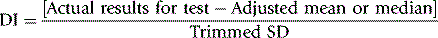Chapter 25 Quality assurance
In the haematology laboratory it is essential to ensure that the right test is carried out on the right specimen and that the correct results are delivered to the appropriate recipient without delay. Quality assurance (QA) is defined as the overall programme for achieving these objectives. It must also ensure adequate control of the pre-analytical and post-analytical stages, i.e. from specimen collection to the timely despatch of an informative report. A QA programme should also include standardization of tests and of instrumentation in order to achieve acceptable levels of precision and accuracy. These objectives represent good laboratory practice (GLP); the mechanism for achieving GLP is encompassed in Total Quality Management (TQM) (Table 25.1).
Table 25.1 Definitions used in total quality management (TQM)
| 1. | Quality assurance (QA) | Overall programme that ensures that the final results reported are correct. QA includes an adequate control of the pre-analytical and post-analytical stages from specimen collection to the timely dispatch of an informative report. A QA programme also includes standardization of procedures and instrumentation. |
| 2. | Quality control (QC) | Measures that must be included during each assay run to verify that the test is working properly. |
| 3. | Proficiency testing (PT) | Procedure to determine the quality of the results generated by the laboratory. Accordingly, PT is a challenge to the effectiveness of the QA and QC programmes and it can be internal or external (also termed ‘quality assessment’). |
| 4. | Internal quality control (IQC) | Monitoring the haematology test procedures to ensure continual evaluation of the reliability of the daily work of the laboratory with validation of tests before reports are released. |
| 5. | External quality assessment (EQA) | Evaluation by an outside agency of the between-laboratory and between-method comparability. It can be organized nationally, regionally or internationally. |
| 6. | Continuous quality improvement (CQI) | Philosophy and attitude for analysing capabilities and processes and improving them repeatedly to achieve the objective of customer satisfaction. |
| 7. | Accreditation | Certification by a duly recognized authority of the facilities, capability, objectivity, competence and integrity of an agency. |
| 8. | Non-compliance | A process which does not comply with a quality system. |
Standardization
Clinical laboratory errors lead to adverse effects on patient diagnosis, therapy and outcomes, also resulting in the inappropriate use of funds. Standardization plays an important role in patient care because it contributes to a decrease in the number of errors, thus improving the harmonization of procedures and comparability between different laboratories. The introduction of the International Standardization Organization (ISO) standards BS EN ISO 9001 (Quality management systems – requirements), BS EN ISO 17025 (General requirements), BS EN ISO 15189 (Medical laboratories – particular requirements for quality and competence) and BS EN ISO 22870 (Point-of-care testing) places quality at the heart of all activities (see also Table 24.8). In the UK, the focus for laboratory practice has been on Clinical Pathology Accreditation (UK) Ltd (CPA), which has evolved to reflect the way service delivery is perceived within pathology and is now linked to UK Accreditation Service. Quality management systems may also include requirements arising from regulations related to blood and tissues and the Good Manufacturing Practice (GMP) guide, while through the ISO standards, there is a move towards international harmonization.
Standardization of procedures and devices used in the haematology laboratory are the concern of the international professional organizations, especially the International Council for Standardization in Haematology (ICSH). The International Organization of Standardization (ISO) and Comité Européen de Normalization (CEN) have also established standards for medical laboratory practice and for the use of in vitro diagnostic medical devices. At a national level, the British Committee for Standards in Haematology (BCSH) publishes guidelines in books, on websites or as journal articles, and in the USA, a wide range of practice guidelines have been published by the Clinical and Laboratory Standards Institute (CLSI) (formerly the National Committee for Clinical Laboratory Standards, NCCLS). Lists of published documents and catalogues from these various organizations can be found on their various websites (see Table 25.2; see also Tables 24.5 and 24.8).
Table 25.2 Some organizations involved in standardization and quality management systems
| Abbreviation | Organization | Website |
|---|---|---|
| AFNOR | Association Française de Normalization | www.afnor.org |
| AENOR | Asociación Española de Normalización y Certificación | www.aenor.es |
| INSTAND | Gesellschaft zur Förderung der Qualitätssicherung in medizinischen Laboratorien | www.instandev.de |
| WHO | WHO EQAS for Haematology | www.who.int/diagnostics_laboratory/quality/haematology/en/index.html |
| AMREF | African Medical and Research Foundation, Nairobi, Kenya | www.amref.org |
| ANCLSH | Asian Network for Clinical Laboratory Standardization and Harmonization | www.ancls.org |
| ASQ | American Society of Quality | www.asq.org |
| BCSH | British Committee for Standards in Haematology | www.bcshguidelines.com |
| CAP | College of American Pathologists | www.cap.org |
| CEN | The European Committee for Standardization | www.cen.eu |
| CLSI | Clinical and Laboratory Standards Institute | www.clsi.org |
| ICSH | International Council for Standardization in Haematology | www.islh.org |
| IRMM | Institute of Reference Materials and Measurements | Irmm.jrc.ec.europa.eu |
| ISO | International Organization for Standardization | www.iso.org |
| JCAHO | Joint Commission for the Accreditation of Healthcare Organizations | www.jointcommission.org |
| PPTC | Pacific Paramedical Training Centre New Zealand | www.pptc.org.nz |
| RCPA | Royal College of Pathologists Australia | www.rcpaqap.com.au |
| UK NEQAS | United Kingdom National External Quality Assessment Scheme | www.ukneqas.org.uk |
| EQALM | European Committee of External Quality Assurance Programmes in Laboratory Medicine | www.eqalm.org |
External quality-assessment schemes have a role in identifying unsatisfactory performance by devices. Some of the terms and definitions generally used in laboratory practice are summarized in Table 25.3.
Table 25.3 Terms relating to quality control in clinical laboratory practice
| Precision | Can be controlled by replicate tests and by repeated tests on previously measured specimens |
| Accuracy | Can be checked only by the use of reference materials that have been assayed by reference methods |
| Standardization | Encompasses both materials and methods. Material standard or reference preparations are used to calibrate analytical instruments and to assign quantitative values to calibrators. Where possible they must be traceable to defined physical or chemical measurement based on the metrological units of length (metre), mass (kilogram), amount of substance (mole) and time (seconds) |
| Reference method | Is an exactly defined technique that is used in association with a reference preparation, when available, to provide sufficiently precise and accurate data for scientific purposes and for assessing the validity of other methods |
| Selected method | Is one that is directly comparable with and traceable to the international reference method; it serves as an alternative to the reference method when an international reference material is not available; it should be used for evaluation and validation of a proposed routine (working) method |
| Working (or recommended) method | Is intended for use in routine practice, taking account of economy of labour and materials and ease of performance and having been shown by a validation study with a reference method to be sufficiently reliable for its intended purpose |
| Controls | Are preparations that are used for either internal quality control or external quality assessment. Some control preparations have assigned values but they should not be used as standards because the assigned values are usually only approximations and they are often stable for a limited time only |
| True value | This is an ideal concept which in general cannot be achieved |
| Accepted true value | The value approximating the true value, the difference between the two values is negligible |
| Error | The discrepancy between the result of a measurement and the true (or accepted true) value |
| Random error | An error which varies in an unpredictable manner, in magnitude and sign, when a large number of measurements of the same quantity are made under effectively identical conditions |
| Systematic error | An error which, in the course of a number of measurements of the same value of a given quantity, remains constant when measurements are made under the same conditions or varies according to a definite law when conditions change |
Control materials and reference standards
Reference Standards
International reference materials relevant to haematology are held at designated institutions (Table 25.4), the relevant websites should be checked for availability of any particular one.
The accessibility of an international reference preparation of haemiglobincyanide (HiCN), first developed by the International Council for Standardization in Haematology (ICSH), has provided improved accuracy of haemoglobin measurement. In some countries, preparations that conform to the international standard are certified by the appropriate national authorities. An important feature of this material is that it is stable for at least several years. A limited quantity of the international standard can be obtained from WHO; a comparable certified reference material is available from IRMM (Table 25.4) and ICSH has recently produced a new preparation with similar specifications.1 Where the use of cyanide reagent for routine haemoglobinometry is prohibited, the haemiglobincyanide standard can still be used to assign a haemoglobin value to a lysate or a whole blood preparation, which is then used as the local secondary standard after appropriate dilution. However, many laboratories are unable to make use of this reference preparation as they no longer have suitable instruments on which to use it. Undiluted lysate is usually stable for up to 6 months, or frozen for several years. Whole blood is stable for about 3 weeks, but for only a few days after dilution. Both whole blood and lysates are useful for quality assurance of haemoglobinometry; whole blood reference samples should be introduced into batches of blood samples and all the samples should be assayed together. This applies to both automated and manual methods.
| General haematology | Erythropoietin, human, urinary Erythropoietin, recombinant DNA-derived Ferritin, human, recombinant Hb A2 Hb F Folate, whole blood C-reactive protein Human serum for immunoassay |
WHO/NIBSC |
| Haemiglobincyanide Haemoglobin Reference Standard |
WHO/NIBSC BCR/IRMM ICSH |
|
| Immunohaematology | Anti-A blood typing serum, anti-B blood typing serum, anti-RhD incomplete blood typing serum, RhD complete blood typing serum, E complete blood typing serum | WHO/CLB |
| Immunology | Human serum immunoglobulin Immunoglobulin G (IgG), A, M, E Antinuclear factor, homogeneous Horseradish peroxidase-conjugate sheep antihuman IgG Human serum complement components C1q, C4, C5, factor B and functional CH50 |
WHO/NIBSC |
| Coagulation | Ancrod, antithrombin plasma, antithrombin concentrate, factor VIII and von Willebrand factor plasma, II, VII, IX, X, VIII, IXa concentrate, plasma fibrinogen, plasmin, plasminogen activator inhibitor, tissue plasminogen activator recombinant, streptokinase, alpha-thrombin, beta-thromboglobulin, antihuman platelet antigen 1a, platelet factor 4, proteins S and C plasma, urokinase high molecular weight, von Willebrand factor concentrate, von Willebrand factor antigen | WHO/NIBSC |
| Thromboplastin bovine combined, thromboplastin human recombinant, thromboplastin rabbit plain | BCR/IRMM WHO/CLB |
|
| WHO/NIBSC | National Institute for Biological Standards and Control, South Mimms EN6 3QH, UK. E-mail: standards@nibsc.ac.uk; www.NIBSC/Catalogue list/Reference standards. See also: World Health Organization/biological standards | |
| BCR/IRMM | Institute for Reference Materials and Measurements, Retieseweg B2440, Geel, Belgium. E-mail: bcr.sales@irmm.jrc.be; www.irmm/reference materials | |
| WHO/CLB | Central Laboratory of Netherlands Red Cross Blood Transfusion Service, 125 Plesmanlaan, 1066 AD Amsterdam, Netherlands. Netherlands Blood Transfusion Service/Central Laboratory |
Assigning Values to Reference Materials
Methods used for assigning values to reference materials must be as accurate and precise as is practical. Standardized reference methods have been described for haemoglobin concentration, red blood cell count, white blood cell count and packed cell volume/haematocrit (see Chapter 3).
Quality assurance procedures
The procedures that should be included in a quality assurance programme vary with the tests undertaken, the instruments used and (especially if these include a fully automatic counting system) the size of the laboratory and the numbers of specimens handled. Also, the computer facilities available and the amount of time that can be devoted to the quality control assurance must be taken into account. At least some form of internal quality control must be undertaken and there must be participation in an external quality assessment scheme where one is available. Some control procedures should be performed daily and other performance checks should be done at appropriate intervals. The latter is particularly important when there is a change in staff and after maintenance service or repair has been carried out on equipment. A comprehensive protocol is summarized in Table 25.5.
| 1. Calibration with reference standards | ||
| Instruments | 6-month intervals or more frequently if control chart or EQA indicates bias or fluctuation in results and after any repair/service | |
| Others: pipettes and scales | Calibration of equipment used for manual techniques should not be overlooked; these should be done annually | |
| Diluting systems | Initially and at 1–2-week intervals | |
| 2. Control chart with control material | ||
| Daily or more frequently with each batch of specimens | ||
| Duplicate tests on two or three patients’ samples: if control chart or delta check shows discrepancies | ||
| 3. Analysis of patients’ results | ||
| Daily to check constancy of mean values for MCV, MCH, MCHC: correlation assessment of test report | ||
| Cumulative results: following previous tests and if changes in clinical state | ||
| Blood film examination if unusual test results and/or counter flags appear | ||
| 4. EQAS performance | ||
| Assessment monthly | ||
Quality design must begin with analytical quality, as it is the essential quality characteristic of any laboratory test. Analytical variations may arise from unsuspected abnormal binding protein(s) in patients, such as heterophile antibodies, anti-animal antibodies and anti-idiotypic antibodies. The exact effect will depend on the site of the interaction with the reaction, leading to falsely raised or lowered measurements.2 More recent data underline the importance of analytical accuracy due to the frequent calibration error that leads to analytical bias affecting the number of patients passing decision thresholds in practice guidelines. The effects of this medically and economically have been demonstrated.3 To ensure reliability in the analytical phase, procedures are required for internal quality control, external quality assessment and standardization. All laboratory staff require training in these various aspects of quality assurance. A useful training manual from WHO* describes the principles and methods, together with practical exercises to illustrate these.4 Another good teaching source is J.O. Westgard’s website: www.westgard.com; this includes a ‘Lesson of the Month’ and other current topics that are regularly updated. The quality control of the analytical stage includes Internal Quality Control (IQC) and External Quality Assessment (EQA).
Internal Quality Control
Control Charts
These were first applied in clinical chemistry by Levey and Jennings.5 They are now widely used in haematology for both automated and manual procedures. Samples of the control specimen are included in every batch of patients’ specimens and the results are checked on a control chart. To check precision, it is not necessary to know the exact value of the control specimen. If, however, its value has been determined reliably by a reference method, the same material can also be used to check accuracy or to calibrate an instrument. If possible, controls with high, low and normal values should be used. It is advisable to use at least one control sample per batch, even if the batch is very small, also at set intervals during a large run, at least once for every 50 patient specimens. Because the controls are intended to simulate random sampling, they must be treated exactly like the patients’ specimens. The results obtained with the control samples can be plotted on a chart as described below.
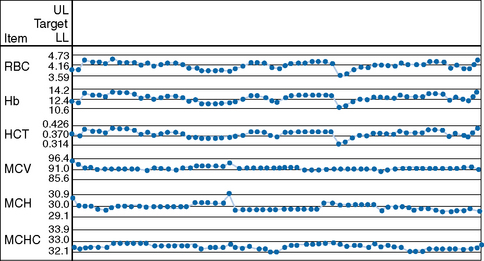
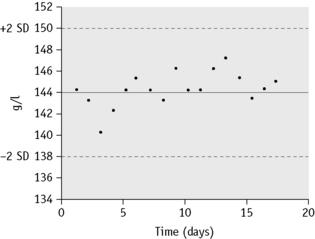
The mean value and standard deviation (SD) of the control specimen should first be established in the laboratory where the tests are performed. Using arithmetic graph paper, a horizontal line is drawn to represent the mean (as a base), and on an appropriate scale of quantity and unit, lines representing +2SD and −2SD are drawn above and below the mean. The results of successive control sample measurements are plotted. If the test is satisfactory, sequential results oscillate about the mean value and <5% of the results fall outside 2SD. Figure 25.1 illustrates a control chart from an automated system; a similar principle can be used for simple methods where the data are plotted manually (Fig. 25.2).
Any of the following indicates a fault in technique or in the instrument or reagent:
Duplicate Tests on Patients’ Specimens
Duplicate tests on patients’ specimens provide another way of checking the precision of routine work.6
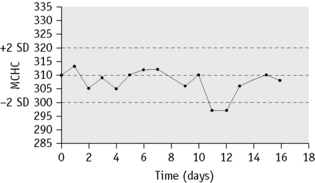
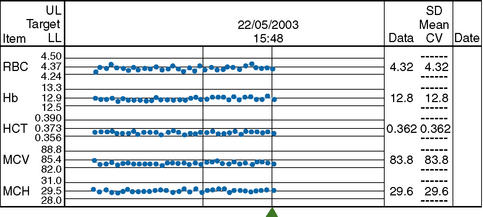
MCHC is used to identify any drift of the three indices and used to identify instrument faults; an increased SD signifies loss of precision. To ensure that each batch is representative, the samples should be randomized before analysis and, if possible, within any batch of 20, no more than seven should come from one clinical source or from patients with the same clinical condition. In laboratories still using manual methods, a simple adaptation of the same principle can be applied, confined to MCHC and excluding results from any special clinic that are likely to be specifically biased. From the daily means for all measurements on 10 consecutive working days, an overall daily mean and SD are established. The mean MCHC is then calculated at the end of each day. If the test does not vary by more than ±2SD, it is considered satisfactory but this may be misleading if there is an error in the same direction in both haemoglobin and PCV. The results may be displayed graphically, as illustrated in Figure 25.3. It is useful in validating successive batches of calibrators. The method is now incorporated in many automated blood counters (Fig. 25.4). A similar instrument-specific procedure is used by some manufacturers who gather the data submitted through network links by users of their instruments. This enables them to maintain a constant check of performance of these instruments overall and to detect any that require recalibration or investigation of faults.
External Quality Assessment
EQA complements internal quality control and is a basic requirement for clinical laboratory certification and accreditation. Certification is a procedure by which a third party gives written assurance that a product, process or service conforms to specific requirements, whereas accreditation is a procedure by which an authoritative body gives formal recognition that a body or person is competent to carry out specific tasks. Accreditation standards related to clinical laboratories place emphasis on having an effective quality assurance system in place, with a commitment to meeting the needs of patients and their doctors as users of laboratory services and a need for continuous cycle of quality improvement at the centre of all policy-making operational decisions (see also Chapter 24, p. 579)
The term EQA, also known as proficiency testing (PT), was adopted in 1979 by a WHO working group on Quality Assurance of Health Laboratories. It is defined as: ‘a system whereby a set of reagents and techniques are assessed by an external source and the results of the testing laboratory are compared with those of an approved reference laboratory or agency’.7 It allows an individual laboratory to compare its performance for one or more tests or techniques against that of other laboratories. Thus, even when all precautions are taken to achieve accuracy and precision in the laboratory, errors arise that are only detectable by objective EQA of the performance of a number of laboratories on material which has been supplied specially for the purpose. EQAS are usually organized, nationally or regionally, as one or PT programmes. National schemes are frequently termed NEQAS (National External Quality Assessment Scheme).
In summary, EQA is primarily intended to check the technical competence of individual laboratories, but it also provides an overview for assessing the state of the art and for identifying problems that have occurred in instruments, reagents or kits that may be affecting an entire group of users through no fault of their own. This provides a means for verification of manufacturers’ conformity to their claimed specifications and for monitoring product performance in the laboratory, a requirement in Europe in the context of the Directive on In-Vitro Diagnostic Medical Devices.8
More information of this type would be useful for determining the priorities for national or international agencies engaged in developing standards. EQA should be used for highlighting shortcomings and for evaluating methods, technologies and reference/standard preparations. It is important for a clinical laboratory to participate in EQAS for accreditation or certification, as clearly stated in ISO 9000, ISO Guides 25 and 58 and also in ISO 15189.9
Standardization of EQA Schemes
Assessment of Participant Performance
Quantitative Tests
Deviation index
From the results returned by the participants, the median or mean and SD are calculated. The deviation index (DI; also termed ‘z-score’) is used by many EQA schemes for assessing performance in quantitative tests. This is the amount of deviation from the mean (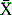 ) or median (m) relative to a unit of 1 standard deviation (SD) (see below). The median is used when there is a non-Gaussian distribution of data with a wide range of results.10 The SD is ‘trimmed’ to exclude any results outside ± 3SD. An individual laboratory can then compare its performance in the survey with that of other laboratories and with its own previous performance from the deviation index (DI) or z-score. This is calculated as the difference between the individual laboratory’s result and the median or mean relative to the SD. Thus,
) or median (m) relative to a unit of 1 standard deviation (SD) (see below). The median is used when there is a non-Gaussian distribution of data with a wide range of results.10 The SD is ‘trimmed’ to exclude any results outside ± 3SD. An individual laboratory can then compare its performance in the survey with that of other laboratories and with its own previous performance from the deviation index (DI) or z-score. This is calculated as the difference between the individual laboratory’s result and the median or mean relative to the SD. Thus,
When results have a Gaussian distribution, the mean and SD are adjusted in a preliminary calculation by excluding all results in the highest and lowest 5%.
The DI provides a simple method for judging performance in a survey and it also indicates whether there have been changes in sequential surveys, thus distinguishing between casual errors and persistent unsatisfactory performance. A limitation of DI is that it is purely statistical. As the state of the art improves, some blood count parameters will have a CV of only 1–2%. Thus, the DI will indicate poor performance with unrealistically small deviations from the median. It may be better to use clinical relevance when determining the acceptable limits of percentage deviation from the target value. There are some differences in these limits as established in the USA for CLIA ′88 requirements, those proposed by ICSH and those by an ISO panel.9–11
Out of consensus method
Target values and bias
The pattern of bias in successive surveys indicates whether there is a constant calibration error or a progressive fault or whether the original defect has been corrected.
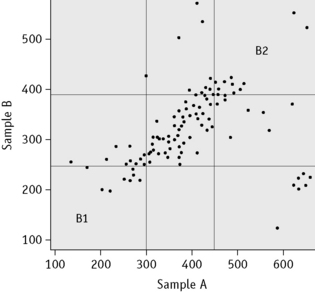
Youden (xy) plot
The Youden plot is a useful method for relating measurements on two samples in a survey to provide a graphic display and, when a participant’s results are unsatisfactory, for distinguishing between a consistent bias and random error. Results for the two samples are plotted on the horizontal (x) and the vertical (y) axis, respectively and the standard deviations (2SD or 3SD) for the two sets are drawn and interpreted as shown in outline in Figure 25.5.
Clinical significance
| Hb and RBC (by cell counter) | 3–4% |
| PCV, MCV, MCH, MCHC | 4–5% |
| Leucocyte count | 8–10% |
| Platelet count | 10–15% |
| Vitamin B12, folate, iron, ferritin | 20% |
| Hb A2 and Hb F quantitation | 5% |
Interpretive Tests
All correct observations are given a positive score and false-positive observations are subtracted in accordance with the grading. The result is expressed as a percentage of the total possible score established by the referees. The more stringent limits have been proposed to provide greater sensitivity in diagnostic discrimination, but they may be impractical, resulting in an overestimate of the numbers of poor performers.12,13
While few EQA schemes provide a bone marrow slide on a regular basis, due to the difficulties of producing large numbers of films from the same donor, the introduction of digital morphology EQAS may be useful in the future. Preparation and reporting on these films should be standardized from conception using guidelines.14
Internal audit for total quality management
Preparation of extended-life material for use in quality assessment
Preparation of Preserved Whole Blood
Method4,15
Preparation of Haemolysate
Method
Preparation of Stabilized Whole Blood Control Material
Method
Preparation of Stable Control Material for EQAS
Preparation of Surrogate Leucocytes
Chicken and turkey red blood cells are nucleated and, when fixed, their size is within the human leucocyte range as recognized on electronic cell counters. They are thus suitable to serve as surrogate leucocytes.4 This material may not be suitable for counting systems that are based on technologies other than impedance cell sizing.
Method
Preparation of QC Material for Platelet Count
Reagent
Alsever’s solution. (A) Trisodium citrate, 16 g; NaCl, 8.2 g to 1 litre with water; (B) dextrose, 41 g to 1 litre with water. Store at 4°C. Immediately before use, mix equal volumes of A and B; filter through 0.2 μm micropore filter. EDTA solution. 100 g/l of K2 EDTA in the Alsever’s solution; stable for 6 months at 4°C.4
Method
A simpler method for preserving platelets by adding prostaglandin E1 to blood in ACD has been reported to provide a control preparation with stability of about 14 days.17
1 Davis B.H., Jungerius B. International Council for Standardization in Haematology technical report 1–2009: new reference material for haemiglobincyanide for use in standardization of blood haemoglobin measurements. International Journal of Laboratory Hematology. 2010;32:139-141.
2 Kazmierczak S.C., Catrou P.G. Analytical interference. More than just a laboratory problem. Am J Clin Pathol. 2000;113:9-11.
3 Klee G.G., Schryver P.G., Kisabeth R.M. Analytical bias specifications based on the analysis of effects on performance of medical guidelines. Scand J Clin Lab Invest. 1999;59:509-512.
4 Lewis S.M. Quality Assurance in Haematology Document LAB/98.4. Geneva: World Health Organization; 1998.
5 Levey S., Jennings E.R. The use of control charts in the clinical laboratory. Am J Clin Pathol. 1950;20:1059-1066.
6 Cembrowski G.S., Lunetsky E.S., Patrick C.C., et al. An optimized quality control procedure for hematology analyzers with the use of retained patient specimens. Am J Clin Pathol. 1988;89:203-210.
7 World Health Organization. EURO report 36: External quality assessment of health laboratories. Copenhagen: WHO Regional Office for Europe; 1981.
8 Directive 98/79/EC of the European Parliament and of the Council on in vitro Diagnostic Medical Devices, October 1998.
9 International Standard ISO 15189 2007 Medical Laboratories. Particular Requirements for Quality and Competence
10 International Council for Standardization in Haematology. Guidelines for organization and management of external quality assessment using proficiency testing. International Journal of Haematology. 1998;68:45-52.
11 International Organization for Standardization. Guide 43–41 proficiency testing by interlaboratory comparisons. In Development and Operation of Proficiency Testing Schemes. Geneva: ISO; 1997.
12 Vives Corrons J.L., Albarede S., Flandrin G., et al. Guidelines for blood smear preparation and staining procedure for setting up an external quality assessment scheme for blood smear interpretation. Part I: Control Material. Clin Chem Lab Med. 2004;42(8):922-926.
13 Vives Corrons J.L., Blerk M Albarede S., et al. Guidelines for setting up an External Quality Assessment Scheme for blood smear interpretation. Part II: Survey Preparation, Statistical Evaluation and Reporting. Clin Chem Lab Med. 2006;44(8):1039-1043.
14 Lee H., Erbert W.N., Porvit A., et al. ICSH guidelines for standardization of bone marrow specimens and reports: International Council for Standardization in Haematology. International Journal of Laboratory Hematology. 2008;30:349-363.
15 Deom A., El Aouad R., Heick C.C., et al. Requirements and Guidance for External Quality Assurance Programmes for Health Laboratories: Document LAB/2000. Geneva: World Health Organization; 2000.
16 Reardon D.M., Mack D., Warner B., et al. A whole blood control for blood count analysers and source material for an external quality assessment scheme. Med Lab Sci. 1991;48:19-26.
17 Zhang Z., Tatsumi N., Tsuda I., et al. Long-term preservation of platelet count in blood for external quality control surveillance using prostaglandin E1. Clin Lab Haematol. 1999;21:71.
* Available on request to the Department of Essential Health Technology, WHO, 1211 Geneva 27, Switzerland.
* A mixing-dispensing unit is recommended for large-scale dispensing. A suitable one can be constructed using standard glassware, etc., except for the specially manufactured flask head which is not commercially available and needs to be made by a company specializing in Medical Engineering. (See Ward PG, Chappel DA, Fox JG, et al 1975 Mixing and bottling unit for preparing biological fluids used in quality control. Laboratory Practice. 24:577–583.)