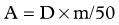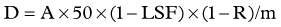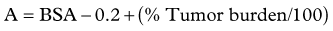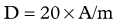Chapter 84A Radioembolization for liver tumors
Overview
Interventional oncology is a field that is establishing its role in the management of liver tumors. The techniques used in this field are targeted to the tumors to allow delivery of toxic chemotherapeutic, radiotherapeutic, and thermal doses with minimal toxicity to normal structures; this minimizes the systemic toxicity associated with these therapeutic options and allows delivery of doses to the tumor that would not be possible using systemic delivery methods. In this chapter, the general concepts associated with radioembolization and its specific use in different primary and secondary malignancies of the liver will be discussed (Table 84A.1)
Vascular Anatomy of the Liver and Its Tumors (See Chapter 1B)
Hepatic Artery
Unlike the normal hepatic parenchyma, liver tumors are supplied primarily by hepatic arterial blood and are hypervascular structures compared with the surrounding uninvolved parenchyma (see Chapter 86). Hepatic tumors may parasitize arterial blood flow from the arteries that supply segments adjacent to the segment the tumor is in and those of surrounding organs, such as the stomach. This relative arterial hypervascularity of the tumors with respect to the normal parenchyma has been the basic principle behind transarterial therapies for targeting liver tumors.
History of Radioembolization
External Radiation (See Chapter 84B)
The use of external radiation for liver tumors has traditionally held limited value because of the sensitivity of the normal hepatic parenchyma to the radiation dose (Ingold et al, 1965; Geschwind et al, 2004). A dose greater than 35 Gy has led to the development of a radiation-induced liver disease (RILD); a clinical syndrome of ascites, anicteric hepatomegaly, and elevation of liver enzymes. Conformal-beam therapy, which uses a three-dimensional approach rather than broad axial plane techniques, has been shown to minimize the toxicity to normal hepatic parenchyma (Dawson et al, 1999). However, even when using conformal-beam therapy, the radiation delivered to normal hepatic parenchyma limits the maximum dose that can be delivered to the tumor without compromising safety.
External-beam radiation had limitations in treating liver tumors in specific locations; this includes tumors near the dome of the liver, which carry the risk of exposing the lungs to radiation and increasing the risk of radiation pneumonitis, and tumors in the caudate lobe in close proximity to the porta hepatis, where risk of damage to the major biliary and vascular structures is a consideration. Dose fractionation, dose delivery to the tumor in fractions, is beneficial in targeting radiosensitive and radioresistant malignant cells at different sessions, but multiple treatments are required. The normal tissue complications probability (NTCP) is an important parameter when calculating radiation dose delivery (Dawson et al, 2002), but its use has been challenged (Langer et al, 1998). Respiratory movement must be considered to minimize the incidence of normal tissue complications, although recent advances in the technology of external-beam radiation have been shown to increase safety and efficacy of this technique, such as stereotactic body radiotherapy, proton radiotherapy, and carbon ion radiotherapy; these are establishing the role of external-beam radiotherapy in the management of liver tumors (Dawson & Guha, 2008; Fukumitsu et al, 2009; Nomiya et al, 2008).
Radioembolization
Radioembolization was first studied by Nolan and Grady (1969) using yttrium-90 oxide (90Y2O3) contained in a metal particle 50 to 100 µm in size. Their study consisted of a small number of patients but showed a favorable response, observed by the reduction in size of palpable masses. The next 90Y study was published in 1982 by Mantravadi and colleagues and studied the effect and distribution of 90Y after whole-liver delivery via the hepatic artery; the authors concluded that patients with hypervascular tumors are much more likely to benefit from this treatment. A dose-escalation study on animals was performed, which formed the basis of the Phase I dose-escalation evaluations in humans (Wollner et al, 1987). Shepherd and colleagues (1992) conducted a Phase I dose-escalation study using 90Y microspheres in 10 patients. Extrahepatic shunting was assessed using technetium-99m–labeled macroaggregated albumin (99mTc-MAA). In contrast to earlier studies, none of the patients in this study experienced hematologic toxicity, a fact that emphasizes the value of assessing angiographic findings and extrahepatic shunting before treatment (Shepherd et al, 1992). The technical aspects of dosimetry, radioassays, safety, and efficacy were discussed by many studies that followed (Andrews et al, 1994; Yan et al, 1993). Lau and colleagues (1994) showed that tumor response was proportional to the dose delivered, with improved survival in patients receiving more than 120 Gy. Recent data on the safety and efficacy of this treatment are discussed below.
90YTTRIUM Microspheres
Pretreatment Evaluation
Pretreatment Angiography and Coil Embolization
Radioembolization requires pretreatment diagnostic mesenteric angiography. The aortogram, superior mesenteric angiogram, and celiac trunk angiogram allow the interventional radiologist an opportunity to study the vascular anatomy of the liver and its surrounding structures in detail. The patency of the portal vein and the presence of arterioportal shunting are assessed. The inadvertent spread of the microspheres is prevented by a meticulous study of the vascular anatomy of the liver and collateral nontarget flow (Covey et al, 2002). Coil embolization of nontarget vessels may be necessary to decrease the unintended deposition of microspheres. Some examples of vessels that may need to be embolized are inferior esophageal, left inferior phrenic, accessory left gastric, supraduodenal, and retroduodenal arteries. The minimal incidence of complications following coil embolization and the grave clinical consequences associated with the inadvertent deposition of microspheres in the stomach, duodenum, or pancreas favor prophylactic coil embolization before radioembolization in select cases.
Available Devices
TheraSphere
SIR-Spheres
Dose Calculation for SIR-Spheres
Three methods for dosimetry of SIR-Spheres are recommended by the manufacturers. The partition method is seldom used, as it is applicable only in special circumstances. The empiric method is outlined in Table 84A.2.
| Tumor Involvement of Liver (%) | Dose (Whole Liver) |
|---|---|
| ≤25 | 2 GBq |
| 25-50 | 2.5 GBq |
| >50 | 3 GBq |
New Concepts
Radiation Segmentectomy
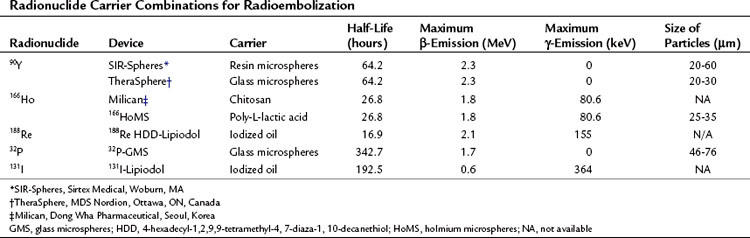
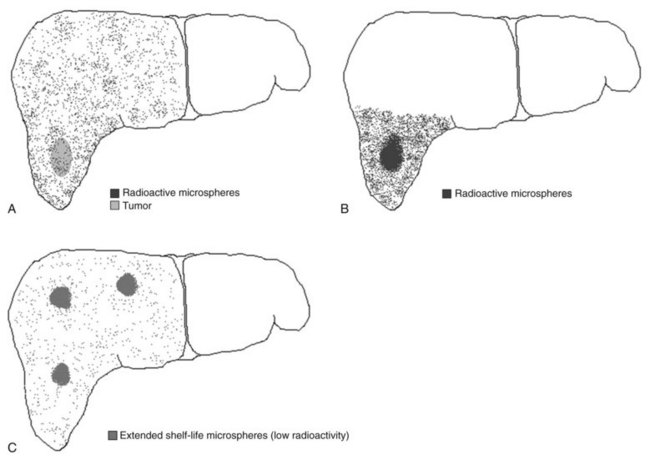
Recently, the concept of radiation segmentectomy has been discussed. This is schematically shown in Figure 84A.1B. The current dosimetry models assume lobar infusion; however, if the tumor is localized to two or fewer liver segments, the interventional radiologist can infuse the dose at the segmental level. This maximizes the dose delivery to the tumor and minimizes delivery of radioactive microspheres to the normal parenchyma (Riaz et al, 2010), an approach that has proven to be a safe and effective method of treatment delivery.
Extended Shelf Life Microspheres
Lewandowski and colleagues (2009b) recently published a report on the concept of extended shelf life microspheres, which can be used for large or multifocal tumors. As stated above, the difference in vials of different activities is the number of microspheres. If the vials of high activities (more microspheres) are allowed to stay on the shelf longer, the activity per microsphere will decrease. This allows the delivery of a higher number of microspheres to larger volumes of the liver with minimal radiotoxicity to the normal parenchyma (Lewandowski et al, 2009b).
Radiation Lobectomy
An animal study concluded that portal fibrosis was seen after the use of radioactive microspheres (Wollner et al, 1988). Gaba and colleagues (2009) recently published a comprehensive analysis on the concept of radiation lobectomy, whereby infusion at the lobar level was used to treat the tumors. Because of fibrosis of the normal parenchyma, decreases in volumes of the treated lobe were seen. Furthermore, compensatory increases in the volumes of the untreated lobes were also observed.
New Concepts
Blood Flow Patterns for Dose Calculation
Kennedy and colleagues (2010) have recently published their analysis on computer modeling of 90Y resin microsphere transport in the hepatic arterial tree. They conclude that computer simulations of both blood flow patterns and microsphere dynamics have the potential to provide insight on methods to optimize microsphere implantation into hepatic tumors while sparing normal tissue.
Transcatheter 90Y Radioembolization
Radioembolization is a transcatheter therapy performed by interventional radiologists. The tumor is approached using its arterial supply, and the vial is injected into the vessel feeding the tumor. The distribution of the tumor is the factor that allows the treatment to be selective, allowing delivery to one lobe, or superselective, allowing delivery to one segment. The apparatus for the administration of 90Y is designed to minimize the radiation exposure to those involved in the procedure, but a physicist should be present throughout the case to ensure that proper protocols are followed to minimize accidental radiation exposure. The procedure is performed on an outpatient basis, and the patient is discharged on the same day (Salem et al, 2002).
Other Radionuclides
Iodine-131–Labeled Iodized Oil and Iodized Oil (Lipiodol)
Overview
The half-life of 131I is 8 days. This radionuclide is a β- and γ-emitter. The use of 131I-Lipiodol has been studied in a prospective trial and compared in efficacy to chemoembolization (Raoul et al, 1997). The iodine moiety of Lipiodol can be substituted for the radionuclide 131I.
Conclusion
The outcomes associated with 131I-Lipiodol are comparable to those of transarterial chemoembolization (TACE; see Chapter 83), but significantly fewer serious adverse events are reported with the use of this radionuclide as compared with using TACE. 131I is expensive, but the isotope has high γ-energy with a short β-range, which decreases its cytotoxic effect. Furthermore, hospitalization after the procedure decreases the cost-effectiveness of 131I (Raoul et al, 1997).
Rhenium-188 HDD–Labeled Iodized Oil
Administration
The quantity of 188Re-HDD iodized oil (Lipiodol) administered is based on the radiation absorbed dose (RAD) to critical organs, which is calculated after transarterial administration of a test dose of the radioconjugate (Kumar et al, 2007). The critical organs include the bone marrow, lung, and normal liver, and the dose limitations to them are 1.5 Gy, 12 Gy, and 30 Gy, respectively. A scout dose of 185 MBq activity of the radioconjugate is injected, and a whole-body nuclear scan is performed to calculate the RAD to the critical organs. The dose is then calculated and administered on the same day.
Conclusion
The incidence of serious adverse events is low with this treatment, and a survival benefit in HCC patients has been reported in published literature (Kumar et al, 2007); Liepe and colleagues (2007) have also published their experience of 10 patients. Its easy availability and published data make TART seem a promising mode of treatment of HCC; however, as of this writing, data are insufficient to recommend use of this radionuclide in metastatic disease to the liver.
Other 188Rhenium Carrier Combinations
There are other 188rhenium carrier combinations under study, such as 188Re-bis-(diethyldithiocarbamato) Lipiodol (188ReN-DEDC Lipiodol), 188Re-(S2CPh)(S3CPh)2 Lipiodol (188Re-SSS Lipiodol), and 188Re-labeled human serum albumin (HSA B20) microspheres (Lambert et al, 2009). 188Re–hyaluronic acid combinations are also being studied as a systemically administered agent for HCC in animals, as hyaluronic acid selectively binds to CD44 receptors (Melendez-Alafort et al, 2009).
Phosphorus-32 Glass Microspheres
Overview
32P is a radioisotope that emits high-energy β-particles during decay. It has a half-life of 14.28 days and a maximum tissue penetration of 8 mm, with an average of 3.2 mm (Wong et al, 1999). It is administered as an integral constituent of nonbiodegradable glass microspheres.
Milican/Holmium-166 Microspheres
166Ho is a β- and γ-emitter. This radionuclide is highly paramagnetic; hence it acts as a negative contrast agent, like iron, on magnetic resonance imaging (MRI; Bult et al, 2009). It is also radiopaque and visible on computed tomography (CT) (Bult et al, 2009).
Holmium/chitosan complex (Milican; Dong Wha Pharmaceutical, Seoul, Korea) has been shown to be effective in treating small HCC in a novel study from Korea (Kim et al, 2006). Chitosan is a unique substance derived from chitin. It has the ability to liquefy in an acidic environment and form a gel in basic environments, and the gel has embolic effects.
Microspheres of 166Ho poly-L-lactic acid are being studied in animal models and have been shown to be safe, but they have yet to be studied in humans (Vente et al, 2010). 166Ho has also recently been studied with hydroxyapatite as the carrier in animal models (Das et al, 2009).
Primary Liver Tumors
Role of Radioembolization in Management of Hepatocellular Carcinoma (See Chapter 80)
Patient Selection
The various diagnostic criteria and staging systems for HCC are beyond the scope of this chapter (Bruix et al, 2001). The management of HCC requires a multidisciplinary approach with the involvement of hepatologists, oncologists, transplant surgeons, and interventional radiologists. Patients should be selected for this treatment modality based on a consensus of the team. As discussed below, the role of radioembolization is not limited by the stage of the disease, but no survival benefit has been seen in patients with distant metastases.
Indications and Efficacy
Patients within Transplant Criteria
The use of surgical options is the gold standard of treatment for these patients. The patients within the Milan criteria, with a single lesion less than 5 cm or three or fewer lesions all less than 3 cm, are eligible for orthotopic liver transplantation (OLT; see Chapter 97D; Mazzaferro et al, 1996). Resection is possible only if liver function is preserved (see Chapter 90F). The limited availability of donor organs for OLT and the dropout of patients as a result of tumor progression limits the number of patients who are able to undergo OLT. Thermal ablation (radiofrequency ablation) has a limited role because of the risk of tract seeding and the size and location of tumor (see Chapter 85A, Chapter 85B, Chapter 85C, Chapter 85D ). Radioembolization has been shown to limit progression of the disease, which allows the patient more time to wait for donor organs and thus increases their chance of undergoing OLT (Kulik et al, 2006). Thus radioembolization has a role in bridging patients to OLT.
Patients Beyond Transplant Criteria
Patients outside the transplant criteria as a result of the size or number of tumors but who do not have malignant PVT or metastatic HCC may also be candidates for radioembolization, which has been shown to downstage the disease in these patients to within transplant criteria. This allows patients who were initially outside Milan criteria to become eligible for OLT with an increase in overall survival reported in these patients as well (Kulik et al, 2006). Lewandowski and colleagues (2009a) recently published their experience of downstaging using transarterial therapies for HCC. Their data suggest a superior ability of radioembolization to downstage HCC compared with chemoembolization. The recurrence-free survival and overall survival after OLT in the downstaged patients has yet to be compared with that of the patients who were already within transplant criteria to determine the efficacy of downstaging.
Patients with Advanced Disease
Patients with PVT have been shown to have a favorable response to treatment after radioembolization (Kulik et al, 2007). The presence of malignant PVT excludes these patients from transplantation, whereas its presence is not a contraindication to radioembolization with 90Y. Systemic therapy with sorafenib has been shown to have a statistically significant improvement in survival in patients with advanced disease (Llovet et al, 2008; see Chapter 88). The hepatic artery is the sole vascular supply to the parenchyma in the presence of PVT, hence embolic therapies are relatively contraindicated. However, 90Y may be used in these cases because of its minimal embolic effect (Kulik et al, 2007). A survival benefit (10.1 to 13.4 months from treatment) has been shown with the use of radioembolization in patients with malignant vascular involvement (Kulik et al, 2007). A survival benefit has not been shown in patients with distant metastases (Salem et al, 2010).
Conclusion
Recently, Salem and colleagues (2010) published a comprehensive analysis on the role of radioembolization in 291 patients with HCC. The data presented suggest that radioembolization is a safe and effective treatment modality. The response rate and promising survival associated with this therapy have established its role in the management of HCC in many centers around the world.
Role of Radioembolization in Management of Intrahepatic Cholangiocarcinoma (See Chapter 50A)
Resection is the best treatment option in patients who have limited disease, and it has been shown to improve survival. The role of chemoembolization for intrahepatic cholangiocarcinoma (ICC) has been studied, and an improvement in survival has been shown, but the rate of toxicity remains high. Radioembolization has been shown to be effective in the treatment of HCC, but its role in the management of ICC has not been extensively studied. A study analyzing the use of 90Y in patients with ICC has shown a favorable response to treatment and favorable survival outcomes (Ibrahim et al, 2008). Patients with a better performance status according to the Eastern Cooperation Oncology Group had a significantly better survival in this study. Saxena and colleagues (2010) recently published their analysis of 25 patients with ICC who underwent radioembolization with resin microspheres. They conclude that 90Y radioembolization is safe and effective, and in the absence of other therapeutic options, this treatment warrants further investigation.
Secondary Liver Tumors
Metastatic disease to the liver is common because of the liver’s unique anatomy and dual vascular supply. The presence of multiple metastases and comorbidities limits the role of surgical resection in these patients (Welsh et al, 2006). The role of radioembolization alone and as a conjunct to systemic chemotherapy has been well established.
Role of Radioembolization in the Management of Metastatic Colorectal Carcinoma (See Chapter 81A)
Overview
Resection is the only curative option available for colorectal cancer (CRC) that has metastasized to the liver, although a small fraction of patients have disease that is amenable to resection (Welsh et al, 2006). Systemic chemotherapy for this disease is beyond the scope of this chapter, but some commonly used chemotherapeutic agents are 5-fluorouracil (5-FU), oxaliplatin, irinotecan (CPT-11), bevacizumab, cetuximab, and capecitabine (see Chapter 86). The favorable role of radioembolization in treatment of metastatic CRC to the liver has been published (Mulcahy et al, 2009).
Efficacy of Radioembolization
The effect of systemic chemotherapy alone has been compared to its combined effect with radioembolization in a randomized control trial. The combination has been shown to have a significantly better tumor response, a longer time to progression, survival benefit, and an acceptable safety profile (Gray et al, 2001). The use of internal radiation alone has also been published in many series and has shown promising results (Kennedy et al, 2005; Mulcahy et al, 2009), and dose-escalation studies have shown better response with increasing doses (Goin et al, 2003). A recent study on the concomitant use of irinotecan and radioembolization in fluorouracil-refractory patients with CRC hepatic metastases showed that the maximum-tolerated dose was not reached (Van Hazel et al, 2009).
Role of Radioembolization in the Management of Metastatic Neuroendocrine Tumors (See Chapter 81B)
Evidence
Radioembolization of metastatic disease to the liver from a neuroendocrine neoplasia has been shown to be effective and safe. A prolonged response to treatment, greater than 2 years, has also been seen (Kennedy et al, 2008; Rhee et al, 2008b). King and colleagues (2008) recently published their experience with 34 patients and concluded that radioembolization can achieve relatively long-term responses in patients with nonresectable NET liver metastases.
Role of Radioembolization in the Management of Metastatic Mixed Neoplasia
Overview
The role of radioembolization in hepatic metastases from primary neoplasia other than the ones listed above is discussed under the heading of metastatic mixed neoplasia. The following secondary tumors have been treated using radioembolization, but only metastatic breast cancer has been studied in detail (see Chapter 81C).
Internal Radiation to Metastatic Breast Cancer to Liver
Breast cancer is the most common cancer in women and has a tendency to metastasize to the liver. Radioembolization presents an efficacious treatment for unresectable breast cancer metastasis to the liver (Coldwell et al, 2005). Radiologic response has been seen following radioembolization in these patients, but the survival benefit of this treatment in this population has not been established (Jakobs et al, 2008b).
Other
Radioembolization has been used to treat secondary liver tumors from various primary sources. This mode of treatment is an effective alternative to patients who have failed chemotherapy or who have become chemorefractory (Sato et al, 2008). The data suggest a similar benefit in survival and tumor response in metastatic liver tumors from the metastatic mixed neoplasia.
Posttreatment Assessment
Clinical and Laboratory Parameters
The response to treatment can be monitored clinically and radiologically. The regular follow-up laboratory workup includes the hepatic panel and tumor markers—such as AFP, CEA, and carbohydrate antigen (CA) 19-9—to look for any toxicity as a result of treatment and to assess clinical improvement in the patient. Decrease in AFP in patients with HCC has recently been shown to be an indicator of prognostic benefit following transarterial locoregional therapies (Riaz et al, 2009a).
Radiologic Parameters
The first radiologic study is done 1 month after treatment. Patients are then followed with scans every 3 months to assess response to treatment or progression of disease. The use of the World Health Organization (WHO) and Response Evaluation Criteria In Solid Tumors (RECIST) are based on decreases in the size of the lesion irrespective of the amount of necrosis seen in the tumor. The European Association for the Study of the Liver (EASL) necrosis criteria are also used to assess response in the target lesions (Ibrahim et al, 2009), and the WHO and EASL guidelines have been shown to correlate well with pathologic necrosis (Riaz et al, 2009c). The conventional anatomic imaging studies are not able to assess tumor response until 6 weeks after treatment. Functional MRI may have a role in earlier detection of tumor response (Rhee et al, 2008b); however, more data are required before functional imaging can replace the conventional imaging guidelines for response assessment.
Complications of Radioembolization
Postradioembolization Syndrome
Patients may experience a mild postradioembolization syndrome, which comprises signs and symptoms that include fatigue, nausea, vomiting, anorexia, fever, abdominal discomfort, and cachexia. These are usually not severe enough to require hospitalization, although some serious adverse events related to radioembolization are explained below and have been discussed in detail previously (Riaz et al, 2009b).
Hepatobiliary Toxicity
Hepatic Injury
Radiation-induced liver disease (RILD) usually occurs between 4 and 8 weeks after radioembolization. Hepatic toxicity is measured by a change in the liver enzymes and metabolites, including ALT, AST, alkaline phosphatase, albumin, and bilirubin. Using the Common Terminology Criteria version 3.0 to assess toxicity to the liver following radioembolization, the grade 3 and 4 toxicity rates following radioembolization have been low (Salem et al, 2010; Sangro et al, 2008; Young et al, 2007). The clinical appearance of ascites and jaundice is very rarely seen.
The histologic hallmark of venoocclusive disease (VOD) may be seen in severe cases (see Chapter 77). Hepatic toxicity is rarely so severe as to lead to significant morbidity and mortality (Sangro et al, 2008). The presence of factors such as a deranged hepatic function at baseline, age, and activity delivered may predispose patients to the hepatotoxic effects of radioembolization.
Biliary Injury
The biliary tract is also susceptible to toxicity by radioembolization. According to Rhee and colleagues (2008a), less than 2% of patients required intervention for the biliary toxicity induced by radioembolization. These included two cholecystectomies, drainage of three bilomas, and one abscess. Radiation-induced cholangitis has also been reported following 90Y administration (Rhee et al, 2008a); however, these are not common, and meticulous technique can minimize their incidence.
Portal Hypertension
Radioembolization has been shown to cause fibrosis that may lead to portal hypertension by changing the volume of the treated lobe. The time it takes for development of portal hypertension is variable (Jakobs et al, 2008a), but it is more often associated with bilobar treatment, and its incidence is increased in patients who have chemotherapy-associated steatohepatitis (CASH; see Chapter 65). The presence of preexisting cirrhosis leading to portal hypertension in most HCC patients makes them more susceptible to the aggravation of this complication as well (Riaz et al, 2009b).
Radiation Pneumonitis
Dose adjustment is required, and caution must be exercised, when the LSF is greater than 13% (Leung et al, 1995). A restrictive pulmonary dysfunction is seen after radioembolization in a few cases with a predisposing high LSF. The LSF is used to calculate the dose that would be administered to the lung, and an absolute contraindication to radioembolization is the predicted administration of a dose greater than or equal to 30 Gy to the lungs in a single treatment or greater than 50 Gy as a cumulative dose after multiple treatments (MDS Nordion, 2004). The presence of radiation pneumonitis can be diagnosed clinically and on finding consolidation on CT, evidenced by a bat-wing appearance.
Gastrointestinal Complications
GI complications after radioembolization have been reported and are due to the inadvertent spread of microspheres to the GI tract (Carretero et al, 2007; Murthy et al, 2007a). Ulceration may occur and may require surgery for treatment; however, it can be prevented by meticulous mapping of the blood vessels to look for aberrant vasculature arising from branches of the hepatic artery that supply the GI tract. The prophylactic use of proton pump inhibitors is also recommended (Riaz et al, 2009b).
Andrews JC, et al. Hepatic radioembolization with yttrium-90 containing glass microspheres: preliminary results and clinical follow-up. J Nucl Med. 1994;35:1637-1644.
Bruix J, et al. Clinical management of hepatocellular carcinoma: conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35:421-430.
Bult W, et al. Microsphere radioembolization of liver malignancies: current developments. Q J Nucl Med Mol Imaging. 2009;53:325-335.
Carretero C, et al. Gastroduodenal injury after radioembolization of hepatic tumors. Am J Gastroenterol. 2007;102(6):1216-1220.
Coldwell D, Nutting C, Kennedy AK, 2005: Treatment of hepatic metastases from breast cancer with Yttrium-90 SIR-Spheres radioembolization. Paper presented at the Society of Interventional Radiology Annual Meeting, March 31-April 5, New Orleans, LA.
Covey AM, et al. Variant hepatic arterial anatomy revisited: digital subtraction angiography performed in 600 patients. Radiology. 2002;224:542-547.
Das T, et al. 166Ho-labeled hydroxyapatite particles: a possible agent for liver cancer therapy. Cancer Biother Radiopharm. 2009;24:7-14.
Dawson LA, McGinn NJ, Ensminger WD, 1999: Preliminary results of escalated focal liver radiation and hepatic artery floxuridine for unresectable liver malignancies. Paper presented at the American Society of Clinical Oncology.
Dawson LA, et al. Analysis of radiation-induced liver disease using the Lyman NTCP model. Int J Radiat Oncol Biol Phys. 2002;53:810-821.
Dawson LA, Guha C. Hepatocellular carcinoma: radiation therapy. Cancer J. 2008;14:111-116.
Fukumitsu N, et al. A prospective study of hypofractionated proton beam therapy for patients with hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2009;74:831-836.
Gaba RC, et al. Radiation lobectomy: preliminary findings of hepatic volumetric response to lobar yttrium-90 radioembolization. Ann Surg Oncol. 2009;16:1587-1596.
Geschwind JF, et al. Yttrium-90 microspheres for the treatment of hepatocellular carcinoma. Gastroenterology. 2004;127(Suppl 1):S194-S205.
Goin JE, et al. Treatment of unresectable metastatic colorectal carcinoma to the liver with intrahepatic Y-90 microspheres: a dose-ranging study. World J Nuc Med. 2003;2:216-225.
Gray B, et al. Randomised trial of SIR-Spheres plus chemotherapy vs. chemotherapy alone for treating patients with liver metastases from primary large bowel cancer. Ann Oncol. 2001;12:1711-1720.
Ibrahim SM, et al. Treatment of unresectable cholangiocarcinoma using yttrium-90 microspheres: results from a pilot study. Cancer. 2008;113:2119-2128.
Ibrahim SM, et al. Radiologic findings following Y90 radioembolization for primary liver malignancies. Abdom Imaging. 2009;34(5):566-581.
Ingold JA, et al. Radiation hepatitis. Am J Roentgenol Radium Ther Nucl Med. 1965;93:200-208.
Jakobs TF, et al. Fibrosis, portal hypertension, and hepatic volume changes induced by intra-arterial radiotherapy with (90)yttrium microspheres. Dig Dis Sci. 2008;53:2556-2563.
Jakobs TF, et al. Radioembolization in patients with hepatic metastases from breast cancer. J Vasc Interv Radiol. 2008;19:683-690.
Kennedy A, et al, 2005: Liver brachytherapy for unresectable colorectal metastases: US results 2000-2004. Paper presented at ASCO GI Symposium; Jan 27-29, Miami, FL.
Kennedy AS, et al. Radioembolization for unresectable neuroendocrine hepatic metastases using resin 90Y-microspheres: early results in 148 patients. Am J Clin Oncol. 2008;31:271-279.
Kennedy AS, et al. Computer modeling of yttrium-90-microsphere transport in the hepatic arterial tree to improve clinical outcomes. Int J Radiat Oncol Biol Phys. 2010;76(2):631-637.
Kim JK, et al. Long-term clinical outcome of phase IIb clinical trial of percutaneous injection with holmium-166/chitosan complex (Milican) for the treatment of small hepatocellular carcinoma. Clin Cancer Res. 2006;12:543-548.
King J, et al. Radioembolization with selective internal radiation microspheres for neuroendocrine liver metastases. Cancer. 2008;113:921-929.
Kulik LM, et al. Yttrium-90 microspheres (TheraSphere) treatment of unresectable hepatocellular carcinoma: downstaging to resection, RFA and bridge to transplantation. J Surg Oncol. 2006;94:572-586.
Kulik LM, et al. Safety and efficacy of (90)Y radiotherapy for hepatocellular carcinoma with and without portal vein thrombosis. Hepatology. 2007;47:71-81.
Kumar A, et al. Inoperable hepatocellular carcinoma: transarterial 188Re HDD-labeled iodized oil for treatment:prospective multicenter clinical trial. Radiology. 2007;243:509-519.
Lambert B, Bacher K, Defreyne L. Rhenium-188 based radiopharmaceuticals for treatment of liver tumours. Q J Nucl Med Mol Imaging. 2009;53:305-310.
Langer M, Morrill SS, Lane R. A test of the claim that plan rankings are determined by relative complication and tumor-control probabilities. Int J Radiat Oncol Biol Phys. 1998;41:451-457.
Lau WY, et al. Treatment of inoperable hepatocellular carcinoma with intrahepatic arterial yttrium-90 microspheres: a phase I and II study. Br J Cancer. 1994;70:994-999.
Leung TW, et al. Radiation pneumonitis after selective internal radiation treatment with intraarterial 90yttrium-microspheres for inoperable hepatic tumors. Int J Radiat Oncol Biol Phys. 1995;33:919-924.
Lewandowski RJ, et al. A comparative analysis of transarterial downstaging for hepatocellular carcinoma: chemoembolization versus radioembolization. Am J Transplant. 2009;9:1920-1928.
Lewandowski RJ, et al. Optimization of radioembolic effect with extended-shelf-life yttrium-90 microspheres: results from a pilot study. J Vasc Interv Radiol. 2009;20:1557-1563.
Liepe K, et al. Feasibility of high activity rhenium-188-microsphere in hepatic radioembolization. Jpn J Clin Oncol. 2007;37:942-950.
Llovet JM, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378-390.
Mantravadi RV, et al. Intra-arterial yttrium 90 in the treatment of hepatic malignancy. Radiology. 1982;142:783-786.
Mazzaferro V, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693-699.
Nordion MDS. TheraSphere Yttrium-90 microspheres package insert. Kanata, Canada: MDS Nordion; 2004.
Melendez-Alafort L, et al. Biokinetic and dosimetric studies of 188Re-hyaluronic acid: a new radiopharmaceutical for treatment of hepatocellular carcinoma. Nucl Med Biol. 2009;36:693-701.
Mulcahy MF, et al. Radioembolization of colorectal hepatic metastases using yttrium-90 microspheres. Cancer. 2009;115:1849-1858.
Murthy R, et al. Gastrointestinal complications associated with hepatic arterial Yttrium-90 microsphere therapy. J Vasc Interv Radiol. 2007;18:553-561.
Murthy R, et al. Hepatic yttrium-90 radioembolotherapy in metastatic colorectal cancer treated with cetuximab or bevacizumab. J Vasc Interv Radiol. 2007;18:1588-1591.
Nolan TR, Grady ED. Intravascular particulate radioisotope therapy: clinical observations of 76 patients with advanced cancer treated with 90-yttrium particles. Am Surg. 1969;35:181-188.
Nomiya T, et al. Carbon ion radiation therapy for primary renal cell carcinoma: initial clinical experience. Int J Radiat Oncol Biol Phys. 2008;72:828-833.
Raoul JL, et al. Prospective randomized trial of chemoembolization versus intra-arterial injection of 131I-labeled-iodized oil in the treatment of hepatocellular carcinoma. Hepatology. 1997;26:1156-1161.
Rhee TK, et al. 90Y radioembolization for metastatic neuroendocrine liver tumors: preliminary results from a multi-institutional experience. Ann Surg. 2008;247:1029-1035.
Rhee TK, et al. Tumor response after yttrium-90 radioembolization for hepatocellular carcinoma: comparison of diffusion-weighted functional MR imaging with anatomic MR imaging. J Vasc Interv Radiol. 2008;19:1180-1186.
Riaz A, et al. Alpha-fetoprotein response after locoregional therapy for hepatocellular carcinoma: oncologic marker of radiologic response, progression, and survival. J Clin Oncol. 2009;27:5734-5742.
Riaz A, et al. Complications following radioembolization with yttrium-90 microspheres: a comprehensive literature review. J Vasc Interv Radiol. 2009;20:1121-1130.
Riaz A, et al. Radiologic-pathologic correlation of hepatocellular carcinoma treated with internal radiation using yttrium-90 microspheres. Hepatology. 2009;49:1185-1193.
Riaz A, et al. Radiation segmentectomy: a novel approach to increase safety and efficacy of radioembolization. Int J Radiat Oncol Biol Phys. 2011;79:163-171.
Salem R, et al. Yttrium-90 microspheres: radiation therapy for unresectable liver cancer. J Vasc Interv Radiol. 2002;13(9 Pt 2):S223-S229.
Salem R, et al. Radioembolization for hepatocellular carcinoma using Yttrium-90 microspheres: a comprehensive report of long-term outcomes. Gastroenterology. 2010;138(1):52-64.
Sangro B, et al. Liver disease induced by radioembolization of liver tumors: description and possible risk factors. Cancer. 2008;112:1538-1546.
Sato KT, et al. Unresectable chemorefractory liver metastases: radioembolization with 90Y microspheres-safety, efficacy, and survival. Radiology. 2008;247:507-515.
Saxena A, et al. Yttrium-90 radiotherapy for unresectable intrahepatic cholangiocarcinoma: a preliminary assessment of this novel treatment option. Ann Surg Oncol. 2010;17:484-491.
Shepherd FA, et al. A phase I dose escalation trial of yttrium-90 microspheres in the treatment of primary hepatocellular carcinoma. Cancer. 1992;70:2250-2254.
Van Hazel GA, et al. Treatment of fluorouracil-refractory patients with liver metastases from colorectal cancer by using yttrium-90 resin microspheres plus concomitant systemic irinotecan chemotherapy. J Clin Oncol. 2009;27:4089-4095.
Vente MA, et al. Holmium-166 poly (L:-lactic acid) microsphere radioembolisation of the liver: technical aspects studied in a large animal model. Eur Radiol. 2010;20:862-869.
Welsh JS, Kennedy AS, Thomadsen B. Selective internal radiation therapy (SIRT) for liver metastases secondary to colorectal adenocarcinoma. Int J Radiat Oncol Biol Phys. 2006;66(2 Suppl):S62-S73.
Wollner I, et al. Effects of hepatic arterial yttrium 90 glass microspheres in dogs. Cancer. 1988;61:1336-1344.
Wollner IS, et al. Effects of hepatic arterial yttrium-90 microsphere administration alone and combined with regional bromodeoxyuridine infusion in dogs. Cancer Res. 1987;47:3285-3290.
Wong JY, et al. Initial clinical experience evaluating Yttrium-90-chimeric T84.66 anticarcinoembryonic antigen antibody and autologous hematopoietic stem cell support in patients with carcinoembryonic antigen-producing metastatic breast cancer. Clin Cancer Res. 1999;5(10 Suppl):3224S-3231S.
Yan ZP, et al. An experimental study and clinical pilot trials on yttrium-90 glass microspheres through the hepatic artery for treatment of primary liver cancer. Cancer. 1993;72:3210-3215.
Young JY, et al. Radiation dose limits and liver toxicities resulting from multiple yttrium-90 radioembolization treatments for hepatocellular carcinoma. J Vasc Interv Radiol. 2007;18:1375-1382.