Chapter 5 Radiofrequency Thermal Ablation
Historical Background
Investigators in the 1960s and 1970s observed third-degree skin burns and saphenous nerve injuries after thermal ablation of the saphenous vein.1,2 Low-wattage, bipolar current, and specific electrode designs, coupled with algorithms governed by frequent sampling of wall temperature and impedance, were expected to mitigate thermal damage to adjacent tissues. The use of bipolar electrodes, which concentrate current density along minimal impedance paths between the poles, helped resolve these problems.3 In addition, early experiences demonstrated that procedural modifications were also needed to minimize complications and early failures.
First-Generation Device
In an industry-sponsored feasibility study: (1) the Restore catheter (VNUS Medical Technologies, Inc., Sunnyvale, CA) induced a short subvalvular constriction to improve the competence of valve leaflets and (2) the Closure catheter applied resistive heating over long vein lengths to cause maximum wall contraction for permanent obliteration.4 Treatment with Restore catheters resulted in recurrent or persistent reflux in 81% of patients followed up for 6 to 12 months. Treatment with Closure catheters resulted in a 6% recurrent reflux rate and a 4% incidence of recurrent varicosities at a mean follow-up of 4.7 months. The authors concluded that treatment with Closure catheters was an effective, less invasive option than saphenous stripping, with complications and early failures that could be mitigated through further procedural modifications.
In 1999, VNUS Medical Technologies, Inc. (Sunnyvale, CA) received approval from the U.S. Food and Drug Administration (FDA) to market and sell a new device for closure of incompetent saphenous veins. The first-generation device, known as the Closure Procedure, used bipolar electrodes mounted on the end of a catheter to deliver radiofrequency (RF) energy to the inner vein wall. The catheter’s collapsible bipolar electrodes included a temperature sensor that provided feedback to a dedicated RF generator. When deployed, the electrodes made direct contact with the endoluminal surface of the vein wall, and RF energy was delivered. The resistive effects of the vein wall tissue caused conversion of RF energy into heat. The principal mechanism of RF ablation has been demonstrated in animal studies.5 Vein wall collagen contraction in response to thermal energy causes immediate vein wall thickening and reduction in the lumen diameter. Endothelial destruction causes an inflammatory response, which results in fibrosis and permanent vein occlusion.
The thermal effect on the vein wall is directly related to both the treatment temperature and the treatment time, the latter being a function of catheter pullback speed. The treatment protocol called for a treatment temperature of 85 °C at a pullback speed of 3 cm/min. The thermal effect produces sufficient collagen contraction to occlude the lumen while limiting heat penetration to perivenous tissue.6,7 To assess the potential of perivenous tissue damage, the adventitial temperature was recorded in an in vitro model.8 With the standard treatment protocol, the average peak adventitial temperature was 64.4° C and usually lasted for approximately 10 seconds at any given position along the length of the vein. Peak adventitial temperature was decreased to 51.3° C in the presence of a 2-mm perivenous saline layer.
Second-Generation Device (VNUS ClosureFAST)
The linear endovenous energy density (LEED) is frequently used to compare energy dosing in endovenous procedures. With the first-generation (bipolar) RF device, the catheter pullback velocity had to be slow enough to allow resistive heating of the vein wall to a target temperature of 85 °C. Measurements of the delivered energy dose to the vein were not displayed to the operator because the power delivered by the generator was subject to regulation by a feedback loop to maintain a constant temperature of 85° C. With CLF, the temperature is kept stable at 120° C during a 20-second treatment cycle. At the saphenofemoral junction (SFJ), two cycles of RF energy are delivered, averaging an LEED of 116.2 ± 11.6 J/cm for the first 7 cm of vein juxtaposed to the SFJ to ensure good vein closure at this critical site.9 Distal to the SFJ, 68.2 ± 17.5 J/cm is delivered to each 7-cm treatment site. Thus, this aggressive “double energy cycle” at the zone of the SFJ is supported by Almeida and Raines’ retrospective analysis.10
Proebstle reported outcomes following CLF in early 2008. The occlusion rate following segmental RFA was 99.6% at 2 years, and 70% of treated patients did not require any analgesia postprocedure.9
Quality-of-Life Changes
Studies of quality of life are scarce, but significant improvements in disease-specific quality-of-life following RFA were reported in the EVOLVeS study, using the CIVIQ-2 questionnaire.11,12 Moreover, these quality-of-life statistics were improved compared to patients treated with traditional venous surgery.
Etiology and Natural History of Disease
Treatment Efficacy
Duplex ultrasound examination has significantly advanced our understanding of venous disease and provides both anatomic and pathophysiologic information. The morphologic and hemodynamic outcomes following RF ablation have been described in detailed ultrasound studies by Pichot et al.13 The pathologic sequelae of a treated vein are reflected by its sonographic progression. Occluded veins were initially hypoechogenic compared with the surrounding tissue and gradually evolved into hyperechogenic and eventually isoechogenic presentations, indicating a healing process. Approximately 60% of veins were hypoechogenic and 40% were hyperechogenic at 1 week. By 6 months, they became either hyperechogenic or isoechogenic.13 Sonographic disappearance of the saphenous vein, the desired endpoint, was observed by Weiss and Weiss in 90% of limbs at 2 years.14
Failures
Incomplete ablation, either segmental or total length of the vein, constitutes anatomic failure. Veins that are patent after treatment represent initial incomplete treatment or subsequent recanalization. Regardless of the presence of an anatomic failure, clinically, symptom improvement was often demonstrated in patients reported in the registry.15
Four types of SFJ morphologies were identified after RF ablation15–17: J-1, defined as complete SFJ obliteration with no SFJ flow; J-2, defined as patent SFJ tributaries draining toward the femoral vein with (J-2b) or without (J-2a) a short patent saphenous stump; and J-3, defined as terminal great saphenous vein (GSV) competence with normal antegrade flow coming from both tributaries and the saphenous vein above a limited GSV obliteration. Two years after RF ablation, the most common findings were either complete SFJ obliteration or a 5-cm or smaller patent terminal stump connecting prograde tributary flow through the SFJ, accounting for approximately 90% of the limbs treated.15,17
The clinical significance of a short patent SFJ stump was analyzed in a study by Merchant et al.15 A total of 319 limbs in the Clinical Registry were followed at 1 week, 6 months, 1 year, and 2 years, with 2-year data available for 121 limbs. Comparison of symptom improvement and varicose vein absence demonstrated no statistically significant differences between patients with complete SFJ obliteration and those with a short patent SFJ stump at any follow-up time point.
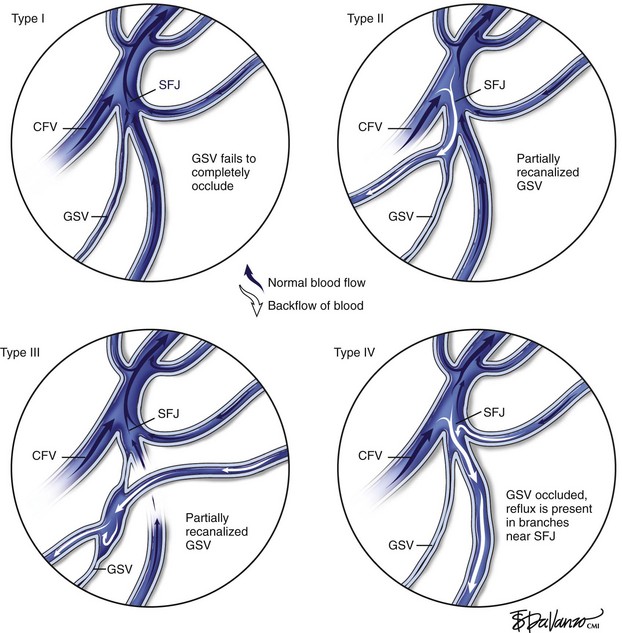
Merchant and Pichot18 categorized anatomic failure after an RF ablation procedure into three types. Type I failure (nonocclusion) refers to a vein that fails to occlude because of suboptimal technique, such as a rapid pullback speed resulting in an insufficient delivery of thermal dose. It has also been observed that in a very small percentage of patients, veins may be nonresponsive to thermal ablation; it has been postulated that the collagen structure might be different in these patients. Of veins that recanalized (Type II failure), 23% were associated with either tributary or perforator incompetence, accounting for 70% of the total anatomic failures.
On the other hand, Type II and Type III failures were risk factors for varicose vein recurrence. Type II failure patients were 3.8 times and Type III failures were 4.0 times as likely to develop varicose vein recurrence compared to patients with anatomic success. Type I failure did not reach statistical significance in this analysis. One possible explanation is lack of follow-up. Most patients had follow-up of less than 3 years. Further, some patients may have been treated with other methods and lost to follow-up. In this setting, the impact of early failure on varicose vein recurrence may not have been identified. Surveillance monitoring, early recognition of anatomic failure, and taking further corrective action that may include RF ablation retreatment may prevent or reduce varicose vein recurrence. However, it should be recognized that disease progression is likely to play a major role in Type III failure and may also account for some of Type II failure. This may contribute to an increase in varicose vein recurrence at 4 and 5 years (Fig. 5-1).
Pearls and Pitfalls
Procedural Technique
The VNUS Closure System includes a computer-controlled RF generator and disposable catheters. The catheters incorporate a central lumen for both fluid infusion and the option of using an over-the-wire approach during catheter placement. Detailed procedure techniques have been described elsewhere14,19,20 and in Chapter 4.
Tumescent Anesthesia
After final positioning of the catheter tip, the electrodes are deployed. Further vein exsanguination is achieved by putting the patient in the Trendelenburg position and applying external compression. On initiation of RF energy delivery to the electrodes, the catheter is slowly withdrawn while maintaining the treatment temperature at 85° C. The feedback mechanism of the system allows controlled energy delivery and appropriate monitoring of the procedure. With the CLF system, the heating element has no electrodes and the treatment temperature is 120° C (procedure details are given in Chapter 4).
Potential Hazards and Adverse Events
As with all endovenous interventions, RF ablation may be associated with technical difficulties in cannulation, guidewire placement, and catheter advancement. These issues are likely to resolve with increasing experience and familiarity with the equipment and technique. Complications, including DVT, skin burns, superficial thrombophlebitis, neuralgia, and bruising, have been reported, but the incidence of these problems appears low. In one study, DVT developed in 12 of 73 limbs (16%) within 30 days of RFA,21 but the incidence of DVT in the majority of studies is in the range of 1%.
Of the 1006 patients recorded in the Closure International Registry, the reported complications were DVT (0.9%), phlebitis (2.9%), and skin burn (1.2%), although the majority of burns occurred in patients who had not received perivenous tumescent anesthesia.18
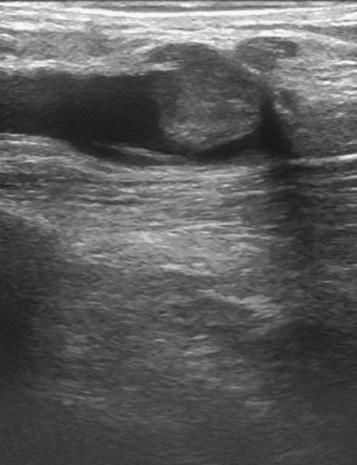
Massive DVT after thermal ablation is almost unheard of, but the surgeon may see a small thrombus extension on the postoperative ultrasound. Although they may look quite alarming on ultrasound imaging, they are usually about 1 cm in size. Anecdotally, these lesions are usually benign and are self-limiting. They are rarely symptomatic and they rarely propagate (Figs. 5-2 through 5-6).
Small Saphenous Vein and Other Veins
In addition to the GSV, RF ablation has also been used to treat small saphenous vein (SSV) and anterior accessory saphenous vein (AASV) incompetence in clinical practice. In this series, 4.3% veins treated were SSVs and 1.3% were AASVs. Although there were not enough samples and follow-up to demonstrate their long-term efficacy, one would not expect a dramatic difference between these treatments and GSV treatment. In this series, no serious adverse event such as motor nerve damage was reported with SSV treatment. The paresthesia rate in the SSV group was 8.9% at 1 week and 9.5% at 6 months; this is similar to the results of GSV treatment. The technical aspects of perivenous tumescent infiltration, catheter tip placement, and patient response monitoring during the procedure demand special attention with SSV treatment to protect the sural nerve and other surrounding nerves. When these elements were applied to SSV procedures, the paresthesia incidence dropped to as low as 0.3% (1 of 30) in one center.11 As has already been emphasized, tumescent infiltration can significantly decrease complications of this procedure.
Treatment efficacy of RF ablation on large veins was also analyzed by Merchant et al.22 There were 39 veins with a diameter greater than 12 mm (maximum, 24 mm) in the registry. Vein occlusion rates were 97.4% within 1 week and 96.2% at 6 months and 1 year.
Comparative Effectiveness of Existing Treatments
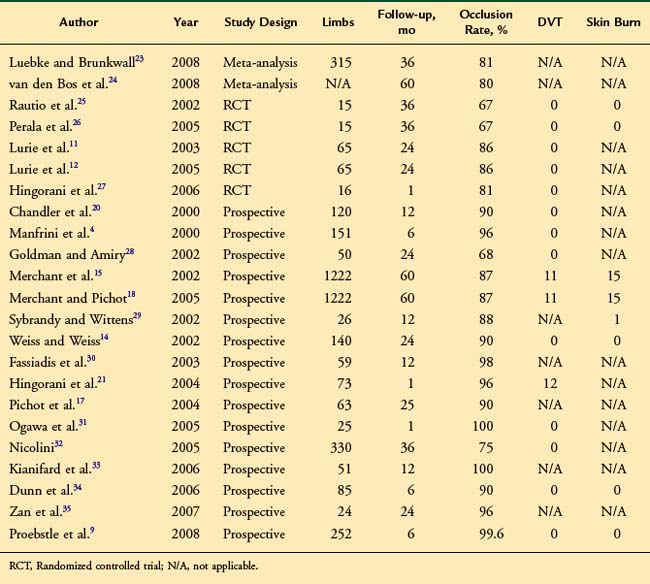
First-generation RF devices had marginally higher recanalization rates than EVL, but the new ClosureFAST catheter results rival those of EVL at about 98% primary closure. The literature database is populated mostly with first-generation device studies. Reported vein closure rates vary between 67% and 100%* (Table 5-1). In a recent meta-analysis, the early technical success following RF ablation was found to be 89% (3 months), reducing to 80% after 5 years.24 These values compared favorably to those for traditional surgery and foam sclerotherapy but were lower than those for EVL ablation. By far, the largest patient series treated by RF ablation was published in 2005 and reported 5-year clinical and anatomic outcomes following RF ablation in 1006 patients (1222 limbs).18 Data were collected from a prospective international registry and occlusion rates of 87.2% were seen at 5 years. Significant improvements in pain, fatigue, and edema were seen up to 5 years; interestingly, these improvements were present despite recurrent truncal reflux. Recurrent varicosities were seen in 27% of patients at 5 years, and anatomic failure of RF ablation was an independent risk factor for varicosity recurrence.18 In randomized studies, recurrent varicosities were seen in 48 of 217 (22%) of patients at 4 years.23
A direct comparison of postoperative recovery endpoints between RF ablation and EVL was conducted in the RECOVERY trial. Results showed reduced pain and ecchymosis scores after CLF.36
Four randomized controlled trials have compared endovenous RF ablation to surgical vein stripping. In the first, Rautio et al. randomized 28 patients to either RF ablation or vein stripping and reported significantly less postoperative pain, fewer postoperative analgesia requirements, and faster recovery in the RF group.37 The second trial, called the EVOLVeS study, was a multicenter, prospective, randomized study comparing quality-of-life factors between RF ablation and vein stripping. In all outcome variables, RF ablation demonstrated superior results compared to vein stripping: faster recovery, less postoperative pain, fewer adverse events, and superior quality-of-life scores.11 EVOLVeS patients seen at 2 years’ follow-up demonstrated virtually identical treatment results between RF ablation and vein stripping, with 91% versus 92% of limbs free of reflux, respectively. In addition, quality-of-life and pain scores were significantly better (p < .05) at 2 years for RF ablation over vein stripping, demonstrating a lasting benefit for patients.12 A third trial compared RF ablation to invaginated stripping and to cryostripping in a single-center trial with 20 patients in each treatment arm. RF ablation showed significantly better quality-of-life score improvement, less pain, and superiority in patient return to normal activity and work.38 The fourth comparative trial involved patients with recurrent GSV reflux after a previous vein stripping. RF ablation treatment resulted in better scores related to bruising and procedure times.27
Vein clinical severity scores have been previously reported with endovenous RF ablation. In the EVOLVeS trial of RF versus vein stripping, mean Venous Clinical Severity Scores were reduced from an average pretreatment score of 4.8 to 2.5 at 3 weeks after treatment.29
Meta-analysis
This meta-analysis of randomized controlled trials reviews the current evidence base, comparing open and endovascular treatment of varicose veins. Systematic review of studies reporting duplex scan follow-up after open surgical, laser (endovenous laser therapy [EVLT]), or radiofrequency (VNUS Closure device; VNUS Medical Technologies Inc.) treatment of refluxing GSVs was completed. Primary outcome measures were occlusion and complication rates and time to resume work. No significant difference were found in recurrence rates at 3 months between open surgery and EVLT (risk ratio [RR] 2.19; 95% confidence interval [CI] 0.99-4.85, p = .05) or VNUS device (RR 7.57; 95% CI 0.42-136.02). Return to work is significantly faster after VNUS device (by 8.24 days; 95% CI 10.50-5.97) or EVLT (by 5.02 days; 95% CI 6.52-3.52). Endovascular treatment of varicose veins is safe and effective and offers the significant advantage of rapid recovery.39
1 Politowski M, Zelazny T. Complications and difficulties in electrocoagulation of varices of the lower extremities. Surgery. 1966;59:932-934.
2 Watts GT. Endovenous diathermy destruction of internal saphenous. Br Med J. 1972;4:53.
3 Pearce JA. Electrosurgery. New York: John Wiley & Sons; 1986.
4 Manfrini S, Gasbarro V, Danielsson G, et al. Endovenous management of saphenous vein reflux. J Vasc Surg. 2000;32:330-342.
5 Weiss RA. RF-mediated endovenous occlusion. In: Weiss RA, Feied CF, Weiss MA, editors. Vein Diagnosis and Treatment: A Comprehensive Approach. New York: McGraw-Hill Medical Publishing Division; 2001:211-221.
6 Weiss RA. Comparison of endovenous radiofrequency versus 810 nm diode laser occlusion of large veins in an animal model. Dermatol Surg. 2002;28:56-61.
7 Goldman MP, Mauricio M, Rao J. Intravascular 1320-nm laser closure of the great saphenous vein: a 6- to 12-month follow-up study. Dermatol Surg. 2004;30:1380-1385.
8 Zikorus AW, Mirizzi MS. Evaluation of setpoint temperature and pullback speed on vein adventitial temperature during endovenous radiofrequency energy delivery in an in-vitro model. Vasc Endovascular Surg. 2004;38:167-174.
9 Proebstle TM, Vago B, Alm J, et al. Treatment of the incompetent great saphenous vein by endovenous radiofrequency powered segmental thermal ablation: first clinical experience. J Vasc Surg. 2008;47:151-156.
10 Almeida JI, Raines JK. Radiofrequency ablation and laser ablation in the treatment of varicose veins. Ann Vasc Surg. 2006;20:547-552.
11 Lurie F, Creton D, Eklof B, et al. Prospective randomized study of endovenous radiofrequency obliteration (Closure) versus ligation and stripping in a selected patient population (EVOLVeS study). J Vasc Surg. 2003;38:207-214.
12 Lurie F, Creton D, Eklof B, et al. Prospective randomised study of endovenous radiofrequency obliteration (Closure) versus ligation and vein stripping (EVOLVeS): two-year follow-up. Eur J Vasc Endovasc Surg. 2005;29:67-73.
13 Pichot O, Sessa C, Chandler JG, et al. Role of duplex imaging in endovenous obliteration for primary venous insufficiency. J Endovasc Ther. 2000;7:451-459.
14 Weiss RA, Weiss MA. Controlled radiofrequency endovenous occlusion using a unique radiofrequency catheter under duplex guidance to eliminate saphenous varicose vein reflux: a 2-year follow-up. Dermatol Surg. 2002;28:38-42.
15 Merchant RF, DePalma RG, Kabnick LS. Endovascular obliteration of saphenous reflux: a multicenter study. J Vasc Surg. 2002;35:1190-1196.
16 Pichot O, Sessa C, Chandler JG, et al. Role of duplex imaging in endovenous obliteration for primary venous insufficiency. J Endovasc Ther. 2000;7:451-459.
17 Pichot O, Kabnick LS, Creton D, et al. Duplex ultrasound scan findings two years after great saphenous vein radiofrequency endovenous obliteration. J Vasc Surg. 2004;39:189-195.
18 Merchant RF, Pichot O. Long-term outcomes of endovenous radiofrequency obliteration of saphenous reflux as a treatment for superficial venous insufficiency, for the Closure Study Group. J Vasc Surg. 2005;42:502-509.
19 Goldman MP, Weiss RA, Bergan JJ. Varicose veins and telangiectasias. Circulation. 1999;12:219-223.
20 Chandler JG, Pichot O, Sessa C, et al. Defining the role of extended saphenofemoral junction ligation: a prospective comparative study. J Vasc Surg. 2000;32:941-953.
21 Hingorani AP, Ascher E, Markevich N, et al. Deep venous thrombosis after radiofrequency ablation of greater saphenous vein: a word of caution. J Vasc Surg. 2004;40:500-504.
22 Merchant RF, Pichot O, Mayers KA. Four-year follow-up on endovascular radiofrequency obliteration of great saphenous reflux. Dermatol Surg. 2005;31:129-134.
23 Luebke T, Brunkwall J. Systematic review and meta-analysis of endovenous radiofrequency obliteration, endovenous laser therapy, and foam sclerotherapy for primary varicosis. J Cardiovasc Surg (Torino). 2008;49:213-233.
24 van den Bos R, Arends L, Kockaert M, et al. Endovenous therapies of lower extremity varicosities: a meta-analysis. J Vasc Surg. 2009;49:230-239.
25 Rautio T, Ohinmaa A, Perala J, et al. Endovenous obliteration versus conventional stripping operation in the treatment of primary varicose veins: a randomized controlled trial with comparison of the costs. J Vasc Surg. 2002;35:958-965.
26 Perala J, Rautio T, Biancari F, et al. Radiofrequency endovenous obliteration versus stripping of the long saphenous vein in the management of primary varicose veins: 3-year outcome of a randomized study. Ann Vasc Surg. 2005;19:669-672.
27 Hinchcliffe RJ, Ubhi J, Beech A, et al. A prospective randomised controlled trial of VNUS closure versus surgery for the treatment of recurrent long saphenous varicose veins. Eur J Vasc Endovasc Surg. 2006;31:212-218.
28 Goldman MP, Amiry S. Closure of the greater saphenous vein with endoluminal radiofrequency thermal heating of the vein wall in combination with ambulatory phlebectomy: 50 patients with more than 6-month follow-up. Dermatol Surg. 2002;28:29-31.
29 Sybrandy JEM, Wittens CHA. Initial experiences in endovenous treatment of saphenous vein reflux. J Vasc Surg. 2002;36:1207-1212.
30 Fassiadis N, Holdstock J, Whiteley MS. Endoluminal radiofrequency ablation of the long saphenous vein (VNUS Closure): a minimally invasive management of varicose veins. Minim Invasive Ther Allied Technol. 2003;12:91-94.
31 Ogawa T, Hoshino S, Midorikawa H, et al. Clinical results of radiofrequency endovenous obliteration for varicose veins. Surg Today. 2005;35:47-51.
32 Nicolini P. Treatment of primary varicose veins by endovenous obliteration with the VNUS closure system: results of a prospective multicentre study. Eur J Vasc Endovasc Surg. 2005;29:433-439.
33 Kianifard B, Holdstock JM, Whiteley MS. Radiofrequency ablation (VNUS Closure) does not cause neovascularisation at the groin at one year: results of a case controlled study. Surgeon. 2006;4:71-74.
34 Dunn CW, Kabnick LS, Merchant RF, et al. Endovascular radiofrequency obliteration using 90 degrees C for treatment of great saphenous vein. Ann Vasc Surg. 2006;20:625-629.
35 Zan S, Contessa L, Varetto G, et al. Radiofrequency minimally invasive endovascular treatment of lower limbs varicose veins: clinical experience and literature review. Minerva Cardioangiol. 2007;55:443-458.
36 Almeida J, Kaufman J, Göckeritz O, et al. Radiofrequency endovenous ClosureFAST versus laser ablation for the treatment of great saphenous reflux: a multicenter, single-blinded, randomized study (RECOVERY study). J Vasc Interv Radiol. 2009;20:752-759.
37 Rautio T, Ohinmaa A, Perälä J, et al. Endovenous obliteration versus conventional stripping operation in the treatment of primary varicose veins: a randomized controlled trial with comparison of costs. J Vasc Surg. 2002;35:958-965.
38 Stötter L, Schaaf I, Bockelbrink A, et al. Radiowellenobliteration, invaginierendes oder Kryostripping. Welches Verfahren belastet den Patienten am wenigsten? Phlebologie (German). 2005;34:19-24.
39 Brar R, Nordon IM, Hinchliffe RJ, et al. Surgical management of varicose veins: meta-analysis. Vascular. 2010;18:205-220.