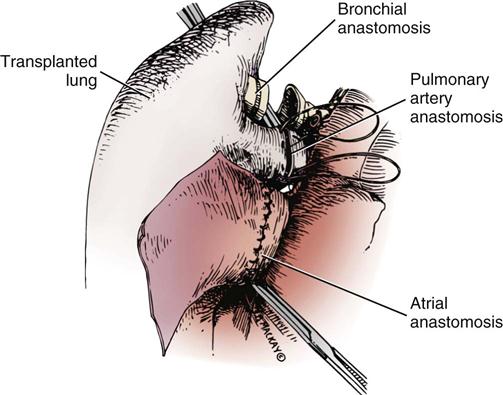
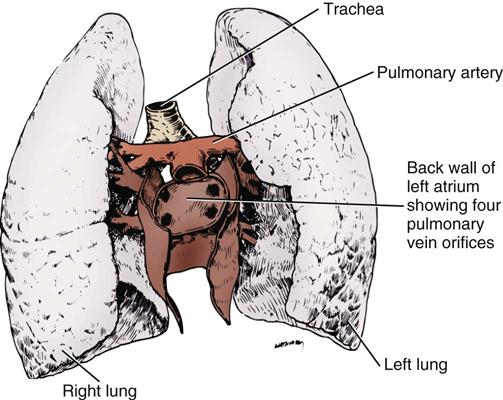
Objectives
• Describe nursing management of a patient receiving oxygen therapy.
• List the indications and complications of the different artificial airways.
• Outline the principles of airway management.
• Discuss the various modes of invasive and noninvasive mechanical ventilation.
• Describe the management of a patient on mechanical ventilation.
• Delineate the care of the postoperative thoracic surgery patient and the lung transplant patient.
![]()
Be sure to check out the bonus material, including free self-assessment exercises, on the Evolve web site at http://evolve.elsevier.com/Urden/priorities/.
Oxygen Therapy
Normal cellular function depends on the delivery of an adequate supply of oxygen to the cells to meet their metabolic needs. The goal of oxygen therapy is to provide a sufficient concentration of inspired oxygen to permit full use of the oxygen-carrying capacity of the arterial blood; this ensures adequate cellular oxygenation, provided the cardiac output and hemoglobin concentration are adequate.1,2
Principles of Therapy
Oxygen is an atmospheric gas that must also be considered a drug, because—like most other drugs—it has detrimental and beneficial effects. Oxygen is one of the most commonly used and misused drugs. As a drug, it must be administered for good reason and in a proper, safe manner. Oxygen is usually ordered in liters per minute (L/min), as a concentration of oxygen expressed as a percentage, such as 40%, or as a fraction of inspired oxygen (FiO2), such as 0.4.
The primary indication for oxygen therapy is hypoxemia.3 The amount of oxygen administered depends on the pathophysiological mechanisms affecting the patient’s oxygenation status. In most cases, the amount required should provide an arterial partial pressure of oxygen (PaO2) of greater than 60 mm Hg or an arterial hemoglobin saturation (SaO2) of greater than 90% during rest and exercise.2 The concentration of oxygen given to an individual patient is a clinical judgment based on the many factors that influence oxygen transport, such as hemoglobin concentration, cardiac output, and arterial oxygen tension.1,2
After oxygen therapy has begun, the patient is continuously assessed for level of oxygenation and the factors affecting it. The patient’s oxygenation status is evaluated several times daily until the desired oxygen level has been reached and has stabilized. If the desired response to the amount of oxygen delivered is not achieved, the oxygen supplementation is adjusted, and the patient’s condition is re-evaluated. It is important to use this dose-response method so that the lowest possible level of oxygen is administered that will still achieve a satisfactory PaO2 or SaO2.2,3
Methods of Delivery
Oxygen therapy can be delivered by many different devices (Table 16-1). Common problems with these devices include system leaks and obstructions, device displacement, and skin irritation. These devices are classified as low-flow, reservoir, or high-flow systems.3
TABLE 16-1
| CATEGORY | DEVICE | FLOW | FiO2 RANGE (%) | FiO2 STABILITY | ADVANTAGES | DISADVANTAGES | BEST USE |
| Low-flow | Nasal cannula | 0.25-8 L/min (adults) ≤2 L/min (infants) |
22-45 | Variable | Use on adults, children, infants; easy to apply; disposable, low cost; well tolerated | Unstable, easily dislodged; high flows uncomfortable; can cause dryness/bleeding; polyps, deviated septum may block flow | Stable patient needing low FiO2; home care patient requiring long-term therapy |
| Nasal catheter | 0.25-8 L/min | 22-45 | Variable | Use on adults, children, infants; good stability; disposable, low cost | Difficult to insert; high flows increase back pressure; needs regular changing; polyps, deviated septum may block insertion; may provoke gagging, air swallowing, aspiration | Procedures where cannula is difficult to use (bronchoscopy); long-term care for infants | |
| Transtracheal catheter | 0.25-4 L/min | 22-35 | Variable | Lower O2 usage/cost; eliminates nasal/skin irritation; improved compliance; increased exercise tolerance; increased mobility; enhanced image | High cost; surgical complications; infection; mucus plugging; lost tract | Home care or ambulatory patients who need increased mobility or who do not accept nasal oxygen | |
| Reservoir | Reservoir cannula | 0.25-4 L/min | 22-35 | Variable | Lower O2 usage/cost; increased mobility; less discomfort because of lower flows | Unattractive, cumbersome; poor compliance; must be regularly replaced; breathing pattern affects performance | Home care or ambulatory patients who need increased mobility |
| Simple mask | 5-12 L/min | 35-50 | Variable | Use on adults, children, infants; quick, easy to apply; disposable, inexpensive | Uncomfortable; must be removed for eating; prevents radiant heat loss; blocks vomitus in unconscious patients | Emergencies, short-term therapy requiring moderate FiO2 | |
| Partial rebreathing mask | 6-10 L/min (prevent bag collapse on inspiration) | 35-60 | Variable | Same as simple mask; moderate to high FiO2 | Same as simple mask; potential suffocation hazard | Emergencies, short-term therapy requiring moderate to high FiO2 | |
| Nonrebreathing mask | 6-10 L/min (prevent bag collapse on inspiration) | 55-70 | Variable | Same as simple mask; high FiO2 | Same as simple mask; potential suffocation hazard | Emergencies, short-term therapy requiring high FiO2 | |
| Nonrebreathing circuit (closed) | 3 × Ve (prevent bag collapse on inspiration) | 21-100 | Fixed | Full range of FiO2 | Potential suffocation hazard; requires 50 psi air/O2; blender failure common | Patients requiring precise FiO2 at any level (21%-100%) | |
| High-flow | Air-entrainment mask (AEM) | Varies; should provide output flow >60 L/min | 24-50 | Fixed | Easy to apply; disposable, inexpensive; stable, precise Fio2 | Limited to adult use; uncomfortable, noisy; must be removed for eating; FiO2 >0.40 not ensured; FiO2 varies with back-pressure | Unstable patients requiring precise low FiO2 |
| Air-entrainment nebulizer | 10-15 L/min input; should provide output flow ≥60 L/min | 28-100 | Fixed | Provides temperature control and extra humidification | FiO2 <28% or >0.40 not ensured; FiO2 varies with back-pressure; high infection risk | Patients with artificial airways requiring low to moderate FiO2 |
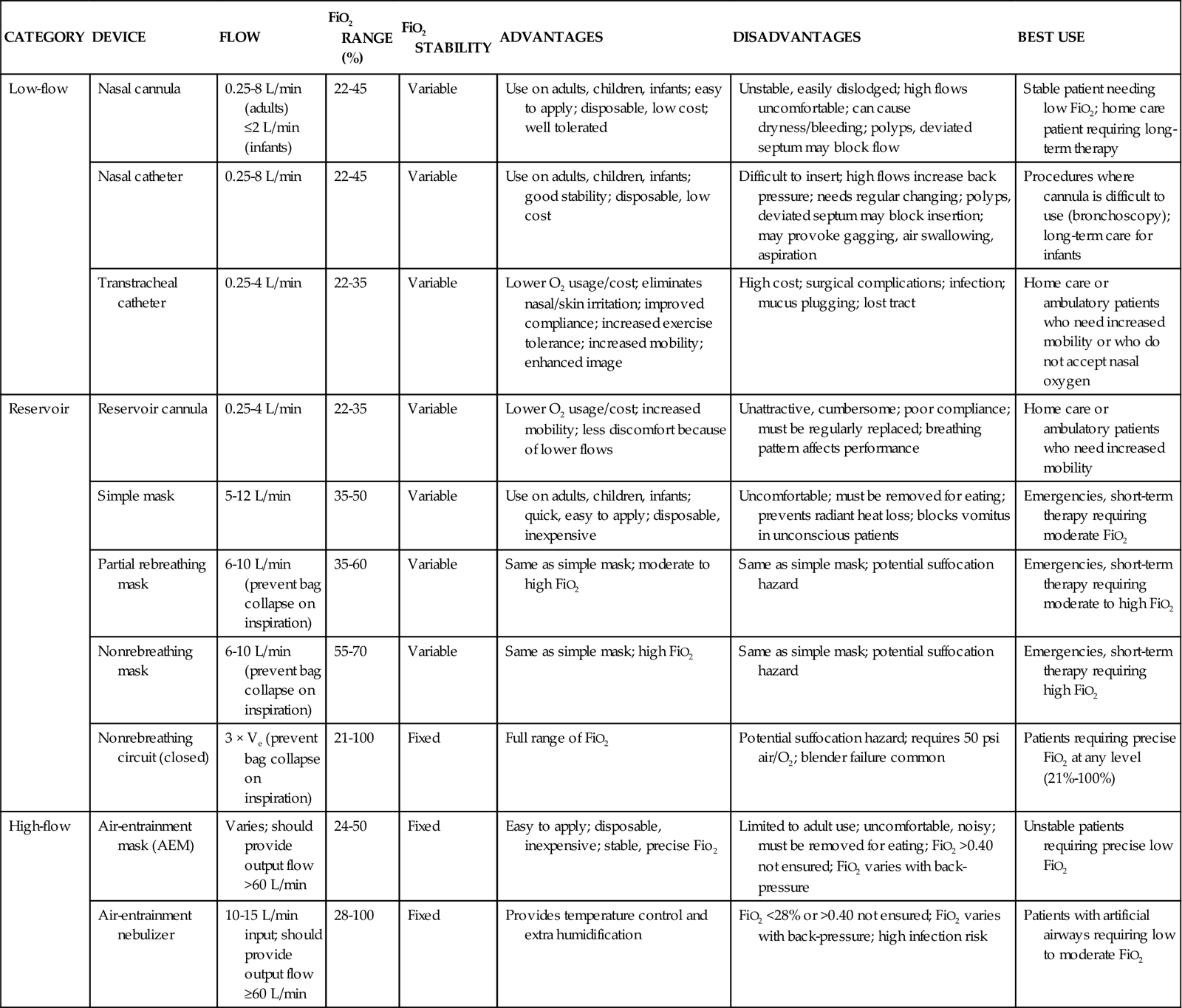
Modified from Wilkins RL, et al, editors: Egan’s fundamentals of respiratory care, ed 8, St Louis, 2003, Mosby.
Low-Flow Systems
A low-flow oxygen delivery system provides supplemental oxygen directly into the patient’s airway at a flow of 8 L/min or less. Because this flow is insufficient to meet the patient’s inspiratory volume requirements, it results in a variable FiO2 as the supplemental oxygen is mixed with room air. The patient’s ventilatory pattern affects the FiO2 of a low-flow system: as the ventilatory pattern changes, differing amounts of room air gas are mixed with the constant flow of oxygen. A nasal cannula is an example of a low-flow device.3
Reservoir Systems
A reservoir system incorporates some type of device to collect and store oxygen between breaths. When the patient’s inspiratory flow exceeds the oxygen flow of the oxygen delivery system, the patient is able to draw from the reservoir of oxygen to meet his or her inspiratory volume needs. There is less mixing of the inspired oxygen with room air than in a low-flow system. A reservoir oxygen delivery system can deliver a higher FiO2 than a low-flow system. Examples of reservoir systems are simple face masks, partial rebreathing masks, and nonrebreathing masks.3
High-Flow Systems
With a high-flow system, the oxygen flows out of the device and into the patient’s airways in an amount sufficient to meet all inspiratory volume requirements. This type of system is not affected by the patient’s ventilatory pattern. An air-entrainment mask is an example of a high-flow system.3
Complications of Oxygen Therapy
Oxygen, like most drugs, has adverse effects and complications resulting from its use. The adage “if a little is good, a lot is better” does not apply to oxygen. The lung is designed to handle a concentration of 21% oxygen, with some adaptability to higher concentrations, but adverse effects and oxygen toxicity can result if a high concentration is administered for too long.4
Oxygen Toxicity
The most detrimental effect of breathing a high concentration of oxygen is the development of oxygen toxicity. It can occur in any patient who breathes oxygen concentrations of greater than 50% for longer than 24 hours. Patients most likely to develop oxygen toxicity are those who require intubation, mechanical ventilation, and high oxygen concentrations for extended periods.3
Hyperoxia, or the administration of higher-than-normal oxygen concentrations, produces an overabundance of oxygen free radicals. These radicals are responsible for the initial damage to the alveolar-capillary membrane. Oxygen free radicals are toxic metabolites of oxygen metabolism. Normally, enzymes neutralize the radicals, preventing any damage from occurring. During the administration of high levels of oxygen, the large number of oxygen free radicals produced exhausts the supply of neutralizing enzymes. Damage to the lung parenchyma and vasculature occurs, resulting in the initiation of acute lung injury (ALI).2,4
A number of clinical manifestations are associated with oxygen toxicity. The first symptom is substernal chest pain that is exacerbated by deep breathing. A dry cough and tracheal irritation follow. Eventually, there is definite pleuritic pain on inhalation, followed by dyspnea. Upper airway changes may include a sensation of nasal stuffiness, sore throat, and eye and ear discomforts. Chest radiographs and pulmonary function tests show no abnormalities until symptoms are severe. Complete, rapid reversal of these symptoms occurs as soon as normal oxygen concentrations are restored.4
Carbon Dioxide Retention
In patients with severe chronic obstructive pulmonary disease (COPD), carbon dioxide (CO2) retention may occur as a result of administration of oxygen in high concentrations. A number of theories have been proposed for this phenomenon. One states that the normal stimulus to breathe (i.e., increasing CO2 levels) is muted in patients with COPD and that decreasing oxygen levels become the stimulus to breathe. If hypoxemia is corrected by the administration of oxygen, the stimulus to breathe is abolished; hypoventilation develops, resulting in a further increase in the arterial partial pressure of carbon dioxide (PaCO2).2,3 Another theory is that the administration of oxygen abolishes the compensatory response of hypoxic pulmonary vasoconstriction. This results in an increase in perfusion of underventilated alveoli and the development of dead space, producing ventilation/perfusion mismatching. As alveolar dead space increases, so does the retention of CO2.2,3,5 One further theory states that the rise in CO2 is related to the ratio of deoxygenated to oxygenated hemoglobin (Haldane effect). Deoxygenated hemoglobin carries more CO2 than oxygenated hemoglobin. Administration of oxygen increases the proportion of oxygenated hemoglobin, which causes increased release of CO2 at the lung level.5 Because of the risk of CO2 accumulation, all chronically hypercapnic patients require careful low-flow oxygen administration.3
Absorption Atelectasis
Another adverse effect of high concentrations of oxygen is absorption atelectasis. Breathing high concentrations of oxygen washes out the nitrogen that normally fills the alveoli and helps hold them open (residual volume). As oxygen replaces the nitrogen in the alveoli, the alveoli start to shrink and collapse. This occurs because oxygen is absorbed into the bloodstream faster than it can be replaced in the alveoli, particularly in areas of the lungs that are minimally ventilated.2,3
Nursing Management
Nursing priorities for the patient receiving oxygen focus on (1) ensuring the oxygen is being administered as ordered and (2) observing for complications of the therapy. Confirming that the O2 therapy device is properly positioned and replacing it after removal is important. During meals, an oxygen mask should be changed to a nasal cannula if the patient can tolerate one. The patient receiving O2 therapy should also be transported with the oxygen. In addition, SpO2 should be periodically monitored using a pulse oximeter.
Artificial Airways
Pharyngeal Airways
Pharyngeal airways are used to maintain airway patency by keeping the tongue from obstructing the upper airway. The two types of pharyngeal airways are oropharyngeal and nasopharyngeal. Complications of these airways include trauma to the oral or nasal cavity, obstruction of the airway, laryngospasm, gagging, and vomiting.6,7
Oropharyngeal Airway
An oropharyngeal airway is made of plastic and is available in various sizes. The proper size is selected by holding the airway against the side of the patient’s face and ensuring that it extends from the corner of the mouth to the angle of the jaw. If the airway is improperly sized, it will occlude the airway.6,7 An oral airway is placed by inserting a tongue depressor into the patient’s mouth to displace the tongue downward and then passing the airway into the patient’s mouth, slipping it over the patient’s tongue.7 When properly placed, the tip of the airway lies above the epiglottis at the base of the tongue. It should be used only in an unconscious patient who has an absent or diminished gag reflex.6,7
Nasopharyngeal Airway
A nasopharyngeal airway is usually made of plastic or rubber and is available in various sizes. The proper size is selected by holding the airway against the side of the patient’s face and ensuring that it extends from the tip of the nose to the earlobe.6,7 A nasal airway is placed by lubricating the tube and inserting it midline along the floor of the naris into the posterior pharynx.7 When properly placed, the tip of the airway lies above the epiglottis at the base of the tongue.6,7
Endotracheal Tubes
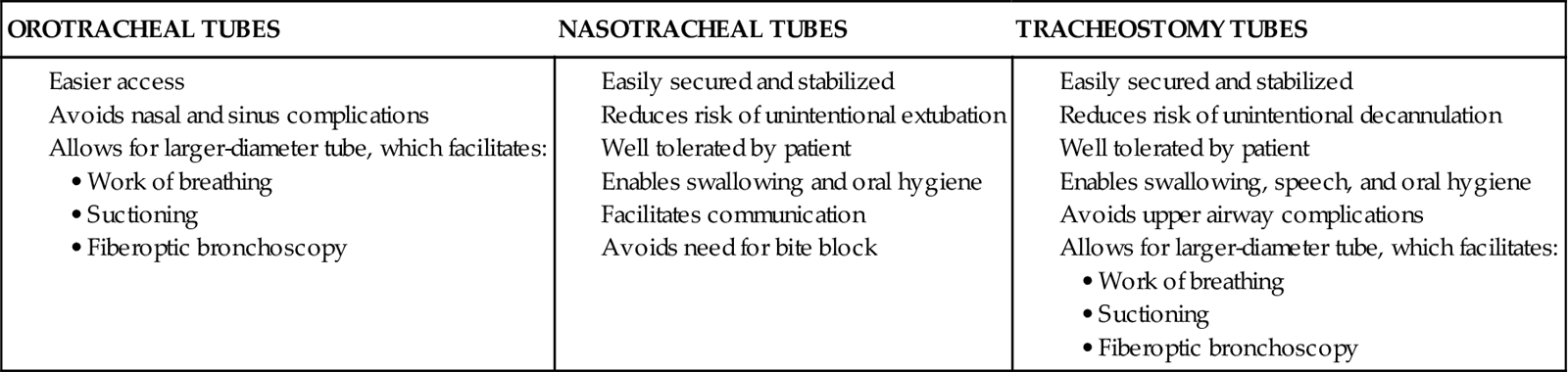
An endotracheal tube (ETT) is the most commonly used artificial airway for providing short-term airway management. Indications for endotracheal intubation include maintenance of airway patency, protection of the airway from aspiration, application of positive-pressure ventilation, facilitation of pulmonary toilet, and use of high oxygen concentrations.8 An ETT may be placed through the orotracheal or the nasotracheal route.9,10 In most situations involving emergency placement, the orotracheal route is used, because it is simpler and allows the use of a larger-diameter ETT.10,11 Nasotracheal intubation provides greater patient comfort over time and is preferred in patients with a jaw fracture.9,11,12 The advantages of orotracheal and nasotracheal intubation are presented in Table 16-2.
TABLE 16-2
ADVANTAGES OF OROTRACHEAL, NASOTRACHEAL, AND TRACHEOSTOMY TUBES
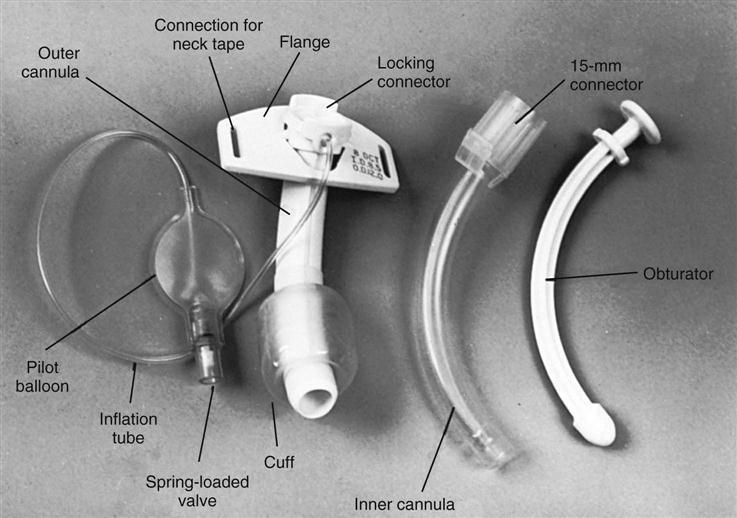
ETTs are available in various sizes, based on the inner diameter of the tube, and have a radiopaque marker that runs the length of the tube. On one end of the tube is a cuff that is inflated with the use of the pilot balloon. Because of the high incidence of cuff-related problems, low-pressure, high-volume cuffs are preferred. On the other end of the tube is a 15-mm adaptor that facilitates connection of the tube to a manual resuscitation bag (MRB), T-tube, or ventilator (Figure 16-1).13
Intubation
Before intubation, the necessary equipment is gathered and organized to facilitate the procedure. Readily available equipment should include a suction system with catheters and tonsil suction, an MRB with a mask connected to 100% oxygen, a laryngoscope handle with assorted blades, a variety of sizes of ETTs, and a stylet. Before the procedure is initiated, all equipment is inspected to ensure that it is in working order. The patient should be prepared for the procedure, if possible, with an intravenous catheter in place, and should be monitored with a pulse oximeter. The patient is sedated before the procedure (as clinical condition allows), and a topical anesthetic is applied to facilitate placement of the tube. In some cases, a paralytic agent may be necessary if the patient is extremely agitated.9,11,14
The procedure is initiated by positioning the patient with the neck flexed and head slightly extended in the “sniff” position. The oral cavity and pharynx are suctioned, and any dental devices are removed. The patient is preoxygenated and ventilated using the MRB and mask with 100% oxygen. Each intubation attempt is limited to 30 seconds. After the ETT is inserted, the patient is assessed for bilateral breath sounds and chest movement. Absence of breath sounds is indicative of an esophageal intubation, whereas breath sounds heard over only one side is indicative of a main stem intubation. A disposable end-tidal CO2 detector is used to initially verify correct airway placement, after which the cuff of the tube is inflated and the tube is secured. Finally, a chest radiograph is obtained to confirm placement.9–11 The tip of the ETT should be approximately 3 to 4 cm above the carina when the patient’s head is in the neutral position.10 After final adjustment of the position is complete, the level of insertion (marked in centimeters on the side of the tube) at the teeth is noted.9,10,14
A number of complications can occur during the intubation procedure, including nasal and oral trauma, pharyngeal and hypopharyngeal trauma, vomiting with aspiration, and cardiac arrest.14 Hypoxemia and hypercapnia can also occur, resulting in bradycardia, tachycardia, dysrhythmias, hypertension, and hypotension.8,12,14
Complications
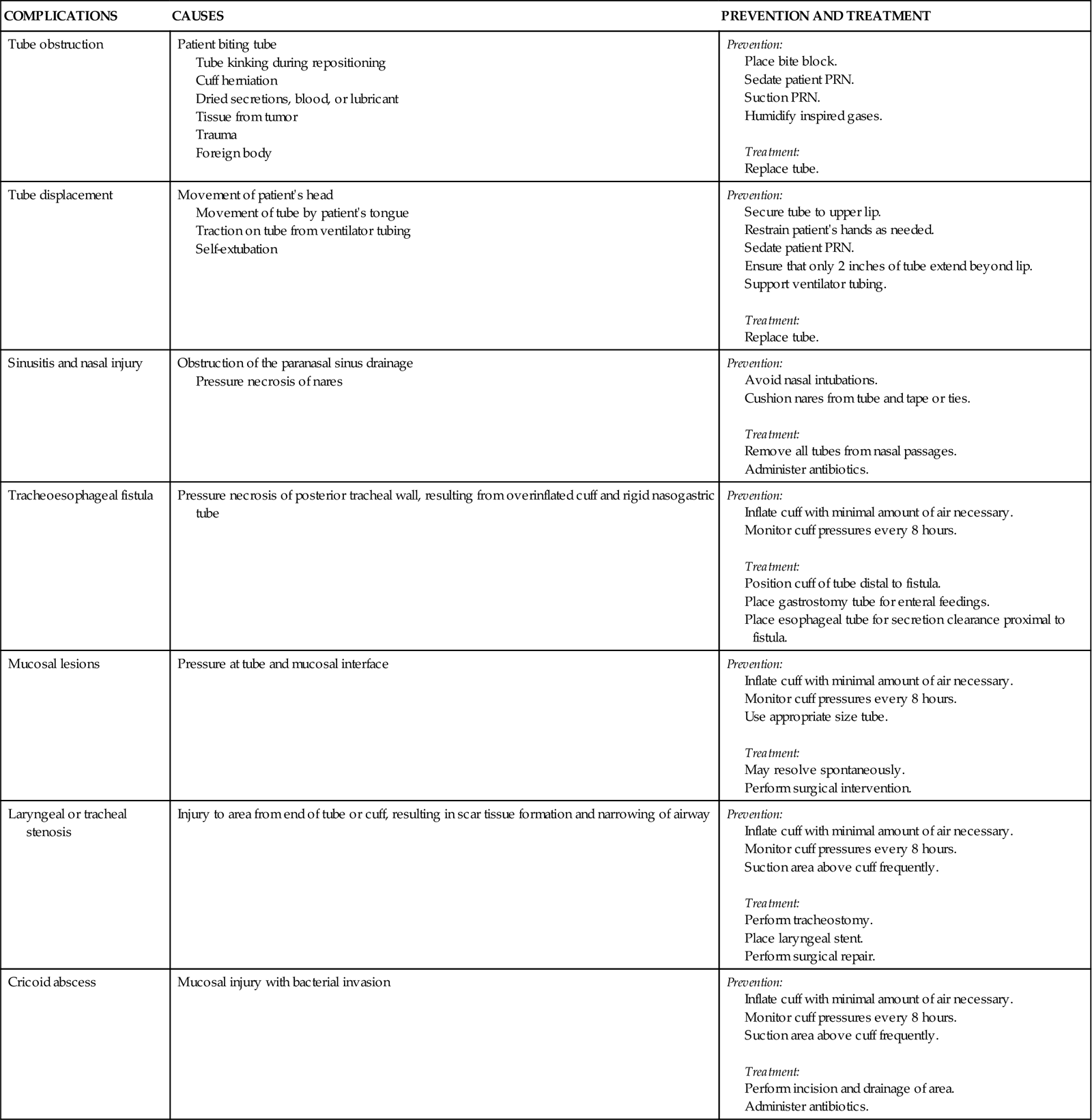
Several complications can occur while the ETT is in place, including nasal and oral inflammation and ulceration, sinusitis and otitis, laryngeal and tracheal injuries, and tube obstruction and displacement. Other complications can occur days to weeks after the ETT is removed, including laryngeal and tracheal stenosis and a cricoid abscess (Table 16-3). Delayed complications usually require some form of surgical intervention.15
TABLE 16-3
COMPLICATIONS OF ENDOTRACHEAL TUBES
| COMPLICATIONS | CAUSES | PREVENTION AND TREATMENT |
| Tube obstruction | Patient biting tube Tube kinking during repositioning Cuff herniation Dried secretions, blood, or lubricant Tissue from tumor Trauma Foreign body |
Prevention: Treatment: |
| Tube displacement | Movement of patient’s head Movement of tube by patient’s tongue Traction on tube from ventilator tubing Self-extubation |
Prevention: Treatment: |
| Sinusitis and nasal injury | Obstruction of the paranasal sinus drainage Pressure necrosis of nares |
Prevention: Treatment: |
| Tracheoesophageal fistula | Pressure necrosis of posterior tracheal wall, resulting from overinflated cuff and rigid nasogastric tube | Prevention: Treatment: |
| Mucosal lesions | Pressure at tube and mucosal interface | Prevention: Treatment: |
| Laryngeal or tracheal stenosis | Injury to area from end of tube or cuff, resulting in scar tissue formation and narrowing of airway | Prevention: Treatment: |
| Cricoid abscess | Mucosal injury with bacterial invasion | Prevention: Treatment: |
Tracheostomy Tubes
A tracheostomy tube is the preferred method of airway maintenance in the patient who requires long-term intubation. Although no ideal time to perform the procedure has been identified, it is commonly accepted that if a patient has been intubated or is anticipated to be intubated for longer than 7 to 10 days, a tracheostomy should be performed.16 A tracheostomy is also indicated in several other situations, such as the presence of an upper airway obstruction due to trauma, tumors, or swelling and the need to facilitate airway clearance due to spinal cord injury, neuromuscular disease, or severe debilitation.17
A tracheostomy tube provides the best route for long-term airway maintenance, because it avoids the oral, nasal, pharyngeal, and laryngeal complications associated with an ETT. The tube is shorter, of wider diameter, and less curved than an ETT; the resistance to air flow is less, and breathing is easier. Additional advantages of a tracheostomy tube include easier secretion removal, increased patient acceptance and comfort, capability of the patient to eat and talk if possible, and easier ventilator weaning.11,17 Table 16-2 presents a list of the advantages of a tracheostomy tube.
Tracheostomy tubes are made of plastic or metal and may have one or two lumens. Single-lumen tubes consist of the tube; a built-in cuff, which is connected to a pilot balloon for inflation purposes; and an obturator, which is used during tube insertion. The double-lumen tubes consist of the tube with the attached cuff, the obturator, and an inner cannula that can be removed for cleaning and then reinserted or, if disposable, replaced by a new sterile inner cannula. The inner cannula can quickly be removed if it becomes obstructed, making the system safer for patients with significant secretion problems. Single-lumen tubes provide a larger internal diameter for airflow, so airflow resistance is reduced, and the patient can ventilate through the tube with greater ease. Plastic tracheostomy tubes also have a 15-mm adaptor on the end (Figure 16-2).17,18
Tracheostomy
A tracheostomy tube is inserted by an open procedure or a percutaneous procedure. An open procedure is usually performed in the operating room, whereas a percutaneous procedure can be done at the patient’s bedside.18
A number of complications can occur during the tracheostomy procedure, including misplacement of the tracheal tube, hemorrhage, laryngeal nerve injury, pneumothorax, pneumomediastinum, and cardiac arrest.15,18
Complications
Several complications can occur while the tracheostomy tube is in place, including stomal infection, hemorrhage, tracheomalacia, tracheoesophageal fistula, tracheoinnominate artery fistula, and tube obstruction and displacement.18
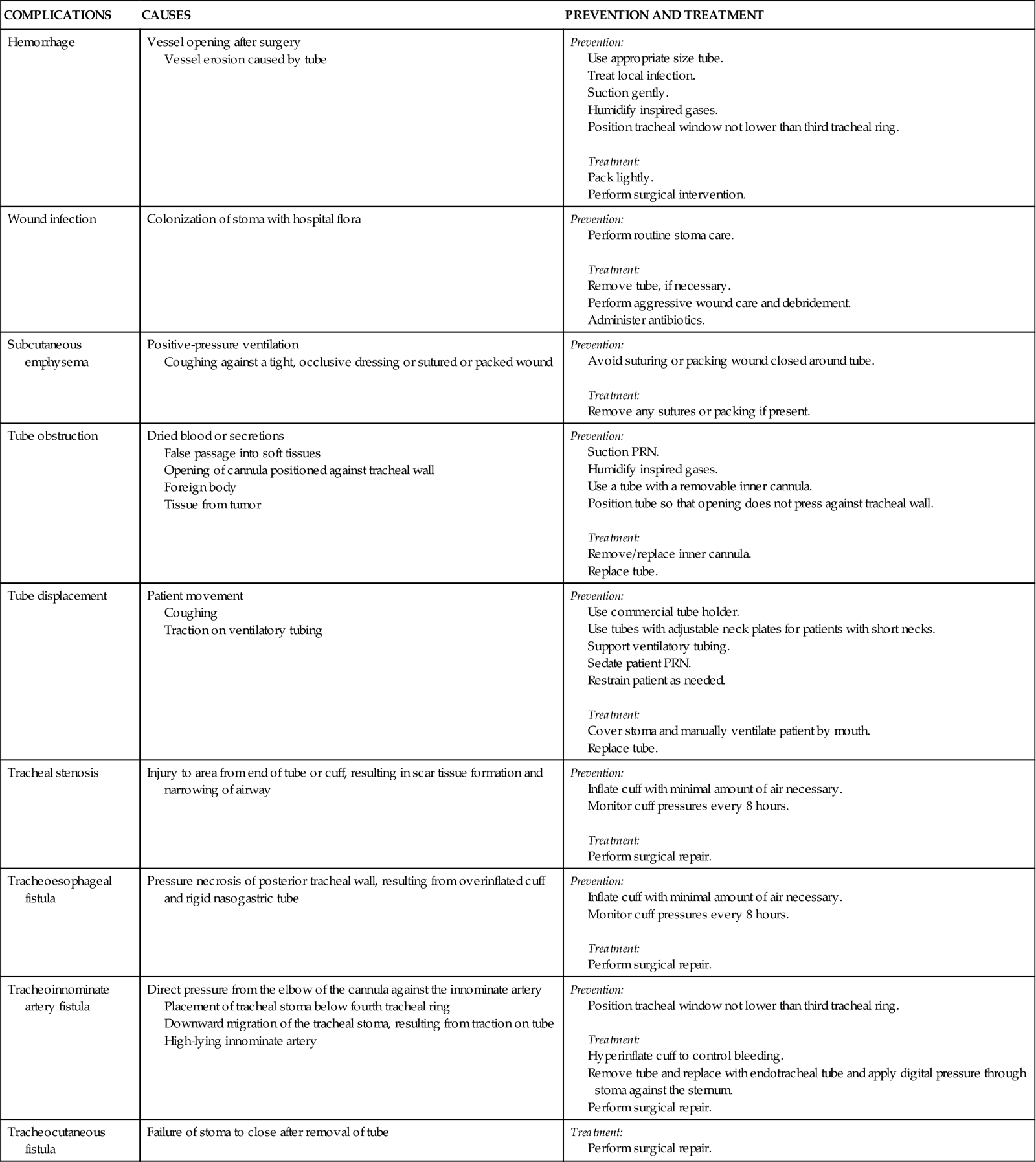
A number of complications can occur days to weeks after the tracheostomy tube is removed, including tracheal stenosis and tracheocutaneous fistula (Table 16-4). Delayed complications usually require some form of surgical intervention.18
TABLE 16-4
COMPLICATIONS OF TRACHEOSTOMY TUBES
| COMPLICATIONS | CAUSES | PREVENTION AND TREATMENT |
| Hemorrhage | Vessel opening after surgery Vessel erosion caused by tube |
Prevention: Treatment: |
| Wound infection | Colonization of stoma with hospital flora | Prevention: Treatment: |
| Subcutaneous emphysema | Positive-pressure ventilation Coughing against a tight, occlusive dressing or sutured or packed wound |
Prevention: Treatment: |
| Tube obstruction | Dried blood or secretions False passage into soft tissues Opening of cannula positioned against tracheal wall Foreign body Tissue from tumor |
Prevention: Use a tube with a removable inner cannula. Position tube so that opening does not press against tracheal wall. Treatment: |
| Tube displacement | Patient movement Coughing Traction on ventilatory tubing |
Prevention: Treatment: |
| Tracheal stenosis | Injury to area from end of tube or cuff, resulting in scar tissue formation and narrowing of airway | Prevention: Treatment: |
| Tracheoesophageal fistula | Pressure necrosis of posterior tracheal wall, resulting from overinflated cuff and rigid nasogastric tube | Prevention: Treatment: |
| Tracheoinnominate artery fistula | Direct pressure from the elbow of the cannula against the innominate artery Placement of tracheal stoma below fourth tracheal ring Downward migration of the tracheal stoma, resulting from traction on tube High-lying innominate artery |
Prevention: Treatment: |
| Tracheocutaneous fistula | Failure of stoma to close after removal of tube | Treatment: |
Nursing Management
The patient with an endotracheal or tracheostomy tube requires some additional measures to address the effects associated with tube placement on the respiratory and other body systems. Nursing priorities for the patient with an artificial airway focus on (1) providing humidification, (2) maintaining the cuff management, (3) suctioning, (4) establishing a method of communication, and (5) providing oral hygiene. Because the tube bypasses the upper airway system, warming and humidifying of air must be performed by external means. Because the cuff of the tube can cause damage to the walls of the trachea, proper cuff inflation and management is imperative. In addition, the normal defense mechanisms are impaired and secretions may accumulate; thus suctioning may be needed to promote secretion clearance. Because the tube does not allow air flow over the vocal cords, developing a method of communication is also very important. Last, observing the patient to ensure proper placement of the tube and patency of the airway is essential. Patient safety issues are addressed in the Patient Safety Priorities box on Artificial Airways.
Humidification
Humidification of air normally is performed by the mucosal layer of the upper respiratory tract. When this area is bypassed, as occurs with ETT and tracheostomy tubes, or when supplemental oxygen is used, humidification by external means is necessary. Various humidification devices add water to inhaled gas to prevent drying and irritation of the respiratory tract, to prevent undue loss of body water, and to facilitate secretion removal.19,20 The humidification device should provide inspired gas conditioned (heated) to body temperature and saturated with water vapor.21
Cuff Management
Because the cuff of the ETT or tracheostomy tube is a major source of the complications associated with artificial airways, proper cuff management is essential. To prevent the complications associated with cuff design, only low-pressure, high-volume cuffed tubes are used in clinical practice.13,22 Even with these tubes, cuff pressures can be generated that are high enough to lead to tracheal ischemia and injury. Proper cuff inflation techniques and cuff pressure monitoring are critical components of the care of the patient with an artificial airway.10,22
Cuff Inflation Techniques
Two cuff inflation techniques are used: the minimal leak (ML) technique and the minimal occlusion volume (MOV) technique. The ML technique consists of injecting air into the cuff until no leak is heard and then withdrawing the air until a small leak is heard on inspiration. Problems with this technique include difficulty maintaining positive end-expiratory pressure (PEEP) and aspiration around the cuff. The MOV technique consists of injecting air into the cuff until no leak is heard at peak inspiration. This technique generates higher cuff pressures than does the ML technique. The selection of one technique over the other is determined by individual patient needs. If the patient needs a seal to provide adequate ventilation or is at high risk for aspiration, the MOV technique is used. If these are not concerns, usually the ML technique is used.10,11,22
Cuff Pressure Monitoring
Cuff pressures are monitored at least every shift with a cuff pressure manometer. Cuff pressures should be maintained at 20 to 25 mm Hg (24 to 30 cm H2O), because greater pressures decrease blood flow to the capillaries in the tracheal wall and lesser pressures increase the risk of aspiration. Pressures in excess of 25 mm Hg (30 cm H2O) should be reported to the physician. Cuffs are not routinely deflated, because this increases the risk of aspiration.10,22
Foam Cuff Tracheostomy Tubes
One tracheostomy tube on the market has a cuff made of foam that is self-inflating. It is deflated during insertion, after which the pilot port is opened to atmospheric pressure (room air), and the cuff self-inflates. After inflation, the foam cuff conforms to the size and shape of the patient’s trachea, thereby reducing the pressure against the tracheal wall. The pilot port can be left open to atmospheric pressure or attached to the mechanical ventilator tubing, allowing the cuff to inflate and deflate with the cycling of the ventilator. Routine maintenance of a foam cuff tracheostomy tube includes aspirating the pilot port every 8 hours to measure cuff volume, to remove any condensation from the cuff area, and to assess the integrity of the cuff. Removal is accomplished by deflating the cuff; this can be complicated if the plastic sheath covering the foam is perforated. If perforation occurs, the foam may not be deflatable because the air cannot be totally aspirated.23
Suctioning
Suctioning is often required to maintain a patent airway in the patient with an ETT or tracheostomy tube. Suctioning is a sterile procedure that is performed only when the patient needs it and not on a routine schedule.10,24 Indications for suctioning include coughing, secretions in the airway, respiratory distress, presence of rhonchi on auscultation, increased peak airway pressures on the ventilator, and decreasing oxygenation saturation.11 Complications associated with suctioning include hypoxemia, atelectasis, bronchospasms, dysrhythmias, increased intracranial pressure, and airway trauma.11,24
Complications
Hypoxemia can result because the oxygen source is disconnected from the patient or the oxygen is removed from the patient’s airways when the suction is applied. Atelectasis is thought to occur when the suction catheter is larger than one half of the diameter of the ETT. Excessive negative pressure occurs when suction is applied, promoting collapse of the distal airways. Bronchospasms are the result of stimulation of the airways with the suction catheter. Cardiac dysrhythmias, particularly bradycardias, are attributed to vagal stimulation. Airway trauma occurs with impaction of the catheter in the airways and excessive negative pressure applied to the catheter.10,11,24
Suctioning Protocol
A number of protocols regarding suctioning have been developed. Several practices have been found helpful in limiting the complications of suctioning. Hypoxemia can be minimized by giving the patient three hyperoxygenation breaths (breaths at 100% FiO2) with the ventilator before the procedure begins and again after each pass of the suction catheter.10,25 If the patient exhibits signs of desaturation, hyperinflation (breaths at 150% tidal volume) should be added to the procedure.10 Atelectasis can be avoided by using a suction catheter with an external diameter of less than one half of the internal diameter of the ETT.24 Using no greater than 120 mm Hg of suction decreases the chances of hypoxemia, atelectasis, and airway trauma.10 Limiting the duration of each suction pass to 10 to 15 seconds10,24 and the number of passes to a maximum of three also helps minimize hypoxemia, airway trauma, and cardiac dysrhythmias.26 The process of applying intermittent (instead of continuous) suction has been shown to be of no benefit.27 The instillation of normal saline to help remove secretions has not proved to be of any benefit24,28 and may actually contribute to the development of hypoxemia10,29 and lower airway colonization, resulting in hospital-acquired pneumonia (HAP).10,30
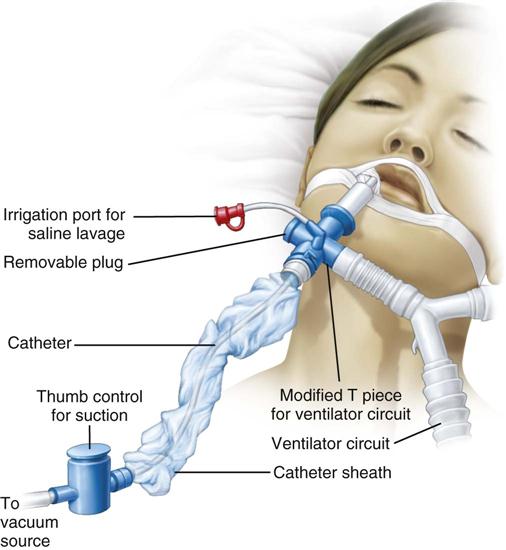
Closed Tracheal Suction System
One device to facilitate the suctioning of a patient on a ventilator is the closed tracheal suction system (CTSS) (Figure 16-3). This device consists of a suction catheter in a plastic sleeve that attaches directly to the ventilator tubing. It allows the patient to be suctioned while remaining on the ventilator. Advantages of the CTSS include maintenance of oxygenation and PEEP during suctioning, reduction of hypoxemia-related complications, and protection of staff members from the patient’s secretions. The CTSS is convenient to use, requiring only one person to perform the procedure.
Concerns related to the CTSS include autocontamination, inadequate removal of secretions, and increased risk of unintentional extubation resulting from the extra weight of the system on the ventilator tubing. Autocontamination has been shown not to be an issue if the catheter is cleaned properly after every use. Inadequate removal of secretions may or may not be a problem, and further investigation is required to settle this issue.11 Although recommendations for changing the catheter vary, one study indicated that the catheter could be changed on an as-needed basis without increasing the incidence of HAP.31
Communication
One of the major stressors for the patient with an artificial airway is impaired communication. This is related to the inability to speak, insufficient explanations from staff members, inadequate understanding, fear of being unable to communicate, and difficulty with communication methods.32 A number of interventions can facilitate communication in the patient with an ETT or tracheostomy tube. These include performing a complete assessment of the patient’s ability to communicate, teaching the patient how to communicate, using a variety of methods to communicate, and facilitating the patient’s ability to communicate by providing the patient with his or her eyeglasses or hearing aid.33
Methods to facilitate communication in this patient population include the use of verbal and nonverbal language and a variety of devices to assist the patient on short-term and long-term ventilator assistance. Nonverbal communication may include the use of sign language, gestures, lip-reading, pointing, facial expressions, or eye blinking. Simple devices available include pencil and paper; Magic Slates; magnetic boards with plastic letters; picture, alphabet, or symbol boards; and flash cards. More sophisticated devices include typewriters, computers, talking ETT and tracheostomy tubes, and external handheld vibrators. Regardless of the method selected, the patient must be taught how to use the device.10,33
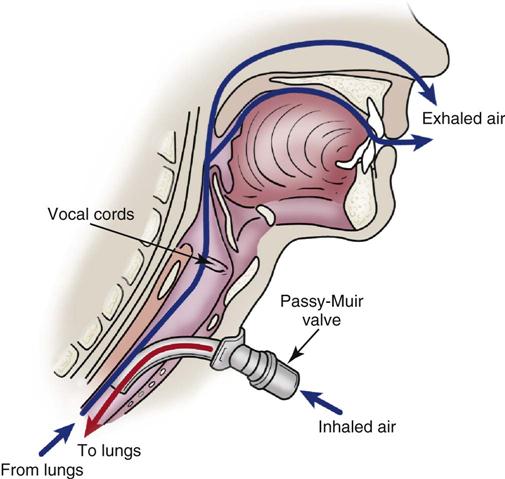
Passy-Muir Valve
One device used to assist the mechanically ventilated patient with a tracheostomy to speak is the Passy-Muir valve. This one-way valve opens on inhalation, allowing air to enter the lungs through the tracheostomy tube, and closes on exhalation, forcing air over the vocal cords and out the mouth, permitting the patient to speak (Figure 16-4). Before the valve can be placed on a tracheostomy tube, the cuff must be deflated to allow air to pass around the tube, and the tidal volume of the ventilator must be increased to compensate for the air leak. In addition to aiding communication, the Passy-Muir valve can assist the ventilator-dependent patient with relearning normal breathing patterns. The valve is contraindicated in patients with laryngeal or pharyngeal dysfunction, excessive secretions, or poor lung compliance.34
Oral Hygiene
Patients with artificial airways are extremely susceptible to developing HAP due to microaspiration of subglottic secretions. Subglottic secretions are fluids from the oropharyngeal area that pool above the inflated cuff of the ETT or tracheostomy tube. These secretions are full of microorganisms from the patient’s mouth. Because the cuff of the artificial airway does not create a tight seal in the patient’s airway, these secretions seep around the cuff and into the patient’s lungs, promoting the development of HAP.35 Although bacteria are normally present in a patient’s mouth, in the critically ill patient there are increased amounts of bacteria and more resistant bacteria. Decreased salivary flow, poor mucosal status, and dental plaque all contribute to this problem.36
Proper oral hygiene has the potential to decrease the incidence of HAP.37 However, recent studies have shown that routine oral care is not a priority intervention for many nurses.38 Currently there is no evidence-based protocol for oral care. Research studies are lacking, particularly with regard to frequency and effectiveness of different procedures.39 Most experts agree, however, that oral care should consist of brushing the patient’s teeth with a soft toothbrush to reduce plaque, brushing the patient’s tongue and gums with a foam swab to stimulate the tissue, and performing deep oropharyngeal suctioning to remove any secretions that have pooled above the patient’s cuff.37–39 One intervention that has evidence supporting its use is rinsing the patient’s mouth with chlorhexidine (15 mL of 0.12% oropharyngeal rinse applied twice daily for 30 seconds). This procedure has been shown to reduce oral colonization of bacteria and to decrease the incidence of ventilator-associated pneumonia, particularly in cardiac surgery patients.40
Extubation and Decannulation
After the airway is no longer needed, it is removed. Extubation is the process of removing an ETT. It is a simple procedure that can be accomplished at the bedside.10,11 Before the cuff of an ETT or tracheostomy tube is deflated in preparation for removal, it is very important to ensure that secretions are cleared from above the tube cuff. Complications of extubation include sore throat, stridor, hoarseness, odynophagia, vocal cord immobility, pulmonary aspiration, and cough.15 Decannulation is the process of removing a tracheostomy tube. It is also a simple process that can be performed at the bedside. After removal of the tracheostomy tube, the stoma is usually covered with a dry dressing, with the expectation that it will close within several days.10,11 Difficulty removing the tracheostomy tube because of a tight stoma is usually the only complication associated with decannulation.15
Invasive Mechanical Ventilation
Indications
Mechanical ventilation is the process of using an apparatus to facilitate the transport of oxygen and carbon dioxide between the atmosphere and the alveoli for the purpose of enhancing pulmonary gas exchange. It is indicated for physiological and clinical reasons. Physiological objectives include supporting cardiopulmonary gas exchange (alveolar ventilation and arterial oxygenation), increasing lung volume (end-expiratory lung inflation and functional residual capacity), and reducing the work of breathing. Clinical objectives include reversing hypoxemia and acute respiratory acidosis, relieving respiratory distress, preventing or reversing atelectasis and respiratory muscle fatigue, permitting sedation and neuromuscular blockade, decreasing oxygen consumption, reducing intracranial pressure, and stabilizing the chest wall.41
Use of Mechanical Ventilators
Types of Ventilators
The two main types of ventilators currently available are positive-pressure ventilators and negative-pressure ventilators. Negative-pressure ventilators are applied externally to the patient and decrease the atmospheric pressure surrounding the thorax to initiate inspiration. They generally are not used in the critical care environment. Positive-pressure ventilators use a mechanical drive mechanism to force air into the patient’s lungs through an ETT or tracheostomy tube.42
Ventilator Mechanics
The ventilator must complete four phases of ventilation to properly ventilate the patient: (1) change from exhalation to inspiration; (2) inspiration; (3) change from inspiration to exhalation; and (4) exhalation. The ventilator uses four different variables to begin, sustain, and terminate each of these phases. These variables are described in terms of volume, pressure, flow, and time.7,43,44
Trigger
The phase variable that initiates the change from exhalation to inspiration is called the trigger. Breaths may be pressure-triggered or flow-triggered, based on the sensitivity setting of the ventilator and the patient’s inspiratory effort; or they may be time-triggered, based on the rate setting of the ventilator. A breath that is initiated by the patient is known as a patient-triggered or patient-assisted breath, whereas a breath that is initiated by the ventilator is known as a machine-triggered or machine-controlled breath.
A time-triggered breath is a machine-controlled breath that is initiated by the ventilator after a preset length of time has elapsed. It is controlled by the rate setting on the ventilator (e.g., a rate of 10 breaths/minute yields 1 breath every 6 seconds). Flow-triggered and pressure-triggered breaths are patient-assisted breaths that are initiated by decreased flow or pressure, respectively, within the breathing circuit. Flow-triggering (also known as flow-by) is controlled by adjusting the flow-sensitivity setting of the ventilator, whereas pressure-triggering is controlled by adjusting the pressure-sensitivity setting. Many ventilators offer the various types of triggers in combination. For example, a breath may be time-triggered and flow-triggered, depending on the patient’s ability to interact with the ventilator and initiate a breath.7,42,43
Limit
The variable that maintains inspiration is called the limit or target. Inspiration can be pressure-limited, flow-limited, or volume-limited. A pressure-limited breath is one in which a preset pressure is attained and maintained during inspiration. A flow-limited breath is one in which a preset flow is reached before the end of inspiration. A volume-limited breath is one in which a preset volume is delivered during the inspiration. However, the limit variable does not end inspiration; it only sustains it.7,42,43
Cycle
The variable that ends inspiration is called the cycle. The classification of positive-pressure ventilators is based on this variable: volume-cycled, pressure-cycled, flow-cycled, and time-cycled. Volume-cycled ventilators are designed to deliver a breath until a preset volume is delivered. Pressure-cycled ventilators deliver a breath until a preset pressure is reached within the patient’s airways. Flow-cycled ventilators deliver a breath until a preset inspiratory flow rate is achieved. Time-cycled ventilators deliver a breath over a preset time interval.7,42,43
Baseline
The variable that is controlled during exhalation is called the baseline. Pressure is almost always used to adjust this variable. The patient exhales to a certain baseline pressure that is set on the ventilator. It may be set at zero (i.e., atmospheric pressure) or above atmospheric pressure (i.e., PEEP).7,42,43
Modes of Ventilation
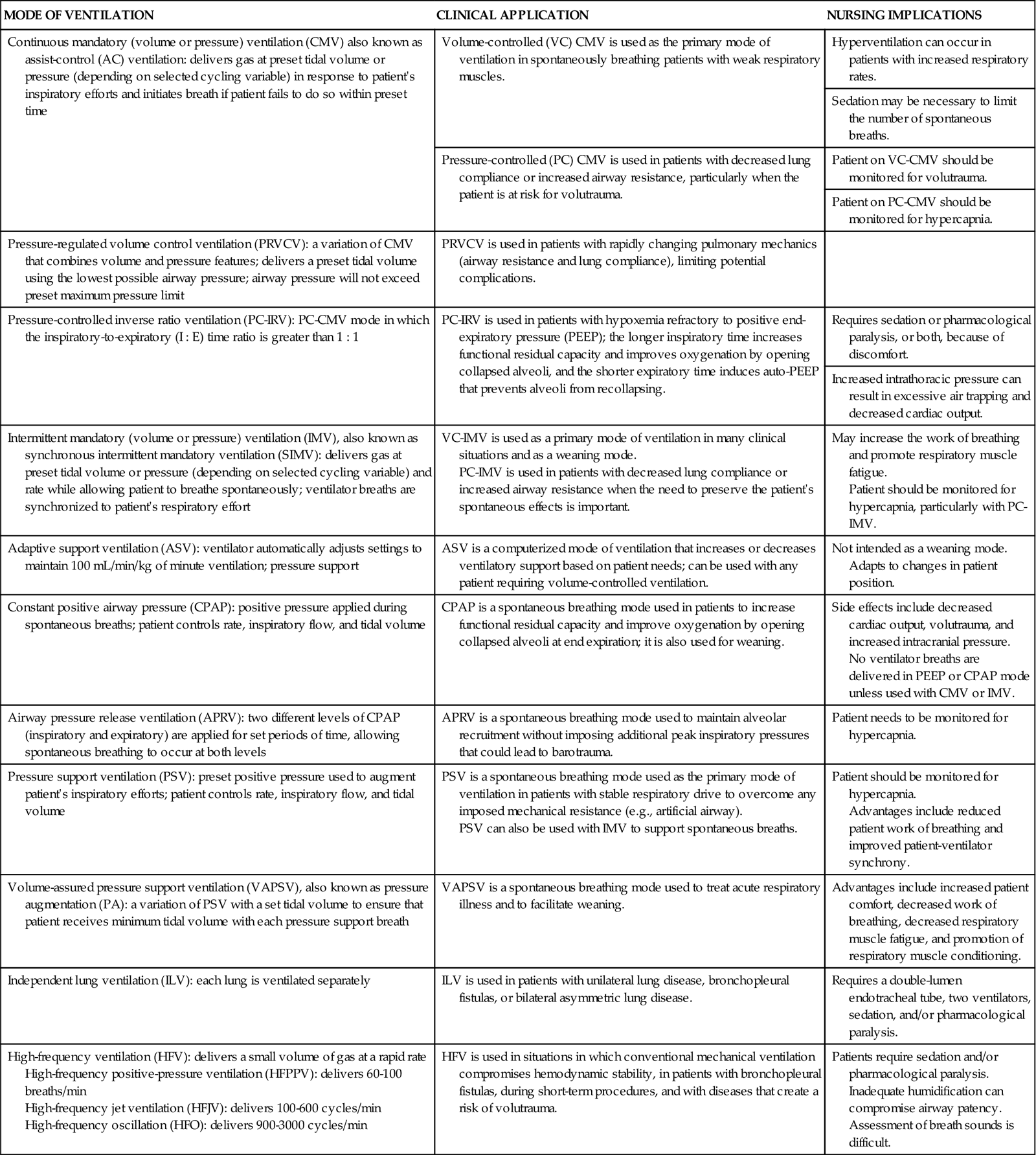
The term ventilator mode refers to how the machine ventilates the patient. Selection of a particular mode of ventilation determines how much the patient will participate in his or her own ventilatory pattern. The choice depends on the patient’s situation and the goals of treatment. The mode is determined by the combination of phase variables selected. Many modes are available (Table 16-5),7,42–44 and some may be used in conjunction with others. Because brands of ventilators vary in their ability to perform certain functions, not all modes are available on all ventilators.43
TABLE 16-5
MODES OF MECHANICAL VENTILATION
| MODE OF VENTILATION | CLINICAL APPLICATION | NURSING IMPLICATIONS |
| Continuous mandatory (volume or pressure) ventilation (CMV) also known as assist-control (AC) ventilation: delivers gas at preset tidal volume or pressure (depending on selected cycling variable) in response to patient’s inspiratory efforts and initiates breath if patient fails to do so within preset time | Volume-controlled (VC) CMV is used as the primary mode of ventilation in spontaneously breathing patients with weak respiratory muscles. | Hyperventilation can occur in patients with increased respiratory rates. |
| Sedation may be necessary to limit the number of spontaneous breaths. | ||
| Pressure-controlled (PC) CMV is used in patients with decreased lung compliance or increased airway resistance, particularly when the patient is at risk for volutrauma. | Patient on VC-CMV should be monitored for volutrauma. | |
| Patient on PC-CMV should be monitored for hypercapnia. | ||
| Pressure-regulated volume control ventilation (PRVCV): a variation of CMV that combines volume and pressure features; delivers a preset tidal volume using the lowest possible airway pressure; airway pressure will not exceed preset maximum pressure limit | PRVCV is used in patients with rapidly changing pulmonary mechanics (airway resistance and lung compliance), limiting potential complications. | |
| Pressure-controlled inverse ratio ventilation (PC-IRV): PC-CMV mode in which the inspiratory-to-expiratory (I : E) time ratio is greater than 1 : 1 | PC-IRV is used in patients with hypoxemia refractory to positive end-expiratory pressure (PEEP); the longer inspiratory time increases functional residual capacity and improves oxygenation by opening collapsed alveoli, and the shorter expiratory time induces auto-PEEP that prevents alveoli from recollapsing. | Requires sedation or pharmacological paralysis, or both, because of discomfort. |
| Increased intrathoracic pressure can result in excessive air trapping and decreased cardiac output. | ||
| Intermittent mandatory (volume or pressure) ventilation (IMV), also known as synchronous intermittent mandatory ventilation (SIMV): delivers gas at preset tidal volume or pressure (depending on selected cycling variable) and rate while allowing patient to breathe spontaneously; ventilator breaths are synchronized to patient’s respiratory effort | VC-IMV is used as a primary mode of ventilation in many clinical situations and as a weaning mode. PC-IMV is used in patients with decreased lung compliance or increased airway resistance when the need to preserve the patient’s spontaneous effects is important. |
May increase the work of breathing and promote respiratory muscle fatigue. Patient should be monitored for hypercapnia, particularly with PC-IMV. |
| Adaptive support ventilation (ASV): ventilator automatically adjusts settings to maintain 100 mL/min/kg of minute ventilation; pressure support | ASV is a computerized mode of ventilation that increases or decreases ventilatory support based on patient needs; can be used with any patient requiring volume-controlled ventilation. | Not intended as a weaning mode. Adapts to changes in patient position. |
| Constant positive airway pressure (CPAP): positive pressure applied during spontaneous breaths; patient controls rate, inspiratory flow, and tidal volume | CPAP is a spontaneous breathing mode used in patients to increase functional residual capacity and improve oxygenation by opening collapsed alveoli at end expiration; it is also used for weaning. | Side effects include decreased cardiac output, volutrauma, and increased intracranial pressure. No ventilator breaths are delivered in PEEP or CPAP mode unless used with CMV or IMV. |
| Airway pressure release ventilation (APRV): two different levels of CPAP (inspiratory and expiratory) are applied for set periods of time, allowing spontaneous breathing to occur at both levels | APRV is a spontaneous breathing mode used to maintain alveolar recruitment without imposing additional peak inspiratory pressures that could lead to barotrauma. | Patient needs to be monitored for hypercapnia. |
| Pressure support ventilation (PSV): preset positive pressure used to augment patient’s inspiratory efforts; patient controls rate, inspiratory flow, and tidal volume | PSV is a spontaneous breathing mode used as the primary mode of ventilation in patients with stable respiratory drive to overcome any imposed mechanical resistance (e.g., artificial airway). PSV can also be used with IMV to support spontaneous breaths. |
Patient should be monitored for hypercapnia. Advantages include reduced patient work of breathing and improved patient-ventilator synchrony. |
| Volume-assured pressure support ventilation (VAPSV), also known as pressure augmentation (PA): a variation of PSV with a set tidal volume to ensure that patient receives minimum tidal volume with each pressure support breath | VAPSV is a spontaneous breathing mode used to treat acute respiratory illness and to facilitate weaning. | Advantages include increased patient comfort, decreased work of breathing, decreased respiratory muscle fatigue, and promotion of respiratory muscle conditioning. |
| Independent lung ventilation (ILV): each lung is ventilated separately | ILV is used in patients with unilateral lung disease, bronchopleural fistulas, or bilateral asymmetric lung disease. | Requires a double-lumen endotracheal tube, two ventilators, sedation, and/or pharmacological paralysis. |
| High-frequency ventilation (HFV): delivers a small volume of gas at a rapid rate High-frequency positive-pressure ventilation (HFPPV): delivers 60-100 breaths/min High-frequency jet ventilation (HFJV): delivers 100-600 cycles/min High-frequency oscillation (HFO): delivers 900-3000 cycles/min |
HFV is used in situations in which conventional mechanical ventilation compromises hemodynamic stability, in patients with bronchopleural fistulas, during short-term procedures, and with diseases that create a risk of volutrauma. | Patients require sedation and/or pharmacological paralysis. Inadequate humidification can compromise airway patency. Assessment of breath sounds is difficult. |
Ventilator Settings
Settings on the ventilator allow the ventilator parameters to be individualized to the patient and also allow selection of the desired ventilation mode (Table 16-6). Each ventilator has a patient-monitoring system that allows all aspects of the patient’s ventilatory pattern to be assessed, monitored, and displayed.42–45
TABLE 16-6
| PARAMETER | DESCRIPTION | TYPICAL SETTINGS |
| Respiratory rate (f) | Number of breaths the ventilator delivers per minute | 6-20 breaths/min |
| Tidal volume (Vt) | Volume of gas delivered to patient during each ventilator breath | 10-12 mL/kg |
| 6-8 mL/kg in acute lung injury (ALI) | ||
| Oxygen concentration (FiO2) | Fraction of inspired oxygen delivered to patient | May be set between 21% and 100%; adjusted to maintain PaO2 level greater than 60 mm Hg or SpO2 level greater than 90% |
| Positive end-expiratory pressure (PEEP) | Positive pressure applied at the end of expiration of ventilator breaths | 3-5 cm H2O |
| Pressure support (PS) | Positive pressure used to augment patient’s inspiratory efforts | 5-10 cm H2O |
| Inspiratory flow rate and time | Speed with which the tidal volume is delivered | 40-80 L/min Time: 0.8-1.2 sec |
| I : E ratio | Ratio of duration of inspiration to duration of expiration | 1 : 2 to 1 : 1.5 unless inverse ratio ventilation is desired |
| Sensitivity | Determines the amount of effort the patient must generate to initiate a ventilator breath; it may be set for pressure-triggering or flow-triggering | Pressure trigger: 0.5-1.5 cm H2O below baseline pressure |
| Flow trigger: 1-3 L/min below baseline flow | ||
| High pressure limit | Regulates the maximal pressure the ventilator can generate to deliver the tidal volume; when the pressure limit is reached, the ventilator terminates the breath and spills the undelivered volume into the atmosphere | 10-20 cm H2O above peak inspiratory pressure |
Complications
Mechanical ventilation is often life-saving, but, like other interventions, it is not without complications. Some complications are preventable, whereas others can be minimized but not eradicated. Physiological complications associated with mechanical ventilation include ventilator-induced lung injury, cardiovascular compromise, gastrointestinal disturbances, patient-ventilator dyssynchrony, and HAP.
Ventilator-Induced Lung Injury
Mechanical ventilation can cause two different types of injury to the lungs: air leaks and biotrauma.44,46 Air leaks related to mechanical ventilation are the result of excessive pressure in the alveoli (barotrauma), excessive volume in the alveoli (volutrauma), or shearing due to repeated opening and closing of the alveoli (atelectrauma).44,47 Barotrauma, volutrauma, and atelectrauma can lead to excessive alveolar wall stress and damage to the alveolar-capillary membrane, resulting in air leakage into the surrounding spaces. The air then travels out through the hilum and into the mediastinum (pneumomediastinum), pleural space (pneumothorax), subcutaneous tissues (subcutaneous emphysema), pericardium (pneumopericardium), peritoneum (pneumoperitoneum), and retroperitoneum (pneumoretroperitoneum). The resultant disorders vary from the fairly benign to the potentially lethal—the most lethal of which is a pneumothorax or pneumopericardium resulting in cardiac tamponade.44,48
Barotrauma, volutrauma, and atelectrauma can also cause the release of cellular mediators and initiation of the inflammatory-immune response. This type of ventilator-induced injury is known as biotrauma.44,49 Biotrauma can result in the development of ALI.50 To limit ventilator-induced lung injury, the plateau pressure (pressure needed to inflate the alveoli) should be kept at less than 32 cm H2O, PEEP should be used to avoid end-expiratory collapse and reopening, and the tidal volume should be set at 6 to 10 mL/kg.46,49
Cardiovascular Compromise
Positive-pressure ventilation increases intrathoracic pressure, which decreases venous return to the right side of the heart. Impaired venous return decreases preload, which results in a decrease in cardiac output. As a secondary consequence, hepatic and renal dysfunction may occur. Positive-pressure ventilation impairs cerebral venous return. In patients with impaired autoregulation, positive-pressure ventilation can result in increased intracranial pressure.44,51
Gastrointestinal Disturbances
Gastrointestinal disturbances can occur as a result of positive-pressure ventilation. Gastric distention occurs when air leaks around the ETT or tracheostomy tube cuff and overcomes the resistance of the lower esophageal sphincter.7 Vomiting can occur as a result of pharyngeal stimulation from the artificial airway.15 These problems can be prevented by inserting a nasogastric tube and ensuring appropriate cuff inflation. Hypomotility and constipation may occur as a result of immobility and the administration of paralytic agents, analgesics, and sedatives.7
Patient-Ventilator Dyssynchrony
Because the ventilatory pattern is normally initiated by the establishment of negative pressure within the chest, the application of positive pressure can lead to patient difficulties in breathing while on the ventilator. To achieve optimal ventilatory assistance, the patient should breathe in synchrony with the machine. The selected mode of ventilation, the settings, and the type of ventilatory circuitry used can increase the work of breathing and lead to breathing out of synchrony with the ventilator. Patient-ventilatory dyssynchrony can result in decreased effectiveness of mechanical ventilation, the development of auto-PEEP, and psychological distress. Patients who are not breathing in synchrony with the ventilator appear to be fighting or “bucking” the ventilator. To minimize this problem, the ventilator is adjusted to accommodate the patient’s spontaneous breathing pattern and to work with the patient. If this is not possible, the patient may need to be sedated or pharmacologically paralyzed.43,52
Ventilator-Associated Pneumonia
Ventilator-associated pneumonia (VAP) is a subgroup of HAP that refers to the development of pneumonia 48 to 72 hours after endotracheal intubation.53 There is great potential for the development of pneumonia after placement of an artificial airway, because the tube bypasses or impairs many of the lung’s normal defense mechanisms. After an artificial airway has been placed, contamination of the lower airways follows within 24 hours. This results from a number of factors that directly and indirectly promote airway colonization. The use of respiratory therapy devices (e.g., ventilators, nebulizers, intermittent positive-pressure breathing machines) also can increase the risk of pneumonia. The severity of the patient’s illness and the presence of ALI or malnutrition significantly increase the likelihood that an infection will ensue. Therapeutic measures such as nasogastric tubes and gastric alkalinization with enteral feedings or medications facilitate the development of pneumonia. Nasogastric tubes promote aspiration by acting as a wick for stomach contents, whereas enteral feedings, antacids, histamine inhibitors, and proton-pump inhibitors increase the pH level of the stomach, promoting the growth of bacteria that can then be aspirated.53 Additional information on managing the patient with pneumonia is provided in Chapter 15.
Prevention of VAP is critical. Several strategies may assist with prevention, including semirecumbent positioning, continuous aspiration of subglottic secretions (CASS), meticulous oral hygiene with antiseptics such as chlorhexidine (discussed earlier), and proper hand hygiene (Evidence-Based Practice box on Hand Hygiene Guidelines in Chapter 15).53–55
Semirecumbency
Positioning of the patient who requires mechanical ventilation is very important. Semirecumbent positioning (elevation of the head of the bed 30 to 45 degrees) reduces the incidence of gastroesophageal reflux and subsequent aspiration and decreases the incidence of VAP. The head of the patient’s bed should be elevated to 30 to 45 degrees at all times unless contraindicated (e.g., hemodynamic instability, presence of intraaortic balloon pump, physician’s order to the contrary).53 However, this intervention does increase the risk of skin shear on the coccyx, and extra surveillance is mandatory for prevention of pressure ulcers.
Continuous Aspiration of Subglottic Secretions
Artificial airways are a significant risk factor for the development of VAP, because they allow for aspiration of bacteria-laden oropharyngeal and gastrointestinal secretions into the lungs. This occurs as a result of pooling of secretions from the mouth and stomach above the cuff of the artificial airway and leaking of the secretions around the cuff into the patient’s airways.35 Removal of the secretions from above the cuff by continuous aspiration has been shown to decrease the incidence of VAP.56 CASS requires the use of a specialized ETT. A CASS tube has an additional lumen, with an opening above the cuff, which is connected to continuous (−20 mm Hg) or intermittent (−100 to −150 mm Hg) suction.35,56 The tubes are recommended for patients who are expected to be intubated for longer than 48 hours. One problem with the CASS tube is that the aspiration lumen can become clogged with thick secretions, food particles, and clots.35
Other Measures to Reduce the Incidence of Ventilator-Associated Pneumonia
Recent studies have shown that use of an ETT with a polyurethane cuff may decrease the incidence of VAP. A traditional ETT has a polyvinyl low-pressure high-volume cuff. When the cuff is inflated, folds form in the cuff, allowing fluids and air to leak around the cuff and into the lungs. This is why subglottic secretion removal is so important. Polyurethane cuffs are much thinner than the traditional polyvinyl cuffs and do not form folds when they are inflated. There is no leakage of fluids into the lungs.57
Another recent study found that the use of silver-coated ETTs significantly reduced the incidence and delayed the onset of VAP, compared with a regular ETT. The tube decreased the incidence of VAP by preventing bacterial colonization and biofilm formation.58 Biofilm is formed when bacteria cling to the inner lumen of the ETT and then secrete an exopolysaccharide substance. This substance forms a gelatinous matrix that allows bacteria to thrive on a nonbiological surface.59
Weaning
Weaning is the gradual withdrawal of the mechanical ventilator and the reestablishment of spontaneous breathing. Weaning should begin only after the original process for which ventilator support was required has been corrected and patient stability has been achieved. Other factors to consider when weaning are length of time on ventilator, sleep deprivation, and nutritional status. Major factors that affect the patient’s ability to wean include the ability of the lungs to participate in ventilation and respiration, cardiovascular performance, and psychological readiness.60 This discussion focuses on weaning of the patient from short-term (≤3 days) mechanical ventilation. Management of weaning in the patient on long-term mechanical ventilation is discussed in Chapter 15.
Readiness to Wean
Patients should be screened every day for their readiness to wean. The screen should include an evaluation of the patient’s level of consciousness, physiological and hemodynamic stability, adequacy of oxygenation and ventilation, spontaneous breathing capability, and respiratory rate and pattern. The rapid, shallow breathing index (RSBI) can predict weaning success. To calculate an RSBI, the patient’s respiratory rate and minute ventilation are measured for 1 minute during spontaneous breathing. The measured respiratory rate is then divided by the tidal volume (expressed in liters). An RSBI of less than 105 is considered predictive of weaning success. If the patient is receiving sedation, the medication should be discontinued at least 1 hour before the RSBI is measured. If the patient meets criteria for weaning readiness and has an RSBI of less than 105, a spontaneous breathing trial can be performed.61 One study showed that implementation of a weaning program that incorporated daily spontaneous-breathing trials had a positive impact on extubation rates and no effect on reintubation rates.62
After readiness to wean has been established, the patient is prepared for the weaning trial. The patient is positioned upright to facilitate breathing and suctioned to ensure airway patency. The process is explained to the patient, and the patient is offered reassurance and diversional activities. The patient is assessed immediately before the start of the trial and frequently during the weaning period for signs of weaning intolerance (Box 16-1).60,61,63,64
Weaning Methods
A number of methods can be used to wean a patient from the ventilator. The method selected depends on the patient, his or her pulmonary status, and length of time on the ventilator. The three main methods for weaning are (1) T-tube (T-piece) trials, (2) synchronized intermittent mandatory ventilation (SIMV), and (3) pressure support ventilation (PSV).60,63,65
T-Piece Trials
T-piece weaning trials consist of alternating periods of ventilatory support (usually assist-control ventilation [ACV] or continuous mandatory ventilation [CMV]) with periods of spontaneous breathing. The trial is initiated by removing the patient from the ventilator and having the patient breathe spontaneously on a T-piece oxygen delivery system. After a set amount of time, the patient is placed back on the ventilator. The goal is to progressively increase the duration of time spent off the ventilator. During the weaning process, the patient is observed closely for respiratory muscle fatigue.60–63,65 Constant positive airway pressure (CPAP) may be added to prevent atelectasis and improve oxygenation.63,65
Synchronized Intermittent Mandatory Ventilation Trials
The goal of SIMV weaning is the gradual transition from ventilatory support to spontaneous breathing. It is initiated by placing the ventilator in the SIMV mode and slowly decreasing the rate, usually one to three breaths at a time, until a rate of zero or near-zero is reached. An arterial blood gas (ABG) sample is usually obtained 30 minutes after the trial. This method of weaning can increase the work of breathing, and the patient must be closely monitored for signs of respiratory muscle fatigue.60,63,65
Pressure Support Ventilation Trials
PSV weaning consists of placing the patient on the pressure support mode and setting the pressure support at a level that facilitates the patient’s achieving a spontaneous tidal volume of 10 to 12 mL/kg. PSV augments the patient’s spontaneous breaths with a positive-pressure boost during inspiration. During the weaning process, the level of pressure support is gradually decreased in increments of 3 to 6 cm H2O, while the tidal volume is maintained at 10 to 15 mL/kg, until a level of 5 cm H2O is achieved. If the patient is able to maintain adequate spontaneous respirations at this level, extubation is considered. PSV also can be used with SIMV weaning to help overcome the resistance in the ventilator system.60,63,65
Nursing Management
Nursing priorities for the patient with invasive mechanical ventilation focus on (1) evaluating the patient for patient-related complications and (2) monitoring the patient for ventilator-related complications. Routine assessment of these patients includes monitoring for patient-related and ventilator-related complications. It includes a total patient assessment, with particular emphasis on the pulmonary system, placement of the ETT, and observation for subcutaneous emphysema and dyssynchrony with the ventilator. Assessment of the ventilator includes a review of all the ventilator settings and alarms. A clear understanding of the alarms and their related problems is important (Table 16-7). The peak inspiratory pressure, exhaled tidal volume, and ABGs are also monitored. Issues regarding patient safety and patient transport are addressed, respectively, in the Patient Safety Priorities box on Invasive Mechanical Ventilation and the Evidence-Based Collaborative Practice box on Guidelines for Intrahospital Transport of Critically Ill Patients.
TABLE 16-7
TROUBLESHOOTING VENTILATOR ALARMS
| PROBLEM | CAUSES | INTERVENTIONS |
| Low exhaled VT | Altered settings; any condition that triggers high- or low-pressure alarm; patient stops spontaneous respirations; leak in system preventing VT from being delivered; cuff insufficiently inflated; leak through chest tube; airway secretions; decreased lung compliance; spirometer disconnected or malfunctioning | Check settings; evaluate patient, check respiratory rate; check all connections for leaks; suction patient’s airway; check cuff pressure; calibrate spirometer. |
| Low inspiratory pressure | Altered settings; unattached tubing or leak around ETT; ETT displaced into pharynx or esophagus; poor cuff inflation or leak; tracheoesophageal fistula; peak flows that are too low; low VT; decreased airway resistance resulting from decreased secretions or relief of bronchospasm; increased lung compliance resulting from decreased atelectasis; reduction in pulmonary edema; resolution of ALI; change in position | Reset alarm; reconnect tubing; modify cuff pressures; tighten humidifier; check chest tube; adjust peak flow to meet or exceed patient demand and correct for the patient’s VT; reposition or change ETT. |
| Low exhaled minute volume | Altered settings; leak in system; airway secretions; decreased lung compliance; malfunctioning spirometer; decreased patient-triggered respiratory rate resulting from drugs, sleep, hypocapnia, alkalosis, fatigue, change in neurological status | Check settings; assess patient’s respiratory rate, mental status, and work of breathing; evaluate system for leaks; suction airway; assess patient for changes in disease state; calibrate spirometer. |
| Low PEEP/CPAP pressure | Altered settings; increased patient inspiratory flows; leak; decreased expiratory flows from ventilator | Check settings and correct; observe for leaks in system; if unable to correct problem, increase PEEP settings. |
| High respiratory rate | Increased metabolic demand; drug administration; hypoxia; hypercapnia; acidosis; shock; pain; fear; anxiety | Evaluate ABGs; assess patient; calm and reassure patient. |
| High-pressure limit | Improper alarm setting; airway obstruction resulting from patient fighting ventilator (holding breath as ventilator delivers VT); patient circuit collapse; tubing kinked; ETT in right main stem bronchus or against carina; cuff herniation; increased airway resistance resulting from bronchospasm, airway secretions, plugs, and coughing; water from humidifier in ventilator tubing; decreased lung compliance resulting from tension pneumothorax, change in patient position, ALI, pulmonary edema, atelectasis, pneumonia, or abdominal distention | Reset alarms; clear obstruction from tubing; unkink and reposition patient off of tubing; empty water from tubing; check breath sounds; reassure patient and sedate if necessary; check ABGs for hypoxemia; observe for abdominal distention that would put pressure on the diaphragm; check cuff pressures; obtain chest radiograph and evaluate for ETT position, pneumothorax, and pneumonia; reposition ETT; give bronchodilator therapy. |
| Low-pressure oxygen inlet | Improper oxygen alarm setting; oxygen not connected to ventilator; dirty oxygen intake filter | Correct alarm setting; reconnect or connect oxygen line to a 50-psi source; clean or replace oxygen filter. |
| I : E ratio | Inspiratory time longer than expiratory time; use of an inspiratory phase that is too long with a fast rate; peak flow setting too low while rate too high; machine too sensitive | Change inspiratory time or adjust peak flow; check inspiratory phase, or hold; check machine sensitivity. |
| Temperature | Sensor malfunction; overheating resulting from too low or no gas flow; sensor picking up outside airflow (from heater, open door or window, air conditioner); improper water levels | Test or replace sensor; check gas flow; protect sensor from outside source that would interfere with readings; check water levels. |
Modified from Flynn JBM, Bruce NP: Introduction to critical care nursing skills, St. Louis, 1993, Mosby.
Bedside evaluation of vital capacity, minute ventilation, ABG values, and other pulmonary function tests may be warranted, according to the patient’s condition. The use of pulse oximetry can facilitate continuous, noninvasive assessment of oxygenation. Static and dynamic compliance should also be monitored to assess for changes in lung compliance (see Appendix B).66
Noninvasive Positive-Pressure Ventilation
Noninvasive positive-pressure ventilation (NPPV) is an alternative method of ventilation that uses a mask instead of an ETT to deliver the therapy. Advantages of this type of ventilation include decreased frequency of HAP, increased comfort, and the noninvasive nature of the procedure, which allows easy application and removal. It is indicated in type I and type II acute respiratory failure, cardiogenic pulmonary edema, and other situations in which intubation is not an option. Contraindications to NPPV include hemodynamic instability, dysrhythmias, apnea, uncooperativeness, intolerance of the mask, recent upper airway or esophageal surgery, and inability to maintain a patent airway, clear secretions, or properly fit the mask.67
NPPV can be applied with a nasal or facial mask and ventilator or with a BiPAP machine (Respironics Inc., Murrysville, Pa.) (Figure 16-5). One study found that a full-face mask is better tolerated than a nasal mask.68 This type of ventilation uses a combination of PSV and PEEP supplied by a ventilator, or inspiratory and expiratory positive airway pressure (IPAP and EPAP, respectively) supplied by a BiPAP machine, to assist the spontaneously breathing patient with ventilation. On inspiration, the patient receives PSV or IPAP to increase tidal volume and minute ventilation, resulting in increased alveolar ventilation, a decreased PaCO2 level, relief of dyspnea, and reduced accessory muscle use. On expiration, the patient receives PEEP or EPAP to increase functional residual capacity, resulting in an increased PaO2 level. Humidified supplemental oxygen is administered to maintain a clinically acceptable PaO2 level, and timed breaths may be added if necessary.69
Nursing Management
Nursing priorities for the patient with noninvasive mechanical ventilation focus on (1) evaluating the patient for patient-related complications and (2) monitoring the patient for ventilator-related complications. As with invasive mechanical ventilation, the patient must be closely monitored while receiving noninvasive mechanical ventilation. Respiratory rate, accessory muscle use, and oxygenation status are continually assessed to ensure that the patient is tolerating this method of ventilation. Continuous pulse oximetry with a set alarm parameter is also performed.69,70
The key to ensuring adequate ventilatory support is a properly fitted mask. A nasal mask or a full-face mask may be used, depending on the patient. A properly fitted mask minimizes air leakage and discomfort for the patient. Transparent dressings placed over the pressure points of the face help minimize air leakage and prevent facial skin necrosis caused by the mask. The BiPAP machine is able to compensate for air leaks.70
The patient is positioned with the head of the bed elevated at 45 degrees to minimize the risk of aspiration and to facilitate breathing. Insufflation of the stomach is a complication of this mode of therapy and places the patient at risk for aspiration. The patient is closely monitored for gastric distention, and a nasogastric tube is placed for decompression as necessary. Often patients are very anxious and have high levels of dyspnea before the initiation of noninvasive mechanical ventilation. After adequate ventilation has been established, anxiety and dyspnea are usually sufficiently relieved. Heavy sedation should be avoided, but if it is needed, it would constitute the need for intubation and invasive mechanical ventilation. It is important to spend 30 minutes with the patient after initiation of noninvasive ventilation, because the patient needs reassurance and must learn how to breathe on the machine.68,70 Patient safety issues are addressed in the Patient Safety Priorities box on Noninvasive Mechanical Ventilation.
Positioning Therapy
Positioning therapy can help match ventilation and perfusion through the redistribution of oxygen and blood flow in the lungs, which improves gas exchange. Based on the concept that there is preferential blood flow to the gravity-dependent areas of the lungs, positioning therapy is used to place the least damaged portion of the lungs into a dependent position. The least damaged portions of the lungs receive preferential blood flow, resulting in less ventilation/perfusion mismatching.71 Currently, there are two approaches to position therapy: prone positioning and rotation therapy.
Prone Positioning
Prone positioning is a therapeutic modality that is used to improve oxygenation in patients with ALI.72 It involves turning the patient completely over onto his or her stomach in the face-down position. Although a number of theories have been proposed to explain how prone positioning improves oxygenation, the discovery that ALI causes greater damage to the dependent areas of the lungs probably provides the best explanation. It was originally thought that ALI was a diffuse, homogenous disease that affected all areas of the lungs equally. It is now known that the dependent lung areas are more heavily damaged than the nondependent lung areas. Turning the patient prone improves perfusion to less damaged areas of the lungs, improves ventilation/perfusion matching, and decreases intrapulmonary shunting. Prone positioning can be used to facilitate the mobilization of secretions and provide pressure relief. Prone positioning is contraindicated in patients with increased intracranial pressure, hemodynamic instability, spinal cord injuries, or abdominal surgery. Patients who are unable to tolerate a face-down position are also not appropriate candidates for this type of therapy.73
No standard has been established for the length of time a patient should remain in the prone position. A review of the research on this subject revealed a wide variation, anywhere from 30 minutes to 40 hours.73 The therapy is considered successful if the patient has an improvement in PaO2 of greater than 10 mm Hg within 30 minutes of being placed in the prone position.73 The positioning schedule (length of time in prone position and frequency of turning) is usually based on the patient’s tolerance of the procedure, the success of the procedure in improving the patient’s PaO2, and whether the patient is able to sustain improvements in PaO2 when turned back to the supine position. Prone positioning is discontinued when the patient no longer demonstrates a response to the position change.73
The biggest limitation to prone positioning is the actual mechanics of turning the patient. A number of procedures have been discussed in the literature that advise using pillows to support the patient or using the Vollman Prone Positioner (Hill-Rom Inc., Batesville, Ind.) The latter is a steel frame with four cushions to support the patient’s forehead, chin, chest, and pelvic area. The device is applied to the patient in the supine position and then used to turn the patient to the prone position (Figure 16-6). Regardless of the method used, the abdomen must be allowed to hang free to facilitate diaphragmatic descent.73
Before the patient is turned to the prone position, his or her eyes are lubricated and taped closed, tubes and drains are secured, and the procedure is explained to the patient and family. A team is organized to implement the turning procedure, and one member is positioned at the head of the bed to maintain the patient’s airway. Complications of the procedure include dislodgment or obstruction of tubes and drains, hemodynamic instability, massive facial edema, pressure ulcers, aspiration, and corneal ulcerations.73
Rotation Therapy
Automated turning beds to provide rotation therapy are often used in the critical care setting. Kinetic therapy and continuous lateral rotation therapy (CLRT) are two forms of rotation therapy. The patient is continuously turned from side to side with a rotation of 40 degrees or greater (kinetic therapy) or with a rotation of less than 40 degrees (CLRT).74 Two types of beds can perform this type of therapy: an oscillation bed, in which the mattress inflates and deflates to provide rotation; and a kinetic bed, in which the entire platform of the bed rotates.75
Rotation therapy is thought to improve oxygenation through better matching of ventilation to perfusion76 and to prevent pulmonary complications associated with bed rest and mechanical ventilation.77 However, to achieve such benefits, rotation must be aggressive, and the patient must be turned at least 40 degrees per side, with a total arc of at least 80 degrees,78 for at least 18 hours a day.77 CLRT has been shown to be of minimal pulmonary benefit to the critically ill patient.74 Kinetic therapy decreases the incidence of VAP, particularly in neurological and postoperative patients.78 In one study, kinetic therapy decreased the incidence of VAP and lobar atelectasis in medical, surgical, and trauma patients.77
Complications of the procedure include dislodgment or obstruction of tubes, drains, and lines; hemodynamic instability; and pressure ulcers. Lateral rotation does not replace manual repositioning to prevent pressure ulcers.79 Repositioning changes the relationship of the patient’s posterior surface to the mattress. This gives the skin a chance to reperfuse and to ventilate. Repositioning shifts weight-bearing points. To prevent pressure ulcers, the patient should be positioned 30 degrees from the surface of the mattress regardless of the degree of rotational turn. One study found that patients receiving rotational therapy still developed pressure ulcers of the sacrum, occiput, and heels.80
Thoracic Surgery
Thoracic surgery refers to a number of surgical procedures that involve opening the thoracic cavity (thoracotomy) and/or the organs of respiration. Indications for thoracic surgery range from tumors and abscesses to repair of the esophagus and thoracic vessels.81 Table 16-8 describes a variety of thoracic surgical procedures and their indications. This discussion focuses only on the surgical procedures that involve the removal of lung tissue.
TABLE 16-8
| PROCEDURE | DEFINITION | INDICATIONS |
Pneumonectomy
 |
Removal of entire lung with or without resection of the mediastinal lymph nodes | Malignant lesions |
| Unilateral tuberculosis | ||
| Extensive unilateral bronchiectasis | ||
| Multiple lung abscesses | ||
| Massive hemoptysis | ||
| Bronchopleural fistula | ||
Lobectomy
 |
Resection of one or more lobes of lung | Lesions confined to a single lobe |
| Pulmonary tuberculosis | ||
| Bronchiectasis | ||
| Lung abscesses or cysts | ||
| Trauma | ||
Segmental resection
 |
Resection of bronchovascular section of lung lobe | Small peripheral lesions |
| Bronchiectasis | ||
| Congenital cysts or blebs | ||
Wedge resection
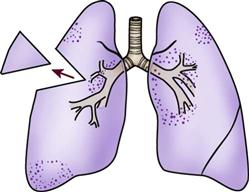 |
Removal of small wedge-shaped section of lung tissue | Small, peripheral lesions (without lymph node involvement) |
| Peripheral granulomas | ||
| Pulmonary blebs | ||
Bronchoplastic reconstruction (also called sleeve resection)
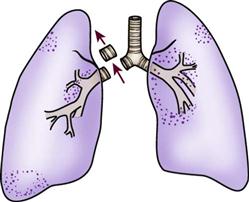 |
Resection of lung tissue and bronchus with end-to-end reanastomosis of bronchus | Small lesions involving the carina or major bronchus without evidence of metastasis |
| May be combined with lobectomy | ||
| Lung volume reduction surgery | Resection of the most damaged portions of lung tissue, allowing more normal chest wall configuration | Severe emphysema |
| Bullectomy | Resection of a large bulla (an airspace that is greater than one centimeter in diameter that formed as a result of pulmonary tissue destruction) | Severe emphysema with large bullae compressing surrounding tissue |
| Open lung biopsy | Resection of a small portion of the lung for biopsy | Failure of closed lung biopsy |
| Removal of small lesions | ||
| Decortication | Removal of fibrous membrane from pleural surface of lung | Fibrothorax resulting from hemothorax or empyema |
| Drainage of empyema | Drainage of pus in the pleural space | Acute and chronic infections |
| Partial rib resection | Removal of one or more ribs to allow healing of underlying lung tissue | Chronic empyemic infections |
| Video-assisted thoracoscopy (VATS) | Endoscopic procedure performed through small incisions in the chest | Evaluation of pulmonary, pleural, mediastinal, or pericardial conditions |
| Biopsy of lung, pleural, or mediastinal lesions | ||
| Recurrent spontaneous pneumothorax | ||
| Evacuation of emphysema, hemothorax, pleural effusion, or pericardial effusion | ||
| Blebectomy/bullectomy | ||
| Pleurodesis | ||
| Sympathectomy | ||
| Closure of bronchopleural fistula | ||
| Lysis of adhesions |
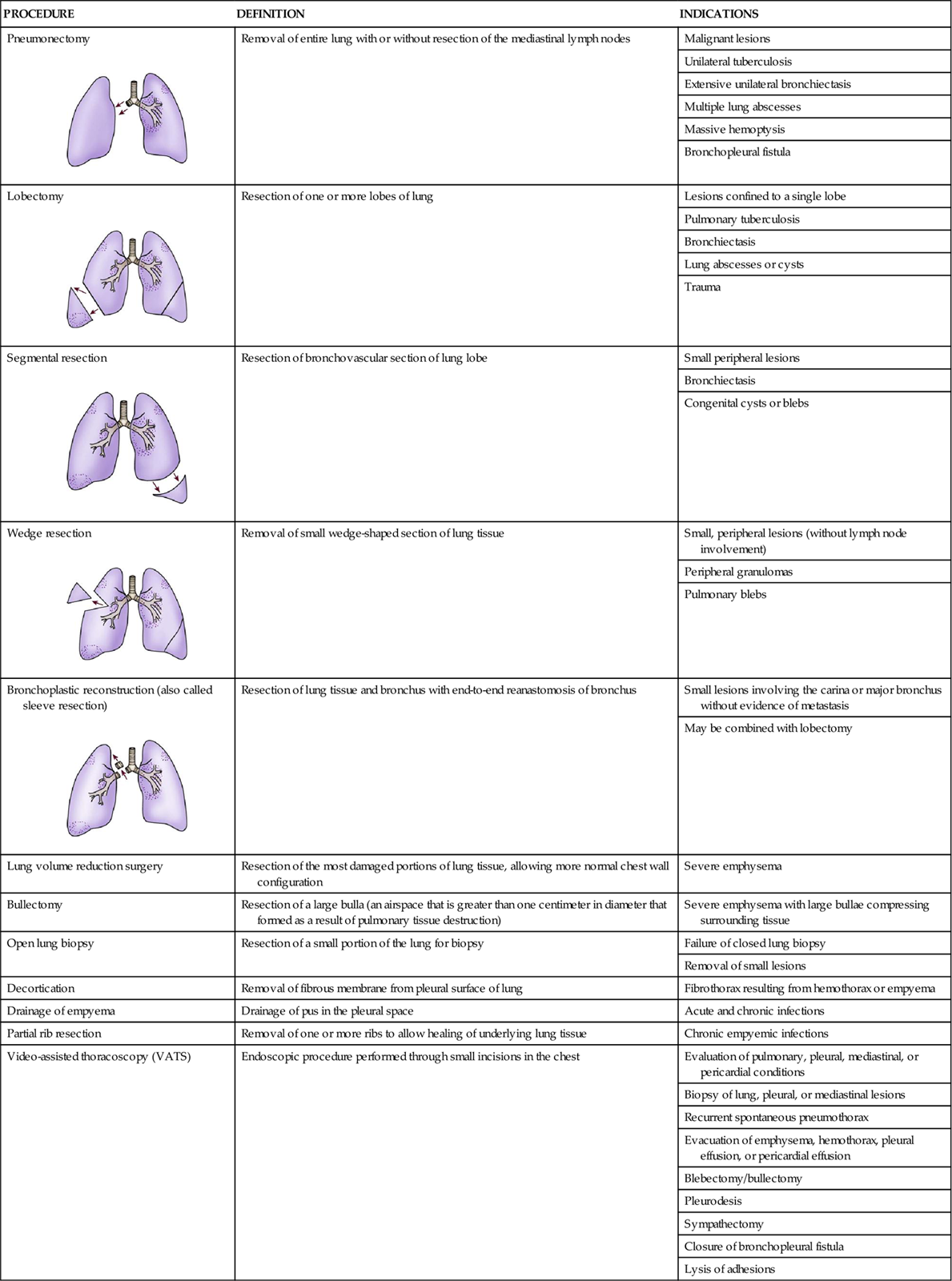
Preoperative Care
Before surgery, a complete evaluation of the patient is needed to determine the appropriateness of surgery as a treatment and to determine whether lung tissue can be removed without jeopardizing respiratory function. This is especially important when a lobectomy or pneumonectomy is being considered. When resection is being undertaken for tumor treatment, preoperative care includes evaluation of the type and extent of the tumor and the physical condition of the patient.81
The evaluation of the patient’s physical status should focus on the adequacy of cardiopulmonary function. The preoperative evaluation should include pulmonary function tests to determine the patient’s ability to manage with less lung tissue. Cardiac function also should be evaluated. Uncontrolled dysrhythmias, acute myocardial infarction, severe chronic heart failure, and unstable angina are all contraindications to surgery.82
Surgical Considerations
The type and location of surgery will dictate the type of surgical approach that is used. The most common approach is the posterolateral thoracotomy, which allows for exposure of both the lung and mediastinum. Other approaches that are used include anterolateral thoracotomy and median sternotomy.81
Special care is taken to avoid drainage of blood or secretions into the unaffected lung during surgery, because such an occurrence could cause hypoxemia and cardiac dysfunction. A double-lumen endotracheal tube is used during the surgery to protect the unaffected lung from secretions and necrotic tumor fragments. To decrease the incidence of hypoxemia during the procedure, 5 to 10 cm H2O of PEEP is maintained to the deflated lung. In addition, the deflated lung is intermittently ventilated during the procedure.83
Complications and Medical Management
A number of complications are associated with a lung resection. These include acute respiratory failure, bronchopleural fistula, hemorrhage, cardiovascular disturbances, and mediastinal shift.
Acute Respiratory Failure
In the postoperative period, acute respiratory failure may result from atelectasis or pneumonia. Atelectasis can occur as a result of anesthesia, the surgical procedure, immobilization, and pain. Treatment should be aimed at correcting the underlying problems and supporting gas exchange. Supplemental oxygen and mechanical ventilation with PEEP may be necessary.83
Bronchopleural Fistula
Development of a postoperative bronchopleural fistula is a major cause of mortality after a lung resection. A bronchopleural fistula develops when the suture line fails to secure occlusion of the bronchial stump and an opening develops into the pleural space. This can result from an imperfect stump closure, perforation of the stump (e.g., with a suction catheter), high pressure within the airways (e.g., caused by mechanical ventilation),84 or infection.85 During surgery, careful attention is given to isolating and closing the bronchus in an attempt to secure a lasting seal with subsequent stump healing.81 In addition, early extubation is encouraged to eliminate the possibility of perforation of the stump and high airway pressures.84 Clinical manifestations of a bronchopleural fistula include shortness of breath and coughing up serosanguineous sputum. Immediate surgery is usually necessary to close the stump and prevent flooding of the remaining lung with fluid from the residual space.85 If this occurs, the patient should be placed with the operative side down (remaining lung up) and a chest tube should be inserted to drain the residual space.81
Hemorrhage
Hemorrhage is an early, life-threatening complication that can occur after a lung resection. It can result from bronchial or intercostal artery bleeding or disruption of a suture or clip around a pulmonary vessel.84 Excessive chest tube drainage can signal excessive bleeding. During the immediate postoperative period, chest tube drainage should be measured every 15 minutes; this frequency should be decreased as the patient stabilizes. If chest tube loss is greater than 100 ml/hour, fresh blood is noted, or a sudden increase in drainage occurs, hemorrhage should be suspected.
Cardiovascular Disturbances
Cardiovascular complications after thoracic surgery include dysrhythmias and pulmonary edema. Resections of a large lung area or a pneumonectomy may be followed by a rise in central venous pressure. With the loss of one lung, the right ventricle must empty its stroke volume into a vascular bed that has been reduced by 50%. This means a higher pressure system is created, which increases right ventricular workload and precipitates right ventricular failure. Depending on previous heart function, acute decompensation of both ventricles can result. Measures are aimed at supporting cardiac function and avoiding intravascular volume excess. These measures include optimizing preload, afterload, and contractility with vasoactive agents.84
Postoperative Nursing Management
Nursing care of the patient who has had thoracic surgery incorporates a number of nursing diagnoses (Nursing Diagnosis box on Thoracic Surgery). Nursing priorities focus on (1) optimizing oxygenation and ventilation, (2) preventing atelectasis, (3) monitoring chest tubes, (4) assisting the patient to return to an adequate activity level, (5) providing comfort and emotional support, and (6) maintaining surveillance for complications.
Optimizing Oxygenation and Ventilation
Nursing interventions to optimize oxygenation and ventilation include positioning, preventing desaturation during procedures, and promoting secretion clearance.
Preventing Atelectasis
Nursing interventions to prevent atelectasis include proper patient positioning and early ambulation, deep-breathing exercises, incentive spirometry (IS), and pain management. The goal is to promote maximal lung ventilation and prevent hypoventilation.
Patient Positioning and Early Ambulation
The nurse should consider the surgical incision site and the type of surgery when positioning the patient. After a lobectomy, the patient should be turned onto the nonoperative side to promote V/Q matching. When the good lung is dependent and blood flow is greater to the area with better ventilation, V/Q matching is better. V/Q mismatching results when the affected lung is positioned down because of the increase in blood flow to an area with less ventilation. The patient should be turned frequently to promote secretion removal but should have the affected lung dependent as little as possible. The patient who has had a pneumonectomy should be positioned supine or on the operative side during the initial period. Turning onto the operative side promotes splinting of the incision and facilitates deep-breathing exercises. Tilting the patient slightly toward the unaffected side is possible, but the surgeon should indicate when free side-to-side positioning is safe.85
When sitting at the bedside or ambulating, patients must be encouraged to keep the thorax in straight alignment while they breathe deeply. This position best accommodates diaphragmatic descent and intercostal muscle action. The sitting or standing position provides enhanced ventilation to areas of the lung that are dependent in the supine position, thus accommodating maximal inflation and promoting gas exchange. Ambulation is essential in restoring lung function and should be initiated as soon as possible.86
Deep Breathing and Incentive Spirometry
Deep breathing and incentive spirometry should be performed regularly by patients who have undergone a thoracotomy. Deep breathing involves having the patient take a deep breath and holding it for approximately 3 seconds or longer. Incentive spirometry involves having the patient take at least 10 deep, effective breaths per hour using an incentive spirometer. These activities help reexpand collapsed lung tissue, thus promoting early resolution of the pneumothorax in patients with partial lung resections. The chest should be auscultated during inflation to ensure that all dependent parts of the lung are well ventilated and to help the patient understand the depth of breath necessary for optimal effect. Coughing, which should be encouraged only when secretions are present, assists in mobilizing secretions for removal.86
Pain Management
Pain can be a major problem after thoracic surgery. Pain can increase the workload of the heart, precipitate hypoventilation, and inhibit mobilization of secretions. Clinical manifestations of pain include tachypnea, tachycardia, elevated blood pressure, facial grimacing, splinting of the incision, hypoventilation, moaning, and restlessness. Several alternatives for pain management after thoracic surgery can be used. The two most common methods are systemic narcotic administration and epidural narcotic administration. Opioids can be administered intravenously or via patient-controlled analgesia (PCA) method. In addition, the patient should be assisted with splinting the incision with a pillow or blanket when deep breathing and coughing. Splinting stabilizes the area and reduces pain when moving, deep breathing, or coughing.87
Maintaining the Chest Tube System
Chest tubes are placed after most thoracic surgery procedures to remove air and fluid. The drainage will initially appear bloody, becoming serosanguineous and then serous over the first 2 to 3 days postoperatively. Approximately 100 to 300 mL of drainage will occur during the first 2 hours postoperatively, which will decrease to less than 50 mL/hour over the next several hours. Routine stripping of chest tubes is not recommended because excessive negative pressure can be generated in the chest. If blood clots are present in the drainage tubing or an obstruction is present, the chest tubes may be carefully milked. The chest tube may be placed to suction or water seal.88
During auscultation of the lungs, air leaks should be evaluated. In the early phase, an air leak is commonly heard over the affected area, because the pleura have not yet tightly sealed. As healing occurs, this leak should disappear. An increase in an air leak or the appearance of a new air leak should prompt investigation of the chest drainage system to discover whether air is leaking into the system from outside or whether the leak is originating from the incision. Increased air leaks not related to the thoracic drainage system may indicate disruption of sutures.84
Assisting Patient to Return to Adequate Activity Level
Within a few days after surgery, range-of-motion exercises for the shoulder on the operative side should be performed. The patient frequently splints the operative side and avoids shoulder movement because of pain. If immobility is allowed, stiffening of the shoulder joint can result. This is referred to as frozen shoulder and may require physical therapy and rehabilitation to regain satisfactory range of motion of the shoulder joint.85
Usually on the day after surgery, the patient is able to sit in a chair. Activity should be systematically increased, with attention to the patient’s activity tolerance. With adequate pulmonary function before surgery and a surgical approach designed to preserve respiratory function, full return to previous activity levels is possible. This may take as long as 6 months to 1 year, depending on the tissue resected and the patient’s general condition.81
Single-Lung and Double-Lung Transplantations
Lung transplantation includes the transplantation of one or two lungs depending on the patient’s underlying condition. Generally speaking, single-lung transplantation (SLT) is most appropriate for patients with restrictive lung diseases such as idiopathic pulmonary fibrosis and sarcoidosis or noninfectious obstructive lung diseases such as emphysema in the absence of significant cardiac dysfunction.81 Pulmonary diseases that typically are associated with chronic lung infections, such as cystic fibrosis and bronchiectasis, require transplantation of both lungs because of the risk of cross-infection from the native lung into the transplanted lung.89
Single-Lung Transplant Surgical Procedure
Transplantation contralateral (opposite side) to a previous thoracotomy is preferable in order to avoid adhesions that require further surgical dissection. The left lung is sometimes preferred because it is easier to expose and has a longer left main bronchus. The longer bronchus gives the surgeon more flexibility in trimming the suture site as needed for anastomosis (Figure 16-7).81 If there is a significant disproportion of ventilation and perfusion to one side, transplantation of the worse side may be the preferred option. With SLT, the remaining native lung, which has either restrictive or obstructive pathophysiology, will have a higher vascular resistance than the transplanted lung. The blood flow is then automatically directed toward the new lung.90
Use of cardiopulmonary bypass (CPB) is becoming less and less common during lung transplantation. However, patients with moderate to severe pulmonary hypertension generally require CPB because the clamping of the pulmonary artery necessary for removal of the diseased lung may cause sudden right heart failure. Inability to maintain adequate oxygenation and ventilation with a single lung, sudden increases in pulmonary artery pressure, poor right ventricular function, and hemodynamic compromise indicate the need for CPB.89
Double-Lung Transplant Surgical Procedure
The surgical procedure for both single- and double-lung transplantation (DLT) is similar, with a few exceptions. In fact, a DLT is performed as a bilateral, sequential SLT. The surgical incision for an SLT can be through either an anterolateral or posterolateral thoracotomy at the level of the fourth or fifth intercostal space. A DLT is performed through bilateral anterior thoracosternotomies extending from the midaxillary line and across the sternum at the fourth intercostal space. This approach is also known as a clamshell incision. The clamshell incision is exquisitely painful for the majority of patients, necessitating frequent pain assessment and intervention by the nurse. A median sternotomy or bilateral anterior thoracotomies are alternative surgical approaches.81,91
The anastomotic sites for DLT include the back wall of the atria (containing the four pulmonary vein orifices), the bronchus, and the main pulmonary artery as illustrated in Figure 16-8. Donor and recipient arteries are trimmed to suitable lengths, and an end-to-end anastomosis is performed. Bronchial anastomosis is performed with a running suture. After the atrial clamp is slowly removed, the patient is assessed for bleeding. In some transplant centers, the omentum is brought through the diaphragm from the abdomen and is wrapped around the bronchus for added stability of the anastomosis and increased vascular supply. Disadvantages of this maneuver include a larger incision and involvement of the abdominal cavity.81
Living-Donor Lung Transplantation
Another alternative to traditional lung transplantation is living-donor lung transplantation. In living-donor transplantation, the lungs are harvested, not from a brain-dead donor, but from two living donors who provide either a right or left lower lobe to the recipient. The two donated lobes essentially function as new lungs. Recipients of this type of transplant tend to be patients with cystic fibrosis or other patients who are smaller in size. Smaller recipients increase the likelihood that two lobes are able to provide adequate pulmonary function. Living-donor lung transplantation is still fairly specialized and is therefore not as commonly practiced as cadaveric lung transplantation.92
Postoperative Medical and Nursing Management
Several nursing diagnoses are associated with the care of single-lung and double-lung transplant patients (Nursing Diagnosis Priorities box on Single-Lung and Double-Lung Transplantation). Nursing priorities focus on (1) optimizing oxygenation and ventilation, (2) providing comfort and emotional support, and (3) maintaining surveillance for complications. SLT patients generally require mechanical ventilation for a shorter duration. Less bleeding can be anticipated because of the brevity of the surgical procedure. A single pleural chest tube usually is sufficient for drainage. A pulmonary artery catheter may be used to measure right ventricular response when significant ventilation/perfusion (V/Q) mismatch occurs. In the event of elevated pulmonary artery pressures, pharmacological vasodilation or afterload reduction can be instituted. Patients with pulmonary hypertension potentially may have a greater V/Q mismatch, resulting in larger alveolar-arterial (A-a)O2 gradients.
Surveillance for rejection and infection is critical. Pulmonary function testing is not initiated until the second or third week postoperatively to allow for surgical recovery. Decreased lung function because of fluid shifts, microatelectasis, and splinting from incisional pain would interfere with accurate testing. In unilateral lung transplantation, the transplanted lung functions in parallel with the native lung, which can be expected to retain any pathology. The patient must be measured by a comparison with his or her own baseline and not with normal standards. This concept also can be applied to the immediate postoperative period of intubation during the evaluation of arterial blood gases. Oxygenation and ventilation occur in both the diseased and transplanted lungs, and parameters for evaluation need to be adjusted accordingly.93
It is important that patient education is provided to cover all aspects of the immunosuppressive medication regimen, signs and symptoms of infection, role of pulmonary function tests, transbronchial biopsy, and clinical signs of pulmonary failure. In addition, a discussion of lifestyle adjustments, long-term considerations, and follow-up visits is included.
Pharmacology
A number of pharmacological agents are used in the care of the critically ill patient with pulmonary dysfunction. Table 16-9 reviews these agents and the special considerations necessary for administering them.94
TABLE 16-9
PHARMACOLOGICAL MANAGEMENT: PULMONARY DISORDERS
| DRUG | DOSAGE | ACTIONS | SPECIAL CONSIDERATIONS |
| Neuromuscular Blocking Agents | Boxed Warning from FDA: Risk of anaphylactic and anaphylactoid type adverse reactions, including fatalities reported in association with use of neuromuscular blockers. |
||
| Vecuronium (Norcuron) | Loading dose: 0.08-0.1 mg/kg IV IV infusion: 0.8-1.2 mcg/kg/min |
Used to paralyze patient to decrease oxygen demand and avoid ventilator dyssynchrony | Administer sedative and analgesic agents concurrently, because NMBAs have no sedative or analgesic properties. |
| Pancuronium (Pavulon) | Loading dose: 0.06-0.1 mg/kg IV IV infusion: 0.02-0.04 mg/kg/hr |
Evaluate level of paralysis q4h using a peripheral nerve stimulator. | |
| Rocuronium (Zemuron) | Loading dose: 0.6mg/kg IV IV infusion: 10-12 mcg/kg/min |
Protect patients from the environment, because they are unable to respond. | |
| Atracurium (Tracrium) | Loading dose: 0.30-0.50 mg/kg IV IV infusion: 4-12 mcg/kg/min |
Prolonged muscle paralysis may occur after discontinuation of the paralytic agent. | |
| Cisatracurium (Nimbex) | Loading dose: 0.15 to 0.2 mg/kg IV IV infusion: 0.5 to 10.2 mcg/kg/min) |
||
| Mucolytics | |||
| Acetylcysteine (Mucomyst) | Nebulizer, 20% solution: 3-5 mL tid-qid Nebulizer, 10% solution: 6-10 mL tid-qid |
Used to decrease viscosity and elasticity of mucus by breaking down disulfide bonds within the mucus | May be administered with a bronchodilator, because drug can cause bronchospasms and inhibit ciliary function. Treatment is considered effective when bronchorrhea develops and coughing occurs. Antidote for acetaminophen overdose. |
| B2-Agonists | |||
| Epinephrine (Adrenalin) | Nebulizer, 1% solution: 2.5-5 mg (0.25-0.5 mL) qid | Used to relax bronchial smooth muscle and dilate airways to prevent bronchospasms | May cause skeletal muscle tremors. |
| Racemic epinephrine | Nebulizer, 2.25% solution: 5.625-11.25 mg (0.25-0.5 mL) qid | Higher doses may cause tachycardia, palpitations, increased blood pressure, dysrhythmias, and angina. | |
| Isoetharine 1% (Bronkosol) | Nebulizer, 1% solution: 2.5-5 mg (0.25-0.5 mL) qid | May increase serum glucose and decrease serum potassium levels. | |
| Terbutaline | MDI, 340 mcg/puff: 1-2 puffs qid MDI, 200 mcg/puff: 2 puffs q4-6h |
Treatment is considered effective when breath sounds improve and dyspnea is lessened. | |
| Metaproterenol (Alupent, Metaprel) | Nebulizer, 5% solution: 15 mg (0.3 mL) tid-qid MDI, 650 mcg/puff: 2-3 puffs tid-qid |
Only approximately 10% of the administered dose reaches the site of action within the lungs. | |
| Albuterol (Proventil, Ventolin) | Nebulizer, 5% solution: 2.5 mg (0.5 mL) tid-qid MDI, 90 mcg/puff: 2 puffs tid-qid |
||
| Levalbuterol (Xopenex) | Nebulizer: 0.63 mg q6-8h | ||
| Anticholinergic Agents | |||
| Ipratropium (Atrovent) | Nebulizer, 0.02% solution: 0.5 mg (2.5 mL) q6-8h | Used to block the constriction of bronchial smooth muscle and reduce mucus production | There are relatively few adverse effects, because systemic absorption is poor. |
| Xanthines | |||
| Theophylline | Loading dose: 4.6 mg/kg IV IV infusion: 0.4-0.8 mg/kg/hr |
Used to dilate bronchial smooth muscle and reverse diaphragmatic muscle fatigue | Administer loading dose over 30 minutes. Monitor serum blood levels; therapeutic level is 10-20 mg/dL. |
| Aminophylline | Loading dose: 5.7mg/kg IV IV infusion: 0.5-1 mg/kg/hr |
Administer with caution to patients with cardiac, renal, or hepatic disease. | |
| Signs of toxicity include central nervous system excitation, seizures, confusion, irritability, hyperglycemia, headache, nausea, hypotension, and dysrhythmias. | |||
| Inhaled Corticosteroids | |||
| Beclomethasone (Vanceril, Beclovent) | MDI, 42 mcg/puff: 2 puffs tid-qid | Used to decrease airway inflammation and enhance effectiveness of beta-agonists | Suppresses inflammatory response and interferes with ability to fight infection. |
| Flunisolide (AeroBid) | MDI, 250 mcg/puff: 2 puffs bid | Oral candidiasis is a side effect that can be minimized by having patients rinse their mouths after treatment. | |
| Triamcinolone (Azmacort) | MDI, 100 mcg/puff: 2 puffs tid-qid | ||
MDI, metered-dose inhaler, NMBAs, neuromuscular blocking agents.