or concave, the MPA may be absent or stenotic when truncus arteriosus, tetralogy of Fallot, pulmonary atresia, or transposition of the great vessels is present.
Left Atrial Border (LAA)
The LAA lies below the left main-stem bronchus in the frontal projection and is border forming with the lung. The LAA forms a smooth and slightly concave portion of the left heart border (see Fig. 5-1). Atrial enlargement should be suspected when the LAA is convex and prominent (Fig. 5-6) but the LA may enlarge significantly, even when the LAA remains small, so that enlargement of the LA may be suspected by other signs of enlargement including a double right atrial-left atrial border on the right side of the mediastinum, elevation and widening of the carinal angle, and a prominent LA bulge on the lateral projection. Nonvascular pathology may simulate enlargement of the left atrial appendage. For example, a pericardial cyst, lymphoma, thymoma, or other mediastinal or pleural neoplasms may appear as a convexity of the left atrial border (Fig. 5-7). Bulging of the LAA border may also be caused by partial absence of the pericardium.
Left Ventricular Border (LV)
The left ventricular border continues downward seamlessly from the left atrial border. The LV is usually mildly convex in relation to the nearby lung. When the LV is hypertrophic as a result of aortic stenosis or hypertrophic obstructive cardiomyopathy, it may be round and slightly enlarged. When the left ventricle is dilated, as may occur with aortic regurgitation or other causes of decompensation, the apex is displaced downward and laterally (Fig. 5-8). An enlarged right ventricle (RV) may displace the LV border downward, thereby simulating LV dilation or aneurysm.
When the LV dilates because of volume overload as in patients with mitral regurgitation, the dimensions of the LV chamber increase markedly, assuming a globular or water flask appearance similar to a large pericardial effusion. The LV border may extend to the left and can reach the lateral ribs in massive LV dilation. As the LV enlarges, the LA border is obscured. In such circumstances, the left anterior oblique (LAO) projection is helpful to separate the two chambers so that their relative sizes can be discerned (Fig. 5-9). Separating the left heart chambers assumes importance when the differential diagnosis lies between ischemic cardiomyopathy (in which case the left ventricle is larger than the left atrium) and mitral regurgitation (in which case the left atrium may be larger than the left ventricle).
Right Mediastinal Border
The right atrial (RA) border is convex in its relationship to the medial segment of the right middle lobe. In the frontal projection, the superior vena cava (SVC) border above the RA is usually straight, and in a good inspiratory film, it can be clearly separated from the RA. The outline of the LA may be visible deep to the RA border as an additional convex shadow and the confluence of the right pulmonary veins directed toward the mid-point of the LA border may also be appreciated. In a well-penetrated film, the LA is also clearly visualized deep to the RA border because of intrusion of lung between the anterior portion of the LA and the more posterior border of the RA. If the LA is markedly enlarged, its border may actually be lateral to the RA. The borders of the right and left atria can be differentiated because the inferior border of the RA blends with the inferior vena cava, whereas the LA crosses the mid-line toward the left side of the heart (Fig. 5-10) The upper right atrial convexity blends superiorly with the SVC, which forms a straight interface with the adjacent lung as it continues into the neck. The RA is considered enlarged when it bulges more than 5.5 cm to the right of the mid-line.
Azygos Vein
The azygos vein forms an elliptical or teardrop structure at the right tracheobronchial angle (see Fig. 5-1A). It ascends in the right paravertebral sulcus and arches forward over the right mainstem bronchus to enter the back of the SVC. The azygos vein and its left-sided equivalent, the hemiazygos vein, receive intercostal veins and act as an important collateral pathway when the deep mediastinal veins are obstructed. Normally measuring 0.7 ± 0.3 cm across in the erect and 1.0 ± 0.3 cm in the supine position, the azygos vein is a good indicator of changing cardiovascular dynamics. It is enlarged in SVC and IVC obstruction, in the absence of the intrahepatic portion of the inferior vena cava, in portal vein obstruction, and in both left- and right-sided cardiac failure. A change in diameter of the azygos vein from film to film will parallel changes in pulmonary venous pressure, which makes it a useful guide to the development of congestive heart failure on plain film radiographs. An azygos fissure can be found in 3% of the population. When an azygos fissure is present, the azygos vein is displaced laterally and superiorly and will dilate under the same conditions as a normally positioned azygos vein.
Lateral View of the Thorax
Proper positioning of the patient in the lateral projection is critical for accurate identification of cardiac structures.5 The need for accurate positioning in the lateral projection is exemplified by the RA. The normal RA is not border forming in this projection, but if the patient is rotated backward, the RA will form part of the lower posterior cardiac border and simulate enlargement. The RV is the border-forming structure in the subxiphoid region and usually extends superiorly about one third the distance between the diaphragm and the suprasternal notch (Fig. 5-11). As the RV enlarges, it encroaches farther into the retrosternal space or lucency. The relationship between the size of the RV and the degree of retrosternal encroachment is affected by the patient’s body habitus and lung volume. For example, in a patient with emphysema, right ventricular enlargement may coexist with an expanded retrosternal space.
Left Atrial Border in the Lateral Projection
The normal LA forms a shallow convex bulge along the upper aspect of the posterior border of the heart on the lateral view. It may be easily identified because the posterior border of the left atrium lies immediately anterior to the pulmonary venous confluence (see Fig. 5-11).
Left Ventricle in the Lateral Projection
In the lateral projection, the normal LV forms a long convexity along the posteroinferior heart border just above the diaphragm. LV enlargement is suggested by the Hoffman-Rigler sign, a measurement determined by drawing a 2.0 cm vertical line upward along the inferior vena cava from the point where the posterior wall of the left ventricle and inferior vena cava cross in the lateral projection. At this point, a second line is drawn parallel to the vertebral bodies. The distance between the LV border and the vertical line should not exceed 1.8 cm. If it does, LV enlargement is suggested. Although this sign can be helpful, it is often inaccurate because of poor positioning of the patient, which adversely influences this measurement.6
Right Anterior Oblique Projection
Chest radiography in the right anterior oblique (RAO) projection (Fig. 5-12) is performed with the patient in a 45-degree obliquity to the film cassette (right shoulder toward the cassette). In this projection, the ventricles are elongated; the long axes of the ventricles are in view, and the atrioventricular groove is in profile, thereby permitting an advantageous view of a calcified mitral or tricuspid valve. This view helps to determine the presence of LA enlargement, a common feature of mitral stenosis or regurgitation. The aortic arch is foreshortened in the RAO projection, so the arch and proximal descending aorta are often superimposed and obscured. The anterior border of the heart in the RAO projection consists of the sinus portion of the right ventricle inferiorly and the right ventricular outflow tract and the main pulmonary artery superiorly. The right-sided or posterior heart border is made up of the right atrium superiorly and the left atrium inferiorly.
Left Anterior Oblique Projection (LAO)
The LAO projection (see Fig 5-9) is performed with the patient positioned in a 60-degree oblique relationship to the cassette. This view is useful to identify the presence of left ventricular enlargement. Because the ventricular septum is in profile in this projection, septal defects and abnormalities of right and left ventricular anatomy can be identified with angiography or with LAO equivalent projection using MR or CT.7 In this projection, the aortic and pulmonary valves are in profile, so that aortic valve calcifications can be clearly visualized and aortic or pulmonary stenosis or regurgitation can be assessed with angiography or cross-sectional imaging. The aortic arch is also in profile in this projection, making it the ideal view for evaluating the presence or absence of aortic dissection or aneurysm for studying the origins of the head and neck vessels as well as identifying aortic coarctation and patent ductus arteriosus (Fig. 5-13).
MEASURING CARDIAC SIZE
Direct measurement of heart size by plain film radiographs is rarely performed because analysis of cardiac anatomy and chamber volume is available with ECHO, CT scan, and MRI. However, because an enlarged heart on the chest radiograph is abnormal, estimation of the cardiothoracic ratio and changes in cardiac size may be a valuable yardstick to assess anatomic changes coinciding with adverse cardiac events. This assessment may be done subjectively by estimating whether a heart is normal in size, enlarged, or grossly enlarged on the basis of an average cardiothoracic ratio of 0.50. The cardiothoracic ratio may also be derived by measuring the ratio between the maximum transverse diameter of the heart divided by the maximum width of the thorax. The transverse cardiac measurement is based first on creating a vertical line on the radiograph through the mid-point of the spine from the sternum to the diaphragm. The transverse cardiac diameter is obtained by adding the widest distance of the right heart border from the mid-line and the left heart border to the mid-line.8 This diameter is then divided by the maximum transverse diameter of the thorax. The normal range of the transverse cardiac measurement is 10 cm in a small, thin individual to 16.5 cm in a tall, heavy person. A measurement 10 percent beyond these values represents the upper limits of normal.
A normal heart may appear larger in the frontal projection when the anterior-posterior dimension is small related to a pectus excavatum deformity or straight back. A large heart may appear smaller than it really is because of a downward displaced cardiac apex in patients with aortic regurgitation or in an elderly patient with a large AP diameter caused by a severe spinal curvature. The cardiac outline will be truly small in patients with Addison disease or anorexia nervosa because of the absence of brown fat. Because of cardiac magnification on AP films (including portable radiographs), visual correction must be made to avoid the over-diagnosis of heart enlargement. A reduction in the calculation of heart size by 10% to 12.5%, depending on the anode-to-tube distance, will correct this discrepancy.9,10 In practice, these calculations are of historical interest and are seldom performed today because more accurate estimation of cardiac size can obtained with cross-sectional techniques.
PERICARDIUM
The incidence of pericardial disease parallels the frequency of cardiac surgery, thoracic irradiation, multisystem inflammatory disease, and the administration of medications that adversely affect the pericardium.11
The Normal Pericardium
Normal pericardium is frequently identified on the lateral chest film as a thin linear opacity separating the anterior mediastinal fat from the subepicardial fat (Fig. 5-14) The pericardium may also be visualized occasionally on the frontal chest film as a lucent stripe paralleling the left heart border. The normal and abnormal pericardium is best appreciated on CT and MRI because of the superior contrast resolution of both techniques. With both CT and MRI, the anterior, lateral, and posterior portions of the pericardium are clearly separated from mediastinal fat, and even subtle areas of pericardial widening may be clearly delineated. One pitfall of both MRI and CT is that pericardial recesses may be confused with aortic dissection or mediastinal lymphadenopathy.
Pericardial Effusion
Pericardial effusions may be a transudate or may be hemorrhagic, gaseous, or chylous, and are due to a wide variety of causes. When a large fluid collection accumulates in the pericardial sac, the cardiac silhouette assumes a flask-like or globular silhouette (Fig. 5-15). The normal indentations and prominences along the left and right heart borders are effaced, so the shape of the cardiac silhouette becomes smooth and featureless. Because the pericardium extends up to the pulmonary bifurcation, when a large pericardial effusion is present, the hilar structures are draped and obscured by the distended pericardial cavity. This radiographic appearance should help distinguish a large pericardial effusion from massive cardiomegaly, which will not obscure the hilar vessels.
In the lateral chest radiograph in a patient with pericardial effusion, the retrosternal space is typically narrowed or obliterated by the expanding cardiac silhouette. Normally, the low-density subepicardial fat merges imperceptibly with the mediastinal fat because the two fat planes are separated by only the 2 mm-thick stripe of the pericardium. When pericardial effusion is present, the subepicardial fat is displaced posteriorly by the higher density fluid, which may be visible as a wide, opaque vertical band between the anterior border of the heart and the mediastinum. This “epicardial fat pad sign” is best visualized on the lateral projection and is highly specific for pericardial effusion.11
Although pericardial effusion may be suggested by plain film findings, ECHO is the most sensitive modality for the detection of pericardial effusion and/or thickening. ECHO has the advantage of ease of performance at the bedside in critically ill patients, is noninvasive, and emits no ionizing radiation when constriction, neoplasm, or hemorrhage is present. If ECHO is inconclusive, CT can be helpful in detecting pericardial thickening, diffuse or loculated effusion, calcification, and adjacent mediastinal and pulmonary disease, as well as neoplasm. MRI not only can clearly detect pericardial effusion with great sensitivity, but also can characterize thrombus, tumor, fibrosis, and hemorrhage. Pericardial fibrosis is characterized by a medium-intensity signal on T1-weighted or dark signal intensity on T2-weighted images. Intrapericardial masses, cysts, and diffuse thickening are well demonstrated with MRI.11
Pericardial Constriction
Pericardial constriction is often confused with restrictive cardiomyopathy, and MRI and CT are helpful in differentiating these two conditions. The pericardium in patients with restrictive cardiomyopathy is normal in thickness and is free of calcification. In addition, diffuse limitation of global cardiac excursion in systole and diastole is present, together with myocardial thickening in hearts with restrictive cardiomyopathy.12
Pericardial Calcification
Pericardial calcification is most often associated with previous inflammation or with blunt cardiac or pericardial trauma. Common causes include viral illness, especially coxsackievirus or influenza infection, tuberculosis, and histoplasmosis. Calcification following trauma or hemopericardium followed by calcification is a frequent scenario (Fig. 5-16).
Pericardial Cyst
A pericardial cyst appears as a smooth convex bulge along the lower right heart border near the cardiophrenic sulcus in 80% of cases of pericardial cysts. However, 20% of pericardial cysts lie along the left heart border, sometimes mimicking a prominent left atrial appendage or left ventricular aneurysm (see Fig. 5-7). Pericardial cysts rarely calcify and do not communicate with the pericardial sac, unlike pericardial diverticula which do communicate with the pericardial sac, Their clinical importance lies in the need to differentiate pericardial cysts from other masses with a similar appearance on plain films, such as thymoma, lymphoma, and postoperative hematoma. Pericardial cysts are best diagnosed by ECHO, CT, or MRI as smoothly marginated, fluid-filled structures adjacent to the right heart border (Fig. 5-17).
NORMAL PULMONARY VASCULATURE
Normal Pulmonary Vascular Anatomy
The main pulmonary artery (MPA) divides within the mediastinum into the left and right main pulmonary arteries. The LPA passes to the left and posterior just above the left main-stem bronchus. Its lateral borders are visible just above the middle of the left hilum. The RPA follows a horizontal course within the mediastinum forming a round or elliptical opacity in front of the right main-stem bronchus on the lateral projection (Fig. 5-18). The LPA, in the lateral view, lies posterior to the airway and parallels the aortic arch. The RPA divides within the mediastinum proximal to the right hilum into the upper branch or truncus anterior and the inferior or interlobar branch. Within the lungs, the pulmonary arteries parallel the bronchi and divide in an orderly manner gradually tapering toward the lung periphery. The bronchi and the pulmonary artery in the same pulmonary segment are approximately the same diameter at any one level with a ratio of about 1.2 to 1.0. Knowledge of this relationship is important to help determine the presence of increased redistributed or reduced blood flow.
In the normal patient, the pulmonary vessels in the outer third of the lung are too small to be identified on the PA chest radiograph. Central pulmonary arteries can usually be distinguished from pulmonary veins because they follow different pathways. Pulmonary veins course within the interlobar septa and converge as horizontal vascular structures into the posterior aspect of the left atrium. The pulmonary arteries radiate outward from the left and right hila several centimeters above the pulmonary venous confluence. Veins to the upper lobes are usually lateral to or are superimposed on their companion pulmonary arteries. For the most part, the veins are larger than their neighboring arteries and branch less frequently. In a normal erect individual, the vessels in the upper lung zones are smaller than vessels at the base of the lungs because of important gravitational differences between the apex and the lung base, causing increased distribution of blood to the base of the lung (Fig. 5-19).
Abnormal Pulmonary Blood Flow
Increased Pulmonary Flow
Enlarging pulmonary branches are found in a variety of conditions including left-to-right intercardiac shunts such as atrial septal defect (ASD), admixture lesions such as total anomalous pulmonary venous return, and situations that increase cardiac output such as pregnancy or chronic anemia (Fig. 5-20). As pulmonary blood flow increases, radiographs will demonstrate enlarged vessels clearly seen to the edge of the pleura.
Decreased Pulmonary Blood Flow
When pulmonary outflow tract obstruction or a right-to-left cardiac shunt is present, pulmonary blood flow is reduced and both veins and arteries are smaller in size. The central vessels narrow and the peripheral vessels are not visible. When the reduced blood flow is generalized or diffuse and when cyanosis is also present, tetralogy of Fallot or other causes of pulmonary outflow tract obstruction should be considered. When the restricted blood flow is regional, other diagnostic possibilities, such as pulmonary embolism, emphysema, tumor invasion, and vasculitis are more likely possibilities (Fig. 5-21). When pulmonary perfusion is significantly reduced, as in pulmonary atresia or extensive thromboembolism, the bronchial and other collateral arteries may increase in size. On a plain chest radiograph, bronchial vessels tend to be tortuous and nontapered, and because they emanate from the descending aorta, they do not radiate from the hilum. Normal-sized or small pulmonary arteries and veins may contribute to pulmonary vascularity in lungs with significant bronchial circulation because pulmonary arteries and bronchial arteries interconnect and blood flow preferentially passes from the higher pressure systemic bronchial arteries to the lower pressure pulmonary arterial bed.
Pulmonary Arterial Hypertension
When the pulmonary vascular reserve is recruited by increased blood flow or is reduced by vasoconstriction, the pressure within the pulmonary circulation will rise. Accompanying increased pressure is vasospasm, vessel wall thickening, and peripheral vasoconstriction. Ultimately, peripheral blood flow is reduced and the outer third of the lungs may appear hyperlucent. In pulmonary arterial hypertension (Fig. 5-22), the central elastic pulmonary arteries will enlarge including the main and central branching vessels. In long-standing and severe pulmonary arterial hypertension, pericardial infusion, abnormal pulmonary parenchymal mosaic perfusion patterns, and calcification of the main pulmonary artery and its proximal branches may be seen.13 Pulmonary arterial hypertension may be primary, particularly in women in the childbearing age group, but is more likely secondary to a variety of systemic disorders, such as chronic hypoxia, collagen vascular disease, chronic pulmonary emboli, sleep apnea syndrome, or cardiovascular causes such as Eisenmenger physiology.13
Pulmonary Venous Hypertension
An increase in pulmonary venous pressure above the normal range of 8 to 12 mm Hg may occur with mitral stenosis, pulmonary venous obstructive disease, obstructing left atrial myxoma, and left ventricular failure. When pressure within the left atrium rises to the level of 12 to 18 mm Hg, pulmonary blood flow is redirected into the upper lobes in the erect position and anteriorly in the supine position, so the normal differences in size between the smaller zone 1 and larger zone 3 vessels are reversed. Enlargement of the mediastinal vessels leads to widening of the mediastinal contour or “vascular pedicle”.14 When there is a further increase of pulmonary venous pressure above 18 mm Hg, pulmonary interstitial edema occurs. When the pulmonary venous pressure rises above 25 mm Hg, alveolar flooding or edema will ensue.
Cephalization or redistribution of pulmonary venous and arterial flow to the upper lobes (zone 1) is one of the earliest signs of pulmonary venous hypertension. One clue to redistributed flow is the diameter of blood vessels at the first anterior costal interspace. Normally, blood vessels at the level of the first anterior interspace measure no more than 3 mm in diameter; however, if they are larger, increased or redirected flow may be the source. The chest radiograph is particularly useful in distinguishing significant from mild pressure elevations, but more precise grading of pulmonary venous pressure levels by plain film radiographs is rarely possible. The exact mechanism of vascular redistribution remains unclear, but one theory has been proposed by several authors. With a pulmonary venous pressure increase, fluid leaks from the pulmonary veins into the surrounding interlobular spaces first in the lower lobes because of gravitational effects. Then fluid leaks from the interlobular spaces decreasing pulmonary compliance, increasing the alveolar-capillary interface causing relative ischemia and resulting in vasoconstriction. This interplay will result in restriction of fluid and increase in venous pressure to the lower lobes. As a result, redirection or diversion of blood flow to the upper lobes will occur (Fig. 5-23A).
Pulmonary Alveolar and Interstitial Edema
On the plain film radiograph, pulmonary alveolar edema secondary to cardiovascular disease will usually be found in the inner two thirds of the lung producing a “bat-wing” or “butterfly” appearance (see Fig. 5-23). One explanation for the development of the central distribution of pulmonary edema in patients with cardiovascular disease is that the outer third of the lung or the pulmonary cortex has better aeration, superior pumping effect during the respiratory cycle, more efficient lymphatic drainage, and better compliance than the inner two thirds of the lung. As a result, fluid concentrates preferentially in the center portion of each lung. It is sometimes difficult to distinguish pulmonary edema caused by heart disease and “congestive heart failure” from that caused by increased pulmonary permeability edema.” In order to distinguish pulmonary edema caused by heart failure from other forms of pulmonary edema, criteria based on heart size, width of the vascular pedicle, blood flow distribution, regional distribution of edema, and interstitial thickening have been developed. In general, cardiovascular pulmonary edema is associated with a large heart, vascular redistribution, septal lines, pleural effusions, increased pulmonary blood volume, and regional distribution of pulmonary edema.14,15
In permeability pulmonary edema, there is generally no cardiac enlargement, little to no pleural effusion, absence of septal lines, and the edema is peripheral rather than central in distribution. The heart and vascular pedicle are not enlarged. When asymmetric pulmonary edema is present, it is usually related to an underlying change in the lung itself. Perhaps the most common cause for asymmetric pulmonary edema is emphysema in heavy cigarette smokers or end-stage sarcoidosis. Other causes of asymmetric pulmonary edema include thromboembolism and irradiation. Plain film radiography has been the most frequently used examination for the diagnosis of pulmonary edema. However, the superior resolution of CT has led to a more precise description of the findings in both interstitial and alveolar edema. Computed tomography may also yield additional insights into the cause and distribution of the edema and the presence of associated abnormalities.16,17
Cardiac Calcification
Myocardial Calcification
Calcification of the myocardium (Fig. 5-24) is most often due to a large myocardial infarction. Myocardial calcification occurs most frequently in true left ventricular aneurysms located at the left ventricular apex or the anterior lateral aspect of the left ventricular wall. Calcifications within left ventricular aneurysms tend to be curvilinear and are usually located in the periphery of the infarct or aneurysm. Calcification may occasionally be homogeneous when the entire infarcted area is affected. A false left ventricular aneurysm will occur when a tear in the myocardial wall occurs and blood leaks into the pericardium, at which time the blood passing into the pericardium is contained by adhesions or pressure. False aneurysms are most likely to occur along the posterolateral wall of the left ventricle.
Valvular Calcification
Visible calcifications within a cardiac valve (Fig. 5-25) suggest the presence of a hemodynamically significant stenosis.18 Calcification in the mitral valve appears thick and irregular or linear, measuring 2 to 4 cm in diameter. It is most frequently caused by rheumatic carditis.
Isolated aortic valve calcifications in patients 40 years of age or younger generally suggests the diagnosis of aortic stenosis owing to bicuspid aortic valve. In older patients, aortic valve calcifications may be related to “senile” sclerosis and degeneration of otherwise normal valve leaflets (Fig. 5-26).
The pattern of the aortic valve calcification may help determine its origin. For example, an irregular, thick, semilunar ring pattern with a central bar or knob is typical of a stenotic bicuspid valve and is seen in more than 50% of patients with congenital aortic stenosis. The distinctive pattern of calcification of a bicuspid valve results from calcification of the valve raphe or dividing ridge and the line of insertion of the shallow conjoint leaflet and the convex unfused leaflet.18 The abundance of calcification in this entity is thought to be due to constant wear and tear from the abnormal injury-producing motion of the valve leaflets. Occasionally, three-leaflet aortic valves will mimic bicuspid valves because of fusion of two of the three leaflets.
Coronary Artery Calcification
Coronary artery calcification can be detected by a number of imaging modalities, including plain radiography, fluoroscopy, and CT. Of these modalities, MDCT and electron beam CT have been studied most intensively (Fig. 5-27).19
Conventional CT is clearly superior to plain film radiography for the detection of coronary calcification. In fact, four times as many patients will be found to have calcified coronary arteries with CT than with plain film. Recent studies with MDCT have emphasized its value as a screening procedure. Because plain film chest radiographs and CT are performed routinely for other disease indications, it is important to make note of coronary calcifications and their distribution on CT to alert the clinician to their presence and significance.20
Calcification of the Great Vessels
Aortic calcification, particularly in the region of the arch, is almost ubiquitous in individuals older than 50 years. It is usually noted on chest radiographs as a thin curvilinear opacity near the lateral border of the arch. When the calcification is located deep to the aortic border, dissection may be present (Fig. 5-28).21 Other causes of aortic calcification are syphilis (usually involving the ascending aorta), sinus of Valsalva aneurysm, calcified ductus arteriosus, and Takayasu arteritis. Calcification of the main pulmonary artery also occurs in severe long-standing pulmonary hypertension.
CONGENITAL HEART DISEASE (CHD) IN THE ADULT
The radiologic manifestations of congenital heart disease in the adult can be classified by the incidence of the abnormalities and by associated findings such as the state of the pulmonary vessels and related skeletal abnormalities, whether the aortic arch is right- or left-sided, and thorax abdominal situs.22 A history of the presence or absence of cyanosis and the time of onset of the clinical manifestations of the disorder should help to narrow the diagnosis. Several of the common cardiovascular disorders in the older patient found with some frequency are described subsequently.
Coarctation of the Thoracic Aorta (COA)
Coarctation of the aorta (Fig. 5-29; also see Fig. 5-13) is due to a deformity of the aortic intima and media with posterior infolding off the aortic lumen at or near the junction of the arch and descending aorta. Coarctation of the aorta accounts for 5% to 10% of childhood congenital heart defects and as many as 6% of CHD in the adult. Of those who escape diagnosis in early childhood, 25% die by the age of 20 years and nearly 50% die by the age of 30 years. The most common associated anomaly is a bicuspid aortic valve, which occurs in as many as 85% of patients with coarctation of the aorta.
The diagnosis of COA can be established on the PA chest film alone in as many as 92% of patients. The most common finding owing to the diagnosis of COA is widening of the LSCA border, but the most useful radiographic sign is pre- and poststenotic dilation at the site of the coarctation, which may appear as a double bulge above and below the usual site of the aortic knob. This pattern has been described as a “figure 3” sign.22,23 This pattern is seen in as many as 60% of adults with untreated coarctation. The upper arc of the “3” is the dilated arch proximal to the coarctation and/or a dilated left subclavian artery. The lower bulge represents poststenotic dilation of the aorta immediately below the coarctation. When the esophagus is filled with barium, a reverse “3” or “E” sign, best visualized in the LAO projection, represents a mirror image of the “figure 3” sign. The “3” sign varies in that the upper arc may be small and the lower arc may be large or vice versa. Widening of the upper mediastinum or scalloping of the sternum caused by large internal mammary collateral arteries may be visible in some patients. A prominent left ventricular border often occurs with COA, especially when there is a bicuspid aortic valve associated with aortic stenosis.23
Computed tomography is diagnostic and demonstrates the site of the coarctation and associated anomalies such as bicuspid aortic valve best seen in the reformatted sagittal oblique images. MRI is particularly valuable to show the site of coarctation, the state of the aortic valve, and with phase-contrast imaging, to calculate the amount of collateral blood flow. CT, MRI, and ECHO are valuable to identify and characterize complications of COA repair such as dissection, postoperative pseudoaneurysm, infective endocarditis, and restenosis at the surgical site.24,25
Ostium Secundum Atrial Septal Defect (ASD) (Fig. 5-30)
ASD, the most common left-to-right shunt first diagnosed in the adult, occurs in more than 40% of adult congenital heart defects. The chest radiograph may appear normal in a patient with a small shunt, but if the ratio is of 1:2 or more, the MPA and the enlarged peripheral pulmonary branches, the RA, and the RV borders are prominent. It is possible in many patients to differentiate ASD from other causes of intracardiac left-to-right shunts. Less pulmonary artery dilation is usually seen in PDA than in ASD, and both PDA and VSD are associated with enlarged left-sided cardiac chambers. In adults older than 50 years with systemic hypertension or left ventricular failure, the radiographic findings of ASD are often atypical and may include left atrial enlargement, vascular cephalization, and pulmonary edema. In uncomplicated ASD without pulmonary hypertension, the MPA, RA, and RV are prominent but the aorta and left-sided chambers are normal.23 The size and the location of the ASD can often be visualized—as well as associated abnormalities such as mitral valve prolapse—with MRI; the size and location of the defect can be important for placement of an occluder device.26
Pulmonary Valvular Stenosis
Pulmonary valvular stenosis (PVS) is usually an isolated anomaly in the adult. Mild-to-moderate enlargement of the MPA is demonstrated because of poststenotic dilation thought to result from the jet effect of blood flow through the narrowed pulmonary valve orifice. As the blood flow is oriented toward the LPA, that vessel is often preferentially enlarged. The differential diagnosis of PVS (Fig. 5-31) includes primary and secondary pulmonary hypertension, idiopathic pulmonary artery dilation, and other causes of pulmonary artery enlargement including left-to-right intracardiac shunts and arteritis.
ACQUIRED HEART DISEASE
Valvular Heart Disease
Aortic Stenosis
In patients with aortic valvular disease,27 the typical radiographic findings include a normal-sized heart with rounding of the left ventricular border or an elongated cardiac silhouette with displacement downward of the cardiac apex as a result of concentric left ventricular hypertrophy. In those patients with aortic stenosis, a characteristic bulge is seen along the right side of the ascending aorta just above the sinus of Valsalva related to poststenotic dilation. Calcification of the leaflets may occur over time so that by the age of 40 years, more than 90% of patients with aortic stenosis caused by a bicuspid valve, will have visible aortic calcification. When aortic stenosis is severe, left ventricular decompensation will occur, enlarging the left ventricular border accompanied by secondary aortic regurgitation.
Subaortic Stenosis
Aortic Regurgitation
Aortic regurgitation may result from a variety of conditions including rheumatic valvulitis, endocarditis, and the sequel of stenotic bicuspid aortic valve. Specific dilation of the aortic annulus is found preferentially in ankylosing spondylitis, psoriatic arthritis, Marfan syndrome, and Reiter syndrome, among others (see Fig. 5-8). In rheumatic heart disease, aortic regurgitation is frequently associated with mitral disease or may, in advanced cases, involve all four cardiac valves. Occasionally, aortic regurgitation will accompany direct trauma to the aortic leaflets or may be found in association with Stanford type A aortic dissection.
Mitral Stenosis
The early radiologic signs of mitral stenosis may be subtle and include mild left atrial enlargement characterized by a prominent bulge at the level of the LAA on the PA projection (see Fig. 5-31). In most patients with mitral stenosis, the left ventricular border is normal. With severe mitral stenosis, characterized by a valve area less than 1 cm2, the LA may increase in size, but there is poor correlation between the degree of stenosis and the size of the LA chamber. In patients with rheumatic carditis, the LAA may be enlarged, but the shape of the appendage may bear no relationship to the degree of absence of associated findings such as thrombosis (Fig. 5-32). As the LA enlarges, the left main-stem bronchus may be displaced upward. The RA is displaced to the right, and pulmonary blood flow is redistributed to the upper lobes because of increased pulmonary venous pressure. The MPA is enlarged related to secondary arterial hypertension and the left ventricle and aorta are usually normal or small. The LA wall and the LAA may be calcified due to carditis or secondary to left atrial thrombus related to atrial fibrillation often found in patients with rheumatic carditis. The findings of pulmonary interstitial edema are common.
Later, hemosiderosis (Fig. 5-33) due to recurrent minute hemorrhages related to elevated capillary pressure may be associated with mitral stenosis. The pattern of pulmonary hemosiderosis is that of interstitial or miliary lung disease, most common in the mid and lower lung zones. Rarely, with chronic pulmonary interstitial edema, small puncture opacities can be found within the alveoli representing islands of bones visible as dense nodules on the chest radiograph.
Mitral Regurgitation
Acute mitral regurgitation may be related to ruptured chordae or papillary muscle dysfunction due to myocardial infarction or infective endocarditis. In acute mitral regurgitation, the heart may not be enlarged, but severe pulmonary edema is frequently visible due to left-sided heart failure. Pulmonary edema due to acute mitral regurgitation is usually symmetric. However, selective right upper lobe pulmonary edema has been described in as many as 9% of patients with either acute or chronic mitral regurgitation.28 More than likely, this phenomenon is related to selective retrograde blood flow from the mitral valve directly into the right upper pulmonary veins.
COMPLICATIONS OF MYOCARDIAL INFARCTION
Cardiac rupture is a catastrophic complication following an acute transmural infarction, Most of these patients die immediately, but in a minority of cases, the rupture is enclosed by surrounding extracardiac soft tissues and a pseudoaneurysm develops. Although a new bulge along the left ventricular border may be observed on the plain film, pseudoaneurysms are best seen with ECHO, CT, or MRI and may be characterized by a wide neck and seen most commonly along the left ventricular posterolateral wall.29
INTENSIVE CARE IMAGING
Technique
Appropriate film-screen combinations, computed and digital imaging, as well as careful patient positioning, are needed to overcome the effects of poor film-screen geometry, low-capacity portable equipment producing low-kilovoltage technique, and high amounts of scatter radiation and motion artifacts compared with conventional PA and lateral chest radiographs. The use of laser beam-aligned antiscatter featureless grids that require less precise geometric alignment with the x-ray source are approaches to obtain higher quality portable chest films than are possible with nongrid systems. Although conventional analog portable chest x-ray equipment continues to be the “norm,” digital images provide a variety of advantages and have largely replaced analog equipment. At present, computed radiology with storage phosphor plates as the radiation detector and laser scanning of conventional film-screen radiographs are the most frequently used methods for producing digital chest images, but fully digital equipment is available and is increasingly used.31 A major advantage of storage phosphor plates is their sensitivity over a wide range of exposures versus the much narrower exposure range of conventional x-ray film. This difference results in more consistent image quality over a wider range of exposures than is possible with analog systems. The advantage of digital systems is particularly important in portable x-ray equipment where overexposure and underexposure are ubiquitous. This improvement helps increase diagnostic accuracy and reduces the need for repeat films.30
After cardiac surgery, a daily morning film exposed in the ICU following a predetermined protocol is often taken. It has been suggested that daily films in these subsets of postoperative patients are justified, especially in those with endotracheal (ET) tubes and Swan-Ganz catheters. In the postoperative period, other critical care films, chest radiographs, a myriad of tubes, wires, catheters, and other devices are present in or overlie the postoperative chest (Table 5-1) (Fig. 5-33).31,32 These devices include ET tube, which should be positioned in the mid-trachea 5 ± 2 cm above the carina to allow excursion with flexion and extension of the neck. If the ET tube lies too close to the carina, flexion of the head or neck may force the distal end of the tube into one of the main-stem bronchi causing varying degrees of atelectasis of the opposite lung and barotrauma if assisted ventilation is used. If the tube is too high, pulmonary aspiration is increased. In spite of close observation, up to 15% of patients have significant malposition of the ET tube following cardiac procedures. It is also important to observe for overinflation of the endotracheal cuff, which may lead to tracheal injury.
The Early Postoperative Chest Radiograph
The portable chest film obtained on the first day following cardiac surgery usually exhibits varying degrees of left and right lower lobe atelectasis, mediastinal widening, pulmonary edema, and pleural effusion.33,34 Unilateral or bilateral lower lobe atelectasis, usually accompanied by small pleural effusions, is the source of the lower lung zone opacities found in almost all cardiac surgery patients within 8 hours after surgery, and these usually clear within 5 to 7 days. Lower lobe atelectasis may be confused with a bacterial pneumonia, but pneumonia is much less common immediately following cardiac surgery.35 Left lower lobe atelectasis is more common than right-sided atelectasis because of paralysis of the phrenic nerve caused by cardioplegic solutions or crushed ice administered for myocardial preservation or retained secretions. Other causes of postoperative left lower lobe atelectasis include dependent pooling of secretions, preferential suctioning of the right main-stem bronchi, and compression from the cardiac insulation pad used during surgery.36
Pleural Effusion
Pleural effusion is manifested radiographically as blunting of the costophrenic angle, loss of sharpness of the diaphragmatic contour, and increased opacity behind the dome of the diaphragm. It may be difficult to identify in a supine film but should be easily recognized on an erect bedside radiograph.37
Other early radiographic findings following cardiac surgery identified on the AP portable chest radiograph include pneumopericardium, sternal dehiscence, pulmonary embolism pneumothorax, mediastinal hematoma, pneumomediastinum, and subcutaneous emphysema. Rib fractures occur in 2% to 4% of patients, causing chest pain which may be confused with angina, pulmonary embolism, or aortic dissection.38
Mediastinal Hemorrhage
Widening of the mediastinum because of postoperative bleeding is common after cardiac surgery. Typically, the mediastinum is widened by up to 35% of the presurgical mediastinal width in comparison with the preoperative PA chest film. Katzberg and associates found that if the mediastinum is widened more than 70%, surgery is usually required to excavate the hematoma.37 It is important to consider that bleeding may be present without visible mediastinal widening, especially if the patient is undergoing positive end-expiratory pressure support, which may compress the mediastinum. Some patients may have a wide mediastinum but remain hemodynamically stable, have no significant bloody drainage, and do not require reoperation.
KEY POINTS
 Plain film imaging of the heart is particularly helpful for the evaluation of the effects of cardiac disease on the nearby lung and pleura.
Plain film imaging of the heart is particularly helpful for the evaluation of the effects of cardiac disease on the nearby lung and pleura.Bettman MA. The chest radiograph. In: Braunwald E, Zipes DP, Libby P, editors. Heart Disease: A Textbook of Cardiovascular Medicine. 8th ed. Philadelphia: WB Saunders; 2008:327-343.
Chen JTT. Essentials of Cardiac Imaging, 2nd ed. Philadelphia: Lippincott-Raven; 1997.
Gatzoulis M, Webb G, Daubeney P. Diagnosis and Management of Adult Congenital Heart Disease. London: Churchill Livingstone; 2003.
Gowda RM, Boxt L. Calcification of the heart. Radiol Clin North Am. 2004;42:603-617.
Jefferson K, Rees S, Clinical. Cardiac Radiology, 2nd ed. London: Butterworth; 1980.
Miller SW. The Requisites: Cardiac Radiology, 2nd ed. Philadelphia: Mosby-Elsevier; 2003.
Milne ENC, Pistolessi M. Reading the Chest Radiograph: A Physiologic Approach. St. Louis: Mosby—Yearbook; 1993. pp 9-50
Perloff JK. Clinical Recognition of Congenital Heart Disease, 5th ed. Philadelphia: WB Saunders; 2003.
Reddy G, Steiner RM. Cardiac Imaging Case Review Series. Philadelphia: Mosby-Elsevier; 2006.
Steiner RM, Rao VM. Radiology of the Pericardium. In: Grainger RG, Allison DJ, editors. Diagnostic Radiology. London: Churchill Livingstone; 1991:675-689.
1 Steiner RM, Gross GW, Flicker S, et al. Congenital heart disease in the adult patient: The value of plain film chest radiology. J Thorac Imaging. 1995;10:1-26.
2 Steiner RM. Radiology of the heart and great vessels. In: Braunwald E, Zipes DP, Libby P, editors. Heart Disease: A textbook of Cardiovascular Medicine. 6th ed. Philadelphia: WB Saunders; 2001:237-272.
3 Henry DA. Radiologic evaluation of the patient after cardiac surgery. Radiol Clin North Am. 1996;34:119-135.
4 Henschke C, Yankelevitz DF, Wand A, et al. Accuracy and efficiency of chest radiography in the intensive care unit. Radiol Clin North Am. 1996;34:21-27.
5 Robinson AE. The lateral chest film: Is it doomed to extinction? Acad Radiol. 1998;5:322.
6 Freeman V, Mutatir C, Pretorius M. Evaluation of left ventricular enlargement in the lateral position of the chest using the Hoffman-Rigler sign. Cardiovasc J S Africa. 2003;14:134-136.
7 Morgan PW, Goodman LR, Aprahamian C, et al. Evaluation of traumatic aortic injury: Does dynamic contrast enhanced CT play a role? Radiology. 1992;182:661-666.
8 Kabala JE, Wilde P. Measurement of heart size in the anteroposterior chest radiograph. Br J Radiol. 1987;60:981-985.
9 Van der Jagt EJ, Smits HJ. Cardiac size in the supine chest film. Eur J Radiol. 1992;14:173-176.
10 Rubinowitz A, Siegel M, Tocino I. Thoracic imaging in the ICU. Critical Care Clinics. 2007;23:539-573.
11 Wang ZJ, Reddy GP, Gotway MB, et al. CT and MR imaging of pericardial disease. Radiographics. 2003;23:S167.
12 Vaitkus P, Kussmaul W. Constrictive pericarditis versus restrictive cardiomyopathy: A reappraisal and update of diagnostic criteria. Am Heart J. 1991;122:1431.
13 Vogel M, Berger F, Kramer A, et al. Incidence of secondary pulmonary hypertension in adults with atrial septal or sinus venosus defects. Heart. 1999;82:30-33.
14 Ely EW, Hoponik EF. Using the chest radiograph to determine intravascular volume status: the role of the vascular pedicle width. Chest. 2002;121:942-950.
15 Ketai LH, Goodwin JD. A new view of pulmonary edema and acute respiratory distress syndrome: state of the art. J Thorac Imaging. 1998;131:147-171.
16 Morgan PW, Goodman LR. Pulmonary edema and acute respiratory distress syndrome. Radiol Clin North Am. 1991;29:943-963.
17 Stern EJ, Muller NL, Swensen SJ, Hartman TE. CT mosaic pattern of lung attenuation: etiologies and terminology. J Thorac Imaging. 1995;10:294-297.
18 Rodan BA, Chen JTT, Halber MD, et al. Chest roentgenographic evaluation of the severity of aortic stenosis. Invest Radiol. 1980;15:416-442.
19 Li J, Galvin HK, Johnson SC, et al. Aortic calcification on plain film radiography increases risk for coronary artery disease. Chest. 2002;121:1468-1471.
20 Feuchtner GM. The utility of computed tomography in the context of aortic valve disease. Int J Cardiovasc Imaging. 2009;6:611-614.
21 Stanford W, Thompson BH. Imaging of coronary artery calcification: Its importance in assessing atherosclerotic disease. Radiol Clin North Am. 1999;37:257-272.
22 Perloff J. Congenital heart disease in the adult: clinical approach. J Thorac Imaging. 1994;9:260.
23 Ferguson EC, Krishnamurthy R, Oldham SAA. Classic imaging signs of congenital cardiovascular abnormalities. Radiographics. 2007;27:1323-1334.
24 Yuan SM, Raanani I. Late Complications of coarctation of the aorta. Cardiol J. 2008;15:517-524.
25 Greenberg SB, Balsara R, Faerber E. Coarctation of the aorta:diagnostic imaging after corrective surgery. J Thorac Imaging. 1995;10:36.
26 Itoey ET, Gopalan D, Ganesh V, et al. Atrial septal defects: magnetic resonance and computed tomography appearances. J Med Imaging. Radiat Oncol. 2009;53:261-270.
27 Boxt LM. CT of valvular heart disease. Int J Cardiovasc Imaging. 2005;21:105-113.
28 Agesilas F, Herblard A, Valentino P, et al. Right upper lobe pulmonary edema as a consequence of mitral regurgitation. Am J Emerg Med. 2007;25:196-197.
29 Oliva P, Hammill S, Edwards WC. Cardiac rupture, a clinically predictable complication of acute myocardial infarction: Report of 70 cases with clinicopathologic correlations. J Am Coll Cardiol. 1993;22:720-726.
30 Nicklason L, Chan H-P, Cascade P, et al. Portable chest imaging: comparison of storage phosphor digital, asymmetric screen-film and conventional screen-film radiography. Radiology. 1993;186:387-392.
31 Krivopal M, Shlobin DA, Schwartzstein RM. Utility of daily portable chest radiographs in mechanically ventilated patients in the medical ICU. Chest. 2003;123:1607-1614.
32 Barge-Caballero E, Estevez-Cid F, Bouzas-Mosquera A, et al. Chest radiography of life supporting interventions. Lancet. 2009;374:476.
33 Graham R, Meziane M, Rice J, et al. Postoperative portable chest radiographs: Optimum use in thoracic surgery. J Thorac Cardiovasc Surg. 1998;115:45.
34 O’Brien W, Karski J, Cheng D, et al. Routine chest roentgenography on admission to the intensive care unit after heart operations: Is it of value? J Thorac Cardiovasc Surg. 1997;113:130.
35 Henry D. Radiologic evaluation of the patient after cardiac surgery. Radiol Clin North Am. 1996;34:119.
36 Landay M, Mootz A, Estrera A. Apparatus seen on chest radiographs after cardiac surgery in adults. Radiology. 1990;174:477.
37 Katzberg R, Whitehouse G, deWeese J. The early radiologic findings in the adult chest after cardiopulmonary bypass surgery. Cardiovasc Radiol. 1978;1:205.
38 Sommer T, Fehske W, Holzknecht N, et al. Aortic dissection: a comparative study of diagnosis with spiral CT, multiplanar transesophageal echocardiography and MR imaging. Radiology. 1996;199:347-352.

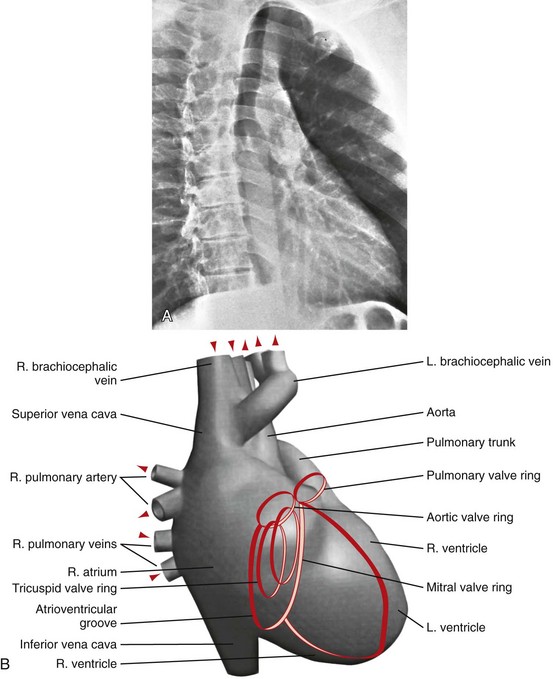
 FIGURE 5-12
FIGURE 5-12
 FIGURE 5-13
FIGURE 5-13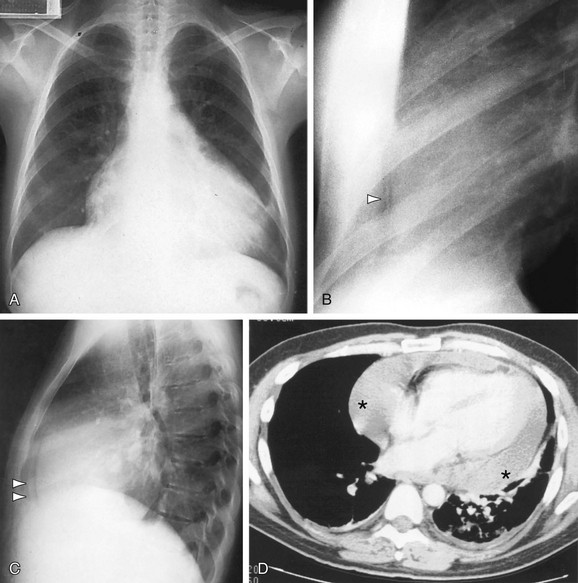
 FIGURE 5-14
FIGURE 5-14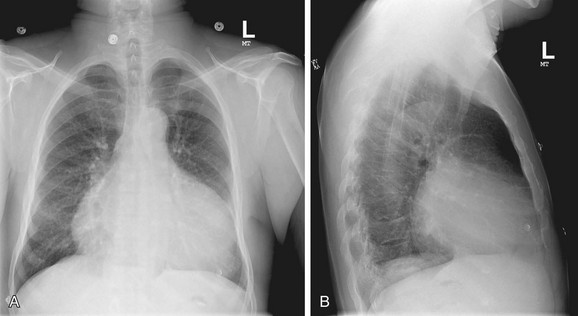
 FIGURE 5-15
FIGURE 5-15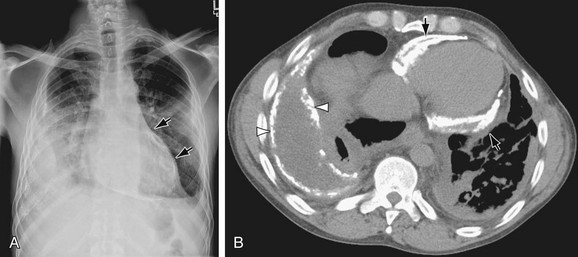
 FIGURE 5-16
FIGURE 5-16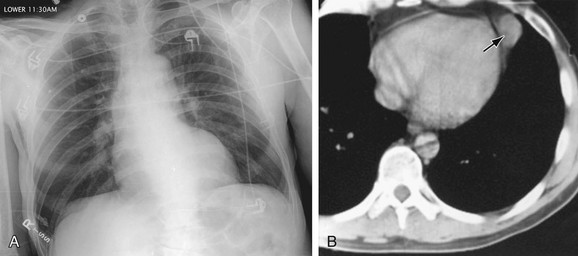
 FIGURE 5-17
FIGURE 5-17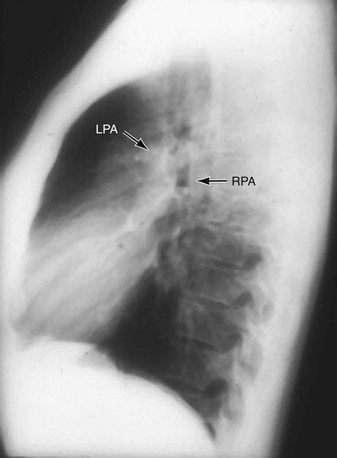
 FIGURE 5-18
FIGURE 5-18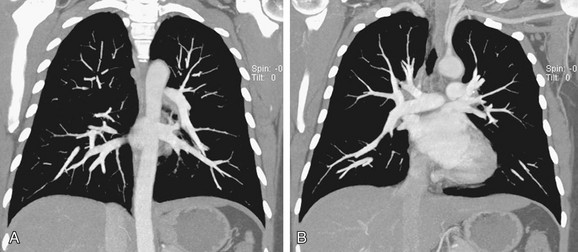
 FIGURE 5-19
FIGURE 5-19
 FIGURE 5-20
FIGURE 5-20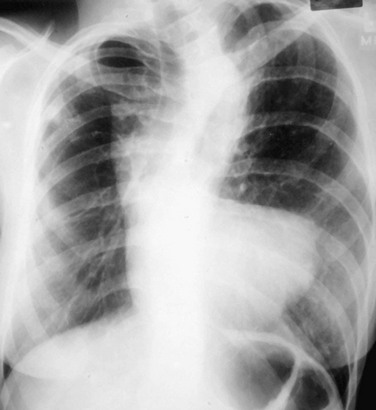
 FIGURE 5-21
FIGURE 5-21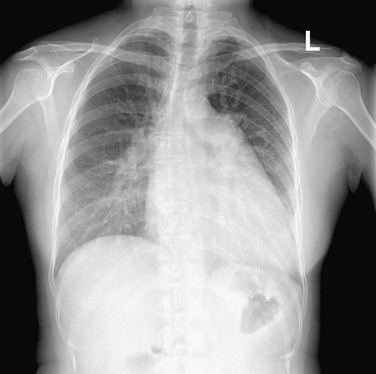
 FIGURE 5-22
FIGURE 5-22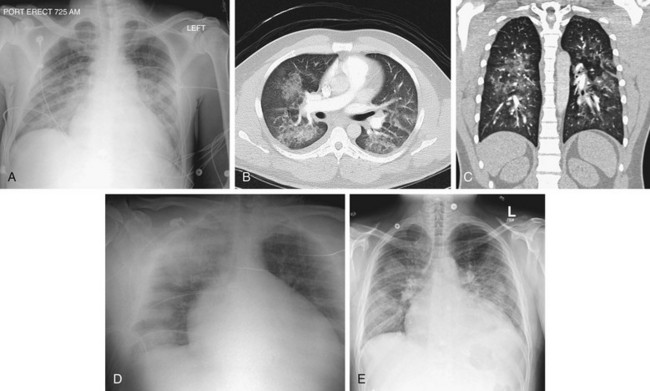
 FIGURE 5-23
FIGURE 5-23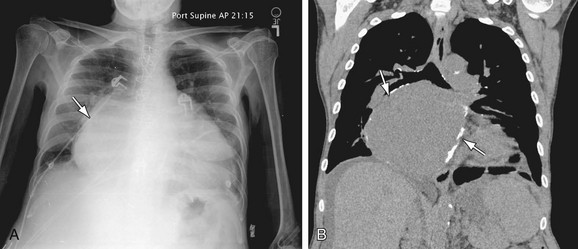
 FIGURE 5-24
FIGURE 5-24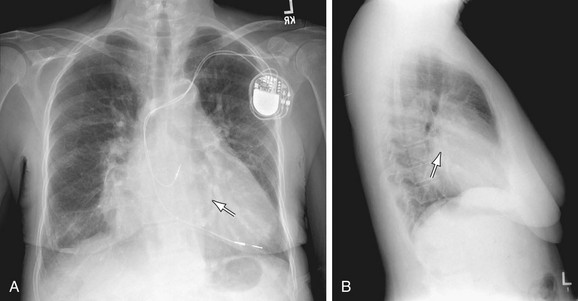
 FIGURE 5-25
FIGURE 5-25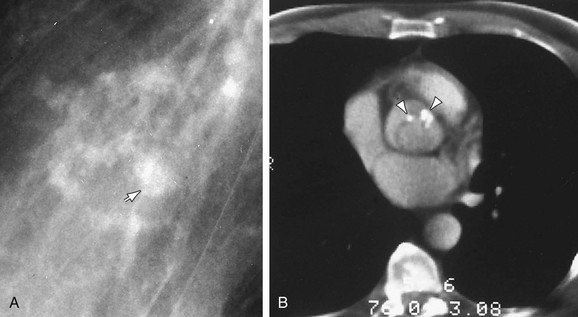
 FIGURE 5-26
FIGURE 5-26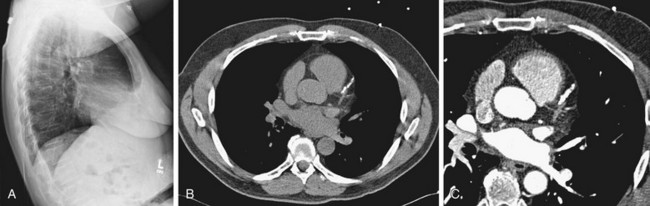
 FIGURE 5-27
FIGURE 5-27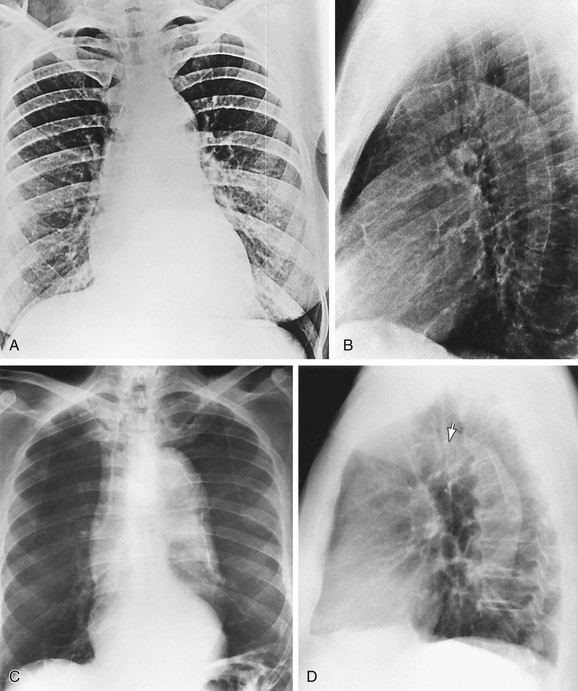
 FIGURE 5-28
FIGURE 5-28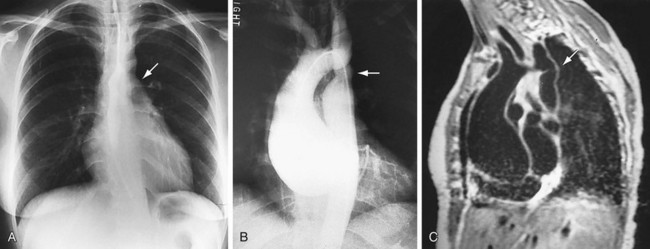
 FIGURE 5-29
FIGURE 5-29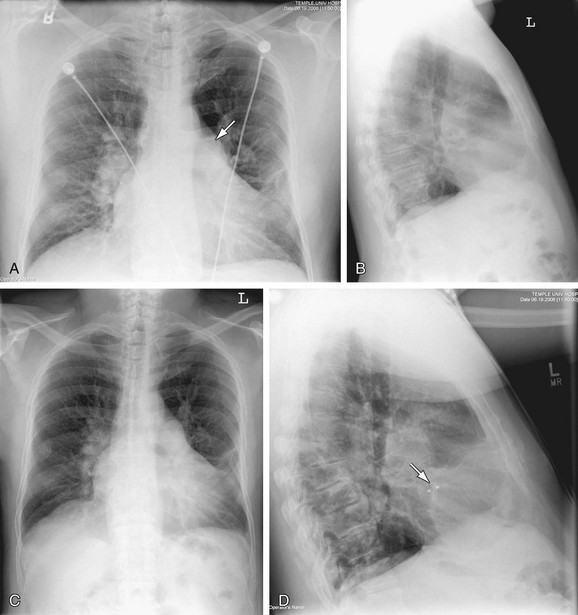
 FIGURE 5-30
FIGURE 5-30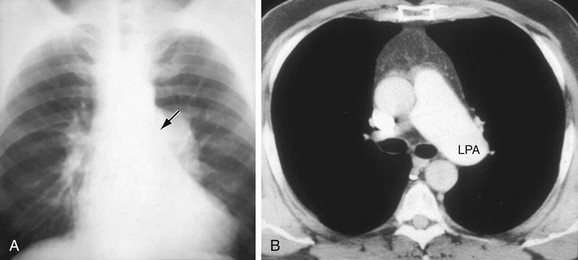
 FIGURE 5-31
FIGURE 5-31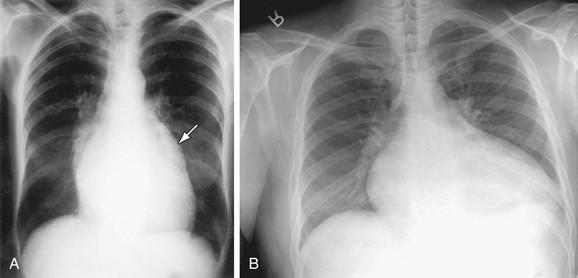
 FIGURE 5-32
FIGURE 5-32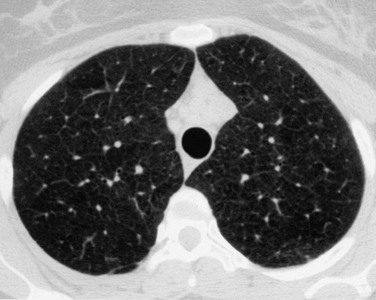
 FIGURE 5-33
FIGURE 5-33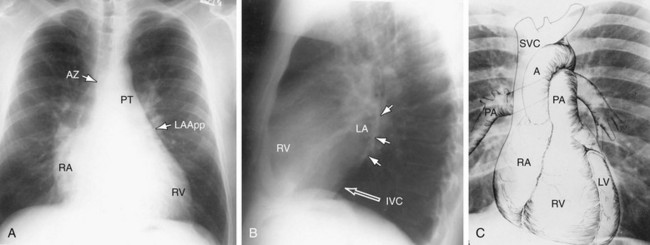
 FIGURE 5-1
FIGURE 5-1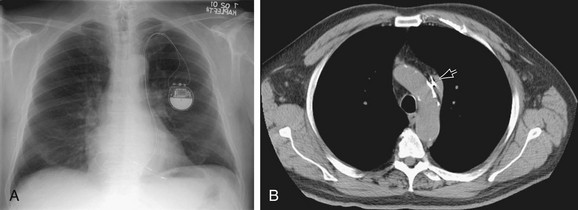
 FIGURE 5-2
FIGURE 5-2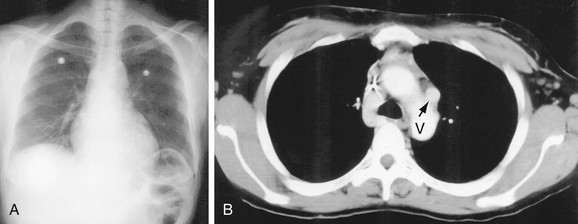
 FIGURE 5-3
FIGURE 5-3
 FIGURE 5-4
FIGURE 5-4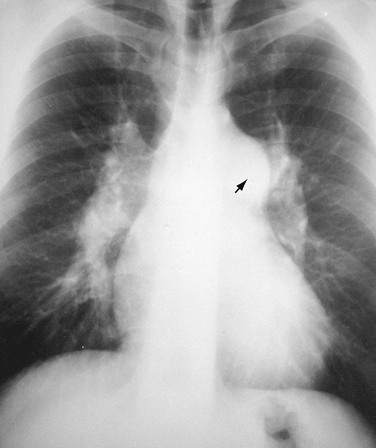
 FIGURE 5-5
FIGURE 5-5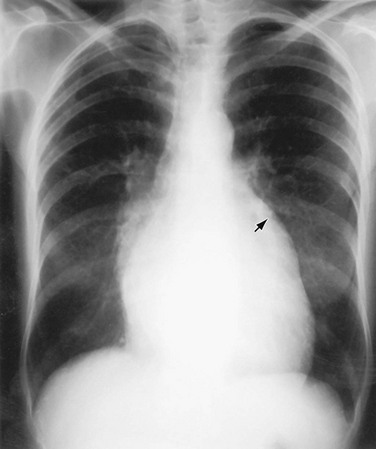
 FIGURE 5-6
FIGURE 5-6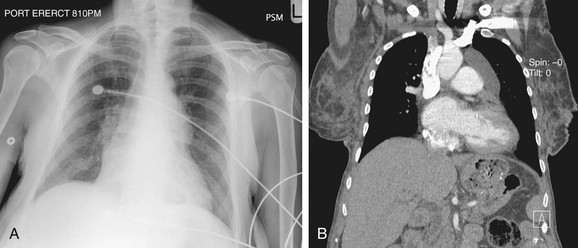
 FIGURE 5-7
FIGURE 5-7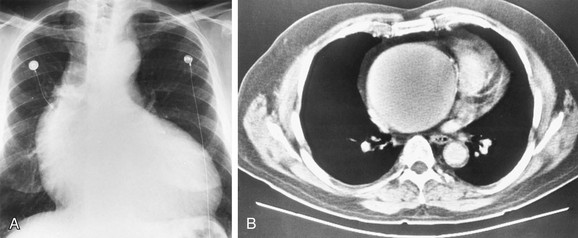
 FIGURE 5-8
FIGURE 5-8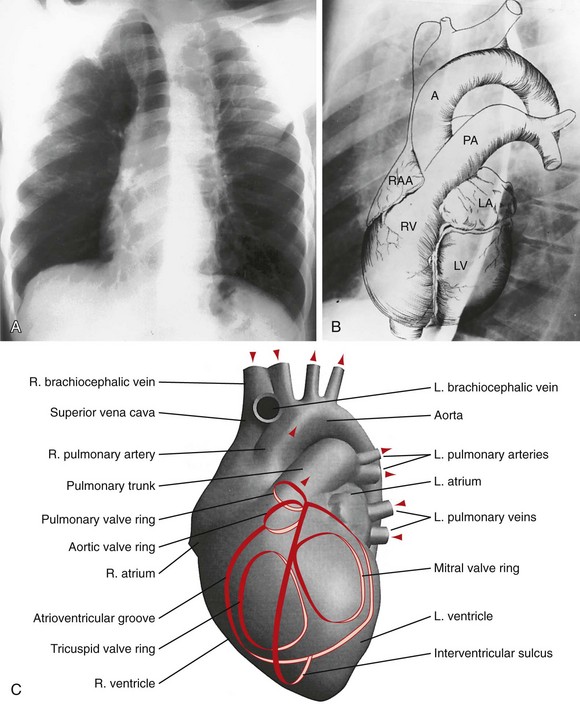
 FIGURE 5-9
FIGURE 5-9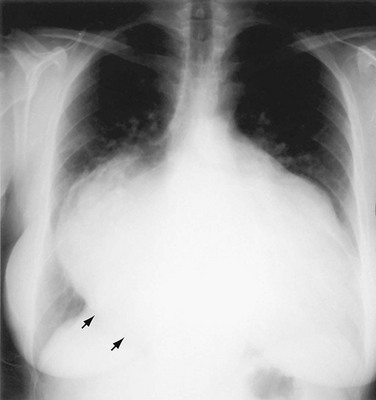
 FIGURE 5-10
FIGURE 5-10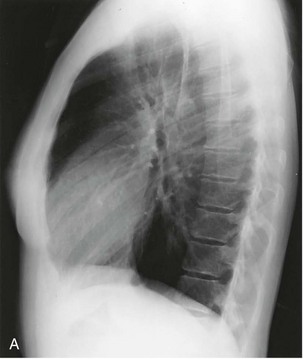
 FIGURE 5-11
FIGURE 5-11

