11 Radial Artery Harvest for Cerebral Revascularization
Technical Pearls
Introduction
Cerebral revascularization remains an important technique in the armamentarium of vascular neurosurgeons. Originally described by Yasargil et al.1 for the treatment of occlusive cerebrovascular disease, the indications for bypass surgery have expanded to include the management of fusiform or giant aneurysms and complex skull-based tumors.2–6 The choice of bypass conduits has evolved concomitantly. The selection criteria for an appropriate conduit is dependent upon a number of factors, including the extent of blood flow augmentation required, graft length, recipient vessel size, long-term occlusion rates, and the ease, availability and safety of the graft’s harvest.6–8 The radial artery is frequently an ideal candidate. Although extensively described for use in coronary circulation bypass, the evaluation of employing radial artery grafts for cerebral revascularization has not been paid a similar service. In this chapter, we focus on the preoperative evaluation and technical nuances of radial artery harvest for cerebrovascular bypass.
Radial artery bypass grafts
The use of the radial artery as a conduit for vascular bypass was first reported by Carpentier et al.9 in the treatment of coronary artery disease. In 1978, Ausman et al.10 described the use of a radial artery interposition graft for PICA distribution revascularization in the neurosurgical literature. However, its subsequent popularity as a bypass conduit diminished initially due to difficulties with vasospasm-induced graft failure and trauma during skeletonized harvesting.11,12 Advancements in calcium channel blockade and the use of the pressure distention technique13 led to its resurgence in the late 1990s.14 The first large series introducing radial artery conduits as a viable alternative to saphenous vein and pedicled arterial grafts for EC-IC bypass was reported by Sekhar et al.13 in 2001. Following that landmark study, it remains the conduit of choice for high flow bypass at many institutions.
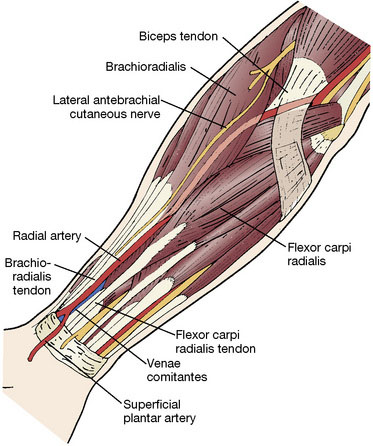
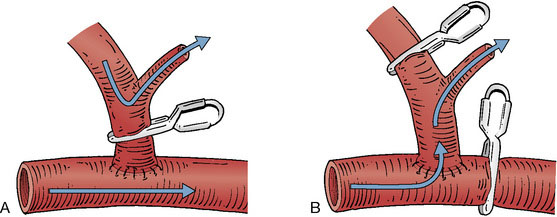
The radial artery is a medium-sized vessel with a diameter of ∼3.5 mm and a flow rate of 40 to 70 ml/min.15 It originates at the bifurcation of the brachial artery in the antecubital fossa, supplies muscular branches to the radial forearm, and terminates in the superficial and deep palmar arcades. Here it anastamoses with the ulnar artery to supply the hand (Figure 11–1). The radial artery is comparable in caliber to common sites of cerebrovascular attachment, namely the M2 and P1 segments, which facilitates anastamosis construction and prevents flow mismatch. It is most commonly used in high flow bypasses, following temporary or permanent occlusion of medium to large vessels, when significant flow augmentation is required. With time and demand, radial artery grafts have been shown to distend appreciably and achieve larger flow rates.3 In comparison to saphenous vein grafts, radial arteries have many advantages. They lack the valves and varices present in venous conduits, which increase the risk of graft thrombosis and induce directionality to the conduit. Arterial walls are thick and facilitate anastamosis construction, minimize kinking, and are physiologically designed to carry arterial blood flow and can adapt to changes in pressure and flow. Radial artery grafts have also been shown to better tolerate temporary occlusion and in the long-term are less susceptible to intimal hyperplasia and graft atherosclerosis than vein grafts.16 They also have improved long-term patency rates as compared to saphenous vein counterparts in both the cardiothoracic17,18 and neurosurgical19,20 revascularization literature. In addition, harvest of the radial artery is relatively straightforward given its constant anatomical location within the radial forearm. Donor site morbidity is minimal and includes vascular insufficiency, forearm or hand dysesthesias and weakness, and a low but measurable harvest site infection rate.21 The major limiting factor for the use of the radial artery is its length. Frequently, lengths of 20 to 25 cm are required to create a tension-free anastamosis from the neck to a cranial attachment. Poor preoperative screening for this limitation can lead to intraoperative abandonment of the graft. Contraindications for its use as a bypass conduit include forearm ischemia on preoperative testing, severe atherosclerosis or calcification within the graft, and dissection from prior cannulation.
Preoperative Diagnostic Evaluation for Radial Artery Harvest
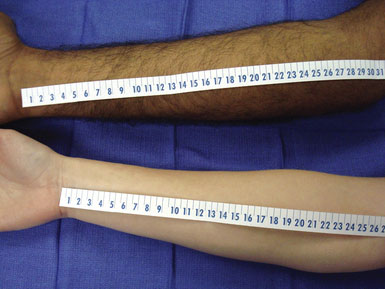
The preoperative assessment of the intended radial harvest graft is crucial to ensure adequate length and prevent postoperative vascular insufficiency within the hand. The nondominant side is preferred; however, either forearm may be used. If appropriate, the arm contralateral to the planned craniotomy allows for multiple teams to proceed simultaneously in the operative room. Short arms may foreshadow a radial artery graft with an inadequate length, requiring more extensive evaluation (Figure 11–2).
The first step in the evaluation of a potential radial artery graft should be an Allen’s test to confirm adequate ulnar collateral circulation to the palmar arcades. This test is inexpensive, easy to perform, and is highly reliable. It is conducted by occluding both the radial and ulnar arteries within the forearm until pallor is appreciated in the hand. The ulnar artery is then released and the pallor should be replaced by hyperemic rubor, which will gradually fade to normal hues. If the ulnar artery supply is insufficient, the hand pallor will remain. The modified Allen’s test employs the use of pulse oximetry while conducting the assessment.22 The probe is placed on the index finger, and a baseline amplitude is measured. Allen’s test is then conducted, and, if the amplitude of the curve is low or the value does not return to baseline within 10 seconds, the harvest of that artery is abandoned. In a prospective study in 2001, Meharwal and Trehan21 used this technique for the preoperative evaluation of 3977 radial artery grafts and reported no postoperative ischemic hand complications. Alternatively, pulse oximetry can be measured intraoperatively following temporary clipping of the radial artery. Digital plethysmography and duplex ultrasonography have also been reported as adjunct measures to assess the relative flow within the radial and ulnar arteries and confirm the patency of the palmar arcade.23,24
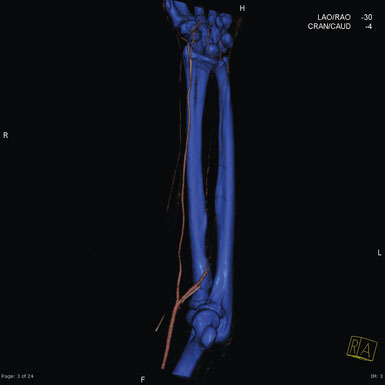
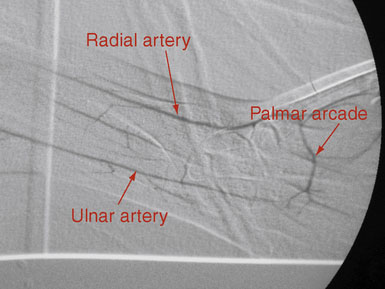
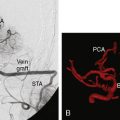
There are many imaging modalities currently available to preoperatively evaluate the caliber and length of the intended radial artery. These include ultrasound, CT angiography, and intraluminal catheter based angiography (Figures 11-3 through 11-5).
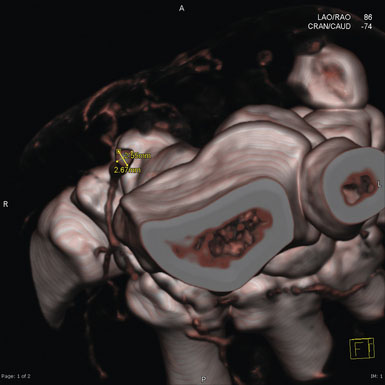
Figure 11–4 Reconstructed CT angiogram of a cross-section of the radial artery in the left distal forearm.
Technique for radial artery harvest and preparation
The technique for radial artery bypass harvest for cerebral revascularization is an evolving process. As detailed above, the preoperative evaluation process is imperative for graft selection and harvest safety profiles. The critical aspect of the procedure involves harvesting a graft of adequate length to enable the creation of a tension-free anastomosis. Various techniques have been reported, including open and endoscopic approaches.25,26 We employ an open technique similar to those previously detailed;8,13 however, over time our technique has evolved to include several new variations.
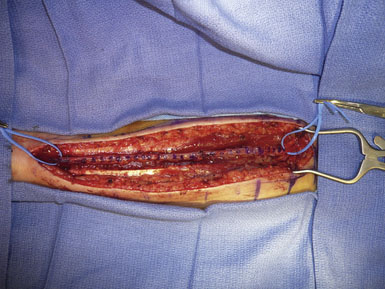
Following extensive preoperative evaluation as described above, the chosen forearm is prepared by the harvesting team. >Assisted by palpation and a Doppler probe, an incision overlying the artery is marked from the proximal transverse furrow of the wrist to the antecubital fossa. The arm is prepped and then opened in distal to proximal fashion, depending upon the length necessary for that particular case. Care is taken to avoid injury to the lateral cutaneous nerve of the forearm, which crosses the artery from lateral to medial near the distal end. The radial artery is identified in the deep fascia, between the brachioradialis and the flexor carpi ulnaris. Muscular branches are coagulated 1 mm from the parent vessel with the use of a harmonic scalpel (Starion CardioForceps, Sunnyvale, CA). Meticulous dissection is employed and care is taken not to injure or manipulate the radial artery. The venae comitantes is left attached to the artery, as it is thought that this may prolong the life of the graft.27 We locate one or two large muscular branches at either end of the graft for use as outlets following anastamosis construction. These branches are divided ∼0.5 to 1 cm from the parent artery and preserved with placement of a temporary aneurysm clip. They will be used later for flushing out clot and evaluation of graft patency (Figure 11–6). The artery is then measured for length and marked on its superficial surface to prevent kinking and rotation during the tunneling process (Figure 11–7). The artery remains in situ, covered in wet gauze until the cranial and cervical dissections are complete. The artery is then stitch ligated proximally and distally, preserving the patency of the ulnar and common interosseous arteries. The most common anomaly of the brachial artery bifurcation is a high division of the radial artery, occurring in up to 15% of cases.28 This can be advantageous for conduit harvest, allowing for increased graft length. The artery is then extracted and flushed with a cocktail of heparinized saline, papaverine, and a calcium channel blocker to remove clots and help prevent vasospasm.13 Various antispasmotic agents have been employed both directly and systemically for this purpose.29 The pressure distention technique as described by Sekhar et al.13 is then undertaken. This involves blunt canalization of the radial artery graft followed by meticulous sequential dilation with heparinized saline. Any remaining patent side branches are cauterized closed with care to avoid injury to the parent vessel. The adventitia is then stripped from both ends of the graft for 1 to 1.5 cm to help facilitate a “clean” anastamosis. The graft remains in a heparinized saline bath until it is ready for implantation. Following proper hemostasis, the forearm is closed in two layers and the skin closed in subcuticular fashion with absorbable suture.
Construction of the extra and intracranial anastomosis and tunneling of the graft are then undertaken with a technique discussed extensively elsewhere.30 Prior to releasing the vascular clamps on the cranial circulation, the preserved large muscular side branches are used to flush out any remaining clot in a manner similar to that of the external carotid artery during a carotid endarterectomy to prevent showering embolic stroke (Figure 11–6A). This technique also ensures patency of the graft and donor vessels immediately following anastomosis construction and can allow for graft evaluation without the use of an additional arteriotomy should micro-Doppler analysis reveal poor flow (Figure 11–6B). Many flow probes exist for evaluation of the graft, including micro-Doppler ultrasonography (Mizuho America, Inc., Ithaca, NY, and Transonic Systems, Inc., Beverly, MA). Pulsatility within the graft is encouraging; however, it is not definitive for patency. On-lay vasodilators can assist in temporarily maximizing graft flow. Intraoperative angiography remains the gold standard to confirm flow. Additionally, ICG video-angiography has become an integral tool within the vascular neurosurgeon’s arsenal and has been shown to be a valid measure of bypass patency.31
Complications
Complications attributable to graft occlusion are limited in the neurosurgical literature. Sekhar and Kalavakonda published their series documenting a 15-year period. A total of 24 radial arteries were harvested in 50 patients with aneurysms and 83 patients with skull base tumors requiring a revascularization procedure. The overall graft patency rate in their cohort was 95.6% with the pressure distension technique credited with overcoming the problem of arterial vasospasm previously encountered with radial artery conduits.32 Similarly, Houkin et al. reported a 100% patency rate in 20 of 43 patients who underwent postoperative MRA and DSA following high-flow EC-IC bypass with radial artery conduits over a 10-year period.19 In the cardiothoracic literature, excellent results have been found with the use of radial artery grafts. The short-, mid-, and long-term patency rates are 96 to 100%, 94 to 97%, and 84 o 96%, respectively;29 however, these grafts are typically shorter in nature and may be prone to striking differences in vascular microenvironments. Saphenous vein grafts patency rates are significantly lower in the neurosurgical literature with short-, mid-, and long-term patency rates of 86%, 82%, and 73%, respectively.33 In 2004, Desai et al.17 enrolled 561 patients in 13 centers into a randomized controlled comparison of radial artery versus saphenous vein conduits for coronary bypass surgery. At 1 year, they found a 5.4% absolute lower occlusion rate in radial arteries as determined by follow-up angiography.
Intraoperative graft occlusions are often secondary to technical issues or hypercoagulable states and are addressed immediately. Postoperative occlusion can occur at any time point and have a number of etiologies related to anastomosis failure, donor or recipient vasculopathy, graft kinking, graft stenosis, flow mismatch, and graft spasm. In addition, intimal hyperplasia and disruption of the vaso vasorum have been implicated as possible etiologies of late occlusion.11,27
The majority of literature describing donor site complications of employing the radial artery as a vascular conduit stems from the thoracic literature as it has been used extensively in coronary bypass surgery. General surgical complications such as wound infection, hematoma formation, and wound dehiscence are encountered rarely. Wound infection of radial artery donor sites range between 2% and 12% with a preoperative diagnosis of diabetes mellitus and a surgical duration of greater than 5 hours identified as independent risk factors.21,34–36 The most common organism encountered is Staphylococcus aureus.36 Postoperative hematomas were observed to develop in 0.3 to 6.4% of cases.21,34,37,38 Wound dehiscence requiring resuturing is encountered very rarely with one study documenting the incidence at 0.16%.21 Neurologic or vascular compromise as a sequelae of radial artery harvest is a rare event and is typically self-limiting in nature. In one study of 560 patients, neurologic complications were reported in 30% with a sensory abnormality in 18% and decreased thumb strength observed in 5%. However, only 12% of patients reported sensory or motor abnormalities that did not improve within 8 months.39 Another study employing the radial artery in 3477 patients found that initially 28% of patients complained of paresthesias and 12% complained of limitation of hand activity that decreased to 3% and 1.22%, respectively, after 6 months.21 In the majority of cases, it appears that the sensory deficits correlate to involvement of the lateral antebrachial cutaneous nerve.37,40 Although hand ischemia has been described in the literature, the incidence of blood flow abnormalities postoperatively was not found to be significant in those patients who had a negative Allen’s test preoperatively.21,40,41
1 Yasargil M.G., Krayenbuhl H.A., Jacobson J.H.2nd. Microneurosurgical arterial reconstruction. Surgery. 1970;67:221-233.
2 Lawton M.T., Hamilton M.G., Morcos J.J., et al. Revascularization and aneurysm surgery: current techniques, indications, and outcome. Neurosurgery. 1996;38:83-92. discussion 92-94
3 Sekhar L.N., Bucur S.D., Bank W.O., et al. Venous and arterial bypass grafts for difficult tumors, aneurysms, and occlusive vascular lesions: evolution of surgical treatment and improved graft results. Neurosurgery. 1999;44:1207-1223. discussion 1223–1224
4 Spetzler R.F., Fukushima T., Martin N., et al. Petrous carotid-to-intradural carotid saphenous vein graft for intracavernous giant aneurysm, tumor, and occlusive cerebrovascular disease. J Neurosurg. 1990;73:496-501.
5 Sundt T.M.Jr, Piepgras D.G., Marsh W.R., et al. Saphenous vein bypass grafts for giant aneurysms and intracranial occlusive disease. J Neurosurg. 1986;65:439-450.
6 Surdell D.L., Hage Z.A., Eddleman C.S., et al. Revascularization for complex intracranial aneurysms. Neurosurg Focus. 2008;24:E21.
7 Baaj A.A., Agazzi S., van Loveren H. Graft selection in cerebral revascularization. Neurosurg Focus. 2009;26:E18.
8 Liu J.K., Kan P., Karwande S.V., et al. Conduits for cerebrovascular bypass and lessons learned from the cardiovascular experience. Neurosurg Focus. 2003;14:e3.
9 Carpentier A., Guermonprez J.L., Deloche A., et al. The aorta-to-coronary radial artery bypass graft. A technique avoiding pathological changes in grafts. Ann Thorac Surg. 1973;16:111-121.
10 Ausman J.I., Nicoloff D.M., Chou S.N. Posterior fossa revascularization: anastomosis of vertebral artery to PICA with interposed radial artery graft. Surg Neurol. 1978;9:281-286.
11 Curtis J.J., Stoney W.S., Alford W.C.Jr, et al. Intimal hyperplasia. A cause of radial artery aortocoronary bypass graft failure. Ann Thorac Surg. 1975;20:628-635.
12 Fisk R.L., Brooks C.H., Callaghan J.C., et al. Experience with the radial artery graft for coronary artery bypass. Ann Thorac Surg. 1976;21:513-518.
13 Sekhar L.N., Duff J.M., Kalavakonda C., et al. Cerebral revascularization using radial artery grafts for the treatment of complex intracranial aneurysms: techniques and outcomes for 17 patients. Neurosurgery. 2001;49:646-658. discussion 658–659
14 Acar C., Jebara V.A., Portoghese M., et al. Revival of the radial artery for coronary artery bypass grafting. Ann Thorac Surg. 1992;54:652-659. discussion 659–660
15 Kamiyama H. [Bypass with radial artery graft]. No Shinkei Geka. 1994;22:911-924.
16 Shi Y., Patel S., Davenpeck K.L., et al. Oxidative stress and lipid retention in vascular grafts: comparison between venous and arterial conduits. Circulation. 2001;103:2408-2413.
17 Desai N.D., Cohen E.A., Naylor C.D., et al. A randomized comparison of radial-artery and saphenous-vein coronary bypass grafts. N Engl J Med. 2004;351:2302-2309.
18 Motwani J.G., Topol E.J. Aortocoronary saphenous vein graft disease: pathogenesis, predisposition, and prevention. Circulation. 1998;97:916-931.
19 Houkin K., Kamiyama H., Kuroda S., et al. Long-term patency of radial artery graft bypass for reconstruction of the internal carotid artery. Technical note. J Neurosurg. 1999;90:786-790.
20 Kocaeli H., Andaluz N., Choutka O., et al. Use of radial artery grafts in extracranial-intracranial revascularization procedures. Neurosurg Focus. 2008;24:E5.
21 Meharwal Z.S., Trehan N. Functional status of the hand after radial artery harvesting: results in 3,977 cases. Ann Thorac Surg. 2001;72:1557-1561.
22 Johnson W.H.3rd, Cromartie R.S.3rd, Arrants J.E., et al. Simplified method for candidate selection for radial artery harvesting. Ann Thorac Surg. 1998;65:1167.
23 Abu-Omar Y., Mussa S., Anastasiadis K., et al. Duplex ultrasonography predicts safety of radial artery harvest in the presence of an abnormal Allen test. Ann Thorac Surg. 2004;77:116-119.
24 Jarvis M.A., Jarvis C.L., Jones P.R., et al. Reliability of Allen’s test in selection of patients for radial artery harvest. Ann Thorac Surg. 2000;70:1362-1365.
25 Connolly M.W., Torrillo L.D., Stauder M.J., et al. Endoscopic radial artery harvesting: results of first 300 patients. Ann Thorac Surg. 2002;74:502-505. discussion 506
26 Gonzalez L.F., Patterson D.L., Lekovic G.P., et al. Endoscopic harvesting of the radial artery for neurovascular bypass. Neurosurg Focus. 2008;24:E10.
27 Dietl C.A., Benoit C.H. Radial artery graft for coronary revascularization: technical considerations. Ann Thorac Surg. 1995;60:102-109. discussion 109–110
28 Doyle J.R., Botte M.J. Surgical Anatomy of the Hand and Upper Extremity. Philadelphia: Lippincott Williams & Wilkins, 2003.
29 Kobayashi J. Radial artery as a graft for coronary artery bypass grafting. Circ J. 2009;73:1178-1183.
30 Abdulrauf S.I. Extracranial-to-intracranial bypass using radial artery grafting for complex skull base tumors: technical note. Skull Base. 2005;15:207-213.
31 Woitzik J., Horn P., Vajkoczy P., et al. Intraoperative control of extracranial-intracranial bypass patency by near-infrared indocyanine green videoangiography. J Neurosurg. 2005;102:692-698.
32 Sekhar L.N., Kalavakonda C. Cerebral revascularization for aneurysms and tumors. Neurosurgery. 2002;50:321-331.
33 Regli L., Piepgras D.G., Hansen K.K. Late patency of long saphenous vein bypass grafts to the anterior and posterior cerebral circulation. J Neurosurg. 1995;83:806-811.
34 Hata M., Shiono M., Sezai A., et al. Comparative study of harvest-site complications following coronary artery bypass grafting between the radial artery and the saphenous vein in identical patients. Surg Today. 2005;35:711-713.
35 Saeed I., Anyanwu A.C., Yacoub M.H., et al. Subjective patient outcomes following coronary artery bypass using the radial artery: results of a cross-sectional survey of harvest site complications and quality of life. Eur J Cardiothorac Surg. 2001;20:1142-1146.
36 Trick W.E., Scheckler W.E., Tokars J.I., et al. Risk factors for radial artery harvest site infection following coronary artery bypass graft surgery. Clin Infect Dis. 2000;30:270-275.
37 Budillon A.M., Nicolini F., Agostinelli A., et al. Complications after radial artery harvesting for coronary artery bypass grafting: our experience. Surgery. 2003;133:283-287.
38 Greene M.A., Malias M.A. Arm complications after radial artery procurement for coronary bypass operation. Ann Thorac Surg. 2001;72:126-128.
39 Denton T.A., Trento L., Cohen M., et al. Radial artery harvesting for coronary bypass operations: neurologic complications and their potential mechanisms. J Thorac Cardiovasc Surg. 2001;121:951-956.
40 Chong W.C., Ong P.J., Hayward C.S., et al. Effects of radial artery harvesting on forearm function and blood flow. Ann Thorac Surg. 2003;75:1171-1174.
41 Fox A.D., Whiteley M.S., Phillips-Hughes J., et al. Acute upper limb ischemia: a complication of coronary artery bypass grafting. Ann Thorac Surg. 1999;67:535-536. discussion 536–537