Chapter 28 Radiologic hepatobiliary interventions
Radiologic Intervention
Radiologic intervention is applicable for a wide range of hepatobiliary diseases (see also Chapters 12, 19, 25, and 32). The use of these techniques in pancreatic disease is referred to in Chapter 25, Chapter 32, Chapter 54, Chapter 55A, Chapter 55B, Chapter 63A, Chapter 63B .
Treatment of Hepatic Cysts
Simple, nonparasitic hepatic cysts (see Chapters 69A and 69B) are the second most common benign lesions that involve the liver, following hemangiomas. They are found in approximately 1% to 2.5% of adults and occur more commonly in women and in the right liver. These cysts are lined with bile duct epithelium, contain clear fluid, and almost never communicate with the biliary tree (Forbes & Murray-Lyon, 1991). Simple cysts can be acquired from processes such as trauma or inflammation, or they may be congenital, such as in polycystic disease (see Chapter 69A, Chapter 69B ), with cysts possibly present in the kidneys and pancreas as well.
On cross-sectional imaging, the requisite feature of a simple hepatic cyst is a circumscribed, thin-walled, nonseptated lesion with lack of enhancement after intravenous contrast medium administration. Imaging modality–specific features include posterior acoustic enhancement on ultrasound (US), low attenuation on computed tomography (CT), and signal intensity similar to that of free water on magnetic resonance imaging (MRI). Wall thickening, the presence of septations, or lack of enhanced through-transmission on US should raise the question of another pathology, such as cystic metastasis, cystadenoma (see Chapter 79B), or hydatid disease (see Chapter 68), which must be thoroughly investigated (Mortele & Ros, 2001; Nisenbaum & Rowling, 1995). This evaluation is especially important before beginning treatment, and periodic imaging follow-up early in the course of therapy is prudent.
In the absence of symptoms or signs, simple liver cysts require no treatment. Traditional therapy of symptomatic cysts has been surgical, with laparoscopic deroofing currently favored by many surgeons (Gall et al, 2009; Mazza et al, 2009; see Chapter 65). Interventional radiologic techniques such as fine needle aspiration (FNA) and catheter drainage without or with cyst sclerosis have been used to treat simple cysts. With these techniques, symptomatic relief usually is achieved, although recurrence is common with aspiration alone.
Percutaneous chemical sclerosis of cysts with a variety of agents—including alcohol, tetracycline antibiotics, and povidone-iodine—either at the time of initial aspiration or after a period of catheter drainage usually provides successful therapy (vanSonnenberg et al, 1994). In the case of alcohol, it fixes the cells lining the cyst cavity, thereby preventing secretion of cyst fluid and subsequent enlargement (Bean & Rodan, 1985). Tikkakoski and colleagues (1996) described single-session treatment with alcohol for 59 symptomatic hepatic cysts in 25 patients. The cysts were evacuated via a 5-Fr drainage catheter, and absolute alcohol was instilled and left in for 20 minutes with the patient turned in various positions to maximize cyst wall exposure to the agent. In some cases, the procedure was repeated once or twice during the same session, after which the entire volume of injected ethanol was aspirated, and the catheter was removed.
Yoshida and colleagues (2003) successfully treated symptomatic solitary hepatic cysts with multiple minocycline injections and noted complete cyst regression without recurrence. Lopes and colleagues (1998) successfully treated seven patients with hepatic cysts by aspiration followed by instillation of tetracycline with a resultant decrease in all lesions. Kimura and colleagues (2005) performed repeated percutaneous instillations of alcohol after cyst aspiration and achieved subtotal or total cyst regression. Another study compared single-session alcohol sclerotherapy versus prolonged catheter drainage with negative pressure; they reported no differences in average volume reduction, final volume, and cyst regression (Zerem et al, 2008).
Laparoscopic cyst fenestration and deroofing are safe and are currently the most effective surgical approaches for symptomatic simple hepatic cysts. Image-guided (US or CT) hepatic cyst aspiration with chemical sclerosis using tetracycline or alcohol should be the treatment of choice for patients who are poor operative risks (Mazza et al, 2009).
Pyogenic Hepatic Abscess Drainage
Pyogenic hepatic abscesses result in considerable morbidity, and mortality occurs if abscesses are left untreated. Advances in cross-sectional imaging and interventional radiology have revolutionized the diagnosis and therapy of liver abscesses with a dramatic improvement in prognosis (see Chapter 66). Mortality has decreased from 65% (1952–1972) to 31% (1973–1993). This decrease occurred in the face of no significant change in patient characteristics, such as age and sex distribution, or incidence of associated conditions, such as diabetes mellitus, steroid use, chronic liver disease, chronic pancreatitis, and pyelonephritis (Huang et al, 1996). Another study from Denmark found the incidence rate of pyogenic abscesses increased from 6 to 18 per 1 million men and from 8 to 12 per 1 million women, and the 30-day mortality rate decreased from 40% for men and 50% for women to around 10% for both genders (Jepsen, 2005).
The liver is the most common site of abdominal visceral abscesses. Liver abscess is a result of either direct spread of infection from a contiguous focus, such as the biliary tree, or hematogenous seeding of the liver via the portal vein or hepatic artery. Biliary tract pathology is the most common cause of liver abscess. Of the nonbiliary diseases that lead to hepatic abscess, appendicitis with rupture and suppurative pylephlebitis, usually from infection in the female genital tract, are frequent causes (Zaleznik & Kasper, 1998).
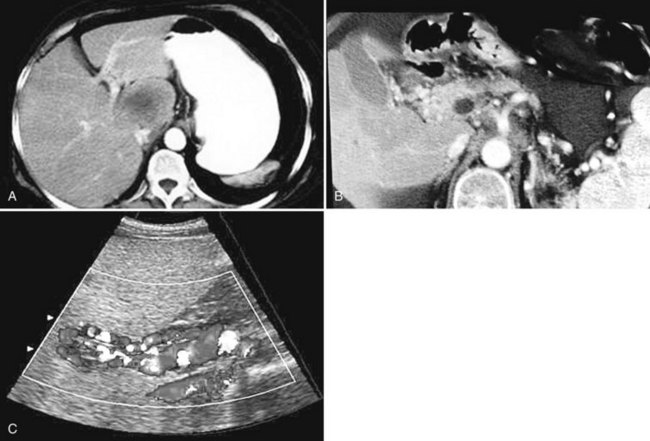
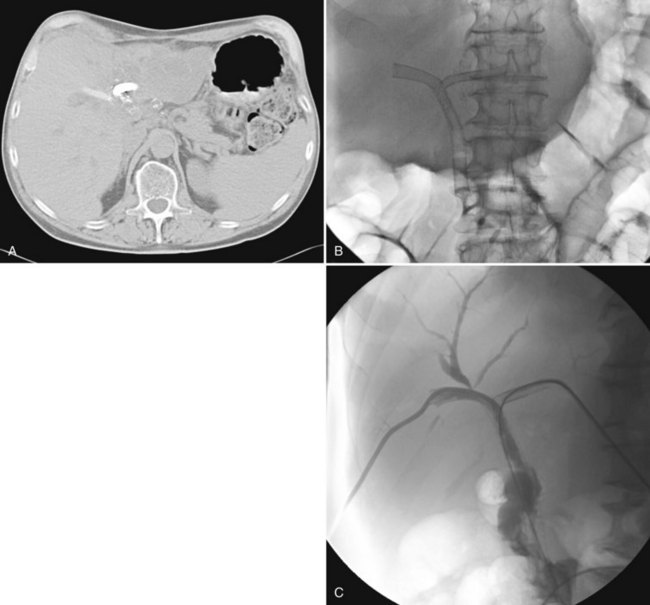
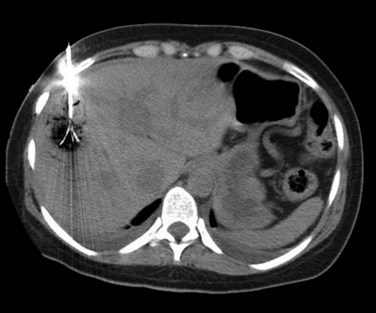
The constellation of a liver abscess and portal vein thrombosis with cavernous transformation of the portal vein may mimic the findings seen in hepatocellular carcinoma (HCC; Fig. 28.1). Clues to the correct diagnosis are the clinical and laboratory parameters that suggest infection and the absence of cirrhosis and portal hypertension, both of which are commonly seen with HCC. With successful treatment, complete resolution of both the abscess (see Fig. 28.1B) and the cavernous transformation of the portal vein (see Fig. 28.1C) is seen in many cases.
The microbial flora often reflect the source of the infectious process (see Chapter 11). In a study of 111 patients with pyogenic liver abscesses, Klebsiella species and Escherichia coli were the most common offenders (Lok et al, 2008). Anaerobes generally are encountered with a previous history of biliary intervention (e.g., surgery or stenting). With nonbiliary gastrointestinal (GI) sources of infection, polymicrobial gram-negative aerobes and anaerobes predominate, whereas systemic bacteremia is more likely to give rise to a single isolate, often S. aureus. Other reported microbes reflect individual patient factors, such as Entamoeba histolytica with a history of residence in or travel to endemic areas, and Candida species in neutropenic patients.
Clinical symptoms and signs are nonspecific. Of 111 patients with pyogenic liver abscess (Lok et al, 2008), 91% were seen initially with fever, 79% with right upper quadrant pain, 18% with anorexia, and 18% with septic shock. Laboratory abnormalities also are nonspecific. In the same study, 93% had hypoalbuminemia, 75% leukocytosis, 72% elevated alkaline phosphatase, and 59% elevated alanine transaminase.
US and CT are the imaging modalities of choice (Zaleznik & Kasper, 1997). Radionuclide scans (gallium, indium, and technetium) and MRI can provide further diagnostic information, but rarely are necessary.
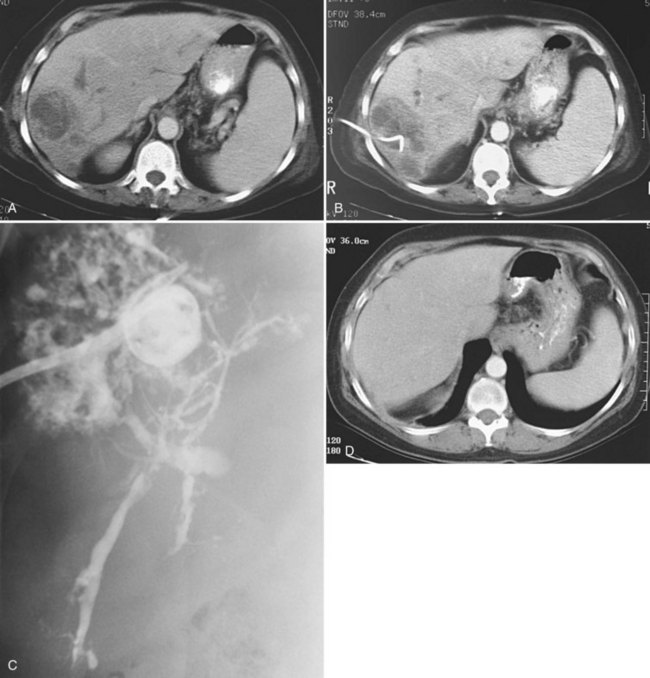
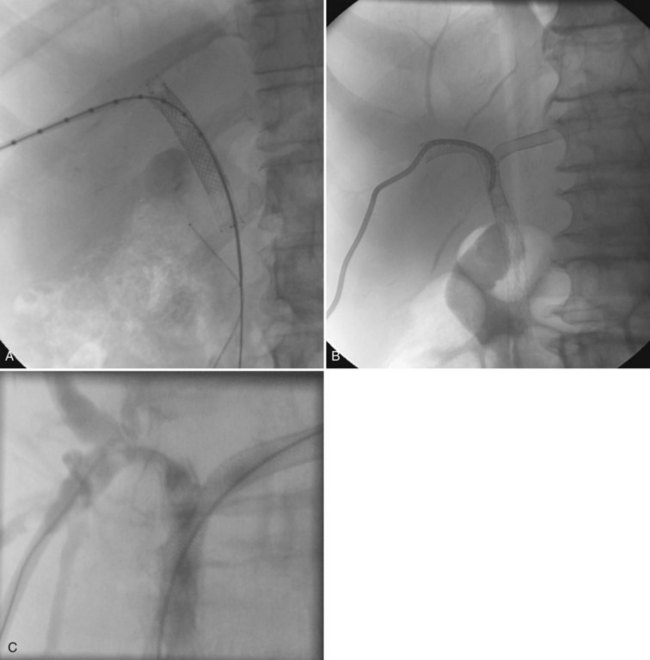
Uncomplicated pyogenic liver abscesses may be single or multiple, often are unilocular, and may be septated (Fig. 28.2). It may be impossible to evacuate complex multiloculated abscesses completely at the time of initial catheter placement (see Fig. 28.2B). Typically, there is free communication between the loculi (see Fig. 28.2C); consequently, most resolve completely when treated with a single catheter (see Fig. 28.2D) and appropriate antibiotics. If in doubt about adequate drainage of multiple locules or multiple abscesses, more than one catheter is indicated. Communication with the biliary tree may be seen when the drainage catheters are injected with contrast material as a part of performing an abscessogram (see Fig. 28.2C). The catheter contrast injections should not be performed until the pus has been evacuated to ensure that septicemia is not provoked iatrogenically.
Regarding the choice of percutaneous needle aspiration versus catheter drainage, opinions vary. Giorgio and colleagues (2006) described their experience with 118 patients treated with US-guided percutaneous needle aspiration and systemic antibiotic therapy. Cure was achieved in all 118 patients, and in 36 instances with only a single puncture. No recurrences were seen at 7 to 42 months of follow-up. The use of percutaneous needle aspiration and antibiotics is simple, less expensive, and more comfortable for the patient than catheter drainage. In practice, however, more than one aspiration is usually necessary for complete treatment with this strategy. Yu et al (2004) performed percutaneous needle aspiration in 64 patients with pyogenic liver abscess and concluded it was probably as effective as continuous percutaneous catheter drainage; however, their data were not statistically significant. Rajak and colleagues (1998) also compared the two methods of percutaneous treatment in 50 patients; they concluded that percutaneous catheter drainage was more effective than percutaneous needle aspiration. A failure of treatment was defined as a lack of response to a second attempt of percutaneous needle aspiration.
A more recent study (Zerem, 2007) also compared the two treatment methods in 60 patients, with the lack of response to percutaneous needle aspiration defined at three attempts. These authors agreed with Rajak and colleagues (1998) and concluded that percutaneous catheter drainage was more effective than percutaneous needle aspiration in the management of liver abscess. They opined that percutaneous catheter drainage is more efficient for multiloculated liver abscesses, and that percutaneous needle aspiration is a valid alternative for abscesses 5 cm or less in greatest diameter. Currently, treatment of pyogenic liver abscess with drainage catheter placement is the most commonly accepted method among interventional radiologists. Percutaneous aspiration without catheter drainage is not recommended except for occasional patients with one or more small liver abscesses that are too small for a catheter.
Surgical intervention for liver abscesses should be reserved for cases with coexisting surgically correctable causes of liver abscess (e.g., biliary tree abnormalities) or for cases of failed percutaneous therapy (see Chapter 66). A recent study demonstrated that partial liver resection is a safe and successful treatment for large, multiloculated complex pyogenic liver abscesses (Hope et al, 2008). Some associated biliary tree abnormalities may be amenable to endoscopic correction. In this subset of patients, endoscopic retrograde cholangiopancreatography (ERCP) might be preferred over surgery (Lam et al, 1999). Another study reported that previous endoscopic sphincterotomy was a good prognostic factor for early resolution of liver abscesses within 6 weeks (Wong et al, 2002).
Amebic Abscess
In a patient with a hepatic abscess and a history of travel to or residence in endemic areas, the possibility of infection with Entamoeba histolytica should be considered (see Chapter 67). Hepatic amebic abscess is the most common extraintestinal manifestation of amebiasis. Conditions that affect cell-mediated immunity—such as extremes of age, pregnancy, corticosteroid therapy, malignancy, HIV infection, and malnutrition—also may increase the chance that enteric E. histolytica infection results in extraluminal disease with liver involvement. The clinical findings are similar to the findings for pyogenic abscess; in addition, amebic abscesses may have superimposed bacterial infection, and they usually are solitary and most commonly located in the right lobe, adjacent to the liver capsule (Brindicci et al, 2006).
Serologic testing is the most widely used method to diagnose hepatic amebic abscess. Aspiration may be used to establish the diagnosis and to exclude secondary bacterial infection. When aspiration is used for diagnosis, the cavity should be aspirated as completely as possible. Amebic abscesses contain acellular, proteinaceous debris and an “anchovy paste,” a chocolate-colored fluid consisting predominantly of necrotic hepatocytes. Antigen tests and polymerase chain reaction on aspirated material may be helpful to confirm the diagnosis. Biopsy of the abscess wall may also establish the diagnosis if the other tests are inconclusive, which is the case in about 10% of patients (vanSonnenberg et al, 1985).
Amebic liver abscesses can generally be treated successfully with antibiotics alone (Chavez-Tapia et al, 2009). With clinical suspicion of amebic liver abscess, empiric treatment with metronidazole and a luminal agent (paromomycin or iodoquinol) may be started while awaiting confirmatory tests. Indications for catheter drainage of amebic abscess include failed medical therapy, secondary pyogenic infection, pregnancy, perforated abscess, false-negative serology, and left lobe abscess because of the proclivity to rupture or spread into the pericardium (vanSonnenberg et al, 1985; Ken et al, 1989; Hanna et al, 2000).
Echinococcal Disease
Echinococcal disease is an endemic zoonosis acquired from ingestion of Echinococcus granulosus eggs from infected animals (see Chapter 68). The most common location of hydatid cysts is the liver. Echinococcal cysts in the liver have a characteristic multiloculated or finely multiseptated appearance, and the right lobe is affected in 60% to 85% of cases.
Traditionally, surgery has been the recommended treatment for hepatic cysts (World Health Organization, 1996), but the combination of percutaneous drainage with drug therapy is a more recent alternative. Although percutaneous aspiration previously was contraindicated because of the risk of anaphylaxis, this complication has been shown to be rare (Akhan et al, 1996). Bret and associates (1988) demonstrated the safety of aspiration or drainage of these cysts in 13 patients, and none of the patients who underwent drainage was premedicated. If a catheter was placed for drainage, a scolicidal agent was injected into the cyst cavity after cystography. No complications or recurrences were reported 6 months to 1 year after treatment. Khuroo and colleagues (1997) compared percutaneous drainage with surgery for hepatic hydatid cysts and reported that percutaneous drainage, combined with albendazole, is an effective alternative to cystectomy. They demonstrated similar efficacy in cyst regression and disappearance, with advantages of a shorter hospital stay and a lower complication rate. Zerem and Jusufovic (2006) treated 72 patients with univesicular and multivesicular hepatic hydatid cysts with US-guided percutaneous drainage combined with albendazole, and 81% of the cysts in the univesicular group and 63% in the multivesicular group disappeared.
Percutaneous evacuation of cyst content (PEVAC) has been reported as a safe and effective treatment of multivesicular echinococcal cysts with or without cystobiliary fistulas, which contain undrainable material (Schipper et al, 2002). Hydatid cysts commonly have a communication with the biliary tree, which is a contraindication to injection of sclerosing agents, because this may cause diffuse injury to the biliary epithelium (Belghiti et al, 1986).
Transjugular Intrahepatic Portosystemic Shunt
The concept of transjugular hepatic intervention dates back to 1967, when Hanafee attempted diagnostic cholangiography via the transjugular intrahepatic route as a safer alternative to the percutaneous technique. At times, he inadvertently punctured portal vein radicles (Hanafee & Weiner, 1967). In 1969, Rosch and colleagues explored the transjugular route to visualize the portal venous system as an alternative to other available methods, such as splenoportography. The same year, Rösch and colleagues created the first transjugular intrahepatic portosystemic shunt (TIPS; see Chapter 76E) in dogs, using coaxial catheters to dilate the intrahepatic tract (Rösch et al, 1969, 1971). They discovered that without support, the tract would close; the plastic tubing used to “stent” the tract either thrombosed or migrated. Balloon dilation (Burgener & Gutierrez, 1979) and cryoprobes (Colapinto et al, 1983) were tried, but keeping the intrahepatic tract from shutting down remained a major problem. In the first human application of TIPS, Colapinto and associates (1982) used prolonged balloon inflation for 12 hours to keep the tract patent, but ultimately without success. The technique became a viable clinical option after refinement of metal stent technology in the late 1980s, when Richter and colleagues (1989) performed a landmark TIPS using a balloon-expandable Palmaz stent to maintain tract patency. The availability of Wallstents led to the rapid expansion and acceptance of the procedure.
Indications
The main indication for TIPS is the treatment of acute or recurrent variceal bleeding that has failed or is refractory to medical or endoscopic therapy, or both (see Chapter 75A, Chapter 75B, Chapter 75C, Chapter 76A, Chapter 76B, Chapter 76C, Chapter 76D, Chapter 76E ). This is especially true for patients who are bleeding from gastric or peristomal varices and those with severe portal hypertensive gastropathy. It is the preferred technique as a bridge to liver transplant, compared with surgical portosystemic shunts, because when performed correctly, TIPS does not alter extrahepatic vascular anatomy. It also is indicated for the control of refractory ascites and hepatic hydrothorax. TIPS may be used to treat Budd-Chiari syndrome in patients with moderate disease who fail to improve with anticoagulation (Boyer & Haskal, 2010).
Contraindications
There are several absolute and relative contraindications to TIPS placement. Absolute contraindications include congestive heart failure, sepsis, unrelieved biliary obstruction, severe pulmonary failure, and as the primary prevention of variceal bleeding (Boyer & Haskal, 2010). Relative contraindications to TIPS include the presence of HCC or hepatic metastases along the expected path of TIPS, hepatic vein obstruction, portal vein thrombosis, severe encephalopathy, polycystic disease of the liver, uncorrectable coagulopathy, and thrombocytopenia.
Technique (See Chapter 76E)
Various techniques and different systems are available for performing TIPS (Fig. 28.3). An imaging study using US, intravenous contrast-enhanced CT, or MRI to show a patent portal vein, hepatic veins, and hepatic venous anatomy is required. In an acutely bleeding patient, upper GI endoscopy confirms esophageal varices, excludes other sources of upper GI bleeding, and allows an attempt at endoscopic treatment. TIPS usually is performed under conscious sedation, but general anesthesia also may be used. Right internal jugular access is preferred, and a vascular sheath is placed. A selective catheter is advanced into one of the hepatic veins (see Fig. 28.3A).
The right hepatic vein is most commonly accessed for TIPS creation. Right atrial and free and wedged hepatic venous pressures are obtained, followed by hepatic and wedged hepatic venography. A specially designed needle is passed via the transjugular route from the right hepatic vein across the liver parenchyma into the right portal vein 2 to 3 cm from the main portal vein confluence. A guidewire is advanced into the superior mesenteric vein or splenic vein and is used to place a catheter for pressure measurements and venography (see Fig. 28.3B). The tract is predilated with a balloon (see Fig. 28.3C); an appropriately sized (8 to 12 mm) self-expanding bare stent or a specially designed covered stent is deployed, and the balloon is dilated to an appropriate size. Pressures are obtained, with the goal being a portal–systemic gradient of less than or equal to 12 mm Hg. If the gradient is greater than 12 mm Hg, certain stents (Wallstents) may be dilated with a 12-mm balloon. If this dilation does not result in a satisfactory pressure gradient, and collaterals fill persistently, or when stents other than Wallstent have been used, parallel TIPS should be considered. If the result is satisfactory (see Fig. 28.3D), the procedure is concluded, and baseline US studies are performed within the following few days.
Results (See Chapter 76E)
In a review of 1750 patients at nine institutions in the United States and abroad (Barton et al, 1995), the technical success rate of TIPS was 97%. Failures were related to portal vein thrombosis, extremely hard livers, and massive ascites. The procedure controlled acute bleeding in 91% of patients. Rebleeding increased with time, with a 6% incidence at 3 months, 17% at 6 months, and 21% at 1 year. Higher Model for End-Stage Liver Disease (MELD), Acute Physiology and Chronic Health Evaluation (APACHE II), and Child-Pugh scores are associated with decreased survival. The Society of Interventional Radiology states technical success for TIPS should be achieved in 95% of patients, and clinical success should be achieved in more than 90% (Haskal et al, 2003).
Complications include TIPS thrombosis or occlusions, transcapsular puncture, stent migration, exacerbation of hepatic encephalopathy, right heart failure, procedure-related hemorrhage, hemobilia, contrast-induced acute tubular necrosis, hypersensitivity reactions to contrast material, fever, and pulmonary edema. Fatal outcomes were reported in 1.7% of patients, caused by intraperitoneal, retroperitoneal, and mediastinal hemorrhage; laceration of hepatic arteries, portal vein branches, and the liver capsule; and right heart failure (Barton et al, 1995). Mortality rates for TIPS are dependent on the indication and the patient’s health status based on the aforementioned scoring systems. The 1-year survival for TIPS performed for bleeding varices ranges from 48% to 90%, with lower survival rates when performed for refractory ascites (Boyer & Haskal, 2010).
Stenoses and occlusions occurred in 35% to 85% of bare stents (Barton et al, 1995). This wide range reflects differences in follow-up protocol, with some centers using US and some using venography. In addition, routine surveillance was done at some centers, whereas at others, patients were not studied unless they became symptomatic. With repeat intervention, assisted patency of TIPS can be maintained in 95% of patients. The use of specially designed covered stents has dramatically improved the primary patency rates of TIPS (Rossi et al, 2004; Saxon, 2004). Bureau and colleagues (2007) composed a randomized controlled study of 80 patients to compare covered stents with bare stents and showed a primary patency rate of 76% and 36% at 2 years, respectively. Rates of being free of encephalopathy were 67% in the covered-stent group compared with 51% in the bare-stent group; thus, performing TIPS with covered stents is the current recommendation (Boyer & Haskal, 2010).
Portal Venous Embolization
Branches of the portal vein may be embolized to induce ipsilateral atrophy and contralateral liver hypertrophy in the preoperative management of select liver and biliary tumors. The resulting hypertrophy of the future liver remnant reduces the risk of postresection liver failure in selected patients. The role and technique of preoperative portal vein embolization are discussed in detail in Chapters 93A and 93B.
Bile Ducts and Gallbladder
Percutaneous Transhepatic Cholangiography
Percutaneous transhepatic cholangiography (PTC) is performed much less commonly for diagnostic purposes than in the past (see Chapter 18). Noninvasive imaging of the biliary tree such as with US (Chapter 13), CT (Chapter 16), and MR cholangiopancreatography (Barish & Soto, 1997; see Chapter 17) has greatly reduced the need for invasive PTC to diagnose biliary tract disorders. Indications for PTC have included defining the level of obstruction in patients with dilated bile ducts, to evaluate for suspected bile duct stones, to determine the etiology of cholangitis, to evaluate suspected bile duct inflammatory disorders, and to demonstrate the site of a bile duct leak (Burke et al, 2003). The complications of the procedure are uncommon and include sepsis, cholangitis, bile leak, hemorrhage, or pneumothorax (Burke et al, 2003).
Percutaneous Cholecystostomy
Percutaneous cholecystostomy is indicated for patients with acute calculous cholecystitis who are too ill to undergo cholecystectomy or for patients with acalculous cholecystitis (see Chapters 31 and 32; Welschbillig-Meunier et al, 2005; Akhan et al, 2002; vanSonnenberg et al, 1992). In the case of acute calculous cholecystitis, management depends on patient-related factors. In a patient with a long life expectancy, percutaneous cholecystostomy is a temporizing method to allow the patient to recover from the critical illness. When the patient’s condition stabilizes, cholecystectomy can be performed on a nonemergent basis. Conversely, for a patient with limited life expectancy and/or multiple comorbidities, if cystic and common bile ducts are patent, stones may be removed from the gallbladder and/or the common bile duct percutaneously or endoscopically.
Before catheter removal, after the tract has matured, it must be understood that a diseased gallbladder will be left behind. The recurrence rate of acute calculous cholecystitis after percutaneous cholecystostomy is approximately 25% (Van Steenbergen et al, 1990). Some patients with extreme debility and obstructed cystic or bile ducts may require long-term percutaneous cholecystostomy. In such cases, the catheter should undergo periodic exchange to avoid clogging and fragmentation.
Acute acalculous cholecystitis usually is considered in elderly patients with an unexplained sepsis syndrome; such patients often are being managed in intensive care units when imaging, usually US, reveals a distended, tender gallbladder. The facility with which the procedure can be performed, even at the bedside, along with the low complication rate allows percutaneous cholecystostomy to serve a diagnostic and therapeutic function. Even patients with gas within the gallbladder wall can be treated with percutaneous cholecystostomy. A 56% to 80% clinical response to percutaneous cholecystostomy has been reported (Boland et al, 1994). A more recent study (Chuang et al, 2007) reported percutaneous cholecystostomy followed by cholecystectomy as a treatment for emphysematous cholecystitis in four patients, none of whom suffered a complication.
Initially, thick, viscous, dark bile is aspirated from the distended gallbladder in these patients. As inflammation subsides and cystic duct patency is restored, normal-appearing bile begins to drain from the catheter. At that point, a contrast study of the catheter can be performed to document patency of the cystic and the common bile ducts, and the percutaneous cholecystostomy catheter may be clamped. It is important not to remove the catheter until a mature tract to the skin has formed to avoid intraperitoneal leakage of bile. Depending on the nutritional status of the patient and whether a transhepatic or transperitoneal approach was used to enter the gallbladder, catheter removal typically occurs no sooner than week 2 or 3 after the procedure (Hatjidakis et al, 1988; vanSonnenberg et al, 1992).
Percutaneous cholecystostomy also may be performed as a means of relieving distal common bile duct obstruction in patients who are too ill to tolerate another, more definitive procedure; in patients who have minimal or no intrahepatic biliary ductal dilation; or in those who have failed or cannot undergo endoscopic retrograde biliary endoscopy, provided that the cystic duct is patent. Percutaneous cholecystostomy is quicker, easier, and safer than percutaneous transhepatic biliary drainage under these circumstances, and it can be performed at the bedside with US guidance. Patients without significant dilation of intrahepatic ducts can have a percutaneous cholecystostomy catheter placed that can be injected with contrast medium at a later time to opacify the intrahepatic ductal system, facilitating conventional transhepatic access to the biliary tree for a variety of interventions (vanSonnenberg et al, 1990a, 1992).
Interventional Radiologic Management of Bile Duct Stones
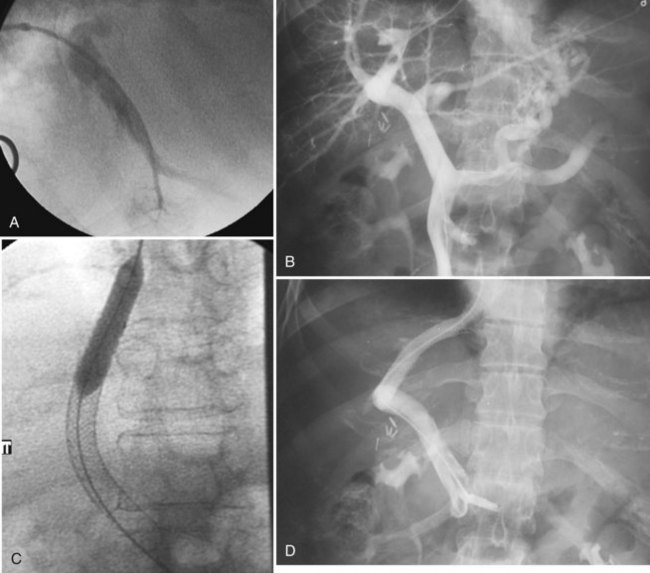
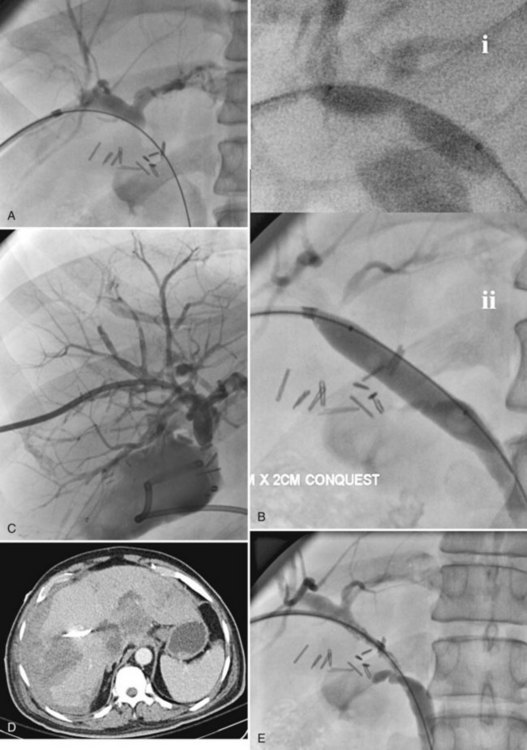
Retained common bile duct stones after surgery may be treated successfully via a T-tube tract in most patients or via a transhepatic approach (Fig. 28.4; see Chapters 35, 36, and 39). Stones can be extracted or crushed with baskets, or they can be swept into the bowel with balloon catheters. Balloon dilation of the sphincter (see Fig. 28.4B) is performed to allow for passage of small fragments, typically with a 10-mm balloon. An older, classic study on stone extraction surveyed 39 institutions and reported 5% morbidity and no deaths in 612 extraction procedures (Burhenne, 1976). With the development of more sophisticated devices for minimally invasive procedures—including steerable catheters, cholangioscopes, and contact lithotripsy probes—the success rate for treatment of retained common bile duct stones is even greater. These techniques and devices also can be applied to the transhepatic removal of intrahepatic stones (see Chapter 39, 44 ).
The principal drawback to T-tube stone extraction is that a 4- to 6-week period is required for tract maturation. In select cases, attempts can sometimes be made earlier with tract protection by sheaths. Endoscopic sphincterotomy and stone extraction (see Chapter 36) may be performed sooner, typically after 7 to 10 days, with 90% to 95% success rate (Becker et al, 1993; O’Doherty et al, 1986). Complication rates are 4% to 19% with a 0% to 2% mortality rate. Christoforidis and colleagues (2004) stated that the endoscopic technique is a safe and efficient treatment for residual stones after common bile duct exploration with a T-tube insertion, offering immediate cure compared with percutaneous techniques.
The location of bile duct stones and previous surgeries are important factors in deciding the method of treatment. Intrahepatic bile duct stones are more readily accessible by interventional radiologists than gastroenterologists. Furthermore, previous operations such as hepaticojejunostomy make an endoscopic approach impossible, thus percutaneous routes by interventional radiologists are indicated for these patients. A series of six patients with extensive and complicated recurrent pyogenic cholangitis (see Chapter 44) who all had previous biliary surgery underwent numerous percutaneous techniques—basketing, flushing, crushing, balloon occlusion, and chemical dissolution—to remove innumerable retained intrahepatic and extrahepatic stones (vanSonnenberg 1986).
Percutaneous Transhepatic Biliary Drainage
Percutaneous transhepatic biliary drainage (PTBD) is indicated for relief of biliary obstruction, especially of the proximal biliary tree, and is associated with pruritus and cholangitis (see Chapter 20, Chapter 26, Chapter 27, Chapter 42A, Chapter 42B , and 50D). In general, distal biliary disease is addressed by biliary endoscopy. PTBD is indicated in some patients as the initial step in the planned treatment of benign and malignant bile duct strictures, biliary stones, or locoregional treatment of primary and metastatic liver neoplasms. PTBD is also indicated for severe cholestasis but should not be performed solely for the relief of jaundice or for the cosmetic treatment of dilated bile ducts; this is due to the invasiveness of the procedure and its associated potential complications, including bleeding, bile peritonitis, and sepsis. Preoperative biliary decompression in jaundiced but otherwise asymptomatic patients is controversial (see Chapter 50A, Chapter 50B, Chapter 50C, Chapter 50D , and 62A). PTBD also may be indicated for lowering serum bilirubin levels to permit the use of chemotherapeutic agents with biliary excretion routes. Lastly, in select complicated situations, the surgeon may request catheterization of the bile ducts to help identify the ducts to avoid injury at surgery.
Preprocedure Evaluation
Preprocedure evaluation should include a complete blood cell count (including platelets), prothrombin time, partial thromboplastin time, international normalized ratio, and liver function tests. Diagnostic imaging of the liver and biliary tree is a prerequisite. US is a safe, cost-effective technique to demonstrate biliary obstruction and the level of obstruction. CT and MR cholangiopancreatography (MRCP; see Chapters 16 and 17) are extremely useful to show hepatobiliary anatomy and pathology in great detail. CT is the procedure of choice for procedural planning. Findings such as liver atrophy are readily identified on any cross-sectional imaging modality, but other anatomic considerations—such as colonic interposition and identification of calcifications or surgical clips that may serve as useful fluoroscopic landmarks—are readily evident on CT. Translation of this information into three-dimensional images greatly facilitates the drainage procedure, and three-dimensional postprocessing CT can be performed if desired.
Patients undergoing biliary drainage have a high incidence of bacterobilia, particularly when there has been previous operation or biliary instrumentation (Brody et al, 1998). Prophylactic antibiotics are indicated in all cases of PTBD. Broad-spectrum agents that cover enterococci, streptococci, and aerobic and anaerobic gram-negative bacilli typically are administered before the interventional radiology procedure commences (Lipsett, 1996; see Chapter 11).
Technique
The choice of a right- or left-sided approach for PTBD is based on many factors. Drainage of an atrophic portion of the liver does nothing to conserve or improve hepatic function or to lower serum bilirubin (see Chapter 5). The only reason to drain an atrophic segment would be for unrelenting biliary sepsis after satisfactory drainage of the functional liver parenchyma. In general, high bile duct obstruction is more likely to result in isolation of the right anterior and right posterior sectoral ducts and to extend to involve second-order branches. Such isolation of segmental ducts occurs later on the left, because the left hepatic duct is typically longer compared with the main right duct, which is shorter and has a more palmate branching pattern. In addition, the right posterior sectoral duct joins the left hepatic duct, rather than its anterior right counterpart, in at least 20% of cases (see Chapter 1B).
In high obstruction, in the absence of left portal vein compromise, a left-sided approach may be preferred. Other advantages of left-sided access are a more favorable angle for biliary intervention; better use of US to guide safe puncture of the duct, which is easier to visualize on the left; avoidance of pleura; less discomfort associated with an intercostal route; and less chance of an ascites leak around the catheter with an anterior left subxiphoid approach rather than a right lateral intercostal approach. The advantages of a right-sided approach are decreased exposure of the operator to radiation and in general more operator familiarity with right-sided access. In certain cases, access from both sides may be needed (vanSonnenberg, 1986). In patients who are being considered for operation, left-sided drainage catheters are often close to or in the line of the intended incision, although this should not be an overriding concern if left-sided drainage is required.
At the time of initial drainage of patients with malignant biliary obstruction and limited life expectancy, placement of an internal biliary endoprosthesis should be considered. Plastic and metal endoprotheses are available. Metal stents have a longer median patency (3.6 to 9.1 months) relative to plastic stents (1.8 to 5.5 months), but there is no difference in median survival (Levy et al, 2004). Tsuyuguchi and colleagues (2008) suggest that metal stents are recommended in patients who are expected to survive longer than 6 months, whereas plastic stents should be used in patients with a suspected survival of less than 6 months. The difference in cost also serves as a factor in this management recommendation, because metal stents are more expensive than plastic stents if reintervention is not required (Davids et al, 1992). Metal stents also may be covered with polyurethane, and these were found to prevent tumor ingrowth and had higher patency, but complications of acute cholecystitis (Isayama et al, 2004) and stent migration also occurred (Yoon et al, 2006). Thus further studies are needed to establish the indications for covered versus bare metal stents.
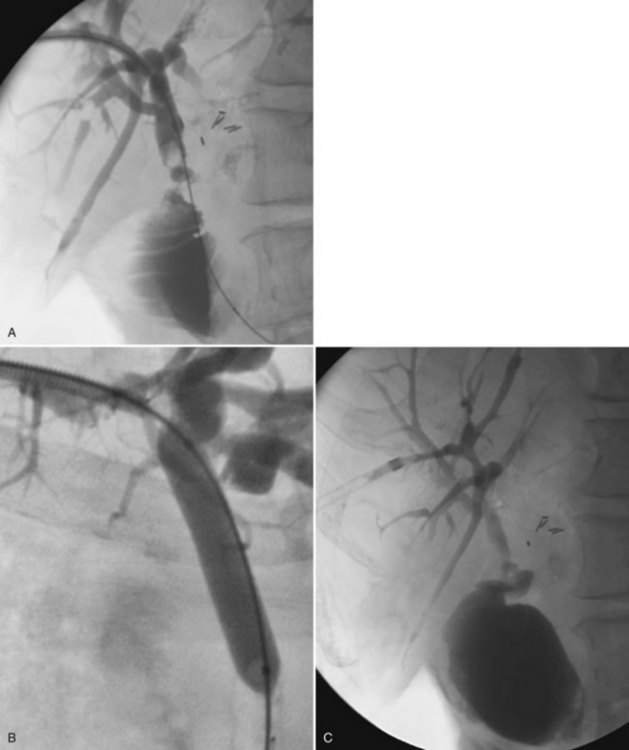
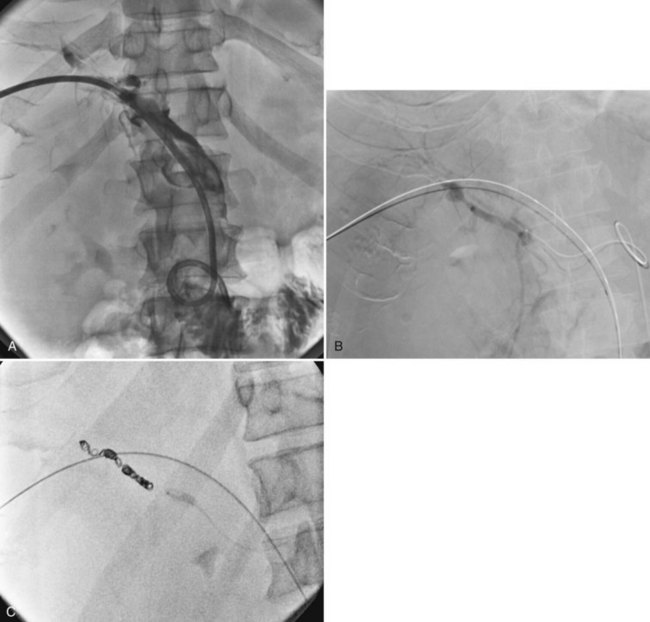
In the case of isolation of the right and left hepatic ducts, management can be accomplished in one of two ways. From one transhepatic approach (Fig. 28.5), a stent can be placed across the confluence of the bile ducts to the contralateral side, and the second stent can be deployed from the ipsilateral side into the common hepatic and/or common bile duct. Alternatively, especially when the patient has failed to respond to a single drainage, the contralateral side can be approached percutaneously (Fig. 28.6), allowing for simultaneous placement of side-by-side stents across the obstruction at the ductal confluence into the distal common bile duct. We agree with Morgan and Adam (1996) that the latter approach is preferable in the event that it is necessary to intervene again for subsequent stent occlusion. For hilar lesions, we are careful to “overstent” (Lammer et al, 1996) in an effort to avoid occlusion related to tumor overgrowth. One way is to predilate and/or postdilate when placing Wallstents. Some patients find balloon dilation to be quite painful, and although general anesthesia is not used routinely, it may be advantageous in select patients. Satisfactory stent expansion typically is achieved within 24 hours (see Figs. 28.5B and 28.6B) without the aid of balloons.
There is a role for balloon dilation if, after deployment, contrast material does not flow promptly across the stent. After stent deployment, the introducer system is removed, and a 4- to 5-Fr end-hole catheter, such as a Kumpe (Cook, Bloomington, IN), is left as a protective catheter. If dual access has been used, two protective catheters are left. These catheters are used for subsequent follow-up cholangiography (see Figs. 28.5C and 28.6C) and to ensure that there has not been asymmetric stent shortening or some other occurrence that would require placement of additional stents or other intervention. In 95% of cases, no other intervention is necessary.
Bile Duct Biopsy
Faced with inoperable, presumed malignant bile duct obstruction, a tissue diagnosis is necessary before initiating brachytherapy or sometimes simply in an effort to give meaningful prognostic information to the patient and family. A diagnosis can be arrived at in several ways. Bile cytology obtained at the time of initial drainage, although insensitive, has been found to be quite specific (Savader et al, 1988), and there is virtually no disadvantage to sending a specimen. Cytology can be obtained also with intraluminal brushes; the reported sensitivity ranges from 44% to 67% (Ryan, 1991; Cropper & Gold, 1983; Mendez et al, 1980). Percutaneous transluminal forceps biopsy has shown better diagnostic capabilities with a sensitivity of 78.4% and specificity of 100% in cases of malignant biliary obstruction (Jung et al, 2002; see Chapter 20). If these methods fail, a cholangiographically guided percutaneous needle biopsy can be performed, which frequently yields a diagnosis. If a mass is visualized on CT or US, these modalities can be used to guide the biopsy. These options will usually yield a biopsy diagnosis, and the choice is tailored to the patient’s situation and the radiologist’s preference.
Brachytherapy
Brachytherapy involves the local delivery of radiation to a tumor and can be used percutaneously to treat unresectable or recurrent cholangiocarcinoma (see Chapters 50A, 50B, 50C, and 50D). We also have used this method to treat intraductal metastatic colorectal carcinoma and bile duct carcinoma. Conventional biliary drainage is performed first, placing one or more internal/external catheters. After the bilirubin has normalized and any underlying infection has subsided, a Wallstent is introduced to bridge the area of obstruction, and a simulation catheter is positioned through the Wallstent. After dosimetry is performed, the radiation source is loaded within 24 hours and remains in place for a predetermined time, depending on dosimetry calculations. When therapy is complete, the catheter and radiation source are removed at the bedside. This method is not only a means for local tumor therapy but also seems to prolong Wallstent patency by decreasing the rate of tumor ingrowth (Eschelman et al, 1996), at least when combined with external-beam therapy.
Bile Duct Injury
Much of what we know about the role of interventional radiology in the management of traumatic injury to the bile ducts has been learned from complications of iatrogenic trauma, particularly after laparoscopic cholecystectomy (Boland et al, 1996; Lillemoe et al, 1997; Lund & Winick, 1996; vanSonnenberg et al, 1990c). Similar principles would apply to the treatment of noniatrogenic injuries, although these are rarely seen. Patients with strictures who do not have a previous history of biliary surgery or significant right upper quadrant trauma should be investigated aggressively in an effort to establish that the stricture is not malignant prior to treatment (see Chapters 38, 42A, 42B, and 102).
In general, small postoperative bile leaks typically heal spontaneously, assuming there is no outflow obstruction, and usually all that is required is percutaneous drainage of any collection that may have formed (see Chapters 42A and 42B). Cystic duct leaks after cholecystectomy may respond best to endoscopic placement of a plastic biliary endoprosthesis. Major bile duct injuries consist of duct transection, laceration, and/or partial or complete ligation (vanSonnenberg et al, 1990c). These injuries are categorized according to the anatomic site of injury. Normal anatomic variants also pose challenges. Christensen et al (1992) discussed how aberrant right hepatic ducts are the most common biliary tract anomaly susceptible to injury at cholecystectomy. Complications include biliary leakage and fistulas, bilomas, pain, fever, and sepsis. An accidentally ligated aberrant right hepatic duct may not be appreciated at ERCP. Prudent evaluation with US, CT, MRCP, and PTC of sequestered posterior right hepatic duct dilation is essential for proper diagnosis. A preoperative biliary catheter may aid the surgeon in subsequent ductal reconstruction.
The length of duct available for surgical reconstruction and the integrity of the hepatic duct confluence greatly influence treatment options. Most surgeons and radiologists agree that the best chance for a trouble-free long-term outcome can be expected after primary surgical repair (Boland et al, 1996; Lillemoe et al, 1997). In the study by Lillemoe and colleagues (1997), patients treated surgically showed improved outcomes compared with patients treated with interventional techniques, including drainage and balloon dilation. All of the patients treated with interventional techniques had previous surgical attempts at reconstruction. In the interventional group, patients who presented late and with low (Bismuth type 1; see Chapters 42A and 42B) injuries fared significantly better. These patients require a team approach. Patients who are poor surgical candidates, those who have portal hypertension, and those who have had multiple previous attempts at surgical repair might be treated best with percutaneous drainage and balloon dilation (see Chapters 27, 42A, and 42B).
Mid to distal common bile duct strictures can be dilated with a balloon catheter. Balloons should be sized slightly larger than the duct being dilated, usually 8 to 12 mm, and inflated to eliminate any “waist.” When dilating strictures at the hilum (Fig. 28.7), balloon dilation may require two or more catheters, which may be used sequentially to dilate from side to side and into the common hepatic duct, across a biliary-enteric anastomosis (see Fig. 28.7B), or simultaneously. When the simultaneous or “kissing balloon” technique is used, with one balloon placed from the right and one from the left, size should be adjusted appropriately. There is no evidence that prolonged inflation or multiple inflations enhance patency, and some authors believe this may promote ductal ischemia. Occasionally, bile duct rupture occurs (see Fig. 28.7C), and we have seen a related arterial vascular injury (see Fig. 28.7D) that required embolization; however, we have not found it necessary to treat bile duct rupture with anything other than continued drainage (see Fig. 28.7E).
How long a catheter should be left in place after balloon dilation is controversial (Boland et al, 1996). We leave a 10- or 12-Fr drainage catheter in place for 2 to 4-weeks, at which time we reevaluate the patient cholangiographically. If the stricture site is widely patent, we exchange for a drainage catheter above the treated area; if the patient tolerates a clamping trial, this catheter is removed in a few days. If the follow-up cholangiogram shows residual or recurrent narrowing, the site is dilated again with a slightly larger balloon, followed by another 4-week observation period and a cholangiogram. Again, a drainage catheter is left above the dilated site and clamped. If the patient tolerates this “capping trial,” the catheter is removed.
Biliary pressure measurements and bile duct perfusion with manometry offer the only real objective information about bile duct dynamics and the status of balloon dilation (vanSonnenberg et al, 1983, 1990b). We utilize manometry and perfusion dynamics if evaluation of the dilatation, patency, and flow dynamics are not straightforward (relatively common). Patients who fail repeated dilations should undergo either continued catheter drainage or surgical treatment. We believe that self-expanding metallic stents should not be used to treat benign strictures, unless the patients are extremely poor surgical candidates or they have limited life expectancy because of comorbid conditions. Surgically treated patients have better outcomes, and when a metallic stent is placed, it makes subsequent surgical repair more difficult (vanSonnenberg et al, 1986). These decisions should always be made with the surgeon and interventional radiologist in concert.
Vascular Injury
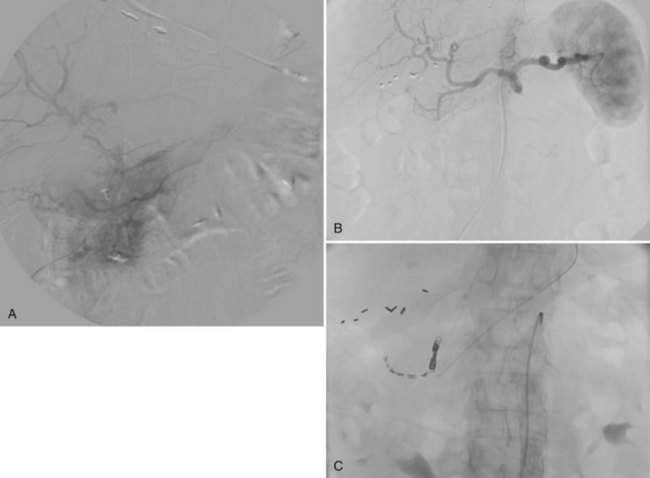
Sclafani (1985) showed that angiographic methods could be used to control hemorrhage from injuries to the liver and spleen (see Chapters 19, 50D, 104, and 105). Hagiwara and colleagues (1997) concurred more recently that this method is an effective alternative to surgery. All but the most severe hepatic venous bleeding is self-limiting, and angiographic methods are suitable to treat hemorrhage secondary to blunt or penetrating trauma, including iatrogenic arterial injuries resulting from biliary interventions (Fig. 28.8). When the site of injury is established—by the presence of pseudoaneurysm formation (Fig. 28.8B), extravasation (Fig. 28.9A), abrupt vessel cutoff, or spasm—the injured vessel may be occluded with absorbable gelatin sponge (Gelfoam), metal coils, or both (Fig. 28.8C). If small coaxial catheters are necessary, microcoils or polyvinyl alcohol particles may be used. The dual blood supply to the liver makes it extremely tolerant of arterial occlusion for hemostasis; however, caution must be exercised in jaundiced patients, because significant compromise of arterial inflow can result in hepatic necrosis.
Percutaneous Tumor Ablation
Many percutaneous methods for tumor ablation are currently in use, including heating, freezing, alcohol injection, and combinations of these techniques. Although radiofrequency ablation (RFA) is the most common ablation method in the United States, treatment should be tailored to the individual patient’s situation (vanSonnenberg et al, 2005). For example, in a patient with metastatic pheochromocytoma to the liver, a combination of alcohol and RFA was used to synergistically enhance tumor kill (Fig. 28.10; Mamlouk et al, 2009; Shankar et al, 2004). Nonresectional ablation of liver tumors is covered further in Chapter 83, Chapter 84A, Chapter 84B, Chapter 85A, Chapter 85B, Chapter 85C, Chapter 85D ).
Akhan O, Akinci D, Ozmen MN. Percutaneous cholecystostomy. Eur J Radiol. 2002;43:229-236.
Akhan O, et al. Liver hydatid disease: long-term results of percutaneous treatment. Radiology. 1996;198:259-264.
Barish MA, Soto JA. MR cholangiopancreatography: techniques and clinical applications. Am J Roentgenol. 1997;169:1295-1303.
Barton RE, et al. TIPS: short- and long-term results: a survey of 1750 patients. Semin Interv Radiol. 1995;12:364.
Bean WJ, Rodan BA. Hepatic cysts: treatment with alcohol. AJR Am J Roentgenol. 1985;144:237-241.
Becker CD, et al. Comparison of percutaneous endoscopic retrograde removal of postoperatively retained bile duct stones. Cardiovasc Interv Radiol. 1993;16:144-149.
Belghiti J, et al. Caustic sclerosing cholangitis: a complication of the surgical treatment of hydatid disease of the liver. Arch Surg. 1986;121:1162-1165.
Boland GW, Müller PR, Lee MJ. Laparoscopic cholecystectomy with bile duct injury: percutaneous management of biliary stricture and associated complications. AJR Am J Roentgenol. 1996;166:603-607.
Boland GW, et al. Percutaneous cholecyctostomy in critically ill patients: early response and final outcome in 82 patients. AJR Am J Roentgenol. 1994;163:339-342.
Boyer TD, Haskal ZJ. American Association for the Study of Liver Diseases. The role of transjugular intrahepatic portosystemic shunt (TIPS) in the management of portal hypertension: update 2009. Hepatology. 2010;51:306.
Bret PM, et al. Percutaneous aspiration and drainage of hydatid cysts in the liver. Radiology. 1988;168:617-620.
Brindicci G, et al. Amoebic hepatic abscesses in an HIV-positive patient. AIDS Patient Care STDS. 2006;20:606-611.
Brody LA, et al. Clinical factors associated with positive bile cultures during primary percutaneous biliary drainage. J Vasc Interv Radiol. 1998;9:572-578.
Bureau C, et al. Patency of stents covered with polytetrafluoroethylene in patients treated by transjugular intrahepatic portosystemic shunts: Long-term results of a randomized multicentre study. Liver Int. 2007;27:742-747.
Burgener FA, Gutierrez OH. Nonsurgical production of intrahepatic portosystemic venous shunts in portal hypertension with the double lumen balloon catheter. Rofo. 1979;130:686-688.
Burhenne HJ. Complications of non-operative extraction of retained common bile duct stones. Am J Surg. 1976;131:260-262.
Burke DR, et al. Society of Interventional Radiology Standards of Practice Committee. Quality improvement guidelines for percutaneous transhepatic cholangiography and biliary drainage. J Vasc Interv Radiol. 2003;14:S243-S246.
Chavez-Tapia NC, et al, 2009: Image-guided percutaneous procedure plus metronidazole versus metronidazole alone for uncomplicated amoebic liver abscess. Cochrane Database Syst Rev 21(1):CD004886.
Christensen RA, et al. Inadvertent ligation of the aberrant right hepatic duct at cholecystectomy: radiologic diagnosis and therapy. Radiology. 1992;183:549-553.
Christoforidis E, et al. Endoscopic management of retained bile stones with an indwelling T-tube. Surg Endosc. 2004;18:1582-1586.
Chuang CH, et al. Management of emphysematous cholecystitis. Chir Gastroenterol. 2007;23:75-78.
Colapinto RF, et al. Creation of an intrahepatic portosystemic shunt with a Gruntzig balloon catheter. Can Med Assoc J. 1982;126:267-268.
Colapinto RF, et al. Formation of intrahepatic portosystemic shunts using a balloon dilatation catheter: preliminary clinical experience. AJR Am J Roentgenol. 1983;140:709-714.
Cropper LDJr, Gold RE. Simplified brush biopsy of the bile ducts. Radiology. 1983;148:307-308.
Davids PH, et al. Randomized trial of self-expanding metal stents versus polyethylene stents for distal malignant biliary obstruction. Lancet. 1992;340:1486-1492.
Eschelman DJ, et al. Malignant biliary duct obstruction: long-term experience with Gianturco stents and combined-modality radiation therapy. Radiology. 1996;200:717-724.
Forbes A, Murray-Lyon IM. Cystic disease of the liver and biliary tract. Gut. 1991;Sept(Suppl):S116-122.
Gall TM, et al. Surgical management and long-term follow-up of non-parasitic hepatic cysts. HPB (Oxford). 2009;11:235-241.
Giorgio A, et al. Percutaneous needle aspiration of multiple pyogenic abscesses of the liver: 13-year single-center experience. AJR Am J Roentgenol. 2006;187:1585-1590.
Hagiwara A, et al. Nonsurgical management of patients with blunt hepatic injury: efficacy of transcatheter arterial embolization. AJR Am J Roentgenol. 1997;169:1151-1156.
Hanafee W, Weiner M. Transjugular percutaneous cholangiography. Radiology. 1967;88:35-39.
Hanna RM, et al. Percutaneous catheter drainage in drug-resistant amoebic liver abscess. Trop Med Int Health. 2000;5:578-581.
Haskal ZJ, et al. Society of Interventional Radiology Standards of Practice Committee. Quality improvement guidelines for transjugular intrahepatic portosystemic shunts. J Vasc Interv Radiol. 2003;14:S265-S270.
Hatjidakis AA, et al. Maturation of the tract after percutaneous cholecystostomy with regard to access route. Cardiovasc Interv Radiol. 1988;21:36-40.
Hope WW, et al. Optimal treatment of hepatic abscess. Am Surg. 2008;74:178-182.
Huang CJ, et al. Pyogenic hepatic abscess: changing trends over 42 years. Ann Surg. 1996;223:600-609.
Isayama H, et al. A prospective randomised study of “covered” versus “un-covered” diamond stents for the management of distal malignant biliary obstruction. Gut. 2004;53:729-734.
Jepsen P, et al. A nationwide study of the incidence and 30-day mortality rate of pyogenic liver abscess in Denmark, 1977-2002. Aliment Pharmacol Ther. 2005;21:1185-1188.
Jung GS, et al. Bile duct: Analysis of percutaneous transluminal forceps biopsy in 130 patients suspected of having malignant biliary obstruction. Radiology. 2002;224:725-730.
Ken JG, et al. Perforated amebic liver abscesses: successful percutaneous treatment. Radiology. 1989;170:195-197.
Khuroo MS, et al. Percutaneous drainage compared with surgery for hepatic hydatid cysts. N Engl J Med. 1997;337:881-887.
Kimura F, et al. Repeated instillation of a limited volume of ethanol for the treatment of symptomatic hepatic cysts. Hepatogastroenterology. 2005;52:1147-1150.
Lam YH, et al. ERCP and pyogenic liver abscess. Gastrointest Endosc. 1999;50:340-344.
Lammer J, et al. Plastic versus metal biliary endoprostheses. Semin Interv Radiol. 1996;13:253-261.
Levy MJ, et al. Palliation of malignant extrahepatic biliary obstruction with plastic versus expandable metal stents: an evidence-based approach. Clin Gastroenterol Hepatol. 2004;2:273-285.
Lillemoe KD, et al. Major bile duct injuries during laparoscopic cholecystectomy. Ann Surg. 1997;225:459-471.
Lipsett PA. Preparation of the patient for biliary surgical or interventional procedures: antibiotic prophylaxis. Semin Interv Radiol. 1996;13:201-206.
Lok KH, et al. Pyogenic liver abscess: clinical profile, microbiological characteristics, and management in a Hong Kong hospital. J Microbiol Immunol Infect. 2008;41:483-490.
Lopes HM, et al. Treatment of benign hepatic cysts by instillation of tetracycline hydrochloride. Hepatogastroenterology. 1998;45:496-499.
Lund GB, Winick AB. Complications from laparoscopic cholecystectomy and the role of interventional radiologic management. Semin Interv Radiol. 1996;13:263-275.
Mamlouk MD, et al. Radiofrequency ablation and biopsy of metastatic pheochromocytoma: emphasizing safety issues and dangers. J Vasc Interv Radiol. 2009;20:670-673.
Mazza OM, et al. Management of nonparasitic hepatic cysts. J Am Coll Surg. 2009;209:733-739.
Mendez GJr, et al. Percutaneous brush biopsy and internal drainage of biliary tree through endoprosthesis. AJR Am J Roentgenol. 1980;134:653-659.
Morgan R, Adam A. Metallic stents in the treatment of patients with malignant biliary obstruction. Semin Interv Radiol. 1996;13:229-239.
Mortele KJ, Ros PR. Cystic focal liver lesions in the adult: differential CT and MR imaging features. Radiographics. 2001;21:895-910.
Nisenbaum HL, Rowling SE. Ultrasound of focal hepatic lesions. Semin Roentgenol. 1995;30:324-346.
O’Doherty DP, Neoptolemos JP, Carr-Locke DL. Endoscopic sphincterotomy for retained common bile duct stones in patients with T-tube in situ in the early post-operative period. Br J Surg. 1986;73:454-456.
Rajak CL, et al. Percutaneous treatment of liver abscesses: needle aspiration versus catheter drainage. AJR Am J Roentgenol. 1998;170:1035-1039.
Richter GM, et al. The transjugular intrahepatic protosystemic stent-shunt: a new nonsurgical percutaneous method. Radiologe. 1989;29:406-411.
Rösch J, Hanafee WN, Snow H. Transjugular portal venography and radiologic portacaval shunt: an experimental study. Radiology. 1969;92:1112-1114.
Rösch J, et al. Transjugular intrahepatic portocaval shunt. Am J Surg. 1971;121:588-592.
Rossi P, et al. Polytetrafluoroethylene-covered nitinol stent-graft for transjugular intrahepatic portosystemic shunt creation: 3-year experience. Radiology. 2004;231:820-830.
Ryan ME. Cytologic brushings of ductal lesions during ERCP. Gastrointest Endosc. 1991;37:139-142.
Savader SJ, et al. Single specimen bile cytology: a prospective study of 80 patients with obstructive jaundice. J Vasc Interv Radiol. 1988;9:817-824.
Saxon RR. A new era for transjugular intrahepatic portosystemic shunts? J Vasc Interv Radiol. 2004;1:217-219.
Schipper HG, et al. Percutaneous evacuation (PEVAC) of multivesicular echinococcal cysts with or without cystobiliary fistulas which contain non-drainable material: First results of a modified PAIR method. Gut. 2002;50:718-723.
Sclafani SJR. Angiographic control of intraperitoneal hemorrhage caused by injuries to the liver and spleen. Semin Interv Radiol. 1985;2:139-147.
Shankar S, et al. Combined radiofrequency and alcohol injection for percutaneous hepatic tumor ablation. AJR Am J Roentgenol. 2004;183:1425-1429.
Tikkakoski T, et al. Treatment of symptomatic congenital hepatic cysts with single-session percutaneous drainage and ethanol sclerosis: technique and outcome. J Vasc Interv Radiol. 1996;7:235-239.
Tsuyuguchi T, et al. Japanese Association of Biliary Surgery; Japanese Society of Hepato-Biliary-Pancreatic Surgery; Japan Society of Clinical Oncology. Stenting and interventional radiology for obstructive jaundice in patients with unresectable biliary tract carcinomas. J Hepatobiliary Pancreat Surg. 2008;15:69-73.
vanSonnenberg, E, McMullen, W, Solbiati, L. Tumor Ablation: Principles and Practice. New York: Springer-Verlag, 2005.
vanSonnenberg E, et al. Biliary pressure: manometric and perfusion studies at percutaneous transhepatic cholangiography and percutaneous biliary drainage. Radiology. 1983;148:41-50.
vanSonnenberg E, et al. Intrahepatic amebic abscesses: indications for and results of percutaneous catheter drainage. Radiology. 1985;156:631-635.
vanSonnenberg E, et al. Oriental cholangiohepatitis: diagnostic imaging and interventional management. AJR Am J Roentgenol. 1986;146:327-331.
vanSonnenberg E, et al. Interventional radiology in the gallbladder: diagnosis, drainage, dissolution and management of stones. Radiology. 1990;174:1-6.
vanSonnenberg E, et al. The benefits of percutaneous cholecystostomy for decompression of selected cases of obstructive jaundice. Radiology. 1990;176:15-18.
vanSonnenberg E, et al. The role of interventional radiology for complications of cholecystectomy. Surgery. 1990;107:632-638.
vanSonnenberg E, et al. Percutaneous gallbladder puncture and cholecystostomy: results, complications, and caveats for safety. Radiology. 1992;183:167-170.
vanSonnenberg E, et al. Symptomatic hepatic cysts: percutaneous drainage and sclerosis. Radiology. 1994;190:387-392.
Van Steenbergen W, et al. Percutaneous transhepatic cholecystostomy for acute complicated cholecystitis in elderly patients. Am J Gastroenterol. 1990;83:1363-1369.
Welschbillig-Meunier K, et al. Percutaneous cholecystostomy for high-risk patients with acute cholecystitis. Surg Endosc. 2005;19:1256-1259.
WHO Informal Working Group on Echinococcosis. Guidelines for treatment of cystic and alveolar echinococcosis in humans. Bull World Health Organ. 1996;74:231-242.
Wong WM, et al. Pyogenic liver abscess: retrospective analysis of 80 cases over a 10-year period. J Gastroenterol Hepatol. 2002;17:1001-1007.
Yoon WJ, et al. A comparison of covered and uncovered Wallstents for the management of distal malignant biliary obstruction. Gastrointest Endosc. 2006;63:996-1000.
Yoshida H, et al. Long-term results of multiple minocycline hydrochloride injections for the treatment of symptomatic solitary hepatic cyst. J Gastroenterol Hepatol. 2003;18:595-598.
Yu SC, et al. Treatment of pyogenic liver abscess: prospective randomized comparison of catheter drainage and needle aspiration. Hepatology. 2004;39:932-938.
Zaleznik DF, Kasper DL. Intra-abdominal abscesses. In: Lamont, JT, editor. Gastrointestinal Infections: Diagnosis and Management. New York: Marcel Dekker; 1997:397.
Zaleznik DF, Kasper DL. Intra-abdominal infections and abscesses. In: Fauci, AS, et al. Harrison’s Principles of Internal Medicine. 14th ed. New York: McGraw-Hill; 1998:792.
Zerem E, et al. Percutaneous treatment of symptomatic non-parasitic benign liver cysts: single-session alcohol sclerotherapy versus prolonged catheter drainage with negative pressure. Eur Radiol. 2008;18:400-406.
Zerem E, Hadzic A. Sonographically guided percutaneous catheter drainage versus needle aspiration in the management of pyogenic liver abscess. AJR Am J Roentgenol. 2007;189:W138-W142.
Zerem E, Jusufovic R. Percutaneous treatment of univesicular versus multivesicular hepatic hydatid cysts. Surg Endosc. 2006;20:1543-1547.