Chapter 85C Radiofrequency ablation for liver tumors
Overview
Hepatic resection and transplantation are the only curative options for patients with liver metastases or primary liver tumors (see Chapter 80, Chapter 81A, Chapter 81B, Chapter 81C, Chapter 97A, Chapter 97B, Chapter 97C, Chapter 97D, Chapter 97E ). Unfortunately, resection is possible in only about 20% of patients; most hepatic malignancies are surgically inaccessible or are associated with a large tumor burden or inadequate hepatic reserve. Liver transplantation for hepatocellular carcinoma (HCC) is limited by a shortage of donor organs (see Chapter 97D). Patients with unresectable disease may be candidates for systemic therapy (see Chapter 88), local ablative techniques (percutaneous ethanol injection, microwave tumor coagulation, interstitial laser photocoagulation, cryosurgical ablation, or radiofrequency ablation [RFA]; see Chapter 85A, Chapter 85B, Chapter 85D ), or hepatic-directed therapy (hepatic artery ligation, chemoembolization, hepatic artery perfusion; see Chapters 83, 86, and 89).
Over the past decade, multiple clinical trials have evaluated RFA for the treatment of primary liver tumors and liver metastases. Animal experiments in the early 1990s determined the size and shape of ablations that could be achieved in the liver (Rossi et al, 1990). Rossi and colleagues (1996) then demonstrated the effectiveness of RFA for the treatment of HCC in humans. The Food and Drug Administration (FDA) approved RFA for generic tissue ablation in 1996 and for ablation of unresectable hepatic metastases in 2000. Since then, randomized trials have been performed in early HCC that demonstrate equivalent survival to resection (Lu et al, 2006), but prospective data in colorectal liver metastases are limited.
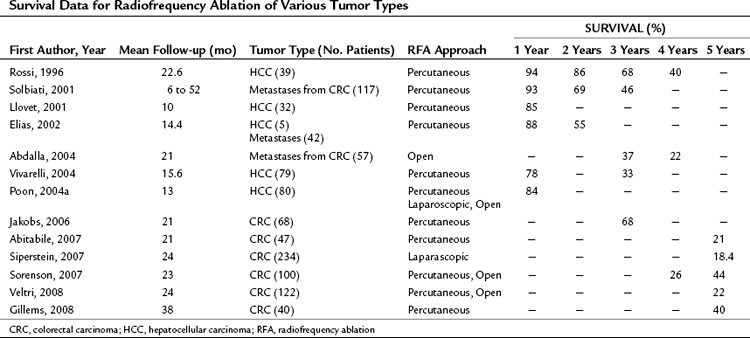
RFA destroys tumor by generating heat within a lesion. During RFA, a high-frequency alternating current changes the direction of ions around an alternating electrode charge. This creates frictional energy and heat conduction. As tissue temperature increases above 45° C, loss of cellular structure and protein denaturing result in tumor cell death. RFA can be performed in the operating room via celiotomy or laparoscopy, or it may be done in the radiology suite by a percutaneous approach. It can be used with other modalities of liver-directed therapy, such as resection and hepatic artery perfusion, and it can be used in conjunction with systemic therapy for other sites of metastatic disease. RFA continues to evolve with the introduction of new technology that increases the field of ablation and simplifies the technique. Long-term survival data have been reported in both HCC and colorectal hepatic metastases (Table 85C.1). A recently published position statement by the Society of Interventional Radiology (Gervais et al, 2009) describes four categories of patients that are preferred candidates for RFA: those with 1) inadequate liver function, 2) comorbid conditions, 3) anatomic distribution, and 4) for local tumor control as a bridge to transplantation.
Radiofrequency Ablation Technology
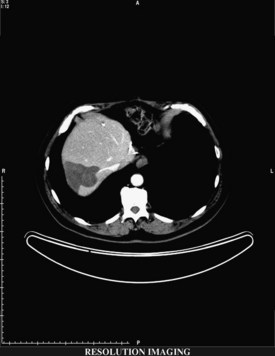
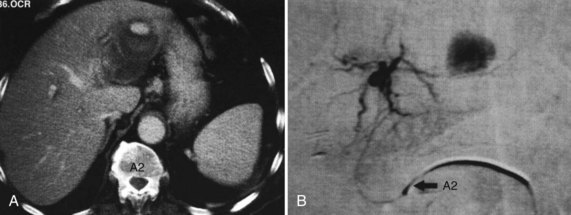
RFA destroys tissue by generating enough heat to denature cell protein and cause cell death (Fig. 85C.1), which can occur instantaneously at 60° C, a temperature achieved by all RFA devices. During RFA an electrical generator produces a current that alternates directions rapidly. Charged molecules in the tissue follow the direction of the changing current: this results in friction, which produces heat. The current around the ablation electrode creates a relatively uniform zone of conductive heat that radiates out from the electrode. If tissue impedance is low, an expanding spherical zone of ablated tissue is created. As the temperature rises, tissue charring and desiccation occur. Also, nitrogen gas forms that can be seen on ultrasound (US) as air bubbles in the tissue. Both of these events raise tissue impedance and inhibit heat conduction, thereby inhibiting further tissue ablation. The spherical size of the ablated tissue is proportional to the square of the RF current (RF power density). It is also dependent on the size of the electrode and the duration of the applied energy. The RF power density decreases in proportion to the square of the distance from the electrode, resulting in a rapid decrease in tissue temperature with increasing distance from the electrode (Strasberg & Linehan, 2003). Changing technology strives to improve ablation zones by limiting impedance and increasing the RF power density.
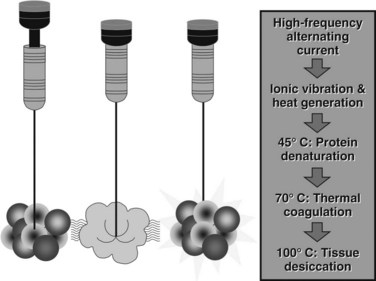
Three different RF electrodes and generators are commercially available. The Radiofrequency Interstitial Tissue Ablation (RITA; AngioDynamics, Latham, NY) and RadioTherapeutics (Boston Scientific, Natick, MA) systems use multiarray electrodes (Fig. 85C.2). The RITA system has a single electrode through which multiple tines are deployed. The ablation is performed at sequential steps of electrode deployment. Thermoprobes measure temperature in the ablation zone, and an automated adjustment is then made to maintain a steady temperature during the ablation. The electrode/generator system measures temperature and time as end points of ablation. This system was recently modified by the addition of saline perfusion to reduce impedance and increase the diameter of the ablation field to 7 cm. The RadioTherapeutics system automatically adjusts current according to tissue impedance; temperature is not measured. The multiarray umbrella-shaped electrode can ablate fields of about 4 cm. The Cool Tip Cluster Electrode, a single or three-pronged electrode, is manufactured by Covidien (Mansfield, MA); each electrode is internally perfused with cold saline, the current is sent in pulses, and the system measures impedance and temperature. Ablation zones can reach 5 cm in diameter with the three-pronged device.
Technical Aspects of Radiofrequency Ablation
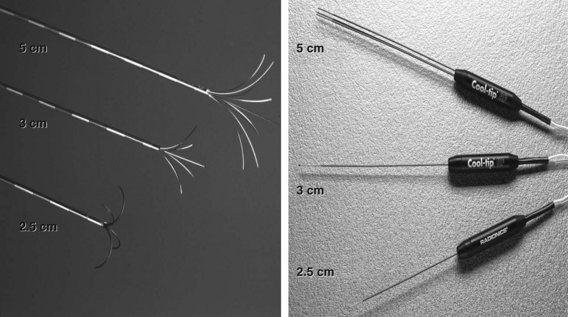
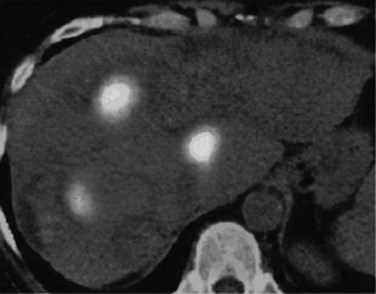
If extrahepatic and/or extensive intrahepatic disease has been detected, ablation is unlikely to be of any benefit. US during surgical or percutaneous RFA or CT during percutaneous RFA is used to guide the probe into the lesion, and the ablation is monitored by real-time US imaging of an expanding hyperechogenic zone (Fig. 85C.3). Depending on the maximal ablation size achievable by the probe, multiple overlapping ablations may be needed to destroy a tumor and produce a surrounding rim of necrosis (Fig. 85C.4). This can be technically challenging. US cannot reliably distinguish between ablated and normal tissue, so ablation should begin at the most posterior portion of the tumor. The probe then can be withdrawn in approximately 2-cm increments to create sequential overlapping zones of ablated tissue. If target temperatures are not reached, as may be the case for lesions near major vascular structures because of a heat-sink effect, the tines are withdrawn slightly or rotated approximately 45 degrees to increase the temperature in the region of ablation. Each ablation should create a 1-cm margin of treated normal parenchyma to ensure complete tumor destruction and reduce the risk of local recurrence. After ablation, as the RFA needle is withdrawn, the probe tract is cauterized to prevent hemorrhage and tumor seeding of the needle tract.
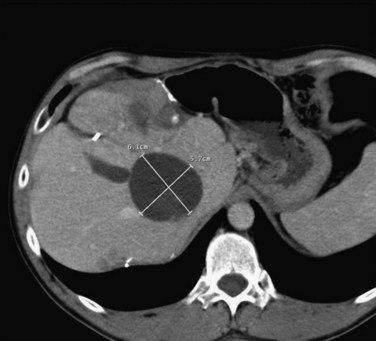
FIGURE 85C.4 Computed tomographic scan demonstrating large-volume ablation achieved with multiple overlapping ablation technique.
Percutaneous Radiofrequency Ablation
The percutaneous approach is somewhat limited, as some liver lesions can only be accessed by a surgical approach. Tumors near the periphery of the liver may be problematic because of the risk of thermal injury to other visceral structures such as the small bowel, stomach, or transverse colon; with an open technique, these structures can be retracted away from the liver. Other lesions can be approached percutaneously but raise technical challenges. For example, ablation of lesions at the dome of the liver might result in thermal injury to the diaphragm. Introduction of an air pocket can be used to separate the diaphragm and liver to avoid diaphragmatic thermal injury (Raman et al, 2004); this requires skill and may result in complications such as diaphragmatic injury or pneumothorax. Injection of saline or use of a balloon catheter has also been reported to separate structures from the liver to avoid thermal injury; however, clinically relevant diaphragmatic injuries are rare in our experience (Fig. 85C.5). Because a percutaneous approach is less invasive than a surgical approach, it may also be preferable in patients with comorbid conditions or poor medical status.
Open or Laparoscopic Radiofrequency Ablation
RFA via celiotomy or laparoscopy is performed in the operating room by a surgeon, while the patient is under general anesthesia. Unlike the percutaneous approach, a surgical approach allows visual inspection of the intraabdominal cavity for extrahepatic disease. Intraoperative US is used to evaluate the liver for intrahepatic lesions not seen on preoperative imaging. In a study of 308 patients undergoing laparoscopic RFA with intraoperative hepatic US, preoperative imaging failed to identify extrahepatic disease in 12% of patients and additional hepatic lesions in 33% of patients (Bilchik et al, 2000). Similarly, Siperstein and colleagues (2000) found that 30% of patients who underwent laparoscopic US of the liver prior to RFA had additional metastatic nodules not apparent on the preoperative CT scan. Larger liver lesions (>5 cm) are more easily treated with an operative approach than percutaneously. These lesions may require multiple overlapping ablations. Intraoperative occlusion of the portal triad can increase the ablation zone by decreasing the heat-sink effect created by nearby vessels. Furthermore, a surgical approach is sometimes necessary when other treatments are used in combination with RFA. For example, a surgical approach is ideal for patients who have bilobar liver metastases, who may benefit from concomitant liver resection. Larger liver lesions may be resected, and smaller lesions in the opposite lobe may be treated by RFA.
Monitoring and Follow-up
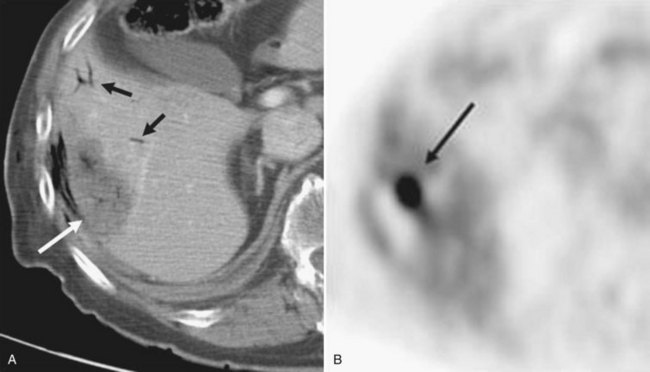
Contrast-enhanced CT scans obtained within a month and then at 3-month intervals after RFA should be compared with preprocedure CT scans. Liver necrosis from RFA can be seen as a nonenhancing hypodense region. When complete necrosis of the tumor is achieved, the resulting necrotic zone exceeds the original tumor size. Postprocedure CT scans are important to determine the success of RFA and to monitor the liver for evidence of recurrence. PET scans may also be particularly sensitive in the assessment of the adequacy of ablation and presence of marginal recurrence (Fig. 85C.6) or to distinguish viable tumor from areas of previous surgical or ablative therapy (Fig. 85C.7).
Current Practice
RFA has mainly been used for unresectable HCC or metastatic disease confined to the liver (see Chapters 80, 81A, and 81B). Tumors may be deemed unresectable based on size, number, location, or doubling time. Therefore, selection of patients is based initially on a thorough preoperative workup that includes imaging to evaluate for other sites of disease, prior response to systemic therapy, and the biology of the disease. If systemic therapy eliminates extrahepatic metastases, and if its effects prove durable over several months, RFA of hepatic metastases may be considered; however, this applies to a small subset of unique patients. Some patients will have resectable disease but limited hepatic reserve. For example, a patient with cirrhosis and HCC might not have enough hepatic reserve for tumor resection but would be able to tolerate RFA. In other patients, resection of larger lesions and RFA of smaller lesions can completely eradicate tumor while maintaining hepatic reserve. Finally, patients who have multiple comorbid factors may not be good candidates for an operation and may be best treated by a less invasive approach such as percutaneous RFA.
Radiofrequency Ablation of Colorectal Hepatic Metastases (See Chapter 81A)
Resection offers the only curative option in patients with colorectal hepatic metastases, with 5-year survival approaching 60% (Ahmad et al, 2007; Pawlik et al, 2008; Tomlinson et al, 2007). The improvement in survival is attributed to more effective systemic therapy and expanded criteria for defining resectability. Despite these, many patients are poor candidates for resection, and RFA provides a locoregional alternative. In a recent review of 21 studies (Stang et al, 2009), the median progression-free survival after RFA ranged between 6 and 13 months; the median and 5-year survival ranged between 24 and 59 months and 18% to 40%, respectively.
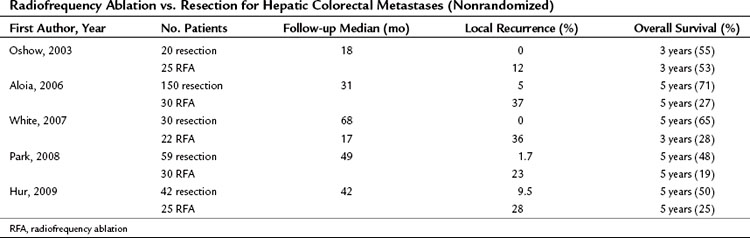
No randomized trials have compared RFA to resection in patients with colorectal hepatic metastases. One retrospective study of 358 patients compared resection, RFA plus resection, RFA alone, and laparotomy with biopsy (Abdalla et al, 2004); RFA was used for cure when complete resection was not possible. All patient-related and tumor-related factors known to influence outcome were similar among the groups. The rate of recurrence was 84% after RFA alone, 63% after RFA plus resection, and 52% after resection alone. Local recurrence in the area treated was more common after RFA plus resection (9%) than after RFA alone (5%) or resection alone (2%). The 3-year rate of overall survival was 73% after resection, 43% after RFA plus resection, and 37% after RFA alone. Patients who underwent RFA had a survival advantage over patients who underwent biopsy with or without chemotherapy. Four recent nonrandomized retrospective studies (Aloia et al, 2006; Hur et al, 2009; Park et al, 2008; White et al, 2007) demonstrated a survival benefit in patients undergoing resection versus RFA; the 5-year survival was between 48% and 71% for resection and between 19% and 27% for RFA (Table 85C.2). The survival analyses in these studies are limited by differences in patient selection, follow-up time, tumor type, and RFA approach, which make them difficult to compare.
Radiofrequency Ablation of Hepatocellular Carcinoma (See Chapter 80)
Locoregional methods for ablation of HCC include percutaneous ethanol ablation (PEI), chemoembolization, cryosurgery, and RFA, although RFA offers many advantages over PEI and cryoablation. In general, PEI requires multiple treatments and needle insertions to treat a single lesion, whereas RFA can treat the entire tumor with one or two probe insertions. Two randomized trials (Lencioni et al, 2003; Lin et al, 2005) have demonstrated superiority over PEI. The 2-year disease-free survival (DFS) was 96% in patients treated with RFA compared with 62% in patients treated with PEI (Lencioni et al, 2003). Similarly, Lin and colleagues (2005) demonstrated that in patients with HCC less than 3 cm in size, the 3-year DFS was superior in those treated with RFA. When compared with cryoablation, RFA is associated with less blood loss, less thrombocytopenia, and a shorter hospital stay (Bilchik et al, 2000). Cryoablation also carries the risk of a systemic inflammatory response, which can result in renal insufficiency, coagulopathy, hypotension, and death. This type of systemic response has not been reported with RFA.
Clinical trials of RFA for HCC have shown promising results (see Table 85C.1). Livraghi and colleagues (2008) demonstrated a local recurrence rate of only 2.8% at a median follow-up of 31 months for tumors smaller than 2 cm. Poon and colleagues (2004a) achieved a complete response rate of 91% for RFA of large (>3 cm) HCC and rates of local recurrence, distant intrahepatic recurrence, and extrahepatic metastasis were independent of tumor size.
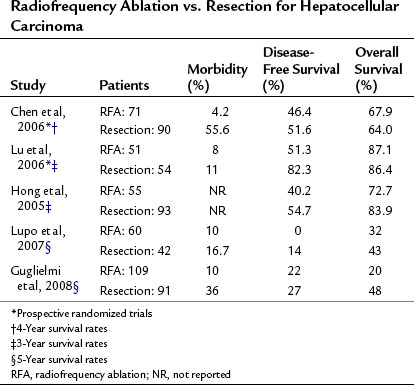
Radiofrequency Ablation Versus Resection for Hepatocellular Carcinoma
Only two prospective randomized controlled trials have been done of RFA versus resection for the treatment of primary liver carcinoma (see Chapter 80). Chen and colleagues (2006) randomized 180 patients with a solitary HCC smaller than 5 cm to either resection or percutaneous RFA. The 4-year overall survival was 68% in the RFA group and 64% in the resection group. Local recurrence rates were also similar, with less morbidity in the RFA group. Lu and colleagues (2006) randomized 105 patients with either a solitary HCC tumor smaller than 5 cm or fewer than three tumors less than 3 cm in size to either a partial hepatectomy or percutaneous RFA and found that 3-year survival rates were similar in both groups (Table 85C.3), but hospital stay and blood transfusions were significantly reduced in the RFA group.
Conflicting result have been reported in several nonrandomized studies (Hong et al, 2005; Lupo et al, 2007; Montorsi et al, 2005) that demonstrate therapeutic equivalence; others report a higher local recurrence rate and reduced survival in the RFA group (Vivarelli et al, 2004). This study was performed at two different centers; RFA was performed at one center and resection at the other. Overall and disease-free survivals were significantly better after resection; however, patients treated with RFA had more liver lesions and a higher CTP classification, both of which may have influenced outcome.
Guglielmi and colleagues (2008) also demonstrated that in HCC larger than 3 cm, significant improvement in survival was seen in patients undergoing surgical resection, but this did not translate to tumors smaller than 3 cm, nor did it apply to patients with multiple tumors.
Radiofrequency Ablation for Recurrent Hepatocellular Carcinoma
After resection or ablative therapies, most patients with HCC will succumb to progressive or recurrent liver disease. Hepatic resection is rarely feasible because of inadequate hepatic reserve, making RFA an effective therapeutic alternative. Taura and colleagues (2006) compared 610 patients who underwent a liver resection before and after 1990. The improved 5-year survival in the latter group (22% vs. 12%) was attributed to percutaneous RFA for intrahepatic recurrence. Similarly, Yang and colleagues (2006) demonstrated a survival benefit in 41 patients with recurrent HCC treated with percutaneous RFA. These studies suggest that RFA is effective in the management of recurrent HCC after resection, and RFA may be the treatment of choice for carefully selected patients with recurrent hepatic disease (Fig. 85C.8).
Radiofrequency Ablation as a Bridge to Transplantation (See Chapter 97D)
Many patients with HCC will succumb to liver failure because of a shortage of donors or will be removed from the transplant waiting list because of tumor progression; indeed, the dropout rate within a year has been reported as high as 38% (Yao et al, 2004). RFA has effectively been used as a bridge to transplantation. At a mean follow-up of 12.7 months, Lu and colleagues (2005) reported a dropout rate of only 5.8 months and 14% at a follow-up of 11.9 months (Brillet et al, 2006).
These encouraging data suggest that RFA is one modality that may be considered for prolonged waiting times. The impact on overall survival is less clear; however, one prospective study of patients with HCC awaiting transplantation undergoing RFA showed no survival benefit compared with the observation group (Porrett et al, 2006).
Tumor Response and Recurrence
CT or MRI is typically used to evaluate response rates (see Chapters 16 and 17); US does not accurately predict the extent of necrosis following RFA and should not be used to determine response. CT with contrast enhancement reveals a hypovascular ablated field with a rim of hypervascular inflammatory tissue; the rim enhancement area should disappear over several months time as inflammation resolves, and the treatment area should encompass the tumor and at least a 1-cm rim of normal tissue. Comparing preoperative imaging to postoperative imaging is important to determine that the tumor was adequately treated. Patients should receive serial CT scans after RFA to identify recurrences, which appear as irregular, nonenhancing areas on contrast CT.
Complete and Incomplete Response to Radiofrequency Ablation
Response rates vary from 48% to 98% across studies, in part because of differences in the size of treated lesions. Livraghi and colleagues (1999, 2000) evaluated percutaneous RFA in patients with HCC. The rate of complete response was 48% for tumors larger than 3 cm (2000), but it jumped to 90% for smaller tumors (1999), and the success rate was lower for infiltrating versus noninfiltrating HCCs. Similarly, Lencioni and colleagues (1998) found a statistically significant difference in response rates to percutaneous RFA for metastatic liver lesions larger than 3 cm (53%) versus those smaller than 3 cm (87%). Larger lesions typically require overlapping ablations, and areas of treated tumor may obscure US imaging of untreated tumor and result in higher treatment failures and recurrences.
The approach to RFA will also influence response rates (see Table 85C.1). Curley and colleagues (2000) reported that 6 of 76 patients who underwent percutaneous RFA had an incomplete response, whereas all 34 patients who underwent laparoscopic or open RFA had a complete response. This difference might be explained by better hepatic imaging with intraoperative US during laparoscopy or celiotomy than with transabdominal US during percutaneous RFA. Another possibility is that intraoperative probe positioning may be more accurate than percutaneous placement of the probe.
Tumor location adjacent to large vessels also influences response rates. Blood flow from large vessels will create a heat-sink effect that cools surrounding tissue and increases the temperature necessary for complete ablation. Large vessels are resistant to high temperatures that can damage surrounding tissue. Lu and colleagues (2002) found that porcine vessels smaller than 3 mm thrombosed or necrosed during RFA, but those larger than 5 mm were not affected. In another porcine model, vascular occlusion during RFA increased the volume of necrosis and prolonged the exposure of tissue to high temperatures (Chinn et al, 2001). De Baere and colleagues (2002) evaluated vascular inflow occlusion in patients undergoing RFA of tumors that were large (>35 mm) or near large blood vessels and found that balloon inflow occlusion significantly increased the area of ablation. These studies support the use of a Pringle maneuver to more effectively ablate tumors near major blood vessels and improve response rates. The use of RFA combined with hepatic artery embolization or chemoembolization is discussed in Chapter 83.
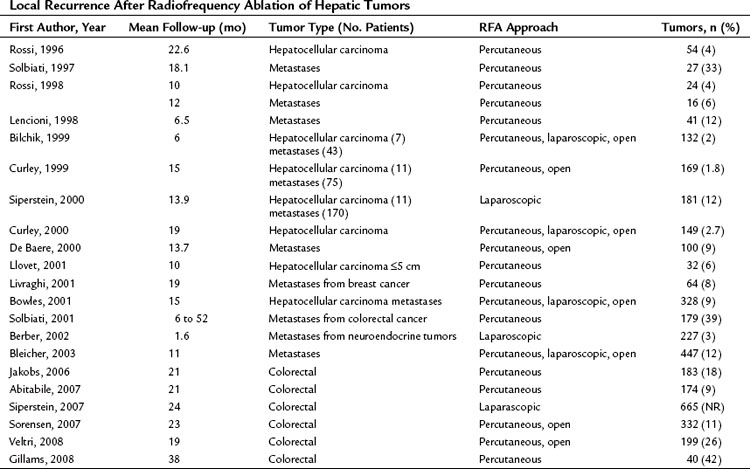
Rates of Recurrence at the Ablation Site
Recurrence rates are difficult to interpret across studies (Table 85C.4). Some authors report recurrences after complete ablation as determined by early postoperative CT or MRI, whereas others do not confirm complete response by postoperative imaging and report recurrences based on follow-up imaging. Investigators may report recurrence rates within the whole liver, rather than recurrence rates at the site of ablation, and the length of follow-up also impacts the recurrence rate. Bowles and colleagues (2001) evaluated 76 patients undergoing RFA of 328 tumors. Sixteen patients underwent repeated ablation for recurrences or new lesions, and 30 recurrences were reported at the site of a prior ablation. Patients with large tumors, tumor vascular invasion, and hepatic dysfunction had a statistically higher recurrence rate. Solbiati et al (2001) evaluated 117 patients undergoing percutaneous RFA of colorectal metastases; time to local recurrence and frequency of recurrences were influenced by lesion size. Similarly, we found that tumor size significantly influenced local recurrence of metastatic disease, independent of RFA technique (Wood et al, 2000; Bleicher et al, 2003). No study has shown that differences in technique influence the rate of recurrence after a complete response.
Complications of Radiofrequency Ablation
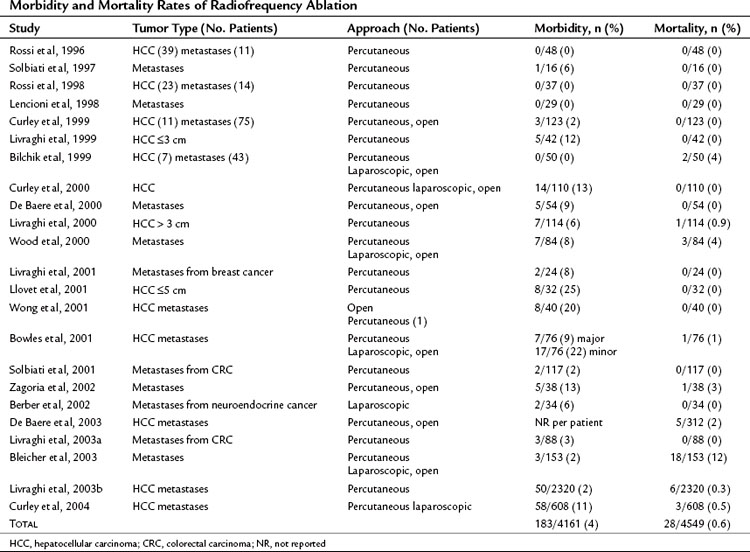
Reported morbidity and mortality rates associated with RFA (Table 85C.5) can be difficult to interpret, in part because technical approaches vary. Some investigators combine RFA with other treatments, such as liver resection, but addition of a second procedure may inflate the complication rate. Ablation of multiple tumors also increases the risk of complications such as bleeding or bile leak. Early studies often used multiple sequential RFAs for treatment of a single hepatic tumor, because the monopolar electrode gave a smaller thermal ablation field than the current cluster electrodes. The multiple ablations required to destroy larger tumors increased the potential for complications.
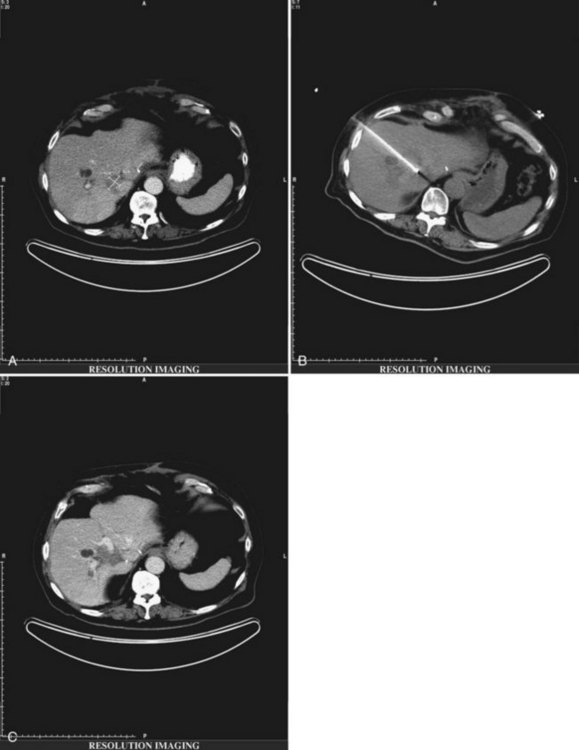
As illustrated by the following two cases, RFA should be undertaken only by skilled physicians able to identify and manage its complications and only at centers equipped with appropriate staff and equipment for acute care. Figure 85C.9 shows a bile duct injury caused by RFA of a colorectal cancer metastasis near the porta hepatis. The injury was treated with endoscopic retrograde cholangiopancreatography (ERCP) and biliary stenting. Figure 85C.10 shows a hepatic artery pseudoaneurysm attributed to RFA of liver metastases. This was successfully treated with embolization by a skilled interventionalist.
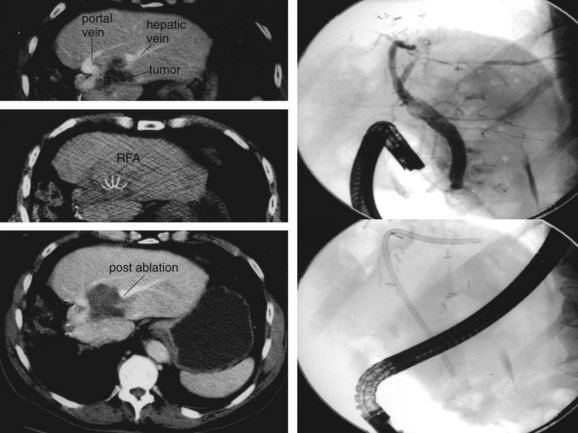
FIGURE 85C.9 Biliary injury following radiofrequency ablation treated with stent placement. RFA, radiofrequency ablation.
The best way to avoid potential complications of percutaneous RFA is to understand its technical limitations. A large multicenter study reported morbidity and mortality rates of 0.3% and 2.2%, respectively (Livraghi, 2003b). In this study, about 33% of all deaths and 10% of morbidities were associated with gastrointestinal thermal injury and perforation. Patients with prior abdominal surgeries resulting in adhesions and those with peripheral liver tumors had an increased risk for gastrointestinal injury during percutaneous ablation. The authors recommended consideration of open or laparoscopic RFA, instead of percutaneous RFA, for tumors within 1 cm of the liver edge adjacent to bowel.
Injury to the diaphragm is also a potential complication during percutaneous RFA, especially when tumors at the dome of the liver are treated. In an animal model, carbon monoxide was introduced into the peritoneal cavity to separate the diaphragm and the liver; although this reduced severe diaphragmatic injury during superficial hepatic RFA (Raman et al, 2004), the technique needs further clinical testing.
Abscess, one of the most frequent complications of RFA, typically occurs 1 week after ablation and requires percutaneous or surgical drainage (de Baere et al, 2003; Wood et al, 2000). Patients with bilioenteric anastomosis or biliary stenting appear to have a higher rate of abscess formation. All patients undergoing RFA should receive periprocedural antibiotics that cover coliforms as well as skin flora, and temperature and leukocyte counts should be followed after the procedure. Normally, low-grade fever and fatigue may occur immediately after RFA for up to 7 to 10 days, a so-called postablation syndrome; however, any persistent significant fever or elevation of white blood cell count should prompt CT imaging for possible hepatic abscess.
Reports of tumor seeding vary from 0.5% to 12% (de Baere et al, 2003; de Sio et al, 2001; Llovet et al, 2001), possibly reflecting differences in follow-up. Llovet and colleagues (2001) found that tumor seeding during RFA was related to subcapsular tumor location and poor tumor differentiation. Recent tumor biopsy, multiple needle insertions, and tumor hemorrhage during treatment may also increase the risk of needle-tract seeding. This complication can be avoided by limiting the number of needle insertions, angling the needle to traverse normal hepatic parenchyma prior to entering the tumor, and cauterizing the tract upon withdrawal of the needle. Nicoli and colleagues (2004) reported rapid diffusion of neoplastic cells after the creation of an arteriovenous fistula following RFA. The authors attributed seeding of tumor cells to the pressure gradient between the high-pressure tumor arteries and the low-pressure portal system; however, this is the only report of this complication in several thousand RFAs to date.
Injury to bile ducts during RFA can result in stenosis and proximal biliary dilation (see Chapter 42A, Chapter 42B ). Most physicians agree that tumors within 15 to 20 mm of a major bile duct should not be treated by RFA (Mulier et al, 2002); however, Elias and colleagues (2001) introduced intraductal cooling to prevent RFA-associated biliary stenosis. They infused cooled (4° C) Ringer’s lactate through a catheter after choledochotomy. Intraductal cooling was undertaken in 13 patients undergoing RFA of tumors within 6 mm of a central bile duct; one patient had a local recurrence, and one developed biliary stenosis (Elias et al, 2004). Biliary stenting also can prevent biliary injury during ablation of tumors near the bile ducts (Wood et al, 2000).
Abdalla EK, et al. Recurrence and outcomes following hepatic resection, radiofrequency ablation, and combined resection/ablation for colorectal liver metastases. Ann Surg. 2004;239:818-827.
Abitabile, et al. Radiofrequency ablation permits an effective treatment for colorectal liver metastasis. Eur J Surg Oncol. 2007;33:67-71.
Ahmad A, Chen SL, Bilchik AJ. Role of repeated hepatectomy in multimodal treatment of hepatic colorectal metastases. Arch Surg. 2007;142:526-532.
Aloia TA, et al. Solitary colorectal liver metastasis: resection determines outcome. Arch Surg. 2006;141:460-466. discussion 466-467
Berber E, Flesher N, Siperstein A. Laparoscopic radiofrequency ablation of neuroendocrine liver metastases. World J Surg. 2002;26:985-990.
Bilchik AJ, et al. Radiofrequency ablation: a minimally invasive technique with multiple applications. Cancer J Sci Am. 1999;5:356-361.
Bilchik AJ, et al. Cryosurgical ablation and radiofrequency ablation for unresectable hepatic malignant neoplasms. Arch Surg. 2000;135:657-664.
Bleicher RJ, et al. Radiofrequency ablation in 447 complex unresectable liver tumors: lessons learned. Ann Surg Oncol. 2003;10:52-58.
Bowles BJ, et al. Safety and efficacy of radiofrequency thermal ablation in advanced liver tumors. Arch Surg. 2001;136:864-869.
Brillet PY, et al. Percutaneous radiofrequency ablation for hepatocellular carcinoma before liver transplantation: a prospective study with histopathologic comparison. AJR Am J Roentgenol. 2006;186:S296-S305.
Chen MS, et al. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann Surg. 2006;243:321-328.
Chinn SB, et al. Effect of vascular occlusion on radiofrquency ablation of the liver: results in a porcine model. AJR Am J Roentgenol. 2001;176:789-795.
Curley SA, et al. Radiofrequency ablation of unresectable primary and metastatic hepatic malignancies: results in 123 patients. Ann Surg. 1999;230:1-8.
Curley SA, et al. Radiofrequency Ablation of hepatocellular cancer in 110 patients with cirrhosis. Ann Surg. 2000;232(3):381-391.
Curley SA, et al. Early and late complications after radiofrequency ablation of malignant liver tumors in 608 patients. Ann Surg. 2004;239:450-458.
De Baere T, Bessoud B, Dromain C. Percutaneous radiofrequency ablation of hepatic tumors during temporary venous occlusion. AJR Am J Roentgenol. 2002;178:53-59.
De Baere T, et al. Radiofrequency ablation of 100 hepatic metastases with a mean follow-up of more than 1 year. Am J Radiol. 2000;175:1619-1625.
De Baere T, et al. Adverse events during radiofrequency treatment of 582 hepatic tumors. Am J Radiol. 2003;181:695-700.
De Sio I, et al. Tumor dissemination after radiofrequency ablation of hepatocellular carcinoma. Hepatology. 2001;34:609-610.
Elias D, et al. Intraductal cooling of the main bile duct during intraoperative radiofrequency ablation. J Surg Oncol. 2001;76:297-300.
Elias D, et al. Percutaneous radiofrequency thermoablation as an alternative to surgery for treatment of liver tumour recurrence after hepatectomy. Br J Surg. 2002;89:752-756.
Elias D, et al. Intraductal cooling of the main bile ducts during radiofrequency ablation prevents biliary stenosis. J Am Coll Surg. 2004;198:717-721.
Gervais D, et al. Society of interventional radiology position statement on percutaneous radiofrequency ablation for the treatment of liver tumors. J Vasc Radiol. 2009;20:S342-S347.
Gillems, et al. Five year survival following radiofrequency ablation of small solitary hepatic colorectal metastases. J Vasc Interv Radiol. 2008;19:712-717.
Guglielmi A, et al. Radiofrequency ablation versus surgical resection for the treatment of hepatocellular carcinoma in cirrhosis. J Gastrointest Surg. 2008;12:192-198.
Hong SN, et al. Comparing the outcomes of radiofrequency ablation and surgery in patients with a single small hepatocellular carcinoma and well-preserved hepatic function. J Clin Gastroenterol. 2005;39:247-252.
Hur H, et al. Comparative study of resection and radiofrequency of solitary colorectal metastases. Am J Surg. 2009;197:728-736.
Jakobs, et al. Radiofrequency ablation of colorectal liver metastases; mid-term results in 68 patients. Anticancer Res. 2006;26:671-680.
Lencioni R, et al. Radio-frequency thermal ablation of liver metastases with a cooled-tip electrode needle: results of a pilot clinical trial. Eur Radiol. 1998;8:1205-1211.
Lencioni RA, et al. Small hepatocellular carcinoma in cirrhosis: randomized comparison of radiofrequency thermal ablation versus percutaneous ethanol injection. Radiology. 2003;228:235.
Lin SM, et al. Randomized controlled trial comparing percutaneous radiofrequency ablation, percutaneous ethanol injection and percutaneous ethanol injection and percutaneous acetic acid injection to treat hepatocellular carcinoma of 3 cm or less. Gut. 2005;54:1151-1156.
Livraghi T, et al. Small hepatocellular carcinoma: treatment with radio-frequency ablation versus ethanol injection. Radiology. 1999;210:655-661.
Livraghi T, et al. Hepatocellular carcinoma: radio-frequency ablation of medium and large lesions. Radiology. 2000;214:761-768.
Livraghi T, et al. Percutaneous radio-frequency ablation of liver metastases from breast cancer: initial experience in 24 patients. Radiology. 2001;220:146-149.
Livraghi T, et al. Percutaneous radiofrequency ablation of liver metastases in potential candidates for resection. Cancer. 2003;97:3027-3035.
Livraghi T, et al. Treatment of focal liver tumors with percutaneous radio-frequency ablation: complications encountered in a multicenter study. Radiology. 2003;226:441-451.
Livraghi T, et al. Sustained complete response and complete response and complication rates of the radiofrequency ablation of very early hepatocellular carcinoma in cirrhosis: is resection still the treatment of choice? Hepatology. 2008;47:82-89.
Llovet JM, et al. Increased risk of tumor seeding after percutaneous radiofrequency ablation for single hepatocellular carcinoma. Hepatology. 2001;33:1124-1129.
Lu DS, et al. Effect of vessel size on creation of hepatic radiofrequency lesions in pigs: assessment of the “heat sink” effect. AJR Am J Roentgenol. 2002;178(1):47-51.
Lu DS, et al. Percutaneous radiofrequency ablation of hepatocellular carcinoma as a bridge to liver transplantation. Hepatology. 2005;41:1130-1137.
Lu MD, et al. Surgical resection versus percutaneous thermal ablation for early stage hepatocellular carcinoma: a randomized clinical trial. Zhonghua Yi Xue Za Zhi. 2006;86:801-805.
Lupo L, et al. Single hepatocellular carcinoma ranging from 3 to 5 cm: radiofrequency ablation or resection? HPB (Oxford). 2007;9:429-434.
Montorsi M, et al. Survival and recurrences after hepatic resection or radiofrequency for hepatocellular carcinoma in cirrhotic patients: a multivariate analysis. J Gastrointest Surg. 2005;9:62-67.
Mulier S, et al. Complications of radiofrequency coagulation of liver tumours. Br J Surg. 2002;89:1206-1222.
Nicoli N, et al. A case of rapid intrahepatic dissemination of hepatocellular carcinoma after radiofrequency thermal ablation. Am J Surg. 2004;188:165-167.
Oshow, et al. Comparison of resection and radiofrequency ablation for treatment of solitary colorectal liver metastases. Br J Surg. 2003;90:1240-1243.
Park IJ, et al. Radiofrequency ablation for metachronous liver metastasis from colorectal cancer after curative surgery. Ann Surg Oncol. 2008;15:227-232.
Pawlik TM, Schulick RD, Choti MA. Expanding criteria for respectability of colorectal liver metastases. Oncologist. 2008;13:51-64.
Poon RT, et al. Effectiveness of radiofrequency ablation for hepatocellular carcinomas larger than 3 cm in diameter. Arch Surg. 2004;139:281-287.
Poon RT, et al. Radiofrequency ablation for subcapsular hepatocellular carcinoma. Ann Surg Oncol. 2004;11(3):281-289.
Porrett PM, et al. Lack of benefit of pretransplant locoregional hepatic therapy for hepato-cellular cancer in the curent MELD era. Liver Transpl. 2006;12:665-673.
Raman SS, et al. Minimizing diaphragmatic injury during radiofrequency ablation: efficacy of intraabdominal carbon dioxide insufflation. Am J Radiol. 2004;183:197-200.
Rossi S, et al. Thermal lesions induced by 480 kHz localized current field in guinea pig and pig liver. Tumori. 1990;76(1):54-57.
Rossi S, et al. Percutaneous RF interstitial thermal ablation in the treatment of hepatic cancer. AJR Am J Roentgenol. 1996;167:759-768.
Rossi S, et al. Percutaneous treatment of small hepatic tumors by an expandable RF needle electrode. AJR Am J Roentgenol. 1998;170:1015-1022.
Siperstein A, et al. Local recurrence after laparoscopic radiofrequency thermal ablation of hepatic tumors. Ann Surg Oncol. 2000;7:106-113.
Siperstein A, et al. Survival after radiofrequency ablation of colorectal liver metastases: 10 year experience. Ann Surg. 2007;246:559-565.
Solbiati L, et al. Percutaneous US-guided RF tissue ablation of liver metastases: results of treatment and follow-up in 16 patients. Radiology. 1997;202:195-203.
Solbiati L, et al. Percutaneous radio-frequency ablation of hepatic metastases from colorectal cancer: long-term results in 117 patients. Radiology. 2001;221:159-166.
Sorenson, et al. Radiofrequency ablation of colorectal liver metastases: long term survival. Acta Radiol. 2007;48:253-258.
Stang A, et al. A systematic review on the clinical benefit and role of radiofrequency ablation as treatment of colorectal liver metastases. Eur J Cancer. 2009;45:1748-1756.
Strasberg SM, Linehan D. Radiofrequency ablation of liver tumors. Curr Prob Surg. 2003;40:451-498.
Taura K, et al. Implication of frequent local ablation therapy for intrahepatic recurrence in prolonged survival of patients with hepatocellular carcinoma undergoing hepatic resection: an analysis of 610 patients over 16 years old. Ann Surg. 2006;244:265-273.
Tomlinson JS, et al. Actual 10 year survival after resection of colorectal liver metastases defines cure. J Clin Oncol. 2007;25:4575-4580.
White RR, et al. Rates and patterns of recurrence for percutaneous radiofrequency ablation and open wedge resection for solitary colorectal liver metastasis. J Gastrointest Surg. 2007;11:256-263.
Wood TF, et al. Radiofrequency ablation of 231 unresectable hepatic tumors: indications, limitations, and complications. Ann Surg Oncol. 2000;7:593-600.
Wong SL, et al. Radiofrequency ablation for unresectable hepatic tumors. Am J Surg. 2001;182:552-557.
Veltri, et al. Radiofrequency ablation of colorectal liver metastases: small size favorably predicts technique effectiveness and survival. Cardiovasc Intervent Radiol. 2008;31:948-956.
Vivarelli M, et al. Surgical resection versus percutaneous radiofrequency ablation in the treatment of hepatocellular carcinoma on cirrhotic liver. Ann Surg. 2004;240:102-107.
Yang W, et al. Radiofrequency ablation of recurrent hepatocellular carcinoma after hepatectomy: therapeutic efficacy on early-and late-phase recurrence. AJR Am J Roentgenol. 2006;186:S275-S283.
Yao FY, et al. Liver transplantation for hepatocellular carcinoma: lessons from the first year under the Model of End-Stage Liver Disease (MELD) organ allocation policy. Liver Transpl. 2004;10:621-630.
Zagoria RJ, et al. Complications of radiofrequency ablation of liver metastases. Am Surg. 2002;68:204-209.