Chapter 106 Radiation Therapy of Epilepsy
History
Experience with stereotactic radiosurgery (SRS) over the 60 years since its development by Lars Leksell1 has demonstrated its efficacy in the treatment of various central nervous system conditions. Although Leksell initially developed this technique to treat functional disorders such as pain and movement disorders, the earliest widespread uses of SRS focused on deep-seated tumors or arteriovenous malformations (AVMs) located in eloquent regions of the brain, using it as a means of treating these lesions while avoiding the risks associated with surgical resection.2 Within these contexts, seizures were treated as secondary manifestations of the primary disorder, rather than as specific conditions. Subsequent to radiosurgical treatment, significant reduction, or even resolution, of the associated seizures has been documented.
The efficacy of SRS in secondary epilepsy has been most evident following the treatment of AVMs.3–9 Several large series of patients with AVMs have found complete seizure remission in 50% to 80% of patients following SRS.5,7–9 Small lesion size,7 shorter duration of epilepsy,5 and absence of prior hemorrhage9 were associated with higher seizure-free outcomes. In contrast, seizure outcome appears to be independent of AVM obliteration.3,4,6,8 Steiner et al. found 3 of 11 patients who became seizure free following SRS did so despite persistence of the AVM,8 while Lim et al. found 40% of patients with partial AVM obliteration to be seizure free.6 A similar phenomenon was described by Schrottner et al. in the treatment of 24 patients with tumor-related intractable epilepsy. Prolonged seizure control was achieved in 54% of patients in a dose-dependent manner, while tumor control was achieved in all patients.10 These studies indicate that radiosurgery exerts an independent antiepileptic effect.
Animal studies have provided additional support for the use of radiosurgery in the treatment of seizures, as well as insight into the potential mechanism underlying its antiepileptic effect. One hypothesis has been that radiation causes destruction of epileptogenic tissue. Dose-dependent cell death and radiation necrosis develop in both normal and epileptic rat models in response to increasing doses of radiation.11–15 Similarly, a dose-dependent effect of radiation on seizure control has been demonstrated in several rat models of epilepsy. However, seizure reduction occurred in the absence of necrosis,11,13,14,16 suggesting necrosis may not be required for antiepileptic effects.
Another hypothesis has been that radiation inhibits seizure-induced neurogenesis and mitosis. In electrically kindled rats, radiation prevented kindling-associated neuroblast proliferation.17,18 Similarly, radiation halted seizure-induced mitosis in flurothyl-kindled mice.19 Despite the clear effect on neurogenesis and mitosis in these models, radiation did not inhibit mossy fiber synaptic reorganization,20 kindling progression, or seizure threshold.17,19 Alternatively, radiation may induce vascular and inflammatory changes that lead to seizure reduction.21 Further animal studies will be critical to understanding the mechanisms underlying radiation-induced seizure control.
Modern Indications
Hypothalamic Hamartomas
Hypothalamic hamartomas (HHs), though rare, are an important cause of debilitating epilepsy in childhood. HHs can cause a progressive epileptic encephalopathy characterized by seizures, endocrine dysfunction, cognitive and behavioral impairment, and developmental delay. Classically, HHs are associated with gelastic seizures, which appear as recurrent bouts of emotionless laughter or grimacing. In addition, most patients develop multiple seizure types, including tonic–clonic, partial complex, and drop attacks.22,23 As devastating is the progressive cognitive decline that accompanies the medically refractory epilepsy.24
These heterotopic masses of mixed neuronal and glial cells arise from the floor of the third ventricle or mammillary bodies and present a significant therapeutic challenge due to their deep location and critical surrounding structures. Both open and endoscopic surgical approaches have been used in the resection of these lesions. While good seizure outcomes have been achieved, resection is associated with high morbidity and mortality.25–27 SRS has therefore emerged as a primary treatment for HHs due to its ability to precisely target and treat HHs without the morbidity of surgical resection.
Arita et al. first applied gamma knife radiosurgery (GKS) to the treatment of a 25-year-old male with HH-associated epilepsy who remained seizure free 21 months after treatment.28 Regis et al. have the largest experience with GKS treatment of HH. They reported seizure cessation in 40% of patients, with an additional 20% experiencing only rare, nondisabling seizures after 3 years.29 Benefits extend to improved sleep, behavior, and learning; these have been documented independent of seizure remission.29–34 No surgical mortality and limited morbidity have been reported in these patients. For these reasons, we advocate SRS as a valid option in the treatment in most patients with HHs, especially when the lesion is confined to the third ventricle.
Mesial Temporal Lobe Epilepsy
The success with secondary epilepsies and HHs has prompted interest in the use of SRS for mesial temporal lobe epilepsy (MTLE) associated with mesial temporal sclerosis. In 1999, Regis et al. reported the first seven cases of amygdalohippocampectomy by GKS. All patients exhibited a reduction in seizure frequency, and all but one patient remained seizure free 2 years after the operation. In addition, magnetic resonance imaging (MRI) changes were noted, specifically in the amygdalohippocampal target, indicating focused destruction.35 Since Regis et al.’s initial study, two large multicenter prospective trials have been completed. In the European trial a 24-Gy marginal dose was used, while in the U.S. trial 20- and 24-Gy marginal doses were compared. In both series, 51 patients in total, a significant reduction in seizure frequency was observed by 1 year and 65% of patients were seizure free at 2 years.36,37 A multicenter prospective trial directly comparing GKS to open surgery (Radiosurgery or Open Surgery for Epilepsy, or the ROSE trial) is under way in the United States.
GKS has also shown efficacy in the treatment of recurrent seizures after incomplete temporal lobectomy. Yen et al. treated four such patients using GKS. This resulted in significant, persistent seizure reduction in all four patients without morbidity.38
Secondary Epilepsies
As discussed earlier, SRS has shown efficacy in the treatment of seizures secondary to AVMs and tumors. SRS has also been applied in the treatment of cavernous malformations (CMs). Seizure control following GKS is less than that found in the treatment of AVMs or tumors, with remission rates ranging from 25% to 53%.39–41 In addition, GKS for CM is associated with an increased risk of radiation-induced complications, including hemorrhage.42 Shih and Pan retrospectively compared surgical resection and GKS of CMs and noted seizure control and complication rates were better with open surgery.40 We therefore feel strongly that the decision to treat seizures due to CMs, as well as due to AVMs or tumors, should be guided primarily by the natural history and the risk–benefit ratio of surgical resection in each particular case.
Nonlesional Epilepsy
Currently, SRS does not offer any advantages over surgical resection in the treatment of nonlesional, cortical epilepsies because of the common need to perform invasive monitoring for seizure localization. Corpus callosotomy (CC), however, reduces the frequency or severity of generalized or multifocal seizures independent of seizure focus and may be a target for SRS. Eder et al. treated three children with SRS callosotomy, one with Lennox-Gastaut syndrome, and two following functional hemispherotomy for cortical dysplasia. The two children with persistent seizures after hemispherotomy became seizure free after GKS.43
Preoperative Evaluation
Patient selection and preoperative evaluation are the same as for patients considered for open resective surgery.44 A complete medical and neurologic history, as well as a physical examination, is critical for understanding the scope of the patient’s disease and identifying the risk factors that may affect treatment. Patients should be refractory to medical therapy. Patients with significant medical disorders (e.g., cardiac, pulmonary, or oncologic), progressive neurologic disorders (e.g., multiple sclerosis), psychiatric disorders, or a history of substance abuse or noncompliance should be excluded. Women of child-bearing age should have a negative pregnancy test and documented use of a reliable birth control method.
Surgical Approaches
Radiosurgery can be performed under either general or local anesthesia, though typically we reserve general anesthesia for children. We recommend administering a small dose of a benzodiazepine sedative (e.g., lorazepam) to reduce the risk of seizures during the procedure. Pin sites are prepared with antiseptic solution, and local anesthetic is injected. The stereotactic frame must be affixed to the skull such that the base sits below the target. We then obtain a high-resolution MRI to include sequences that highlight the target (see specifics later). While the practices at individual centers vary, we find consultation among the neurosurgeon, radiation oncologist, and physicist beneficial in treatment planning. Advances in the gamma knife system and planning software (Leksell GammaPlan, Elekta, Stockholm, Sweden) allow more conformal radiation delivery using multiple isocenters. There is no predefined number of isocenters recommended for treatment. Treatment duration ranges from 1 to 2 hours, during which the patient’s blood pressure, oxygen saturation, and neurologic status should be monitored. After treatment, the frame is removed. Most patients can be discharged to home within 2 hours of treatment completion, depending on their baseline neurologic function and the amount of sedatives given during the procedure.
Hypothalamic Hamartomas
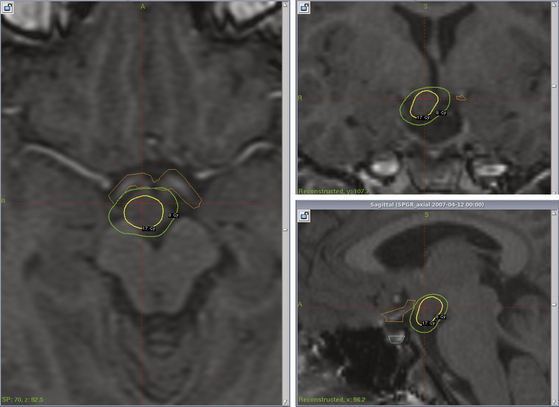
T2 and gadolinium-enhanced T1 coronal and axial magnetic resonance images must be carefully studied to delineate lesion boundaries and adjacent critical structures. HHs tend to be hyperintense on T2 and hypointense on T1 as compared to normal gray matter, and they do not enhance.45 The planning software can be used to minimize radiation to the optic nerves and tracts, as well as normal hypothalamus, using a beam-blocking approach (Fig. 106-1). For most patients we set the 50% isodose line to be the marginal isodose line, corresponding to the tumor margin. As seizure remission is greater among patients receiving a marginal dose of at least 17 Gy,30 every attempt should be made to achieve this marginal dose while preventing dosing greater than 8 Gy to the optic pathways. We typically use 18 or 19 Gy as our marginal dose.
Mesial Temporal Lobe Epilepsy
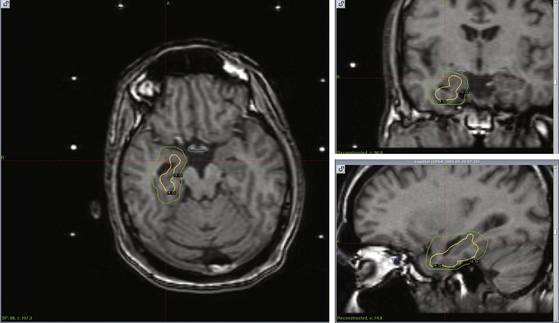
Planning is most direct when the frame is applied with the base parallel to the long axis of the temporal horn. Coronal T2 fluid-attenuated inversion recovery, three-dimensional spoiled gradient recalled, and gadolinium-enhanced T1 magnetic resonance images are obtained, in addition to axial fast spin echo and gadolinium-enhanced T1 images. Planning software is then used to delineate the target defined as the head and anterior body of the hippocampus (approximately 2 cm), amygdala, and parahippocampal gyrus. As with HHs, the 50% isodose line is set to be the marginal dose (Fig. 106-2). Marginal doses ranging from 20 to 24 Gy have been reported to reduce seizure frequency,36,37 though the optimal dose has yet to be clearly established. The optimal target volume also remains in debate.46 The current multicenter ROSE trial uses 24 Gy for the margin and has a range of treatment volumes between 5.5 and 7.5 ml. Care must be taken to limit radiation to the neighboring brain stem and optic pathways (less than 10 and 8 Gy, respectively).
Corpus Callosotomy
GKS for CC remains in the investigational phase, because few patients have been treated to date. The group from Austria has the largest experience, having treated both adults and children with GKS. They describe using two to five isocenters along the anterior or posterior corpus callosum with a marginal dose (equal to the 50% isodose line) of 55 to 85 Gy.43,47,48 Future work will be necessary to define the optimal treatment parameters.
Other Radiosurgical Systems
GKS has been the predominant technology applied to the radiosurgical treatment of epilepsy. While there is no reason to believe that other technologies cannot be utilized, experience has been limited thus far. Using linear accelerator particle accelerator (LINAC) technology, Liang et al. administered a total of 12 Gy (85% isodose line) in two fractions to seven patients with MTLE.49 All patients in their cohort had poor seizure control at 2 years, including two patients who experienced a doubling of their seizure frequency. The maximum seizure reduction documented was 50% (two patients). Cmelak et al. also used LINAC to treated one patient with MTLE using a single fraction of 15 Gy. While the patient experienced good seizure control for 3 months after treatment, his seizure frequency then increased to the preceding pretreatment levels. He subsequently underwent surgical resection due to intractable seizures 1 year after SRS.50 Poor seizure control in these studies is likely due to insufficient dosing, rather than limitations of the technology, as seizure outcome has been equivalent in the instances when LINAC or proton beam radiation were used to treat secondary epilepsies.3,51,52 We recommend the specific SRS parameters be worked out for each system at each facility.
Surgical Morbidity
The most significant benefit of GKS in the treatment of epilepsies may be the avoidance of morbidity and mortality related to open surgical procedures. This benefit has already been documented in patients with HH. Several highly experienced centers have reported serious complications to occur in 30% to 60% of patients following open resection.53 Such complications include ischemic stroke, endocrine dysfunction, cranial nerve damage, and a high rate of short-term memory impairment.25,53–56 In contrast, morbidity associated with GKS treatment of HHs has been low. No deaths or serious neurologic deficits have been reported, though a few patients experienced transient poikilothermia.29,31,32,34,53,57
Data from two multicenter trials have indicated that GKS is also safe for the treatment of MTLE.36,37 In our series of 30 patients, 1 patient developed headaches, papilledema, and blind spots associated with edema on MRI. As these symptoms progressed despite steroids, a temporal lobectomy was performed.37 Regis et al. reported 1 case mortality due to myocardial infarction in their series of 21 patients that was deemed unrelated to GKS.36 The most frequent side effect following GKS is headache (70%), often requiring steroid treatment.37 In addition, 50% of patients were noted to have a quadranopsia identified by formal visual field testing, though less than 10% were symptomatic.36,37 This is similar to the rate reported after surgical resection of the mesial temporal lobe structures.58
The risk of long-term, or delayed, complications must also be considered. Fractionated radiation treatment has been associated with gradual cognitive decline and secondary malignancies59–62; however, these complications have only rarely been reported with SRS.63 Furthermore, the small, theoretical risk of cognitive decline due to SRS must be weighed against the well-documented association between uncontrolled seizures and cognitive function.64
Outcomes
Seizure Control
Unlike the immediate effect of surgical resection, seizure remission following GKS is a process that takes months to years. Patients with HH often experience an immediate, though short-lived improvement, in seizures. This is followed by seizure return at pretreatment frequency (2-6 months after treatment). A third phase of acute increase in seizures lasts for days to weeks but seems to herald the progressive resolution of seizures.53 Overall, at 3 years, 60% of patients have significant seizure reduction, as well as improved cognition and behavior.53
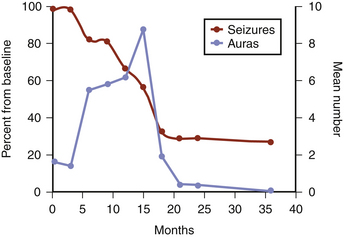
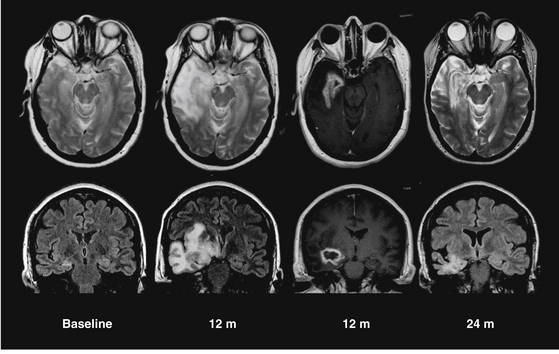
Patients with MTLE experience a dramatic increase in auras 9 to 15 months after GKS, coinciding with the reduction, or cessation, of complex partial seizures, as illustrated in Fig. 106-3.36,37 Also during this period, MRI-documented temporal lobe edema reaches its peak (Fig. 106-4). Data from Chang et al. indicated that this process of radiation-induced temporal lobe damage is necessary for seizure remission. Two MRI markers of edema (volume of T2 hyperintensity and contrast enhancement), when measured 12 months post-treatment, correlated with seizure remission documented 24 to 36 months post-treatment.65 Though the edema and increased aura frequency may persist for several months, they subsequently resolve. After a minimum of 2 years follow-up, 65% to 67% of patients were seizure free,36,37,66 with 60% seizure free at 5 years.66 The ongoing multicenter trial direct comparison between radiosurgery and open surgery will offer the best determination of equivalence.
Cognition and Memory
As discussed previously, cognition and behavior significantly improve after SRS for HH, even before meaningful seizure reduction occurs.29–34 This presents a major benefit of treatment, particularly given the severe epileptic encephalopathy caused by persistent gelastic seizures.67
In the case of MTLE, SRS may provide benefit with respect to verbal memory preservation. Open surgical resection presents a well-characterized risk of significant decline in verbal memory. Significant verbal memory decline is documented to occur in 38% of patients after temporal lobectomy.68 Such decline is more consistent among patients who undergo dominant hemisphere resection.69 Stroup et al. found that 60% of patients declined in at least one measure of verbal memory (either the Long Delay Free Recall score of the California Verbal Learning Test or the Rey Delay Recall score of the Wechsler Memory Scale) after dominant temporal lobe resection.68 In contrast, only 25% of patients who underwent dominant temporal lobe SRS in the U.S. multicenter pilot study experienced a significant decline in either measure of verbal memory.37
Barbaro N.M., Quigg M., Broshek D.K., et al. A multicenter, prospective pilot study of gamma knife radiosurgery for mesial temporal lobe epilepsy: seizure response, adverse events, and verbal memory. Ann Neurol. 2009;65(2):167-175.
Bartolomei F., Regis J., Kida Y., et al. Gamma knife radiosurgery for epilepsy associated with cavernous hemangiomas: a retrospective study of 49 cases. Stereotact Funct Neurosurg. 1999;72(Suppl 1):22-28.
Brisman J.L., Cole A.J., Cosgrove G.R., et al. Radiosurgery of the rat hippocampus: magnetic resonance imaging, neurophysiological, histological, and behavioral studies. Neurosurgery. 53(4), 2003. 951,61: discussion 961-962
Chang E.F., Quigg M., Oh M.C., et al. Predictors of efficacy after stereotactic radiosurgery for medial temporal lobe epilepsy. Neurology. 2010;74(2):165-172.
Chen Z.F., Kamiryo T., Henson S.L., et al. Anticonvulsant effects of gamma surgery in a model of chronic spontaneous limbic epilepsy in rats. J Neurosurg. 2001;94(2):270-280.
Feichtinger M., Schrottner O., Eder H., et al. Efficacy and safety of radiosurgical callosotomy: a retrospective analysis. Epilepsia. 2006;47(7):1184-1191.
Gerszten P.C., Adelson P.D., Kondziolka D., et al. Seizure outcome in children treated for arteriovenous malformations using gamma knife radiosurgery. Pediatr Neurosurg. 1996;24(3):139-144.
Heikkinen E.R., Konnov B., Melnikov L., et al. Relief of epilepsy by radiosurgery of cerebral arteriovenous malformations. Stereotact Funct Neurosurg. 1989;53(3):157-166.
Kida Y., Kobayashi T., Tanaka T., et al. Seizure control after radiosurgery on cerebral arteriovenous malformations. J Clin Neurosci. 2000;7(Suppl 1):6-9.
Kondziolka D., Lunsford L.D., Flickinger J.C. The application of stereotactic radiosurgery to disorders of the brain. Neurosurgery. 62(Suppl 2), 2008. 707,19: discussion 719–720
Leksell L. The stereotaxic method and radiosurgery of the brain. Acta Chir Scand. 1951;102(4):316-319.
Liang S., Liu T., Li A., et al. Long-term follow up of very low-dose LINAC-based stereotactic radiotherapy in temporal lobe epilepsy. Epilepsy Res. 2010;90(1-2):60-67.
Liscak R., Vladyka V., Novotny J.Jr., et al. Leksell gamma knife lesioning of the rat hippocampus: the relationship between radiation dose and functional and structural damage. J Neurosurg. 2002;97(5 Suppl):666-673.
Maesawa S., Kondziolka D., Dixon C.E., et al. Subnecrotic stereotactic radiosurgery controlling epilepsy produced by kainic acid injection in rats. J Neurosurg. 2000;93(6):1033-1040.
Mathieu D., Kondziolka D., Niranjan A., et al. Gamma knife radiosurgery for refractory epilepsy caused by hypothalamic hamartomas. Stereotact Funct Neurosurg. 2006;84(2-3):82-87.
Mori Y., Kondziolka D., Balzer J., et al. Effects of stereotactic radiosurgery on an animal model of hippocampal epilepsy. Neurosurgery. 46(1), 2000. 157,65: discussion 165-168
Quigg M., Barbaro N.M. Stereotactic radiosurgery for treatment of epilepsy. Arch Neurol. 2008;65(2):177-183.
Raedt R., Boon P., Persson A., et al. Radiation of the rat brain suppresses seizure-induced neurogenesis and transiently enhances excitability during kindling acquisition. Epilepsia. 2007;48(10):1952-1963.
Regis J., Bartolomei F., de Toffol B., et al. Gamma knife surgery for epilepsy related to hypothalamic hamartomas. Neurosurgery. 47(6), 2000. 1343,51: discussion 1351-1352
Regis J., Bartolomei F., Rey M., et al. Gamma knife surgery for mesial temporal lobe epilepsy. Epilepsia. 1999;40(11):1551-1556.
Regis J., Rey M., Bartolomei F., et al. Gamma knife surgery in mesial temporal lobe epilepsy: a prospective multicenter study. Epilepsia. 2004;45(5):504-515.
Rheims S., Fischer C., Ryvlin P., et al. Long-term outcome of gamma-knife surgery in temporal lobe epilepsy. Epilepsy Res. 2008;80(1):23-29.
Schrottner O., Eder H.G., Unger F., et al. Radiosurgery in lesional epilepsy: brain tumors. Stereotact Funct Neurosurg. 1998;70(Suppl 1):50-56.
Schulze-Bonhage A., Trippel M., Wagner K., et al. Outcome and predictors of interstitial radiosurgery in the treatment of gelastic epilepsy. Neurology. 2008;71(4):277-282.
Yen D.J., Chung W.Y., Shih Y.H., et al. Gamma knife radiosurgery for the treatment of recurrent seizures after incomplete anterior temporal lobectomy. Seizure. 2009;18(7):511-514.
1. Leksell L. The stereotaxic method and radiosurgery of the brain. Acta Chir Scand. 1951;102(4):316-319.
2. Kondziolka D., Lunsford L.D., Flickinger J.C. The application of stereotactic radiosurgery to disorders of the brain. Neurosurgery. 62(Suppl 2), 2008. 707,19: discussion 719-720
3. Heikkinen E.R., Konnov B., Melnikov L., et al. Relief of epilepsy by radiosurgery of cerebral arteriovenous malformations. Stereotact Funct Neurosurg. 1989;53(3):157-166.
4. Gerszten P.C., Adelson P.D., Kondziolka D., et al. Seizure outcome in children treated for arteriovenous malformations using gamma knife radiosurgery. Pediatr Neurosurg. 1996;24(3):139-144.
5. Kurita H., Kawamoto S., Suzuki I., et al. Control of epilepsy associated with cerebral arteriovenous malformations after radiosurgery. J Neurol Neurosurg Psychiatry. 1998;65(5):648-655.
6. Lim Y.J., Lee C.Y., Koh J.S., et al. Seizure control of gamma knife radiosurgery for non-hemorrhagic arteriovenous malformations. Acta Neurochir Suppl. 2006;99:97-101.
7. Schauble B., Cascino G.D., Pollock B.E., et al. Seizure outcomes after stereotactic radiosurgery for cerebral arteriovenous malformations. Neurology. 2004;63(4):683-687.
8. Steiner L., Lindquist C., Adler J.R., et al. Clinical outcome of radiosurgery for cerebral arteriovenous malformations. J Neurosurg. 1992;77(1):1-8.
9. Kida Y., Kobayashi T., Tanaka T., et al. Seizure control after radiosurgery on cerebral arteriovenous malformations. J Clin Neurosci. 2000;7(Suppl 1):6-9.
10. Schrottner O., Eder H.G., Unger F., et al. Radiosurgery in lesional epilepsy: brain tumors. Stereotact Funct Neurosurg. 1998;70(Suppl 1):50-56.
11. Chen Z.F., Kamiryo T., Henson S.L., et al. Anticonvulsant effects of gamma surgery in a model of chronic spontaneous limbic epilepsy in rats. J Neurosurg. 2001;94(2):270-280.
12. Liscak R., Vladyka V., Novotny J.Jr., et al. Leksell gamma knife lesioning of the rat hippocampus: the relationship between radiation dose and functional and structural damage. J Neurosurg. 2002;97(5 Suppl):666-673.
13. Maesawa S., Kondziolka D., Dixon C.E., et al. Subnecrotic stereotactic radiosurgery controlling epilepsy produced by kainic acid injection in rats. J Neurosurg. 2000;93(6):1033-1040.
14. Mori Y., Kondziolka D., Balzer J., et al. Effects of stereotactic radiosurgery on an animal model of hippocampal epilepsy. Neurosurgery. 46(1), 2000. 157,65: discussion 165-168
15. Brisman J.L., Cole A.J., Cosgrove G.R., et al. Radiosurgery of the rat hippocampus: magnetic resonance imaging, neurophysiological, histological, and behavioral studies. Neurosurgery. 53(4), 2003. 951,61: discussion 961-962
16. Sun B., DeSalles A.A., Medin P.M., et al. Reduction of hippocampal-kindled seizure activity in rats by stereotactic radiosurgery. Exp Neurol. 1998;154(2):691-695.
17. Pekcec A., Lupke M., Baumann R., et al. Modulation of neurogenesis by targeted hippocampal irradiation fails to affect kindling progression. Hippocampus. 2011;21(8):866-876.
18. Raedt R., Boon P., Persson A., et al. Radiation of the rat brain suppresses seizure-induced neurogenesis and transiently enhances excitability during kindling acquisition. Epilepsia. 2007;48(10):1952-1963.
19. Ferland R.J., Williams J.P., Gross R.A., et al. The effects of brain-irradiation-induced decreases in hippocampal mitotic activity on flurothyl-induced epileptogenesis in adult C57BL/6J mice. Exp Neurol. 2003;179(1):71-82.
20. Parent J.M., Tada E., Fike J.R., et al. Inhibition of dentate granule cell neurogenesis with brain irradiation does not prevent seizure-induced mossy fiber synaptic reorganization in the rat. J Neurosci. 1999;19(11):4508-4519.
21. Mizumatsu S., Monje M.L., Morhardt D.R., et al. Extreme sensitivity of adult neurogenesis to low doses of x-irradiation. Cancer Res. 2003;63(14):4021-4027.
22. Castro L.H., Ferreira L.K., Teles L.R., et al. Epilepsy syndromes associated with hypothalamic hamartomas. Seizure. 2007;16(1):50-58.
23. Mullatti N., Selway R., Nashef L., et al. The clinical spectrum of epilepsy in children and adults with hypothalamic hamartoma. Epilepsia. 2003;44(10):1310-1319.
24. Frattali C.M., Liow K., Craig G.H., et al. Cognitive deficits in children with gelastic seizures and hypothalamic hamartoma. Neurology. 2001;57(1):43-46.
25. Palmini A., Chandler C., Andermann F., et al. Resection of the lesion in patients with hypothalamic hamartomas and catastrophic epilepsy. Neurology. 2002;58(9):1338-1347.
26. Nishio S., Shigeto H., Fukui M. Hypothalamic hamartoma: the role of surgery. Neurosurg Rev. 1993;16(2):157-160.
27. Rosenfeld J.V., Harvey A.S., Wrennall J., et al. Transcallosal resection of hypothalamic hamartomas, with control of seizures, in children with gelastic epilepsy. Neurosurgery. 2001;48(1):108-118.
28. Arita K., Kurisu K., Iida K., et al. Subsidence of seizure induced by stereotactic radiation in a patient with hypothalamic hamartoma. Case report. J Neurosurg. 1998;89(4):645-648.
29. Regis J., Scavarda D., Tamura M., et al. Gamma knife surgery for epilepsy related to hypothalamic hamartomas. Semin Pediatr Neurol. 2007;14(2):73-79.
30. Regis J., Bartolomei F., de Toffol B., et al. Gamma knife surgery for epilepsy related to hypothalamic hamartomas. Neurosurgery. 47(6), 2000. 1343,51: discussion 1351-1352
31. Regis J., Hayashi M., Eupierre L.P., et al. Gamma knife surgery for epilepsy related to hypothalamic hamartomas. Acta Neurochir Suppl. 2004;91:33-50.
32. Unger F., Schrottner O., Feichtinger M., et al. Stereotactic radiosurgery for hypothalamic hamartomas. Acta Neurochir Suppl. 2002;84:57-63.
33. Mathieu D., Kondziolka D., Niranjan A., et al. Gamma knife radiosurgery for refractory epilepsy caused by hypothalamic hamartomas. Stereotact Funct Neurosurg. 2006;84(2-3):82-87.
34. Barajas M.A., Ramirez-Guzman M.G., Rodriguez-Vazquez C., et al. Gamma knife surgery for hypothalamic hamartomas accompanied by medically intractable epilepsy and precocious puberty: experience in Mexico. J Neurosurg. 2005;102(Suppl):53-55.
35. Regis J., Bartolomei F., Rey M., et al. Gamma knife surgery for mesial temporal lobe epilepsy. Epilepsia. 1999;40(11):1551-1556.
36. Regis J., Rey M., Bartolomei F., et al. Gamma knife surgery in mesial temporal lobe epilepsy: a prospective multicenter study. Epilepsia. 2004;45(5):504-515.
37. Barbaro N.M., Quigg M., Broshek D.K., et al. A multicenter, prospective pilot study of gamma knife radiosurgery for mesial temporal lobe epilepsy: seizure response, adverse events, and verbal memory. Ann Neurol. 2009;65(2):167-175.
38. Yen D.J., Chung W.Y., Shih Y.H., et al. Gamma knife radiosurgery for the treatment of recurrent seizures after incomplete anterior temporal lobectomy. Seizure. 2009;18(7):511-514.
39. Liu K.D., Chung W.Y., Wu H.M., et al. Gamma knife surgery for cavernous hemangiomas: an analysis of 125 patients. J Neurosurg. 2005;102(Suppl):81-86.
40. Shih Y.H., Pan D.H. Management of supratentorial cavernous malformations: craniotomy versus gamma knife radiosurgery. Clin Neurol Neurosurg. 2005;107(2):108-112.
41. Bartolomei F., Regis J., Kida Y., et al. Gamma knife radiosurgery for epilepsy associated with cavernous hemangiomas: a retrospective study of 49 cases. Stereotact Funct Neurosurg. 1999;72(Suppl 1):22-28.
42. Karlsson B., Kihlstrom L., Lindquist C., et al. Radiosurgery for cavernous malformations. J Neurosurg. 1998;88(2):293-297.
43. Eder H.G., Feichtinger M., Pieper T., et al. Gamma knife radiosurgery for callosotomy in children with drug-resistant epilepsy. Childs Nerv Syst. 2006;22(8):1012-1017.
44. Engel J.Jr., Wiebe S., French J., et al. Practice parameter: temporal lobe and localized neocortical resections for epilepsy: report of the Quality Standards Subcommittee of the American Academy of Neurology, in association with the American Epilepsy Society and the American Association of Neurological Surgeons. Neurology. 2003;60(4):538-547.
45. Freeman J.L., Coleman L.T., Wellard R.M., et al. MR imaging and spectroscopic study of epileptogenic hypothalamic hamartomas: analysis of 72 cases. AJNR Am J Neuroradiol. 2004;25(3):450-462.
46. Quigg M., Barbaro N.M. Stereotactic radiosurgery for treatment of epilepsy. Arch Neurol. 2008;65(2):177-183.
47. Feichtinger M., Schrottner O., Eder H., et al. Efficacy and safety of radiosurgical callosotomy: a retrospective analysis. Epilepsia. 2006;47(7):1184-1191.
48. Pendl G., Eder H.G., Schroettner O., et al. Corpus callosotomy with radiosurgery. Neurosurgery. 45(2), 1999. 303,7: discussion 307-308
49. Liang S., Liu T., Li A., et al. Long-term follow up of very low-dose LINAC-based stereotactic radiotherapy in temporal lobe epilepsy. Epilepsy Res. 2010;90(1-2):60-67.
50. Cmelak A.J., Abou-Khalil B., Konrad P.E., et al. Low-dose stereotactic radiosurgery is inadequate for medically intractable mesial temporal lobe epilepsy: a case report. Seizure. 2001;10(6):442-446.
51. Selch M.T., Gorgulho A., Mattozo C., et al. Linear accelerator stereotactic radiosurgery for the treatment of gelastic seizures due to hypothalamic hamartoma. Minim Invasive Neurosurg. 2005;48(5):310-314.
52. Eisenschenk S., Gilmore R.L., Friedman W.A., et al. The effect of LINAC stereotactic radiosurgery on epilepsy associated with arteriovenous malformations. Stereotact Funct Neurosurg. 1998;71(2):51-61.
53. Regis J., Scavarda D., Tamura M., et al. Epilepsy related to hypothalamic hamartomas: surgical management with special reference to gamma knife surgery. Childs Nerv Syst. 2006;22(8):881-895.
54. Delalande O., Fohlen M. Disconnecting surgical treatment of hypothalamic hamartoma in children and adults with refractory epilepsy and proposal of a new classification. Neurol Med Chir (Tokyo). 2003;43(2):61-68.
55. Harvey A.S., Freeman J.L., Berkovic S.F., et al. Transcallosal resection of hypothalamic hamartomas in patients with intractable epilepsy. Epileptic Disord. 2003;5(4):257-265.
56. Ng Y.T., Rekate H.L., Prenger E.C., et al. Transcallosal resection of hypothalamic hamartoma for intractable epilepsy. Epilepsia. 2006;47(7):1192-1202.
57. Schulze-Bonhage A., Trippel M., Wagner K., et al. Outcome and predictors of interstitial radiosurgery in the treatment of gelastic epilepsy. Neurology. 2008;71(4):277-282.
58. Egan R.A., Shults W.T., So N., et al. Visual field deficits in conventional anterior temporal lobectomy versus amygdalohippocampectomy. Neurology. 2000;55(12):1818-1822.
59. Kondziolka D., Niranjan A., Flickinger J.C., et al. Radiosurgery with or without whole-brain radiotherapy for brain metastases: the patients’ perspective regarding complications. Am J Clin Oncol. 2005;28(2):173-179.
60. Glosser G., McManus P., Munzenrider J., et al. Neuropsychological function in adults after high dose fractionated radiation therapy of skull base tumors. Int J Radiat Oncol Biol Phys. 1997;38(2):231-239.
61. McCord M.W., Buatti J.M., Fennell E.M., et al. Radiotherapy for pituitary adenoma: long-term outcome and sequelae. Int J Radiat Oncol Biol Phys. 1997;39(2):437-444.
62. Niranjan A., Kondziolka D., Lunsford L.D. Neoplastic transformation after radiosurgery or radiotherapy: risk and realities. Otolaryngol Clin North Am. 2009;42(4):717-729.
63. McIver J.I., Pollock B.E. Radiation-induced tumor after stereotactic radiosurgery and whole brain radiotherapy: case report and literature review. J Neurooncol. 2004;66(3):301-305.
64. Roulet-Perez E., Davidoff V., Mayor-Dubois C., et al. Impact of severe epilepsy on development: recovery potential after successful early epilepsy surgery. Epilepsia. 2010;51(7):1266-1276.
65. Chang E.F., Quigg M., Oh M.C., et al. Predictors of efficacy after stereotactic radiosurgery for medial temporal lobe epilepsy. Neurology. 2010;74(2):165-172.
66. Rheims S., Fischer C., Ryvlin P., et al. Long-term outcome of gamma-knife surgery in temporal lobe epilepsy. Epilepsy Res. 2008;80(1):23-29.
67. Striano S., Striano P., Coppola A., et al. The syndrome gelastic seizures—hypothalamic hamartoma: severe, potentially reversible encephalopathy. Epilepsia. 2009;50(Suppl 5):62-65.
68. Stroup E., Langfitt J., Berg M., et al. Predicting verbal memory decline following anterior temporal lobectomy (ATL). Neurology. 2003;60(8):1266-1273.
69. Hermann B.P., Wyler A.R., Bush A.J., et al. Differential effects of left and right anterior temporal lobectomy on verbal learning and memory performance. Epilepsia. 1992;33(2):289-297.