Chapter 16 Pupillary and Eyelid Abnormalities
Pupillary Abnormalities
Pupil Anatomy and Neural Control
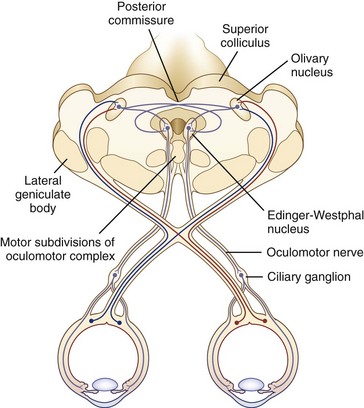
The size of the pupil is determined by the balance of action between two muscles embedded in the iris: the sphincter pupillae, primarily under parasympathetic control, and the dilator pupillae, primarily under sympathetic control. The sphincter is located circumferentially around the pupil and constricts the pupil on exposure to light. The dilator is situated radially and dilates the pupil in darkness. The resting tone of the pupil is primarily determined by the degree of baseline parasympathetic innervation. On exposure to light, the pupil constricts as a result of the pupillary light reflex (Fig. 16.1). The afferent limb of the light reflex originates in the retinal ganglion cells and travels via the optic nerve, chiasm, and optic tract to the dorsal midbrain pretectum just rostral to the superior colliculus, from which neuronal signals are relayed bilaterally to the paired parasympathetic Edinger-Westphal nuclei of the oculomotor nerve (Akert et al., 1980; Nester et al., 2010; Papageorgiou, Wermund, and Wilhelm, 2009). In primate studies, the pretectal olivary nucleus is identified as the primary relay between retinal ganglion cells and the Edinger-Westphal nuclei (see Fig. 16.1) (Kourouyan and Horton, 1997; Pong and Fuchs, 2000; Warwick, 1954). The efferent limb of the light reflex consists of the preganglionic parasympathetic fibers traveling from the Edinger-Westphal nuclei in both oculomotor nerves to the intraorbital ciliary ganglion and the postsynaptic, postganglionic short ciliary nerves carrying the parasympathetic innervation from the ciliary ganglion to the sphincter muscle (see Chapter 70 for a more extensive discussion of oculomotor nucleus and nerve anatomy). The pupillary near reflex consists of pupillary constriction as a response to viewing of a near target. Such miosis is a component of the near triad, along with lens accommodation and ocular convergence. The anatomic substrate of the pupillary near reflex is less well defined than that of the light reflex.
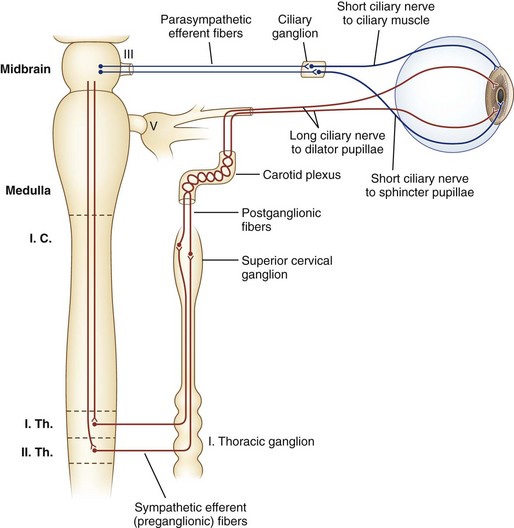
Sympathetic nerves destined for the dilator muscle consist of a chain of three neurons: first-, second-, and third-order neurons (Fig. 16.2). First-order neurons originate in the posterolateral hypothalamus and descend in the dorsolateral brainstem and in the intermediolateral cell column of the spinal cord to the thoracic level (T2). After the first-order neurons synapse in the spinal cord, second-order neurons exit to the paravertebral cervical sympathetic chain via the ventral horns. They ascend near the lung apex and then with the common and internal carotid arteries to reach the superior cervical ganglion in the neck at the angle of the jaw, where they synapse with the third-order neurons. At this point, sudomotor fibers related to facial sweating separate anatomically from those fibers serving pupillary dilation. From the superior cervical ganglion, third-order neurons continue their ascent with the carotid artery through the skull base and into the cavernous sinus, where they temporarily travel with the abducens nerve. They then join branches of the trigeminal nerve, with which they enter the orbital apex, bypass the ciliary ganglion, and reach the dilator muscle via long ciliary nerves (see Fig. 16.2).
Normal Pupil Phenomena
Hippus, or pupillary unrest, is a nonrhythmical, small-amplitude (<1 mm) variation in pupil size that occurs in healthy eyes after light stimulation and is not triggered by accommodation (Hunter et al., 2000; Thompson et al., 1971). After a light stimulus, the pupil constricts, redilates, and then oscillates. The role (if any) of these oscillations in pupillary or visual function is unclear.
With age, pupils become smaller and less reactive to light. Such pupils generally do not require diagnostic evaluation. Although not a normal condition, diabetes similarly affects the pupils sufficiently often as to make small and poorly reactive pupils “normal” in that clinical setting in the absence of any other pathological pupil state. Both parasympathetic and sympathetic pupillary dysfunction are reported in diabetes, and pupillary abnormalities are correlated with a number of other disease processes, including the presence of cardiovascular autonomic dysfunction, peripheral neuropathy, and retinopathy (Bremner and Smith, 2006; Bremner, 2009).
Afferent Pathological Conditions of the Pupils
The relative afferent pupillary defect (RAPD), or Marcus Gunn pupil, is a hallmark of optic nerve disease. It is a manifestation of a relative unilateral disruption of the afferent limb of the pupillary light reflex via the optic nerve and occurs as a result of the consensual and bilateral nature of the light reflex. When a light stimulus is applied to one eye, both pupils constrict as a result of the bilateral connections between pretectal nuclei and the Edinger-Westphal nuclei. When the swinging flashlight test is performed to evaluate for RAPD, the light will be transmitted normally via one optic nerve and to a lesser extent by the diseased optic nerve. This results in the appearance of a brisk bilateral pupillary constriction when the light stimulus is applied to the normal eye and a lesser constriction with initial relative dilation when the light stimulus is transferred to the eye with the optic neuropathy, thus the RAPD. The greater the extent of retinal ganglion cell and optic nerve damage, the larger the relative dilation of the pupil will appear (Lagreze and Kardon, 1998). See Chapter 36 for a more detailed description and a table with step-by-step instructions on how to evaluate for an RAPD, and see Chapter 15 for an extensive discussion of optic nerve disease.
Efferent Pathological Conditions of the Pupils
Clinical Presentation and Examination
The pupil examination (see Chapter 36 for additional discussion) of a patient being evaluated for a pupillary abnormality should begin with observation of the resting positions of the pupils in the ambient room light and of resting eyelid positions. If anisocoria is present, the degree of anisocoria in the light versus in the dark should be evaluated. Pupil evaluation in the dark is done by having the patient look straight ahead in a dark room while the examiner shines just enough light indirectly from below to allow visualization of the pupils. Assessment in the light versus in the dark will help determine which pupil, if either, is the pathological pupil. Anisocoria that is more pronounced in the light suggests that the large pupil is the abnormal pupil, because the small pupil will constrict normally to the light, enhancing the difference in size between the small pupil and the large, nonconstricting pupil. The differential diagnosis for this includes parasympathetic outflow damage (tonic pupil, oculomotor palsy), iris sphincter injury or ischemia, pharmacological pupil dilation, and excessive sympathetic activation. Anisocoria that is more pronounced in the dark suggests that the small pupil is the pathological pupil, because the large pupil will dilate normally in the dark, enhancing the difference in size between the large pupil and the small, nondilating pupil. A caveat to the suggestion that the small pupil is pathological in this situation is that the anisocoria will generally be slightly enhanced in the dark with physiological anisocoria, a normal situation in which neither pupil is pathological (Lam et al., 1987). The differential diagnosis for a pathologically small pupil includes sympathetic inhibition (Horner syndrome), local iris pathology such as inflammation, and parasympathetic stimulation (e.g., pharmacological stimulation).
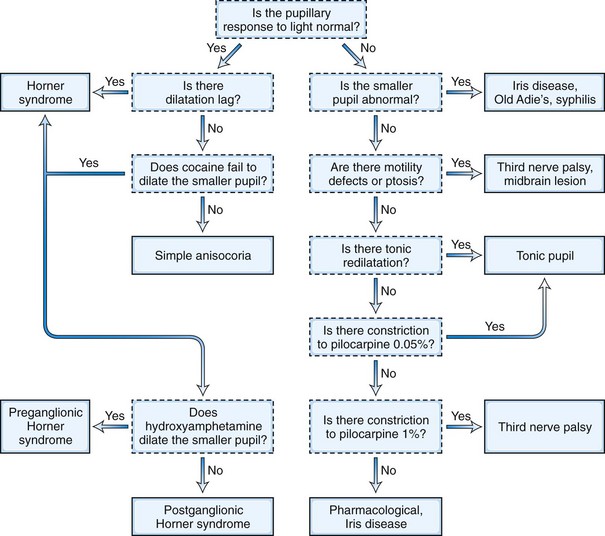
The next step in the examination is evaluation of the direct and consensual pupillary light reflexes, followed by evaluation of the pupillary near response (Kasthurirangan and Glasser, 2006). The near response is best assessed by having the patient hold the thumb several inches in front of the eyes while looking across the room and then to have the patient shift and maintain gaze on the thumb. In certain abnormal conditions, the pupils may have light-near dissociation with poor direct light reflexes but relatively preserved constriction to near stimuli. Once the abnormal pupil is identified, pharmacological testing with a number of topical eye drops is often used for confirmation and assistance in localization (Table 16.1). The general method is application of a drop of the pharmacological agent into each eye, followed by reexamination of the pupils 30 to 45 minutes later. Sensitivity of accurate response detection is increased with before-and-after photographic documentation. Diagnostic use of topical pharmacological agents and additional helpful examination findings for each of the specific disorders are described in detail in the respective sections of this chapter covering the disorders and outlined in a systematic guideline in Fig. 16.3. The presence of a slight degree of ptosis in the eye with the small pupil may indicate sympathetic dysfunction; ptosis in the eye with the larger pupil may indicate oculomotor nerve dysfunction. Careful examination of vision, ocular motility, facial strength and sensation, and ocular fundus should also be performed.
| Testing | Mechanism of Action | Diagnostic Utility and Expected Response |
|---|---|---|
| ANISOCORIA GREATER IN THE LIGHT (ABNORMAL LARGER PUPIL) | ||
| Dilute pilocarpine (0.0625% or 0.1%) | Parasympathomimetic; direct sphincter stimulation | Tonic pupil will constrict (denervation supersensitivity) |
| Normal pupil and pupil affected by oculomotor palsy or pharmacological blockade will not respond | ||
| Pilocarpine (1%) | Parasympathomimetic; direct sphincter stimulation | Normal pupil and pupil affected by oculomotor palsy will constrict |
| Pupil affected by pharmacological blockade will not respond | ||
| ANISOCORIA GREATER IN THE DARK (ABNORMAL SMALLER PUPIL) | ||
| Cocaine (2% to 10%) | Inhibits norepinephrine reuptake at the sympathetic terminus | Horner pupil will not dilate |
| Normal pupil will dilate | ||
| Hydroxyamphetamine (1%) | Induces third-order sympathetic neuron to release any stored norepinephrine | Preganglionic (first- or second-order neuron) Horner pupil will dilate |
| Postganglionic (third-order neuron) Horner pupil will not dilate | ||
| Apraclonidine (0.5%) | Weak sympathetic agonist | Horner pupil will dilate (denervation supersensitivity) |
| Normal pupil will not change or will constrict slightly |
Anisocoria Greater in the Light
Postganglionic Parasympathetic Dysfunction—Tonic Pupil
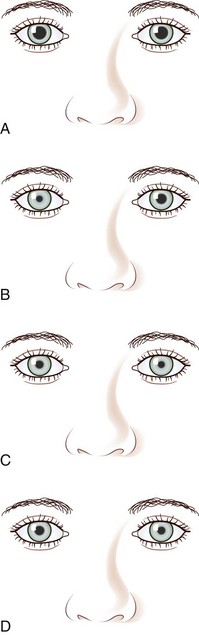
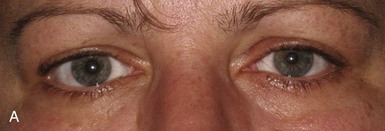
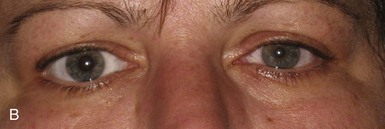
A tonic pupil is large and reacts poorly to light and slowly to near stimuli; after distance refixation, it exhibits a slow, tonic redilation (Fig. 16.4). After removal of the near stimulus, such tonic redilation transiently reverses the anisocoria, making the tonic pupil smaller than the normal pupil, because the normal pupil quickly redilates, and the tonic pupil does not (see Fig. 16.4, D). In the ophthalmology office, evaluation of a tonic pupil at the slit lamp reveals wormlike segmental constriction of some portions of the pupil and absent constriction of other portions. Because of parasympathetic denervation of the iris sphincter muscle, denervation supersensitivity may occur, and instillation of a dilute solution of the cholinergic agent, pilocarpine, may confirm the diagnosis (Fig. 16.5; see also Table 16.1). A 0.1% solution is often suggested, but false-positive results may be more frequent than with a 0.0625% solution (Ashker et al., 2008; Leavitt et al., 2002). Dilute pilocarpine will constrict the tonic pupil, but not a normal pupil or a dilated pupil from another cause such as oculomotor palsy or pharmacological toxicity. Solutions of pilocarpine less than 1% are not commercially available and must be prepared by dilution with preservative-free normal saline solution.
Tonic pupils result from damage to the intraorbital ciliary ganglion or short ciliary nerves from any etiology, including focal infectious (herpes zoster, syphilis) or noninfectious (giant cell arteritis) inflammation, malignant infiltration, paraneoplastic processes, and trauma. Tonic pupils may also be seen as a component of a systemic autonomic neuropathy (see Chapter 77) (Bremner and Smith, 2006; Yamashita et al., 2010), Guillain-Barré syndrome or its Miller Fisher variant, and botulism. Perhaps the most common and most easily recognizable tonic pupil is the benign Holmes-Adie pupil, which often presents with acute painless enlargement of the pupil and may be accompanied by complaints of photophobia and blurred near vision due to involvement of fibers serving the ciliary body for lens accommodation. In 80% of patients, the condition is unilateral. It is most common in healthy young women and is thought to be due to a viral ciliary ganglionitis. Additional examination findings may include decreased deep tendon reflexes and decreased corneal sensation. The affected pupil tends to become smaller over time and may even eventually become smaller than the unaffected pupil. Ross syndrome is the triad of tonic pupils, hyporeflexia, and segmental anhidrosis (Ross, 1958). It is unclear whether this syndrome is a variant of the Holmes-Adie syndrome or a mechanistically distinct disorder, but impaired thermoregulation is the distinguishing element (Nolano et al., 2006; Shin et al., 2000). Harlequin syndrome, failure of facial flushing to thermal or emotional stress, may also occur with tonic pupils and hyporeflexia, although Horner syndrome is more common (Bremner and Smith, 2008).
Preganglionic Parasympathetic Dysfunction—Oculomotor Palsy
Pupillary enlargement from oculomotor palsy may be accompanied by ocular motor deficits due to impairment of the oculomotor-innervated medial, inferior, and superior rectus and inferior oblique muscles, and by ptosis due to involvement of the levator superioris muscle. Isolated pupillary involvement may occur as an early sign of neurovascular compression by a posterior communicating artery aneurysm because the pupillary fibers are located superficially on the superomedial surface of the nerve. It may also be a sign of herniation of the temporal lobe uncus from increased intracranial pressure, known as Hutchinson pupil (Ropper, 1990). A fixed and dilated Hutchinson pupil on initial presentation of head trauma, stroke, or intracranial mass lesion is associated with 75% mortality (Clusmann et al., 2001). Although dilute pilocarpine (0.1%) does not typically result in constriction of the pupil in this situation, it should constrict after administration of 1% pilocarpine (see Table 16.1). This may help in differentiating isolated pupillary enlargement from an oculomotor palsy from pharmacological pupillary enlargement, because the pharmacological pupil should not constrict. See Chapter 70 for a complete discussion of oculomotor nerve anatomy and clinical lesions; Fig. 70.2 shows an example of oculomotor palsy.
Pharmacological Mydriasis
Pharmacological mydriasis usually occurs after accidental or intentional instillation of anticholinergic agents such as atropine. Accidental mydriasis usually occurs by hand-to-eye contact in individuals who have contact with dilating agents; examples include use of a scopolamine skin patch for motion sickness, administration of eye drops to a family member with eye disease, or exposure to plants with parasympathetic blocking activity such as angel’s trumpet (Havelius and Asman, 2002). Inadvertent administration of nebulized ipratropium into the eye via an ill-fitting face mask in a critically ill patient may result in unilateral mydriasis that mimics a Hutchinson pupil from intracranial hypertension and uncal herniation (Eustace et al., 2004). Pharmacologically dilated pupils tend to be larger than the dilated pupil of third cranial nerve palsy. Sympathomimetic agents, such as phenylephrine, cause mydriasis that is less extensive and prolonged than that caused by anticholinergic agents. Pharmacologically dilated pupils do not constrict after administration of 1% pilocarpine (see Table 16.1).
Anisocoria Greater in the Dark
Horner Syndrome
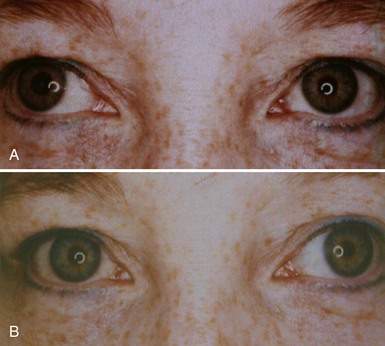
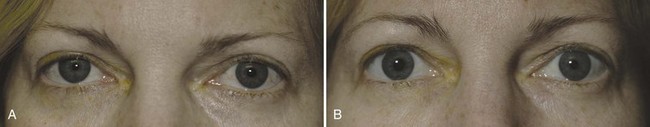
The classic Horner syndrome triad of sympathetic dysfunction consists of ipsilateral ptosis, miosis, and facial anhidrosis. The lesion may be anywhere along the three-neuron sympathetic pathway (described in Pupillary Anatomy and Neural Control). Anhidrosis is only present with lesions of the first- or second-order (preganglionic) neurons because the fibers serving facial sweating take a pathway distinct from those destined for the pupillary dilator muscle after synapsing in the superior cervical ganglion. The ptosis results from impaired innervation of the Müller muscle, which contributes only slightly to maintenance of eyelid opening. As a result, the maximum ptosis possible from sympathetic dysfunction is only 1 to 2 mm. Apparent enophthalmos (an illusion of a smaller, “sunken” eye) due to palpebral fissure narrowing from involvement of the Müller muscle in combination with subtle elevation of the lower lid (“reverse” or “upside down” ptosis) from involvement of lower lid smooth muscle may occur. An additional finding on examination is dilation lag, the delayed dilation of the Horner pupil in darkness (Pilley and Thompson, 1975) (Fig. 16.6).
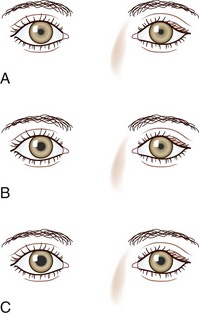
The differential diagnosis for Horner syndrome varies for a preganglionic lesion (first- or second-order neurons) versus a postganglionic (third-order neuron) lesion, and differentiation between the two will dictate the diagnostic approach. Determination of preganglionic versus postganglionic lesion can be made with pupillary diagnostic eye drops (Mughal and Longmuir, 2009). Hydroxyamphetamine (1%) is the drop most commonly used for this purpose. However, some diagnosticians prefer to utilize cocaine (2% to 10%) eye drops to confirm the presence of Horner syndrome rather than proceeding directly to hydroxyamphetamine to determine a preganglionic versus postganglionic lesion. Administration of both cocaine and hydroxyamphetamine cannot be done on the same day; at least 48 hours must separate the two diagnostic eye drop tests. From a practical standpoint, both medications are difficult to obtain. Cocaine is a controlled substance, must be kept under lock and key, and results in a positive urine toxicology screen for 2 days after ocular administration (Jacobson et al., 2001). Hydroxyamphetamine eye drops are no longer marketed in the United States but are usually obtainable from compounding pharmacies such as Leiter’s Park Avenue Pharmacy, 1756 Park Avenue, San Jose, CA 95126 (www.leiterrx.com; phone 800-292-6773). Cocaine inhibits the reuptake of norepinephrine at the sympathetic terminus, thereby causing accumulation of norepinephrine that is continuously released from sympathetic nerve terminals (Kardon et al., 1990). The result is dilation of a normal pupil but impaired dilation in the sympathetically denervated pupil, with a resultant increase in anisocoria. Lack of dilation after cocaine administration occurs for both preganglionic and postganglionic Horner syndrome. Hydroxyamphetamine induces the third-order neuron to release any stored norepinephrine. It will dilate a normal pupil and a pupil with an intact third-order sympathetic neuron. Therefore, if pupillary dilation occurs in a Horner pupil, the lesion is localized to the preganglionic first- or second-order neurons. If pupillary dilation fails to occur, a third-order neuron Horner syndrome is likely (Fig. 16.7, A-B).
Apraclonidine, in a 0.5% solution, is a recent addition to the diagnostic armamentarium for Horner syndrome (Koc et al., 2005; Morales et al., 2000). It is an α-adrenergic agonist approved for lowering intraocular pressure during certain ophthalmologic surgical procedures. After bilateral administration of apraclonidine in a patient with Horner syndrome, there is reversal of the anisocoria caused by dilation of the Horner pupil and either constriction or no change in the normal pupil (Fig. 16.8). This occurs because apraclonidine is a weak α-1 agonist with no direct dilating activity in the normal pupil; the Horner pupil is dilated due to denervation supersensitivity. In addition to pupil dilation, the Horner-induced ptosis is resolved; however, this alone cannot be used to confirm diagnosis, because elevation of the eyelid may also be seen in normal eyes. Apraclonidine as a replacement for cocaine in the diagnosis of Horner syndrome is promising, but rigorous study is needed; it is unclear whether all interruptions of sympathetic outflow will result in denervation sensitivity and how much time is necessary for this to occur (Kardon, 2005). Positive apraclonidine testing is reported within 36 hours of lesion onset; however, false-negative testing is also reported up to 16 days after carotid dissection (Dewan et al., 2009; Lebas et al., 2010).
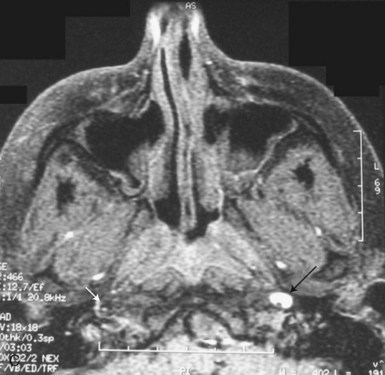
Any inflammatory, compressive, neoplastic, traumatic, or other lesion along the course of the three-neuron sympathetic pathway may cause Horner syndrome. Two lesions in particular deserve detailed description, owing to their clinical importance and potential ominous prognosis if left undetected. These are carotid dissection, with presentation as an isolated Horner syndrome, and the Pancoast tumor. Acute onset of a painful Horner syndrome leads to suspicion of a carotid dissection until proven otherwise. Such presentation requires a very high degree of suspicion because it is often the intracranial precavernous portion of the carotid artery that is affected. It is not uncommon for this lesion to be missed on magnetic resonance angiography (MRA) and detected only by careful examination of non–contrast-enhanced axial T1-weighted magnetic resonance images (MRIs) through the base of the skull, looking for a crescent of hyperintense signal surrounding the carotid artery flow void (see Fig. 16.7, C). Treatment most typically consists of anticoagulation with heparin, followed by warfarin for 3 to 6 months, to minimize the risk of emboli from the dissected artery. A preganglionic Horner syndrome, particularly in a patient with a history of tobacco use, should prompt a search for a pulmonary apical neoplasm, or Pancoast tumor. Outside of these two clinical settings, the workup for Horner syndrome consists of neuroimaging of the affected sympathetic pathways: brain and spine (the entire cervical and first two thoracic segments) MRI with gadolinium for a preganglionic lesion, and brain MRI with gadolinium and MRA for a postganglionic lesion (Almog et al., 2010; Digre et al., 1992). In children, the majority of Horner syndromes are congenital; however, birth trauma, vascular malformations, carotid dissection, and neoplasm are also possible causes. The recommended evaluation in children includes neuroimaging and urinary catecholamine testing to screen for neuroblastoma (Smith et al., 2010).
Episodic Anisocoria
Anisocoria may be intermittent. Physiological anisocoria can vary from week to week and occasionally from hour to hour. A rare condition known as tadpole pupil results from intermittent spasms of segments of the pupillary dilator muscle; often these patients have an underlying Horner syndrome (Thompson et al., 1983). A related phenomenon is oculosympathetic spasm associated with lesions of the cervical spinal cord.
Benign episodic unilateral mydriasis is a diagnosis of exclusion in which episodes of pupillary dilation last from minutes to a few days (Jacobson, 1995). Some patients have typical migraine headaches or a trigeminal autonomic cephalgia, but many patients have isolated monocular visual blurring or no symptoms at all (Antonaci et al., 2010). The frequency of episodes varies from a few per week to one every few years. Some patients appear to have parasympathetic insufficiency; others, sympathetic hyperactivity of the pupil, but no other neurological problems develop. Cyclical oculomotor palsy is a rare condition in which periodic oculomotor spasms occur in a patient with third cranial nerve palsy. During the spasms, the eyelid rises, the exotropic eye moves to the midline, and the pupil constricts. In some cases, the spasms are limited to the pupil. Intermittent spasm of portions of the pupillary sphincter may occur in traumatic third cranial nerve paralysis and with aberrant oculomotor regeneration. Unilateral pupillary dilation and other pupillary signs can also occur during seizures, and rhythmic pupillary oscillation is reported in Creutzfeld-Jakob disease (Nagasaka et al., 2010).
Pupillary Light-Near Dissociation
The term light-near dissociation refers to pupils that have marked diminution of constriction to light, with a much better constriction to near stimuli. The differential diagnosis includes tonic pupils, Argyll-Robertson pupils, and the dorsal midbrain syndrome (Han et al., 2010). Tonic pupils were described earlier (Anisocoria Greater in the Light). Argyll-Robertson pupils are small and irregular. This condition is historically most commonly seen in the setting of syphilis, but currently diabetes is likely the most common etiology, although the pupils are not miotic. Localization of the lesion causing such pupils is unclear, but possibilities include the ciliary ganglion and the dorsal midbrain (Thompson and Kardon, 2006). The dorsal midbrain syndrome, or Parinaud syndrome (see Chapter 19) consists of impaired upgaze, convergence-retraction nystagmus, and lid retraction (Collier sign), in addition to pupillary light-near dissociation. It is generally due to pineal gland lesions or hydrocephalus. Light-near dissociation in this centrally mediated mechanism may be due to destruction of the dorsally located olivary pretectal nuclei involved in the pupillary light reflex (see Pupillary Anatomy and Neural Control), with relative sparing of the fibers serving the pupillary near response, which seem to approach the pupillomotor center from a more ventral pathway.
Eyelid Abnormalities
Eyelid Anatomy and Neural Control
The width of the palpebral fissure is determined by the activity balance of the orbicularis oculi muscles, the levator palpebrae superioris muscles, smooth muscle called Müller muscle, and periorbital and eyelid connective tissues. The orbicularis oculi is innervated by the facial nerve, the levator palpebrae superioris by the oculomotor nerve, and Müller muscle by the sympathetic nervous system. Activation of the orbicularis oculi results in eye closure. The levator palpebrae and, to a lesser extent, Müller muscle are responsible for maintaining eye opening. See Chapter 70 for a complete discussion of the anatomy of the facial and oculomotor nerves.
Pathological Conditions of the Eyelids
Clinical Presentation and Examination
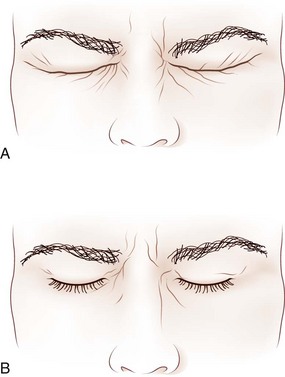
Steps in the examination of the eyelids are summarized in Box 16.1. The normal palpebral fissure is between 12 and 15 mm. At rest, the upper eyelid normally covers the upper 1 to 2 mm of the iris, and the upper border of the lower eyelid just touches the lower border of the iris (Fig. 16.9). In an eye with mild upper lid retraction, the lid just touches the upper limit of the iris, or a small amount of sclera is visible between the iris and the upper lid margin (superior scleral show) (Fig. 16.10). With lower lid retraction, sclera is visible between the iris and the lower lid margin (inferior scleral show). Patients with factitious (voluntary) ptosis display contraction of the orbicularis oculi with wrinkling of the skin near the lid margin and a lowered eyebrow. An example of true upper lid ptosis is seen in Fig. 16.10. In an effort to elevate the lids, patients with bilateral ptosis often have associated frontalis contraction with eyebrow elevation. Proptosis (exophthalmos, or eye protrusion) can be evaluated by inspecting the globe position with respect to the orbital rim by looking tangentially across the orbital margin from above, below, or laterally.
Box 16.1 Clinical Examination of the Eyelids
Assessment of lid position in different gaze positions may reveal lid retraction on downgaze, which suggests lid lag from orbital disease such as thyroid ophthalmopathy or aberrant regeneration of the oculomotor nerve. The latter condition may also cause lid retraction on adduction (see Fig. 70.2, A–B). There are many potential lid abnormalities with neuromuscular junction disease such as myasthenia gravis, including increased ptosis or induction of ptosis with prolonged upgaze, Cogan sign, enhanced ptosis, and the peek sign. Cogan sign is induced by rapid return of the eyes to central position after sustained downgaze; the upper eyelid may elevate excessively, twitch, and then return to its ptotic position. Enhanced ptosis is increased ptosis in a less or nonptotic eyelid on manual elevation of the more ptotic lid. The peek sign is positive when prolonged eye closure increases orbicularis oculi weakness and causes lid separation and globe exposure despite initial complete eye closure. Incomplete gentle eye closure may also suggest facial weakness from causes other than myasthenia gravis, including facial nerve palsy or a myopathic process such as chronic progressive external ophthalmoplegia (CPEO). More subtle weakness of the orbicularis oculi muscles may be detected with forced eye closure (Fig. 16.11). Forced lid closure with rapid reopening is an excellent technique for evaluating hemifacial spasm, apraxia of lid opening, and blepharospasm—conditions that are often worsened by this maneuver. Patients with apraxia of lid opening contract their forehead and elevate their brows when asked to open their eyes, but the eyes remain closed. Patients with blepharospasm have persistent orbicularis oculi contraction after being asked to open their eyes.
The final step in evaluation is to look for abnormalities of the pupil and ocular motility and to perform a full neurological examination. Ophthalmological consultation may be helpful from a diagnostic standpoint if the etiology of the lid abnormality is not readily apparent; it will also help ensure the corneal health of patients with lid abnormalities. After completion of these examination steps (outlined in Table 16.2), it should be possible to determine whether the abnormal palpebral fissure is the one that is too wide or the one that is too narrow, and to determine whether the dynamics of eyelid opening and closure are normal.
Pathologically Widened Palpebral Fissures
When the abnormal palpebral fissure is too wide, the differential diagnosis includes lid retraction from orbital disease (e.g., thyroid eye disease) or facial nerve dysfunction with resultant orbicularis oculi weakness (Bartley, 1996). Less common, but also to be considered in the differential diagnosis, are the stare and decreased blinking associated with parkinsonism; Summerskill sign, lid retraction associated with end-stage hepatic disease (Meadows et al., 2001); and Collier sign, or midbrain-induced lid retraction seen as a component of the dorsal midbrain syndrome. These conditions may result in exposure keratitis, causing the patient to complain of eye pain and blurred vision.
Pathologically Narrowed Palpebral Fissures
When the abnormal palpebral fissure is too narrow, the differential diagnosis includes pseudoptosis and true ptosis (Figueiredo, 2010). Pseudoptosis may result from dermatochalasis (redundancy of eyelid skin) or contralateral lid retraction. True ptosis may be congenital or acquired via mechanical, myopathic, or neurogenic factors. Congenital ptosis can be caused by abnormalities of the levator muscle or its innervation and can be unilateral or bilateral. Additional congenital anomalies resulting in ptosis include trigeminal-levator synkinesis (Marcus Gunn jaw-wink phenomenon), in which unilateral ptosis occurs with jaw movements; Duane syndrome with paresis of abduction, adduction, (or both), associated with ptosis and globe retraction on attempted adduction; congenital oculomotor palsies; and congenital Horner syndrome (Demirci et al., 2010).
Acquired mechanical causes of ptosis include dehiscence of the levator aponeurosis secondary to aging (involutional blepharoptosis, or senile ptosis) or trauma and inflammation or infiltration of the lid with amyloid or malignancy (Klapper et al., 1998). Dehiscence of the levator aponeurosis is likely the most common cause of ptosis in the elderly and is accompanied by an elevated superior eyelid crease. Such ptosis typically worsens with downgaze (Olson and Putterman, 1995). The most common myopathic cause of ptosis is CPEO, which is most frequently a manifestation of a mitochondrial myopathy (see Chapter 63). CPEO may occur in isolation or as a component of a wider mitochondrial defect such as Kearns-Sayre syndrome, with pigmentary retinopathy and cardiac conduction defects. Oculopharyngeal muscular dystrophy and myotonic dystrophy are additional myopathic causes of ptosis. Myasthenia gravis is a frequent cause of acquired ptosis—either in isolation or accompanied by ocular motor defects or generalized weakness. Diagnosis is most reliably confirmed with acetylcholine receptor antibodies, a decremental response with electromyographic repetitive stimulation, or single-fiber electromyography. Neurogenic etiologies include Horner syndrome, oculomotor paresis, and occasionally, disease of the cerebral hemispheres (cerebral ptosis)—particularly right hemispheric lesions (Averbuch-Heller et al., 2002; Manconi et al., 2006) (Table 16.2). Rightward gaze deviation and upgaze paresis often accompany cerebral ptosis. Horner syndrome and oculomotor paresis are described in detail earlier in this chapter. Lower lid elevation may accompany upper lid ptosis in Horner syndrome and may occur secondary to enophthalmos (e.g., from orbital blowout fractures), local lid edema, excessive lid closure, or factitious ptosis.
Table 16.2 Lid Abnormalities Associated with Cerebral Hemisphere Lesions
| Lid Abnormality | Pathological Findings |
|---|---|
| Unilateral ptosis | Contralateral hemisphere lesions; contralateral and ipsilateral hemisphere lesions |
| Bilateral ptosis | Bilateral frontal lobe lesions; unilateral and bilateral hemisphere lesions |
| Impairment of voluntary lid opening and closure | Dominant hemisphere or bilateral hemisphere lesions or basal ganglia disease |
| Impairment of voluntary and reflex lid opening (apraxia of lid opening) | Basal ganglia disease; bilateral hemisphere lesions; nondominant cerebral lesion |
| Difficulty maintaining lid closure (motor impersistence) | Nondominant hemisphere or bilateral hemisphere lesions |
| Difficulty maintaining lid opening (reflex blepharospasm) | Nondominant hemisphere or bilateral hemisphere lesions |
Modified with permission from Nutt, J.G., 1977. Lid abnormalities secondary to cerebral hemisphere lesions. Ann Neurol 1, 149-151; and from Johnston, J.C., Rosenbaum, D.M., Picone, C.M., et al., 1989. Apraxia of eyelid opening secondary to right hemisphere infarction. Ann Neurol 25, 622-624.
Dynamic Eyelid Abnormalities
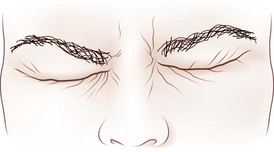
Dynamic lid abnormalities include blepharospasm, apraxia of lid opening, hemifacial spasm, myokymia, and myotonia. Blepharospasm consists of uncontrolled bilateral orbicularis oculi contraction causing eyelid closure (Fig. 16.12) (Etgen et al., 2006; Hallett, 2002). Secondary blepharospasm may occur as a result of photophobia from ocular disease such as dry eyes, corneal disease (abrasion, keratitis), and ocular inflammation. When the condition is bilateral with no associated ocular or neurological abnormalities, the diagnosis is benign essential blepharospasm, which is a focal dystonia. When there are dystonic movements of the lower face, jaw, tongue, or neck, the designation is oromandibular dystonia with blepharospasm (Meige syndrome), a segmental dystonia. When orbicularis contraction occurs only with lid manipulation or other stimulation, the term reflex blepharospasm is sometimes used. Most patients with parkinsonism have reflex blepharospasm, and all types of blepharospasm are made worse by lid manipulation and photophobia. Factitious (voluntary) blepharospasm is rare. The term apraxia of lid opening describes inappropriate inhibition of the levator palpebrae muscle that occurs in some patients with bilateral or nondominant cerebral lesions or in association with benign essential blepharospasm (Fig. 16.13) (Dewey and Marion, 1994; Krack et al., 1994).
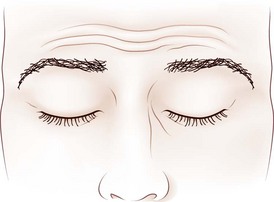
Fig. 16.13 Apraxia of lid opening. Elevated eyebrows and forehead contraction with persistent eye closure.
Hemifacial spasm is characterized by paroxysmal, involuntary, synchronous contraction of all muscles innervated by the facial nerve on one side (Fig. 16.14) (see Chapter 70). Involuntary twitches of portions of the orbicularis oculi muscle (orbicularis myokymia) are common in normal individuals; these generally affect the lower eyelid. In facial myokymia, these muscular contractions involve other facial muscles. Occasionally, facial myokymia is associated with spastic paretic hemifacial contracture, a condition characterized by tonic contraction of facial muscles on one side with associated weakness of the same muscles. Facial myokymia may be unilateral or bilateral. This sign indicates brainstem disease; the most common causes are multiple sclerosis and brainstem neoplasm (usually gliomas), but Guillain-Barré syndrome and extraaxial neoplasms may be causal. Myotonia of lid closure may occur in myotonic dystrophy, hypothyroidism, and hyperkalemic (and more rarely, hypokalemic) familial periodic paralysis.
Akert K., Glicksman M.A., Lang W., et al. The Edinger-Westphal nucleus in the monkey. A retrograde tracer study. Brain Res. 1980;184:491-498.
Almog Y., Gepstein R., Kesler A. Diagnostic value of imaging in Horner syndrome in adults. J Neuroophthalmol. 2010;30:7-11.
Antonaci F., Sances G., Loi M., et al. Sunct syndrome with paroxysmal mydriasis: clinical and pupillometric findings. Cephalalgia. 30, 2010. epub ahead of print
Ashker L., Weinstein J.M., Dias M., et al. Arachnoid cyst causing third cranial nerve palsy manifesting as isolated internal ophthalmoplegia and iris cholinergic supersensitivity. J Neuroophthalmol. 2008;28:192-197.
Averbuch-Heller L., Leigh R.J., Mermelstein V., et al. Ptosis in patients with hemispheric strokes. Neurology. 2002;58:620-624.
Bartley G.B. The differential diagnosis and classification of eyelid retraction. Ophthalmology. 1996;103:168-176.
Bremner F. Pupil evaluation as a test for autonomic disorders. Clin Auton Res. 2009;19:88-101.
Bremner F.D., Smith S.E. Pupil abnormalities in selected autonomic neuropathies. J Neuroophthalmol. 2006;26:209-219.
Bremner F., Smith S. Pupillographic findings in 39 consecutive cases of harlequin syndrome. J Neuroophthalmol. 2008;28:171-177.
Clusmann H., Schaller C., Schramm J. Fixed and dilated pupils after trauma, stroke, and previous intracranial surgery: management and outcome. J Neurol Neurosurg Psychiatry. 2001;71:175-181.
Demirci H., Frueh B.R., Nelson C.C. Marcus Gunn jaw-winking synkinesis: clinical features and management. Ophthalmology. 2010;117:1447-1452.
Dewan M.A., Harrison A.R., Lee M.S. False negative apraclonidine testing in acute Horner syndrome. Can J Ophthalmol. 2009;44:109-110.
Dewey R.B.Jr. Maraganore DM: Isolated eyelid-opening apraxia: report of a new levodopa-responsive syndrome. Neurology. 1994;44:1752-1754.
Digre K.B., Smoker W.R., Johnston P., et al. Selective MR imaging approach for evaluation of patients with Horner’s syndrome. AJNR Am J Neuroradiol. 1992;13:223-227.
Etgen T., Muhlau M., Gaser C., et al. Bilateral grey-matter increase in the putamen in primary blepharospasm. J Neurol Neurosurg Psychiatry. 2006;77:1017-1020.
Eustace N., Gardiner C., Eustace P., et al. Nebulised ipratropium causing a unilateral fixed dilated pupil in the critically ill patient: a report of two cases. Crit Care Resusc. 2004;6:268-270.
Figueiredo A.R.P. Blepharoptosis. Semin Ophthalmol. 2010;25:39-51.
Hallett M. Blepharospasm: recent advances. Neurology. 2002;59:1306-1312.
Han S.W., Ryu J.H., Baik J.S., et al. Early dorsal midbrain syndrome mimicking an Adie’s tonic pupil. J Clin Neurol. 2010;6:38-40.
Havelius U., Asman P. Accidental mydriasis from exposure to angel’s trumpet (Datura suaveolens). Acta Ophthalmol Scand. 2002;80:332-335.
Hunter J.D., Milton J.G., Ludtke H., et al. Spontaneous fluctuations in pupil size are not triggered by lens accommodation. Vision Res. 2000;40:567-573.
Jacobson D.M. Benign episodic unilateral mydriasis: clinical characteristics. Ophthalmology. 1995;102:1623-1627.
Jacobson D.M., Berg R., Grinstead G.F., et al. Duration of positive urine for cocaine metabolite after ophthalmic administration: implications for testing patients with suspected Horner syndrome using ophthalmic cocaine. Am J Ophthalmol. 2001;131:742-747.
Kardon R. Are we ready to replace cocaine with apraclonidine in the pharmacologic diagnosis of Horner syndrome? J Neuroophthalmol. 2005;25:69-70.
Kardon R.H., Denison C.E., Brown C.K., et al. Critical evaluation of the cocaine test in the diagnosis of Horner’s syndrome. Arch Ophthalmol. 1990;108:384-387.
Kasthurirangan S., Glasser A. Age related changes in the characteristics of the near pupil response. Vision Res. 2006;46:1393-1403.
Klapper S.R., Jordan D.R., Pelletier C., et al. Ptosis in Waldenstrom’s macroglobulinemia. Am J Ophthalmol. 1998;126:315-317.
Koc F., Kavuncu S., Kansu T., et al. The sensitivity and specificity of 0.5% apraclonidine in the diagnosis of oculosympathetic paresis. Br J Ophthalmol. 2005;89:1442-1444.
Kourouyan H.D., Horton J.C. Transneuronal retinal input to the primate Edinger-Westphal nucleus. J Comp Neurol. 1997;381:68-80.
Krack P., Marion M.H. “Apraxia of lid opening,” a focal eyelid dystonia: clinical study of 32 patients. Mov Disord. 1994;9:610-615.
Lagreze W.D., Kardon R.H. Correlation of relative afferent pupillary defect and estimated retinal ganglion cell loss. Graefes Arch Clin Exp Ophthalmol. 1998;236:401-404.
Leavitt J.A., Wayman L.L., Hodge D.O., et al. Pupillary response to four concentrations of pilocarpine in normal subjects: application to testing for Adie tonic pupil. Am J Ophthalmol. 2002;133:333-336.
Lam B.L., Thompson H.S., Corbett J.J. The prevalence of simple anisocoria. Am J Ophthalmol. 1987;104:69-73.
Lebas M., Seror J., Debroucker T. Positive apraclonidine test 36 hours after acute onset of Horner syndrome in dorsolateral pontomedullary stroke. J Neuroophthalmol. 2010;30:12-17.
Manconi M., Cesnik E., Casetta I., et al. Cerebral blepharoptosis: two new cases, literature review and proposal for diagnostic criteria. Neurol Sci. 2006;27:161-165.
Meadows A., Raynor M., Luff A. Lid retraction in primary hepatocellular carcinoma. Br J Ophthalmol. 2001;85:754.
Morales J., Brown S.M., Abdul-Rahim A.S., et al. Ocular effects of apraclonidine in Horner syndrome. Arch Ophthalmol. 2000;118:951-954.
Mughal M., Longmuir R. Current pharmacologic testing for Horner syndrome. Curr Neurol Neurosci Rep. 2009;9:384-389.
Nagasaka K., Ohta E., Nagasaka T., et al. Rhythmic pupillary oscillation in Creutzfeld-Jakob disease associated with the glu/lys mutation of prion protein codon 200. Mov Disord. 2010;25:112-116.
Nester S., Ohayon S., Gutfreund Y. Multiple manifestations of microstimulation in the optic tectum: eye movements, pupil dilations, and sensory priming. J Neurophysiol. 2010;104:108-118.
Nolano M., Provitera V., Perretti A., et al. Ross syndrome: a rare or a misknown disorder of thermoregulation? A skin innervation study on 12 subjects. Brain. 2006;129:2119-2131.
Olson J.J., Putterman A. Loss of vertical palpebral fissure height on downgaze in acquired blepharoptosis. Arch Ophthalmol. 1995;113:1293-1297.
Papageorgiou E., Wermund T., Wilhelm H. Pupil perimetry demonstrates hemifield pupillary hypokinesia in a patient with a pretectal lesion causing a relative afferent pupil defect but no visual field loss. J Neuroophthalmol. 2009;29:33-36.
Pilley S.F., Thompson H.S. Pupillary “dilatation lag” in Horner’s syndrome. Br J Ophthalmol. 1975;59:731-735.
Pong M., Fuchs A.F. Characteristics of the pupillary light reflex in the macaque monkey: discharge patterns of pretectal neurons. J Neurophysiol. 2000;84:964-974.
Ropper A.H. The opposite pupil in herniation. Neurology. 1990;40:1707-1709.
Ross A.T. Progressive selective sudomotor denervation; a case with coexisting Adie’s syndrome. Neurology. 1958;8:809-817.
Shin R.K., Galetta S.L., Ting T.Y., et al. Ross syndrome plus: beyond Horner, Holmes-Adie, and harlequin. Neurology. 2000;55:1841-1846.
Smith S.J., Diehl N., Leavitt J., et al. incidence of pediatric Horner syndrome and the risk of neuroblastoma. Arch Ophthalmol. 2010;128:324-329.
Thompson H.S., Franceschetti A.T., Thompson P.M. Hippus. Semantic and historic considerations of the word. Am J Ophthalmol. 1971;71:1116-1120.
Thompson H.S., Kardon R.H. The Argyll Robertson pupil. J Neuroophthalmol. 2006;26:134-138.
Thompson H.S., Zackon D.H., Czarnecki J.S. Tadpole-shaped pupils caused by segmental spasm of the iris dilator muscle. Am J Ophthalmol. 1983;96:467-477.
Warwick R. The ocular parasympathetic nerve supply and its mesencephalic sources. J Anat. 1954;88:71-93.
Yamashita F., Hirayama M., Nakamura T., et al. Pupillary autonomic dysfunction in multiple system atrophy and Parkinson’s disease: an assessment by eye-drop tests. Clin Auton Res. 2010;20:191-197.