Chapter 13 Pulmonary rehabilitation in chronic respiratory disease
INTRODUCTION
In the early 1980s there was scepticism regarding the value of physical training, due partly to one study that demonstrated negative results after an exercise training programme in patients with chronic obstructive pulmonary disease (COPD) (Belman & Kendregan 1981). These negative results may in part be explained by inadequate intensity of training. Fortunately, scientific advances have led to widespread recognition of the value of exercise in COPD. There is now a strong body of evidence showing that exercise training by itself or as part of a pulmonary rehabilitation programme results in improvements in disease-related problems of dyspnoea, reduced exercise intolerance, muscle weakness and poor health-related quality of life (Lacasse et al 2002). Despite the strong evidence supporting the benefits of physical training, survey data suggest that less than 2% of appropriate patients receive pulmonary rehabilitation (British Lung Foundation 2002). It is hoped that this chapter, publications, reviews and the impending National Service Framework for COPD will help to redress the balance and improve delivery of pulmonary rehabilitation to all patients with COPD.
Pulmonary rehabilitation is defined as:
Pulmonary rehabilitation programmes are delivered ideally by a multidisciplinary team whose structure varies according to patient population, programme budget, availability of team members and resources (Nici et al 2006). For many years, physiotherapists have been an important part of this multidisciplinary approach and have played an important role in the management of patients with respiratory disease. Effective positioning, mobilization, functional exercises, relaxed breathing and techniques to aid the removal of secretions are recognized physiotherapeutic treatment interventions for these patients. Physiotherapists have also traditionally been active in the education of patients with respiratory disease. The aim now must be to promote healthy attitudes and recognition of the benefits of exercise.
RATIONALE FOR REHABILITATION IN CHRONIC OBSTRUCTIVE PULMONARY DISEASE
COPD is a major cause of morbidity and mortality worldwide, and individuals with COPD constitute the largest proportion of patients referred for pulmonary rehabilitation. One of the major limiting symptoms reported by patients with COPD is dyspnoea: the distressing and fearful sensation of breathlessness. Patients with COPD report significant limitations during daily life and reductions in exercise tolerance. One study has shown high levels of disability in patients with COPD, with 50% of patients studied requiring assistance with household chores (Garrod et al 2000a). Almost all the patients investigated reported some degree of breathlessness during washing and dressing. Other studies have shown that most patients with severe COPD are breathless even when performing simple activities of daily living (ADL) or walking around at home (Bestall et al 1999, Restrick et al 1993). Reduced tolerance to exercise is also a feature of COPD, and may be attributable to the illness itself (e.g. ventilatory limitation), cardiac dysfunction, gas exchange limitations, pre-existing levels of cardiovascular fitness and muscle dysfunction. In fact, while dyspnoea remains a striking and important symptom of COPD, weakness in the peripheral muscles (and possibly respiratory muscles) contributes strongly to exercise inefficiency (Gosselink et al 1996, Koppers et al 2006, Lotters et al 2002). It is increasingly apparent that muscle weakness and muscle fatigue play an important part in the disability evidenced in COPD patients. Fat-free mass in particular has been identified as an important predictor of muscle mass and associated with peak oxygen uptake during exercise (Gosker et al 2003). Peripheral muscle dysfunction in COPD is characterized by reduced muscle strength, reduced muscle endurance, impaired muscle oxidative capacity, and a shift toward a glycolytic fibre-type distribution; that is, a decrease in type I (slow oxidative) fibres and an increase in type IIb (fast glycolytic) fibres (Allaire et al 2004, Gosker et al 2002, Gosselink et al 2000, Janaudis-Ferreira et al 2006, Mador et al 2003, Maltais et al 1999 , Whittom et al 1998). Janaudis-Ferreira and co-workers compared quadriceps muscle strength and endurance in 42 patients with COPD with 53 age-matched healthy controls and showed significantly reduced muscle strength in the COPD group (Janaudis-Ferreira et al 2006). In addition, endurance was lower in the COPD group compared with their healthy age-matched controls. This supports earlier observations that leg fatigue is experienced at lower work intensities in COPD patients compared with healthy subjects (Killian et al 1992). The quadriceps muscle may therefore be significantly weaker and more prone to fatigue in patients with COPD compared with healthy subjects.
Patients with COPD are exposed to a number of factors that may contribute to peripheral muscle dysfunction. An extremely sedentary lifestyle is observed in this population (Pitta et al 2005a, Sandland et al 2005). Donaldson and co-workers showed that time spent outdoors declines markedly over time and deteriorates acutely during exacerbations (Donaldson et al 2005). Pitta and co-workers showed that patients with COPD are severely inactive not only during hospitalization for an acute exacerbation but also after discharge (Pitta et al 2006a). While it is clear that physical inactivity is an important factor, other factors such as the use of corticosteroids, malnutrition, disequilibrium in protein balance, chronic hypoxia and hypercapnia, oxidative stress, muscle apoptosis and genotype profile contribute to muscle impairment (American Thoracic Society/European Respiratory Society (ATS/ERS) 1999, Troosters et al 2005). There is now much interest in the place of inflammation in COPD and the part it plays in the development of muscle dysfunction. It has been shown that systemic inflammation is associated with loss of fat-free mass and muscle weakness both in stable patients and in patients during an acute exacerbation (Schols et al 1996, Spruit et al 2003). Importantly, data have shown relationships between inflammatory markers such as C-reactive protein (CRP), interleukin 6 (IL-6) and tumour necrosis factor alpha (TNFa) and health status, muscle weakness and exercise tolerance (Broekhuizen et al 2006, de Torres et al 2006, Garrod et al 2005a, Yende et al 2006). The mechanisms and contribution of inflammation to disability, reduced exercise tolerance and response to training are not yet clear (Spruit et al 2005), although it has been suggested that the intensity of systemic inflammation is linked to the severity of airflow obstruction (Gan et al 2004, Takabatake et al 2000). Furthermore, inactivity, oxidative stress, lactic acidosis and inflammatory cytokines may work congruently to disrupt the local anabolic/catabolic mechanisms (Debigare et al 2001). The current body of evidence demonstrates that peripheral muscle dysfunction in COPD is multifactorial, and factors mentioned above may act in combination. Owing to this multidimensional character, determining the severity of the disease based only on pulmonary function measurements has been questioned and a multidimensional index has been used to characterize COPD patients. Besides pulmonary function, this index includes also body composition, level of dyspnoea and functional exercise capacity (6 minute walking test — 6MWT) (Celli et al 2004). These developments in our understanding of the causes of myopathy in COPD reinforce the fact that strategies aimed at maximizing functional performance and peripheral muscle strength are of utmost importance in the pathophysiology of COPD.
EXERCISE PRESCRIPTION
Exercise training is considered the cornerstone of a pulmonary rehabilitation programme (Lacasse et al 1997). Reconditioning of patients with respiratory disease reflects the same principles as those applied in healthy subjects, although programmes should be adapted to the individual limitations of the patient and take into consideration ventilatory, cardiovascular and muscular abnormalities (Troosters et al 2005). Based on solid evidence, it is now widely accepted that exercise training is beneficial to patients with chronic respiratory disease. It is also known that the training effects depend on different factors including duration and frequency of the training programme, training intensity and training modality. The extent of the benefits obtained will depend on the management of these factors.
Duration and frequency of the training programme
There is as yet no consensus as to the optimal duration of an exercise training programme for patients with COPD. Evidence suggests that longer programmes yield larger and more endurable training effects (Lacasse et al 2002). Twenty sessions of pulmonary rehabilitation have been shown to produce better results than 10 sessions in outcomes such as exercise tolerance and health-related quality of life (Rossi et al 2005). Furthermore, various studies have demonstrated that exercise programmes lasting at least 7 weeks (7–12 weeks) result in greater benefits than programmes of shorter duration (Bendstrup et al 1997, Carrieri-Kohlman et al 2005, Green et al 2001, Lake et al 1990). It has therefore been suggested that programmes of at least 8 weeks are advisable to result in substantial positive effects (Fabbri & Hurd 2003, Troosters et al 2005). A meta-analysis has shown strong trends for improved results in functional exercise capacity from longer programmes with close supervision (Lacasse et al 2002). Programmes of 6 months or longer also seem to result in better long-term effects (Berry et al 2003, Guell et al 2000, Salman et al 2003, Troosters et al 2000).
Ideal training frequency is also a topic that has been much debated but there is as yet insufficient evidence to identify the optimal frequency. The scarce evidence available suggests that patients should exercise at least three times per week, and that regular supervision is fundamental (Puente-Maestu et al 2000, Ringbaek et al 2000, Wadell et al 2005). However, many programmes with twice-weekly supervision and encouragement of ‘home exercise’ have shown good results (Lacasse 2006). One intensive programme with 20 sessions condensed into 3–4 weeks showed positive results (Fuchs-Climent et al 1999), suggesting that frequency may be as important as duration of the programme.
Training intensity
Determining training intensity
A further element of exercise prescription concerns the intensity of exercise. A number of early studies of pulmonary rehabilitation had methodological flaws concerning the description of exercise intensity. Before considering appropriate levels of intensity, it is necessary to revisit relevant assessment tools. Two common methods of prescribing intensity are used: symptom-limited exercise prescription and physiological testing derived from maximal oxygen consumption (or related measures). In the first method patients are instructed to exercise to a prescribed symptom level, for example ‘moderately or somewhat short of breath’ (scores of 4–6) on the Borg breathlessness score (Borg 1982, Horo-witz et al 1996). Although this provides an effective training stimulus for most patients, problems may occur when patients demonstrate very high levels of dysp-noea, thus limiting the intensity of training. Dyspnoea is very much a subjective perception, meaning that fear and anxiety at the start of a programme may heighten scores. If this method is used, it may be necessary to reassess dyspnoea levels halfway through the programme and set a higher training target if appropriate. Calculating the exercise intensity from maximum oxygen consumption (VO2max) is probably more reliable and is easily performed using cycle ergometry or derived from an associated measure such as the shuttle walk test (Dyer et al 2002). However, for patients with COPD, a true VO2max may be unattainable due to ventilatory limitations. An effective compromise is to determine the initial exercise prescription at 70–80% of the derived VO2max and then to use breathlessness scores to monitor the training and adjust accordingly (Mahler et al 2003).
Power output has also been used as an option to determine training intensity, and an intensity of 60–80% of the maximal workload has been frequently used with positive results. However, the peak work rate obtained during the maximal incremental exercise test is determined by the work rate increment used in the test and this must be taken into consideration (Debigare et al 2000). Another option to determine training intensity is using a percentage of the maximal heart rate. Caution is necessary because this may result in inadequate training stimulus (Brolin et al 2003, Pitta et al 2004, Zacarias et al 2000). This inadequacy may occur because maximal exercise capacity is often not affected by the cardiocirculatory system in COPD patients, and heart rate is also influenced by various medications commonly prescribed to COPD patients.
Ideal training intensity
Casaburi and colleagues (1991) demonstrated greater physiological and cardiovascular benefits in patients who exercise at higher intensities when compared with patients exercising for a longer duration but at a lower intensity. The same research group, in another study, targeted patients with more severe COPD and obtained similar results (Casaburi et al 1997). Puente-Maestu and colleagues have also shown that supervised higher- intensity training programmes were more effective than lower-intensity self-monitored training (Puente-Maestu et al 2000). Others studies have also highlighted the positive effects of high-intensity exercise training (Gimenez et al 2000, Punzal et al 1991). These studies recommend training intensities of 60–80% of peak work rate or maximal oxygen consumption in order to achieve the greatest effects, although the rate of work increment during the maximal incremental test is an important factor in determining whether a patient is able to achieve a training target of 80% of peak work rate (Debigare et al 2000, Maltais et al 1997, Neder et al 2000). Low-intensity training has also been shown to result in significant improvements in symptoms and quality of life (Normandin et al 2002) and even in exercise tolerance (Clark et al 1996, Roomi et al 1996). Wedzicha and colleagues stratified a group of COPD patients according to their degree of dyspnoea and instructed them to exercise until moderately to severely shortness of breath (Wedzicha et al 1998). They reported that the improvement in exercise performance and health status following an exercise programme depends on the initial degree of dyspnoea. Therefore, a cautionary note concerns the relative severity of the patients.
Findings from a systematic review highlight the fact that in very severe patients, there is a lack of evidence to indicate that high-intensity exercise is the ideal mode of training. (Puhan et al 2005). Applicability of high-intensity training in these most severe and symptomatic COPD patients requires further study in order to determine the ideal training intensity and to achieve better results. In summary, the most recent consensus statement by the American Thoracic Society and European Respiratory Society states that:
In cases where high-intensity exercise is advocated for the more symptomatic patients with severe COPD, interval training may prove to be more comfortable (Vogiatzis et al 2005).
Training modality
A variety of training modalities has been employed in the management of patients with COPD, all with generally good results. The British Thoracic Guidelines recommend including functional exercises (Morgan et al 2001). Most programmes use continuous (or endurance) exercise training, incorporating an element of walking and/or cycling for 20–30 minutes per session. An alternative approach is interval training, where the 20- or 30-minute exercise session is divided into short bouts of high-intensity exercise for 30 seconds to 2–3 minutes, interspersed with equal periods of rest. One study compared interval training with continuous training in COPD patients and showed a different pattern of physiological response (Coppoolse et al 1999). Continuous training resulted in an improvement in maximal oxygen consumption, reduction in minute ventilation and a more pronounced decrease in lactic acid production, whereas interval training resulted in improvement in peak workload and a decrease in leg pain. This difference in training response may be a reflection of specific training effects in either oxidative or glycolytic muscle metabolic pathways. Vogiatzis and colleagues (2005) compared high-intensity interval training (bouts of 30 seconds at 125% maximal cycle ergometry and 30 seconds rest for 45 minutes) with equivalent constant load (at 75% of maximum for 30 minutes constantly) 3 times per week for 10 weeks. Both groups showed significant training effects; however, during the training sessions, symptoms of dyspnoea and leg discomfort were significantly lower for the high-intensity interval group. These data suggest that in order to minimize discomfort associated with exercise training (and hence aid long-term adherence) patients may be advised to exercise in short, high-intensity bursts of activity. Furthermore, interval training may allow more severe patients to achieve higher work rates and to exercise for longer with fewer symptoms, due to less dynamic hyperinflation and a higher stable ventilation (Sabapathy et al 2004, Vogiatzis et al 2004). When using interval training, it is important that the total exercise time is not reduced but kept to 20–30 minutes.
The relative benefits of generalized training programmes have been compared with individualized training programmes (Sewell et al 2005). General exercises consisted of three strength training activities for the lower limbs (step-ups, sit to standing and stationary cycling), thoracic exercises and upper limb activities (wall pushes, arm circling and shrugging). Individualized exercises were based on patient goals identified using the Canadian Occupational Performance Measure (2005). After the 7-week programme, there were no differences in any outcome measure between the 59 patients randomized to general training and the 64 randomized to individualized training. The sample size was large in this study with adequate power suggesting that targeting exercise specifically to patient-identified goals is unnecessary assuming that adequate attention is made to ensure that both upper and lower limb exercises are included at an appropriate intensity.
As data have become available from studies investigating the relative merits of different training regimens in pulmonary rehabilitation, it may be concluded that the most important aspect of exercise is that strengthening exercises are routinely incorporated into programmes. Strength training has the potential to increase muscle mass and muscle force, both of which are common therapeutic aims in COPD patients. Training is generally performed with 2 to 4 sets of 6 to 12 repetitions, at intensities ranging from 50% to 85% of the one repetition maximum (O’Shea et al 2004). A systematic review evaluating a number of comparative study designs concluded that strength training resulted in greater improvements in health-related quality of life than endurance training, although the benefits of strength over endurance are equivocal when considering the relative change in exercise tolerance (Puhan et al 2005). Interestingly, a randomized controlled trial reported no difference in change in muscle strength, distance walked or health-related quality of life between those who performed a strength training regimen compared with endurance training (Spruit et al 2002). In addition, Probst and colleagues showed that a major advantage of strength training is that the cardiopulmonary stress during this kind of exercise is lower than during whole-body endurance exercise and results in fewer symptoms (Probst et al 2006). As muscle weakness contributes to reductions in maximal walking test but not to endurance walking, there is probably a place for both types of training (Steiner et al 2005). Guidelines for pulmonary rehabilitation in COPD patients currently recommend a combination of endurance and strength training as it has multiple beneficial effects and is well tolerated (Nici et al 2006).
Although the focus of most studies of exercise training in COPD patients has been the lower limbs, it has been shown that upper limbs are also affected (Franssen et al 2005, Gosselink et al 2000) and that upper limb activity influences dynamic hyperinflation and pulmonary mechanics (Dourado et al 2006, Gigliotti et al 2005, McKeough et al 2003). Due to the fact that improvement is specific to the muscles trained, the inclusion of exercise for the upper limbs in a programme is justified, particularly exercises that reflect activities of daily living. Examples of such exercises include arm cycle ergometer, multigyms, free weights and elastic bands.
When training the upper limbs, it is important to consider the principles of positioning during exercise. Exercise endurance is less during unsupported upper limb compared with supported upper limb work (Astrand et al 1968), especially when the arms are elevated above the head such as in ‘reaching’ or ‘arching the arms’. Stabilization of the accessory muscles occurs only during movements where the shoulder girdle is fixed. These principles can be utilized during training. Unsupported upper limb work may achieve greater desensitization of dyspnoea, while strength training will be performed best with the upper limbs supported in order to minimize dyspnoea and maximize the number of repetitions possible. Guidelines for exercise prescription in pulmonary rehabilitation for patients with COPD are outlined in Box 13.1.
Box 13.1 Practice guidelines for exercise prescription in pulmonary rehabilitation for patients with COPD
 A training programme consisting of between 14 and 24 sessions, supervised at least twice a week, should be offered: longer programmes generally result in better long-term effects.
A training programme consisting of between 14 and 24 sessions, supervised at least twice a week, should be offered: longer programmes generally result in better long-term effects. 20–30 minutes of high-intensity endurance exercise training (walking, cycling) generally produces greater physiological benefit. Intensity of 60–80% of the peak work rate is a useful target. However, low-intensity training can also be effective for the more severe and symptomatic patients who cannot achieve this intensity level.
20–30 minutes of high-intensity endurance exercise training (walking, cycling) generally produces greater physiological benefit. Intensity of 60–80% of the peak work rate is a useful target. However, low-intensity training can also be effective for the more severe and symptomatic patients who cannot achieve this intensity level. In more severe patients, interval training (i.e. short bouts of high-intensity exercise interspersed by rest) is a valid alternative to endurance training in order to allow patients to exercise at higher intensities, but total exercise time should be kept to 20–30 minutes.
In more severe patients, interval training (i.e. short bouts of high-intensity exercise interspersed by rest) is a valid alternative to endurance training in order to allow patients to exercise at higher intensities, but total exercise time should be kept to 20–30 minutes.PHYSIOLOGICAL TRAINING RESPONSES
Physiological training effects differ according to the training regimen. The first aspect of training that occurs is a learning effect or improved neuromuscular coordination. This is not associated with physiologi-cal training effects per se, but may result in improved gait efficiency and increased stride length after a programme involving repeated walks (McGavin et al 1977).
Improved mechanical efficiency
Mechanical efficiency is reduced in patients with chronic respiratory disease when compared with a healthy elderly population (Baarends et al 1997, Richardson et al 2004). The explanation for this seems to be linked to the elevated number of less efficient type II fibres (Richardson et al 2004) and the increased oxygen cost of breathing observed in COPD (Baarends et al 1997). Arm efficiency seems to be relatively preserved in comparison to the lower limbs (Franssen et al 2002).
Much of the improvement in exercise tolerance following pulmonary rehabilitation is likely to be a result of improvements in mechanical efficiency (O’Donnell 1994). Measures that may suggest an improvement in efficiency include stride length and gait coordination. McGavin and co-workers (1977) showed improvements in exercise tolerance after a 12-week home programme of low-intensity exercise. They reported a modest increase of approximately 8% in walking distance which was probably attributable to improvements in mechanical efficiency rather than cardiovascular changes per se. Similarly a group of patients housebound because of dyspnoea, showed some improvement in exercise tolerance after an 8-week home-based programme although this was not significant when compared with a control group (Wedzicha et al 1998). Another programme of home exercise, continued over a period of 1 year showed larger changes, suggesting that when exercise intensity is low a longer period of time may be needed to achieve true physiological training effects (Sinclair & Ingram 1980). Improvements in efficiency of the skeletal muscles after exercise training may lead to reduced alveolar ventilation during exercise, therefore reducing dynamic hyperinflation and reducing exertional dysp-noea (Nici et al 2006).
Cardiovascular adaptations
Cardiovascular adaptations that might be expected in a normal subject after training include a reduction in the heart rate after training for a given level of work, reductions in minute ventilation, a lowering of the onset of lactic acidosis and a lower maximum oxygen uptake (VO2max) for a given work rate. Numerous studies show evidence of these changes in patients with COPD, with both moderate and severe obstruction (Casaburi et al 1991, Griffiths et al 2000, Ries et al 1995).
Muscle changes
Studies have shown that the peripheral muscles in COPD respond to training in a similar manner to muscles in healthy individuals (Casaburi et al 1991). This suggests that the contractile mechanism of the peripheral muscles in patients with COPD remains intact and muscle strength can be improved with an appropriate training programme (Bernard et al 1999, Maltais et al 1997, Simpson et al 1992). Recently Vogiatzis and colleagues performed muscle biopsies of COPD patients before and after pulmonary rehabilitation (Vogiatzis et al 2005) and demonstrated that training, both interval and continuous, achieved physiological changes in muscle fibres of COPD patients. The improved oxidative capacity of muscles, evident by changes in cross-sectional area of both type I and type II fibres and by a shift from type II b fibres (glycolytic) to type IIa (oxidative) fibres, supports previous observations that showed delayed onset of lactic acidosis with training. The result of this is an improvement in oxygen uptake and the ability to maintain aerobic muscle metabolism for a prolonged period (Casaburi et al 1991).
In addition, there is evidence that type I fibres increase in size and number and that the concentration of mitochondrial enzymes is greater after training (Maltais et al 1996). Strength training is predominantly associated with an increase in size of muscle cells and number of myofibrils. Most importantly, muscle capillaries and myoglobin levels within a trained muscle are higher after training, thus improving the transport of oxygen to exercising muscles. In summary, adequate exercise training leads to improvements in the capacity to generate and to sustain contraction. However, it is important to remember that peripheral muscles of patients with COPD are responsive to training but factors other than deconditioning also contribute to dysfunction, namely nutritional status, hypoxia and hyper capnia, inflammatory mediators and circulating hormones.
PRACTICAL ASPECTS OF TRAINING
Location
There are arguments in favour of rehabilitation in a number of settings, from the hospital inpatient setting to the outpatient, home or community setting. Hospital inpatient programmes (Goldstein et al 1994) are better suited to patients with severe deconditioning and/or limited transportation resources. These programmes may offer a multidisciplinary approach and intensive training, but are costly and may lack insurance coverage in some countries. Outpatient programmes seem to be the most cost-effective in producing optimal effects especially in moderate to severe patients, as a multidisciplinary approach, adequate equipment and careful supervision are possible at more reasonable costs (Nici et al 2006). Most studies involving rehabilitation programmes that resulted in significantly positive effects were developed in hospital-based outpatient settings (Fig. 13.1) (Lacasse et al 2002, Nici et al 2006). The advantage of home-based programmes relates to improved adherence and prolonged benefits with an additional focus on functional and meaningful activities (Strijbos et al 1996). The disadvantages relate to the lack of peer group support (Wedzicha et al 1998), the potentially limited space for mobilization and the limited availability of a multidisciplinary team, exercise equipment and proper supervision. Individual supervision is required and patients may need input for a longer period of time when compared with outpatient programmes (Sinclair & Ingram 1980). The pros and cons of pulmonary rehabilitation at home are reviewed (Garrod 1998) and summarized in Table 13.1.
Table 13.1 The pros and cons of pulmonary rehabilitation at home
| Pros | Cons |
|---|---|
(Adapted from Garrod 1998)
Other avenues are being explored, from community care settings (Cambach et al 1997) to primary care interventions in local surgeries and sports centres, but further trials will be needed to evaluate the role of rehabilitation in primary care. Recent advances in ‘exercise on prescription’ schemes run in conjunction with local sports facilities and general practitioner surgeries point towards greater community involvement and better use of private sector resources. In recognition of patient wishes and significant underresourcing of pulmonary rehabilitation, future developments will need to make greater use of community facilities (Garrod & Backley 2006). Physiotherapists are ideally placed to lead the way with referrals, support and training of local members.
Timing
Perhaps of more importance than where pulmonary rehabilitation should take place, is the issue of when rehabilitation is initiated. Pitta and co-workers showed that physical activity is markedly reduced not only during hospitalization for an acute COPD exacerbation, but also continued to be low after discharge (Pitta et al 2006a). In this study, the amount of time spent being active was related to the degree of muscle weakness. A study by Man et al (2004) has shown positive benefits of a training programme delivered within 10 days of discharge from hospital. In this randomized controlled trial a large clinical and statistically significant difference was seen in change in exercise tolerance after rehabilitation, compared with usual care only. No adverse events were reported and the drop out rate was similar in each group. Although the trial was small there were trends towards a reduction in hospital admissions and a significant difference in emergency room visits was observed. These are important findings, since they highlight two factors: firstly that rehabilitation early after discharge is safe and secondly that with usual care alone, exercise tolerance at 3 months following discharge does not improve (and in fact shows a small decline), suggesting that patients may deteriorate over time rather than improve without rehabilitation. A study involving pulmonary rehabilitation and neuromuscular electrostimulation initiated immediately after a hospital admission for acute exacerbation has also highlighted the benefits of early initiation of rehabilitation after exacerbation (Vivodtzev et al 2006). A higher level of physical activity has also been shown to be associated with a 46% reduction in readmission in COPD (Garcia-Aymerich et al 2003), further emphasizing the clinical importance of early rehabilitation. Preliminary data from Probst et al (2005) have shown that an exercise protocol based on strength training can be safely applied even during hospitalization, resulting in improvement in muscle force and counteracting the deleterious effects of immobilization.
Whether rehabilitation programmes should be repeated for individuals is an important clinical question. At the moment data suggest there is little value in repeating a programme 1 year after attendance (Foglio et al 2001), although there has been some suggestion that the number of hospitalizations may be reduced as a result of repeat rehabilitation. Another trial also showed that in severe and disabled COPD patients, a more frequently repeated inpatient programme resulted in only modest additional benefits over 1 year (Romagnoli et al 2006). These benefits were mostly linked to self-reported symptoms and health-related quality of life. The issue is made more complex when consideration is given to the fact that patients often request additional sessions, while many more known people with COPD do not have the opportunity to attend even one course of rehabilitation.
Equipment
As mentioned previously, functional exercise programmes are of the utmost importance to the success of pulmonary rehabilitation. Simple exercises aid clarity, and practical measures to include exercise in daily life may aid long-term adherence. Moreover, for older patients with COPD exercise must be seen as ‘appropriate’; for patients with less severe disease, swimming, bike riding, golfing, bowling and walking are all appropriate forms of exercise. The type of equipment will depend primarily on the type of training to be performed and local financial resources. Equipment requirements may be as simple as a mat for floor exercises, dumbbells or hand weights and space to perform aerobic training. Where endurance training is the main objective, equipment such as cycle ergometry (Fig. 13.2A) and a treadmill may be helpful. However, walking practice, devices to simulate stair climbing (Fig. 13.2B), or actual stairs have high functional applicability. In order to achieve long-term benefits, patients must be able to continue exercising effectively after the programme has ended. For strength training a multigym may be ideal but simple hand and ankle weights will also be sufficient. It is important to note the necessity of training both the upper and lower limbs (Lake et al 1990, Sivori et al 1998) and the use of breathing control throughout exercise (see Other adjuncts to rehabilitation later in this chapter).
Changing attitudes towards exercise and dyspnoea
Fear and anxiety influence the breathing pattern. For many patients, the thought of exercise exacerbates dysp-noea. The underlying philosophy of pulmonary rehabilitation is that exercise is beneficial. During the programme the therapist attempts to help the patient to replace negative thought processes with positive ones. Teaching patients to perceive breathlessness as a positive effect of ‘good’ exercise rather than a negative effect of their health may enhance the training effect (Atkins et al 1984). This philosophy must, of course, extend to relatives and demands the education and involvement of the families of those with respiratory disease.
Supplemental oxygen during exercise training
The use of supplemental oxygen during training remains a complex question, one that in many cases is further complicated by the issue of available resources. It is widely accepted that oxygen supplementation leads to significant acute improvement in exercise tolerance in hypoxaemic patients (O’Donnell et al 2001), even in patients without appreciable exercise desaturation (Somfay et al 2001). Despite this, studies in which oxygen was provided during an exercise training programme have not been able to show additional benefits directly linked to oxygen supplementation. An early study by Zack & Palange (1985) showed an improvement in exercise tolerance after a 12-week outpatient training programme in which all patients trained with supplemental oxygen. However, there was no control group and it is not known whether additional benefits resulted from the use of supplemental oxygen. One randomized study investigated the role of oxygen in patients with severe COPD and exercise desaturation (Garrod et al 2000a). This showed an improvement in dyspnoea after rehabilitation that was greater in the patients who trained with oxygen compared with those who did not. However, as in an earlier study (Rooyackers et al 1997), there was no difference in the changes in exercise tolerance between the two groups. This suggests that although additional oxygen may augment desensitization to dyspnoea, it does little to enhance changes in exercise tolerance. Further data from 2001 showed similar results and concluded there were minimal benefits of oxygen as a training adjunct (Wadell et al 2001).
Hoo (2003) has undertaken a comprehensive review of this issue. Emtner and colleagues (2003) performed a randomized evaluation of the effects of oxygen on training in 29 non-hypoxaemic COPD patients. Breathing oxygen significantly increased endurance time compared with the air-trained group. Furthermore the rate of improvement was greater in the oxygen-trained patients. These data support previous observations that supplemental oxygen has a greater effect on sub-maximal exercise, improving endurance rather than intensity and reinforcing the likelihood that oxygen benefits are accrued largely through reductions in dynamic hyperinflation rather than correction of hypoxia per se (Somfay et al 2001). At the present time it is prudent to advise that patients who are on long-term oxygen therapy (LTOT) should exercise with supplemental oxygen. In addition, training with oxygen both for hypoxaemic and non-hypoxaemic patients may allow them to exercise at higher intensity and with less dyspnoea, although it is still unknown whether this translates into significant clinical benefits following a training programme.
Safety issues in rehabilitation
Many elderly people perceive exercise at ‘their age’ to be dangerous (O’Brien et al 1995). Issues of safety are obviously compounded in older people with respiratory disease and considerable reassurance may be required concerning safety. The issues of safety are somewhat unknown in the field of pulmonary rehabilitation. Although full exercise testing with ECG heart monitoring is recommended as routine for patients with COPD (American Thoracic Society 1999), a maximal incremental cycle ergometry test is unrealistic for many patients with severe disease. Even unloaded cycling can be exhausting for these patients, while adding incremental loads may cause distressing dyspnoea, ultimately preventing further exercise and disheartening the patient.
Most programmes exclude patients with unstable angina. For most patients, a field walking test with pulse oximetry (Fig. 13.3) and heart rate monitoring will identify oxygen needs and enable prescription of exercise intensity. In the hospital setting, resuscitation equipment and oxygen should be readily available and the personnel involved trained in the use of this equipment. However, a more pragmatic approach is required in the community setting where patients may be exercising at home or in local centres. There is evidence that patients with COPD demonstrate arterial desaturation during routine activities. The long-term effects of temporary falls in arterial saturation are unknown and warrant further investigation (Schenkel et al 1996). Patients with COPD often demonstrate ventilatory limitation or report fatigue before there is significant cardiovascular stress. However, this will not be the same for all groups of patients with respiratory disease and further research is required in this area. Anecdotally the only complications of exercise in these patients have been related to minor musculoskeletal injuries.
RESPIRATORY MUSCLE TRAINING
A considerable amount of research has focused on specific training for the respiratory muscles, hypothesizing that increased respiratory muscle strength will translate into increased exercise tolerance via a reduction in dysp-noea (Belman et al 1994). In theory, by improving the strength or endurance of the diaphragm, greater inspiratory loads may be tolerated, thereby prolonging exercise tolerance. Inspiratory muscle training can be performed by inspiratory resistive training, threshold loading and normocapnic hyperpnoea, and currently there is no sufficient evidence to support one method over another. Studies have found that inspiratory muscle training (IMT) significantly increases inspiratory muscle strength and endurance (Belman & Shadmehr 1988, Chen et al 1985 , Harver et al 1989), whereas others did not (Belman et al 1986, Guyatt et al 1992). Positive results on muscle force and dyspnoea have been reported using high-intensity IMT, that is IMT performed at the highest tolerable inspiratory threshold load (Hill et al 2006). Other studies have evaluated the place of respiratory muscle training together with general body training and once again variable results have been reported. Wanke and co-workers (1994) showed an additional effect of IMT in COPD patients compared with cycle endurance alone. In contrast, studies by Berry et al (1996) and Larson et al (1999) showed no significant additive effects of IMT compared with general training alone. The differences in these results may be due to the different characteristics of the training, i.e. type, duration and intensity of programmes, since these characteristics will influence the response. In many studies training intensity is below that required to achieve physiological effects. In addition, changes in breathing pattern during training alter the resistance provided (Reid & Samrai 1995). Goldstein (1993) reported that in many studies no attempt to control breathing pattern was made and measurements of endurance were therefore often unreliable. Future work in respiratory muscle training must ensure standardization of breathing frequency and pattern with training regimens of sufficient intensity to achieve a training effect.
The most recent meta-analysis of inspiratory muscle training has shown that, overall, improvements in muscle strength, endurance and dyspnoea can be achieved (Lotters et al 2002). However, these benefits are not always translated into improvement in functional outcomes such as exercise tolerance. Furthermore, this meta-analysis identified that patients with inspiratory muscle weakness improve significantly more in comparison to patients without inspiratory muscle weakness. This suggests that patients with low inspiratory pressures are the preferential target for inspiratory muscle training, since positive results are mostly linked to the presence of inspiratory muscle weakness.
Specific training of the expiratory muscles has been reported to increase exercise performance, symptoms and health-related quality of life (Mota et al 2006, Weiner et al 2003a). However, Weiner et al (2003b) showed no additional benefits in terms of functional exercise capacity and dyspnoea sensation when comparing expiratory and inspiratory muscle training in comparison with inspiratory muscle training alone.
EDUCATION AS PART OF REHABILITATION
There are few trials specifically evaluating the benefit of education programmes as part of pulmonary rehabilitation. Education alone (i.e. outside the context of a rehabilitation programme) appears to do little to reduce the sensation of dyspnoea (Hunter & Hall 1989), improve quality of life (Gallefoss et al 1999, Ries et al 1995) or exercise tolerance (Sassi-Dambron et al 1995, Wedzicha et al 1998). Moreover, a review by Folgering and co-workers (1994) suggested that education programmes in COPD patients show ambiguous results and compare poorly with asthmatic patient education on the grounds of cost-effectiveness. Control groups for research studies often comprise groups of patients receiving education alone on the grounds that they have little effect on exercise tolerance (Ries et al 1995, Wedzicha et al 1998).
Appropriate assessment of health education requires evaluation of the benefits on patient knowledge, therapeutic compliance, cost-effectiveness and dyspnoea. Mackay (1996) reported that patients with COPD displayed improved knowledge and understanding after receiving dietary advice at home. However, no follow-up assessment was made of the change in eating habits. In a review of patient education Mazzuca (1982) stated that although general education may have little benefit:
Regimen-orientated instruction includes interactive sessions such as medical management with instruction on inhaler technique, teaching relaxation techniques, practical demonstrations of energy conservation techniques and stress management. These approaches clearly have beneficial effects on rescue medication, use of steroids and antibiotics and yet the data do not always show any reduction in hospitalizations or exacerbation rate, nor any improvements in other outcomes such as health-related quality of life and lung function (Monninkhof et al 2003). While it seems strange that better use of medication does not impact on morbidity it is likely that the combined approach, which includes physical training, is more successful. This has been demonstrated by a multicentre randomized trial that showed evidence of effectiveness of a self-management programme including an exacerbation action plan and home exercise training (Bourbeau et al 2003). The programme significantly improved health-related quality of life, reduced hospital admissions due to an exacerbation of COPD (by 40%), reduced admissions for other health problems (by 57%), emergency department visits (by 41%) and unscheduled physician visits (by 58%). It is therefore not surprising that this form of self-management programme is considered cost-effective (Bourbeau et al 2006).
Psychosocial education
A meta-analysis of psychoeducational components of COPD management has shown a significant beneficial effect of behavioural education on inhaler use and a small non-significant effect on healthcare utilization (Devine & Pearcy 1996). Previous studies have shown reductions in dyspnoea after training in relaxation skills (Renfroe 1988) and coping strategies (Sassi-Dambron et al 1995). Eiser and colleagues (1997) reported improvements in exercise tolerance after group psychotherapy. In another study investigating psychosocial interventions, a combined behavioural and cognitive approach to exercise in COPD patients achieved greater improvements in walking distance than single interventions alone (Atkins et al 1984). Since the prevalence of depression and anxiety in COPD patients is very high (van Manen et al 2002), psychological counselling in addition to pulmonary rehabilitation renders additional benefits to patients with these characteristics (i.e. 20–40% of the patients) (Nguyen & Carrieri-Kohlman 2005, Withers et al 1999). Behaviour-orientated approaches to pulmonary rehabilitation such as goal setting may improve adherence and task performance (Locke et al 1981). Education programmes must be task-orientated, specific to the population and provided in a manner that is accessible to the patient. Analysis of the effects of education should focus on patient knowledge and its translation into improved self-management, prompt identification of problems and reductions in hospitalization. An educational programme, combined with physical training, optimizes functional ability as well as self-mastery. The main targets for self-management education programmes are patients with reduced health-related quality of life and frequent exacerbations.
OTHER ADJUNCTS TO REHABILITATION
Breathing techniques
Breathing control is defined as:
Patients should be encouraged to breathe slowly and naturally. Appropriate terminology may help instruction, with words such as ‘let the air flow in’ rather than ‘breathe in’ which implies that a forced breath in is required. Effective positioning can reduce the work of breathing (O’Neill & McCarthy 1983) and should be utilized during exercise. Patients may use the ‘lean forward position’ in standing, against walls or equipment or in sitting (Bott 1997). Breathing control can be used during stair climbing (inhale while climbing up one step, exhale while climbing up the next two steps) to reinforce a rhythmical breathing pattern and minimize breath holding during activities. Although there is little empirical evidence for the value of these techniques, anecdotal and physiological evidence supports their use (Sharp et al 1980).
More recently investigators have studied the role of pursed lips breathing (PLB) as a component of rehabilitation. The subject performs a moderately active expiration through the half-opened lips, inducing expiratory mouth pressures of about 5 cmH2O (van der Schans et al 1995). Data from Garrod and colleagues (2005b) show significant reductions in respiratory frequency and a speedier recovery after a maximal walk test when exercise is performed using PLB compared with tidal flow breathing. Other studies show evidence that during both rest and exercise PLB is associated with an increase in tidal volume and a reduction in minute ventilation (Jones et al 2003, Mueller et al 1970). Confirmation that PLB promotes a slower and deeper breathing pattern both at rest and during exercise, but has a variable effect on dyspnoea sensation when performed volitionally during exercise by patients with COPD, has been demonstrated by Spahija et al (2005).
Diaphragmatic breathing (DB) is a technique in which the patient is encouraged to allow forward movement of the abdominal wall during inspiration and to reduce upper rib cage motion. When performing DB, patients are able to voluntarily change their breathing pattern to a more abdominal movement, improve their blood gases, increase tidal volume, and decrease breathing frequency (Vitacca et al 1998). However, this happens at a high cost: DB can lead to increased asynchronous and paradoxical breathing movements, increased work of breathing, enhanced oxygen cost of breathing, reduced mechanical efficiency and ultimately to a worsening of dyspnoea sensation (Gosselink et al 1995, Sackner et al 1984, Vitacca et al 1998, Willeput et al 1983). In addition, no permanent changes of the breathing pattern are observed when DB training is stopped. Currently, there is no evidence from controlled studies to support the use of DB in COPD patients (Gosselink 2003).
Specific training of activities of daily living
Specific training of activities of daily living (ADL) mainly concerns the optimization of the most common movements and activities performed in daily life, through energy conservation techniques. Physiotherapists and occupational therapists can teach patients how to perform their ADL in a way that induces less dysp-noea and fatigue, with the aim of maximal function and independence. Patients can also be taught to organize their home space and time schedule in order to facilitate ADL and functionality. Velloso and colleagues showed that the use of these energy conservation techniques lowers energy cost and dyspnoea perception (Velloso & Jardim 2006). In another study, addition of these techniques improved self-reported performance in ADL, although no additional benefits in exercise capacity (6MWD) were observed (Lorenzi et al 2004). Similar results were shown by Norweg and coworkers (Norweg et al 2005). They also found evidence for additional benefits in dyspnoea, fatigue and activity involvement after activity-specific training combined with exercise compared with exercise training alone, although the improvements were greater in the oldest patients. Conversely, Sewell and colleagues found no significant differences in exercise capacity, amount of physical activity performed in daily life and self-perceived domestic function when comparing a general exercise group with an individually targeted exercise group (Sewell et al 2005). The authors concluded that general exercise training is as effective as an ADL-targeted training in improving domestic function and physical activity in daily life. Evidence from the literature is contradictory and further research is required to determine the real benefits of adding specific ADL training to a pulmonary rehabilitation programme.
Walking aids
In order to improve walking ability, a rollator may be a useful option. A rollator is a walker with four wheels, equipped with swivel castors on the front wheels, brakes, a basket for carrying objects and a seat that allows sitting for rest (Fig. 13.4A). An alternative is a three-wheeled walker (Fig. 13.4B). Various studies have shown the acute benefits of using a rollator in more impaired individuals in terms of improved walking distance, ventilation, reduced dyspnoea and provision of a greater sense of safety (Probst et al 2004, Solway et al 2002). Despite these positive acute effects, the provision of a rollator for long-term use at home did not result in a significant effect on quality of life or exercise capacity (Gupta et al 2006). This was possibly because a large proportion of the patients did not use the rollator on a regular basis at home, that is less than three times per week. Regular users demonstrated better results than infrequent users. Therefore, the choice of which patient is most likely to use the rollator regularly is crucial in order to obtain positive results. It is important to bear in mind that the most disabled patients are the ones who benefit most from the use of a rollator, and specific coaching should be provided in order to maximally increase walking efficiency when using the device (Probst et al 2004, Solway et al 2002).
Non-invasive positive pressure ventilation
The place of non-invasive positive pressure ventilation (NIPPV) as an adjunct to rehabilitation is based on the theory that unloading of the respiratory muscles during activity will enable higher work intensities to be reached, accruing greater benefit from exercise. For many patients with severe problems, dyspnoea may significantly limit the ability to exercise and NIPPV may be a valuable addition to rehabilitation. In a systematic review, rehabilitation experts from the Netherlands pooled data from seven critically appraised studies (van’t Hul et al 2002) and found there was a significant benefit on exercise-induced dyspnoea and endurance time when NIPPV was applied during an acute exercise test.
However, there are practical difficulties to providing ventilation during a training session in terms of limiting the type of exercise and adherence to exercise. In a randomized controlled trial of overnight application of NIPPV plus daytime non-assisted rehabilitation, significant improvements in exercise tolerance and quality of life were found after rehabilitation in the home ventilated group compared with a non-ventilated group (Garrod et al 2000b). Improvement in exercise tolerance may have resulted from overnight relief of low-level fatigue of respiratory muscles caused during exhaustive exercise. Valid criticisms of this work concern the relatively short period of time spent using the ventilator (mean 2.5 hours) and the lack of placebo ventilation.
Pharmacological agents
The effects of anabolic steroids and exercise training, especially in male patients with COPD, have been studied. The use of steroids was shown to increase muscle strength, enhancing the effects of strength training programmes (Casaburi et al 2004). A gain in bodyweight was also identified and this was said to be due mainly to an increase in fat-free mass rather than fat mass (Creutzberg et al 2003, Schols et al 1995). The main targets for this kind of intervention are male hypogonadal patients with COPD and patients receiving oral corticosteroids (Creutzberg et al 2003). The ideal dose of anabolic steroids, to balance the risks and benefits, deserves further investigation.
The effects of growth hormone administration have been investigated in patients with COPD as it had been hypothesized that hormone therapy may enhance muscle changes following rehabilitation. However, Burdet et al (1997) did not show a significant effect of added growth hormone on either exercise tolerance or muscle strength. Currently, the lack of evidence of functional benefit does not support the use of human growth hormone combined with a pulmonary rehabilitation programme.
Guidelines recommend the initiation of maintenance long-acting bronchodilator therapy in patients with moderate-to-severe disease (Fabbri & Hurd 2003). Evidence from a meta-analysis suggested that initiation of long-acting bronchodilator therapy with tiotropium, at earlier stages of the disease, may result in improvements in lung function and health-related quality of life (Barr et al 2006). In addition, there is evidence that the effects of exercise training may be amplified in patients with COPD receiving the long-acting anticholinergic agent tiotropium (Casaburi et al 2005). Cautious interpretation of this data is required since the placebo group were not previously optimized for pharmacological therapy. In addition, other classes of drugs show benefits on hyperinflation and exercise endurance such as combined corticosteroid and bronchodilator therapy (O’Donnell et al 2006). This is a promising approach for respiratory rehabilitation.
There is still a need to identify those patients within the large heterogeneous group of patients with COPD who may benefit from corticosteroid administration. Complications from long-term corticosteroid use are important considerations (e.g. muscle weakness, osteoporosis) but they appear to be less of a problem when the corticosteroid is given via the inhaled route (Goldstein et al 1999, MacIntyre 2006). Guidelines recommend the use of inhaled corticosteroids in patients with severe disease and frequent exacerbations (Fabbri & Hurd 2003). There is strong clinical evidence to support the use of inhaled corticosteroids to prevent exacerbations and oral corticosteroids to reduce the duration and impact of exacerbations (MacIntyre 2006).
Nutrition
It has been demonstrated that there is a significant relationship between exercise tolerance and nutritional status in patients with COPD (Schols et al 1991). Low bodyweight and low body mass index (BMI) are each known to be poor prognostic factors of disease severity (Landbo et al 1999). Weight loss, muscle wasting and reduced fat-free mass often occur, and are linked to the lack of balance between ingestion and energy expenditure (decreased dietary intake, high energy requirements, and disturbances in metabolism caused by altered anabolic and catabolic mediators such as hormones, cytokines and growth factors).
A systematic review of randomized controlled trials showed no significant effects of nutritional supplementation on anthropometric measures, lung function or exercise capacity in patients with stable COPD (Ferreira et al 2005). Therefore, currently there is insufficient evidence of benefit of nutritional support for outpatients. However, patients who are non-responsive to nutritional support seem to have a profile of older age, relative anorexia and elevated systemic inflammatory response (Creutzberg et al 2000), and future research should therefore focus on the potential positive effects in subgroups of patients.
Neuromuscular electrical stimulation
Using transcutaneous low-intensity currents, muscle contraction can be induced by neuromuscular electrical stimulation (NMES) and specific muscle groups can be trained. In stable patients with muscle weakness, NMES applied to the lower limbs muscles improved muscle strength, exercise tolerance and peak oxygen uptake (Bourjeily-Habr et al 2002, Neder et al 2002). The use of NMES has also been reported to result in a fast functional recovery in severely disabled patients receiving mechanical ventilation who were bedbound for more than 30 days (Zanotti et al 2003). In patients with well-preserved functional status, however, the results were very modest (Dal Corso et al 2007). The use of NMES as an adjunct to rehabilitation in severely disabled patients with low BMI has been shown to result in improvements in dyspnoea, exercise tolerance and muscle strength (Vivodtzev et al 2006). This suggests that NMES may provide an additional stimulus for changes in muscle physiology for malnourished patients or those with severe ventilatory limitation. The intervention was provided early following discharge from a hospital admission for acute exacerbation. It is possible that malnourished patients show greater hospital-induced sarcopenia than those with adequate BMI and that this may have contributed to the improved effects found in this study. One cautionary point is the fact that the rehabilitation programme lasted only 4 weeks rather than the recommended minimum of 8 weeks, and these early differences may not be evident in the longer term or after prolonged training.
Smoking cessation
There are different approaches for sustained smoking cessation, from counselling to pharmacological tools (nicotine replacement therapy and non-nicotine agents such as bupropion) (Marlow & Stoller 2003). Although smokers are more likely to decline invitations to rehabilitation programmes and may be less adherent (Young et al 1999), there is no evidence that continued smoking reduces the response to pulmonary rehabilitation. There has been much debate over whether patients who refuse to stop smoking should be eligible for pulmonary rehabilitation. However, in clinical practice, it is common to observe that for many smokers, the combined positive influence of pulmonary rehabilitation and the effect of peer pressure have helped a number of patients to stop smoking. Until trials show clearly that the benefit of training is reduced in current smokers, it is recommended that smokers be supported throughout pulmonary rehabilitation programmes and offered appropriate smoking cessation help (Kawane 1997).
Acupressure
One interesting study has evaluated the effect of acupressure practised daily in conjunction with a 6-week exercise programme (Maa et al 1997). The study was randomized with the control group receiving ‘sham’ acupressure. Significant benefits of acupressure on dysp-noea were reported although these were not reflected in differences in exercise tolerance. Unfortunately, the study was single blinded and the investigator, who met with the patients weekly to reinforce ‘sham’ or ‘real’ acupressure, was aware of the randomization. A repeat of this study with a double-blind design would be of interest.
ASSESSMENT TOOLS IN PULMONARY REHABILITATION
Assessment of exercise tolerance and muscle function
Assessment of exercise tolerance is required in order to assess risks, characterize initial disability, set targets of training intensity, assess the benefit of rehabilitation programmes and motivate patients to continue with training regimens. All patients should perform a standardized test of exercise capacity before and after training. Evidence-based guidelines indicate that patients undergoing a period of rehabilitative training show improvements in exercise tolerance without evidence of adverse complications (American College of Chest Physicians (ACCP)/ American Association of Cardiovascular and Pulmonary Rehabilitation (AACVPR) 1997). These improvements are observed both in laboratory tests and field tests.
Laboratory incremental maximal tests versus field tests
Laboratory tests measuring maximal oxygen consumption (VO2max), heart rate, workload, arterial oxygenation and blood lactate levels remain the gold standard in exercise testing and are appropriate in patients with COPD for having the potential to provide a variety of vital information (e.g. the cause of exercise limitation) in this rather limited population (Palange et al 1994). However, where resources are limited, ‘field tests’ (tests of exercise tolerance applied in the clinical setting rather than the laboratory) may be performed, but these tests may be more susceptible to bias. Laboratory tests may have limited application, particularly in patients with severe disease where work capacity is very reduced, resulting in an inability to reach ventilatory threshold levels (Midorikawa et al 1997). The functional ability of patients will not necessarily reflect the true daily activities they can perform (Pitta et al 2005a). Field tests may have advantages over laboratory measurements due to simplicity and functional appropriateness, but disadvantages include the effects of motivation and the lack of physiological correlates. Overall, maximal incremental tests and field tests provide different and complementary information concerning exercise capacity in patients with COPD (Ong et al 2004, Turner et al 2004). Measurements of muscle strength and endurance have a central place in the assessment of patients with COPD (American Thoracic Society/European Respiratory Society 1999). These measurements are useful to identify muscle dysfunction, determine training load and assess improvements after interventions such as pulmonary rehabilitation.
The 6-minute walking test
In early rehabilitation trials, the 12-minute walking test (12MWT) was used to assess exercise tolerance (Cockcroft et al 1981, McGavin et al 1977, Sinclair & Ingram 1980). Over the years it has been replaced by a shorter version, the 6-minute walking test (6MWT). The 6MWT has been widely used in pulmonary rehabilitation (American Thoracic Society 2002). It is a simple test that measures the distance walked over 6 minutes. Subjects undergoing the test typically increase their oxygen consumption until the 3rd minute, when a plateau is observed (Troosters et al 2002). Pinto-Plata and co-workers (2004) showed that, in severe COPD, the 6MWT predicts mortality better than other traditional markers of disease severity such as spirometric variables. Furthermore, it also reflects the patient’s functional capacity (Carter et al 2003) and is the best correlate of time spent actively in daily life, especially in those patients with reduced exercise capacity (Pitta et al 2005a). However, its limitations include dependence upon patient motivation, the susceptibility to practice effects and the lack of standardization of the test procedure throughout studies in the literature. To reduce these disadvantages, an American Thoracic Society guideline statement (2002) suggests a number of useful standardization measures in order to minimize the test’s shortcomings. These measures include the length of the hallway (minimum of 30 metres), the need for standardized encouragement and the value of a practice test. Predicted normal values according to age, gender, height and weight are available (Troosters et al 1999).
Statistically significant changes in the 6MWT need to be interpreted in the light of clinically significant changes: ‘how big is big?’ (Guyatt et al 1991). The threshold for clinical differences is a measured value that equates to a level of change at which the patient perceives either an improvement in symptoms or deterioration. Redelmeier et al (1997) have identified the clinical threshold of the 6MWT as an increase or decrease of 54 metres following an intervention. Many results of rehabilitation trials in COPD patients fall short of this threshold (Cambach et al 1997, Wijkstra et al 1996), although many others have reached clinically important levels of improvement (de Torres et al 2002, Spruit et al 2002). Improvements in the 6MWD after pulmonary rehabilitation programmes vary considerably depending on the duration, frequency, intensity and location of the exercise training, as well as the modalities applied.
The shuttle walking tests
A ‘field walking’ test that is less susceptible to motivation is the shuttle walking test (SWT) (Singh et al 1992). This incremental test is externally paced, enhancing standardization, does not include patient stops and correlates to VO2max (Singh et al 1994). The SWT demonstrates validity with other measures of exercise tolerance and is reliable on test—retest, requiring only one practice walk (Singh et al 1992). It has been shown to be a sensitive measure of change after rehabilitation in patients with severe COPD (Wedzicha et al 1998) and has been validated for use in elderly patients with COPD (Dyer et al 2002). A further test from this group of researchers measures endurance in a standardized manner, the endurance shuttle walk test (ESWT) (Revill et al 1999). Comparisons between the 6MWT and ESWT suggest that ESWT may be more responsive to change after pulmonary rehabilitation than the 6MWT (Eaton et al 2006) but much will depend upon individual programmes, resources and assessor preference.
In summary, field tests (Chapter 3) are an appropriate measure of exercise capacity in these patients and, although they may not provide full understanding with respect to the physiological mechanisms of improvement, when combined with other functional assessments they help to provide a practical evaluation of pulmonary rehabilitation. A comparison between the SWT and the 6MWT is summarized in Table 13.2.
Table 13.2 Comparison between the shuttle walking test (SWT) and the 6-minute walking test (6MWT)
| SWT | 6MWT |
|---|---|
| Standardized | Partially standardized |
| Facility to extrapolate V.O2max from results |
Evidence of validity and weak association with V.O2max |
| Externally paced | Susceptible to patient motivation |
| Requires one practice walk | One practice test advised |
| Maximal test | Submaximal timed test |
| Requires tape and recorder | Requires no equipment |
| No clinical threshold | Clinical threshold identified |
Peripheral muscle strength and endurance
Muscle strength
Electromagnetic stimulation avoids the volitional effects of other motivation-dependent techniques by assuring maximal activation of the muscle (Man et al 2003), but is methodologically complex and not used in everyday practice. In clinical practice, muscle strength is commonly assessed by maximal voluntary contraction, and different types of dynamometers (handheld, motorized, electronic) are available. If adequate standardization and encouragement are used, the measurements should be appropriate for the clinical assessment of individuals before and after training. Grip strength, measured by simple handgrip devices, may be useful to assess upper limbs. In repetition maximum (RM) tests, subjects lift progressive loads until they are unable to complete the movement across a range of motion or use compensatory mechanisms to undertake the movement. This test can be performed with free weights or in multigyms.
Muscle endurance
The classical test for assessment of muscle endurance is that of the subject performing regular repetitions of movement against a determined weight, equivalent to a percentage of their maximal strength. The test is stopped when the subject is unable to sustain the repetitions, and the duration of the test (or the number of the repetitions) is the main outcome (Coronell et al 2004). Another endurance index is the total work (e.g. in Joules) executed during a certain time (Neder et al 2002). Exogenous electrical stimulation can also be used when assessing muscle endurance, in order to induce fatigue of limb muscles from which mechanical output can be measured.
Assessment of activities of daily living, health-related quality of life and dyspnoea
Activities of daily living (ADL)
Increasing participation in daily activities is one of the main goals of pulmonary rehabilitation programmes (Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2001). Currently the improvement in daily activities following rehabilitation programs remains poorly studied in patients with COPD, although there have been significant advances in the development of assessment tools both subjective and objective.
Subjective tools (questionnaires and scales)
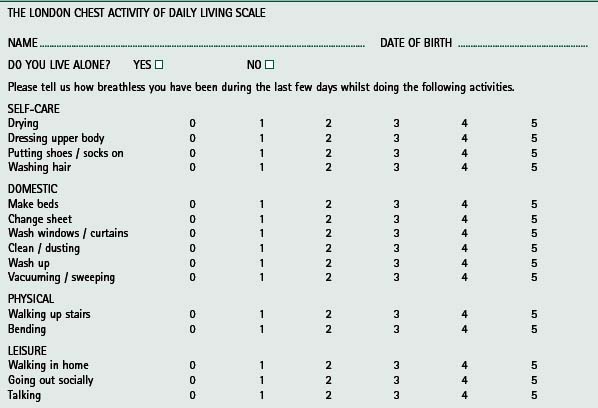
Lareau and coworkers (1994) developed a 70-item activities of daily living (ADL) assessment tool, employed as a measure of outcome following pulmonary rehabilitation. The tool was modified to form a shorter questionnaire of 40 items and has been successful in identifying improvements in daily activities after pulmonary rehabilitation (Lareau et al 1998). Using this questionnaire, Trappenburg and colleagues (2005) showed significant improvements in functional status after 3 months of pulmonary rehabilitation. The short 15-item questionnaire, the London Chest Activity of Daily Living Scale has been validated in patients with COPD (Garrod et al 2000c) (Table 13.3). Significant improvements in dyspnoea during ADL were documented following pulmonary rehabilitation and the change in ADL was associated with change in exercise tolerance (Garrod et al 2002). Self-reported performance in ADL has also been shown to improve after pulmonary rehabilitation in other studies (Bendstrup et al 1997, Yohannes et al 2000).
Improvement in ADL, assessed by subjective methods, provides a useful and recognizable measure of the benefit of pulmonary rehabilitation to an individual’s daily functioning. However, the abov-ementioned ADL questionnaires and scales do not aim primarily at quantifying the amount and intensity of activity performed in daily life. In other words, these subjective tools assess whether the patient is less impaired by dysp-noea, more independent and able to perform better in daily activities, but this does not necessarily equate to the patient being more active in quantitative terms. Quantification of the amount and intensity of physical activity assessed by subjective methods has been shown to be inaccurate in patients with COPD (Pitta et al 2005b). The reasons for this are that questionnaires quantifying physical activity in daily life depend on factors such as cognitive capacity, age, cultural factors, accurate perception and memory, in addition to the questionnaire design (Pitta et al 2006b).
Objective tools (motion sensors)
In contrast to ADL assessment by subjective tools, literature concerning objective quantification of improvement in physical activity in daily life after rehabilitation yields conflicting results. Two studies demonstrated very modest or no improvement in the motion sensors’ output after programmes lasting 3 and 8 weeks (Coronado et al 2003, Steele et al 2003). It is not clear whether these disappointing results derived from actual lack of improvement by the patients due to characteristics of the rehabilitation programme (e.g. duration, intensity, frequency) or from methodological issues of the assessment method (e.g. the outcomes used). In contrast, two other studies found significant improvements after programmes of 7 and 8 weeks of duration (Mercken et al 2005, Sewell et al 2005). Differences in the populations involved and the types of programmes may explain the conflicting results among these studies. It is not yet possible to identify which patients are more physically active following pulmonary rehabilitation or the characteristics of the programmes that will induce changes in day-to-day physical activity behaviour. This is an area in which further research is required.
Health-related quality of life
A broad definition of quality of life includes factors that health care may not affect directly (although there may be indirect effects on health). Such factors include financial status, housing, employment and social support. Health-related quality of life (HRQoL) instruments can vary from disease-specific questionnaires, which measure a single item such as dyspnoea or numerous items, to generic measures intended for use in any disease. Generic questionnaires were originally designed to define the health of populations and not to measure therapeutic efficacy. They are considered useful when comparing different populations of patients. Disease-specific questionnaires were designed to detect and quantify health gain following treatment (Table 13.4).
Table 13.4 Examples of disease-specific and generic health-related quality of life tools
| Disease-specific | Generic |
|---|---|
| Chronic Respiratory Disease (CRQ) | Sickness Impact Profile Questionnaire (SIP) |
| St George’s Respiratory Questionnaire (SGRQ) | MOS Short-Form 36 Disease (SF36) |
| Breathing Problems Questionnnaire (BPQ) | Quality of Well-Being Scale (QWB) |
| Oxygen Cost Diagram (OCD) | Nottingham Health Profile (NHP) |
HRQoL instruments have been useful in assessing benefits of pulmonary rehabilitation programmes. There have been numerous reports of improvements in health status after such programmes (Garrod et al 2000a, 2000c, Goldstein et al 1994, Griffiths et al 2000, Lacasse et al 2002, Wedzicha et al 1998). In a study from the Netherlands (Wijkstra et al 1994), HRQoL was measured using the disease-specific Chronic Respiratory Questionnaire (CRQ) which measures four aspects of HRQoL: dyspnoea, mastery, emotion and fatigue (Guyatt et al 1987). Another commonly used questionnaire is the St George’s Respiratory Questionnaire (SGRQ) (Jones et al 1991), which comprises symptoms, activities and impact domains and has provided strong evidence of a change in health status after rehabilitation (Griffiths et al 2000). The clinical threshold for the CRQ has been identified as requiring a change of at least 0.5 point per item (Jaeshchke et al 1989) while a change of 4 points or more, in the total score of the SGRQ, is required to achieve a clinical effect (Jones 2005). An empirical comparison between the SGRQ and CRQ showed strong similarities between the questionnaires in terms of validity and reliability, suggesting that either would be an appropriate measurement tool (Rutten-van Mölken et al 1999). Two studies have shown that these two disease-specific questionnaires are more responsive to changes than generic questionnaires, and suggest that the CRQ has superior responsiveness (Puhan et al 2007). It should also be acknowledged that informing patients about their pre-treatment scores does not significantly improve the responsiveness of the CRQ or the SGRQ after rehabilitation (Schunemann et al 2002).
Dyspnoea
Repeated trials of rehabilitation have shown improvement in dyspnoea using validated measures (Goldstein et al 1994, Reardon et al 1994, Ries et al 1995). A meta-analysis by Lacasse and colleagues (1997) concluded that training resulted in significant relief of dyspnoea. Dyspnoea can be measured in a variety of ways and graded scales such as the Baseline and Transition Dyspnoea Index (BDI) (Mahler et al 1984) and the Medical Research Council (MRC) Breathlessness Score (Fletcher 1960) are examples.
The MRC Breathlessness Score, which is self-administered, grades dyspnoea during walking according to five levels, with grade 5 representing patients who consider themselves to be housebound due to breathlessness. It is a valid and reliable questionnaire that has been found to be useful in stratification of patients before entry to a pulmonary rehabilitation programme (Bestall et al 1999). However, the problem with such scales concerns the wide variation of disability evident between the grades, which — although improving the reliability of the questionnaire — reduces its sensitivity (Jones 1992).
Other scales used to measure dyspnoea include the Borg Scale of Perceived Dyspnoea (Borg 1982), the dysp-noea component of the CRQ (Guyatt et al 1987), the University of California, San Diego (UCSD) Shortness of Breath Questionnaire (Eakin et al 1998) and the visual analogue scale (VAS) (Aitken 1969).
DO ALL PATIENTS BENEFIT FROM PULMONARY REHABILITATION?
This is a contentious question and to some extent remains unanswered. It is probably fair to say that all patients have the potential to gain benefit in specific outcomes, albeit modest in some cases, and the majority of patients benefit significantly in terms of improvement in exercise tolerance (Troosters et al 2001). As yet, it has not been possible to describe fully the specific predictive factors. ZuWallack and co-workers (1991), investigating 50 patients with COPD following rehabilitation, were unable to show a relationship between improvements and age, gender, pulmonary function or initial walking distance. However, they did show that patients with the greatest ventilatory reserve at baseline had the greatest improvement in walking distance following training. More recent robust evidence, concerning response to rehabilitation, comes from Troosters et al (2001) who showed that the patients most likely to respond well to rehabilitation were those with relatively weak peripheral muscles and preserved ventilatory reserve on starting the programme. This reinforces the importance of quadriceps assessment before rehabilitation. Moreover, this study also showed that about 30% of the patients may not show a significant improvement in exercise capacity, but may improve their health-related quality of life to a clinically significant extent. Garrod and colleagues (2006) recently performed a similar study aiming to identify predictors of response to rehabilitation. The authors purposefully included patients with mild disease (Grade 1 & 2 on MRC Scale) since there had been little evaluation of rehabilitation in this group, meaning that previous regression analysis may not have included all relevant stages of disease. It was not possible to identify clear predictors of response, but the authors did report two important findings. First, patients with mild disease do very well with rehabilitation and, secondly, patients with more severe disease (grade 5) demonstrate a smaller magnitude of change. This is in accordance with earlier data (Wedzicha et al 1998) but contrasts with findings from Berry and colleagues, who showed that patients with mild, moderate and severe disease all have similar improvements in physical function and HRQoL (Berry et al 1999). Garrod et al’s (2006) work also highlighted an important finding concerning drop-out of pulmonary rehabilitation: patients who were depressed at baseline were eight times more likely to drop out compared with those who were not depressed. This further highlights the need for optimal medicalization of the condition before entry to pulmonary rehabilitation.
LONG-TERM EFFECTS OF PULMONARY REHABILITATION — IS BENEFIT MAINTAINED?
Many studies have assessed the short-term benefits of pulmonary rehabilitation. These studies have shown that patients can gain significant benefits in exercise capacity, health status and dyspnoea immediately following a rehabilitation programme. However, full assessment of pulmonary rehabilitation requires an evaluation of the long-term benefits of such programmes. As previously mentioned, one of the ways to obtain longer-lasting effects is to increase the programme’s duration and another is to provide a follow-up programme. Vale et al (1993) assessed the maintenance of improvements in exercise tolerance and health status approximately 1 year after training. They compared two groups of patients; the first group had participated in a structured exercise maintenance follow-up programme, whereas the second group had no follow-up programme. Both groups showed a decline in outcome measures with no significant difference evident between the groups. The authors suggested that follow-up programmes were of little benefit and that other strategies are required to prolong the beneficial effects. However, interpretation of these findings should be made with caution as no control group (those who did not receive rehabilitation) was included.
Contrasting results were found in a study performed by Berry and coworkers (2003), in which patients had an initial programme of 3 months and were divided in two groups: a follow-up programme comprising sessions three times per week for 15 months and an exercise advice programme for the same period of time (Berry et al 2003). The exercise group showed superior results, demonstrating the value of long-term follow-up including regular exercise training. Another study has reported that participation in regular walking, after completing a pulmonary rehabilitation programme, is associated with slower declines in overall HRQoL and walking self-efficacy as well as less progression of dyspnoea during ADL (Heppner et al 2006). The conflicting results between these different studies may be due to the characteristics of the exercise follow-up programme. Repeated short-term programmes do not seem to achieve effective results, except for a reduction in exacerbation rates (Foglio et al 2001). Regular telephone support and monthly visits or meetings with health professionals have very modest effects (Brooks et al 2002, Ries et al 2003).
HRQoL improvements are still evident 12 months following rehabilitation (Griffiths et al 2000), and seem to be preserved for longer than improvements in exercise tolerance (Foglio et al 1999, Guell et al 2000, Troosters et al 2000). Wijkstra and colleagues (1995) demonstrated that positive results in HRQoL can be maintained for up to 18 months after a 2-month home-based programme followed by a programme comprising monthly home physiotherapy sessions. One of the most extensive follow-up studies has been carried out by Ries et al (1995), who reported on patients receiving an 8-week rehabilitation programme followed by monthly reinforcement sessions for 1 year and patients were followed for 6 years. They showed that improvements in exercise tolerance, dyspnoea and daily activity obtained following the initial 8-week programme were partially maintained for 1 year, but tended to diminish after that. Improvements in self-efficacy and perceived breathlessness were maintained for a few months longer. Overall the literature is conflicting and the available evidence does not allow definitive conclusions to be drawn on the ideal design of a follow-up programme, although periodic supervised exercise sessions seem advisable.
Aitken RB. A growing edge of measurement of feelings. Procedings of the Royal Society of Medicine. 1969;62:989-993.
Allaire J, Maltais F, Doyon JF, et al. Peripheral muscle endurance and the oxidative profile of the quadriceps in patients with COPD. Thorax. 2004;59:673-678.
American College of Chest Physicians (ACCP)/American Association of Cardiovascular and Pulmonary Rehabilitation (AACVPR). Pulmonary rehabilitation guidelines panel. Pulmonary rehabilitation. Joint ACCP/AACVPR Evidence-based guidelines. Chest. 1997;112:1363-1396.
American Thoracic Society. Pulmonary rehabilitation: an official statement. American Journal of Respiratory and Critical Care Medicine. 1999;159:1666-1682.
American Thoracic Society Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. American Journal of Respiratory and Critical Care Medicine. 2002;166(1):111-117.
American Thoracic Society and European Respiratory Society. Skeletal muscle dysfunction in chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine. 1999;159(4):S1-S40.
Astrand I, Guharay A, Wahren J. Circulatory responses to arm exercise with different arm positions. Journal of Applied Physiology. 1968;25:528-532.
Atkins CJ, Kaplan RM, Timms RM, et al. Behavioural exercise programmes in the management of chronic obstructive pulmonary disease. Journal of Consulting and Clinical Psychology. 1984;52(4):591-603.
Baarends EM, Schols AM, Akkermans MA, et al. Decreased mechanical efficiency in clinically stable patients with COPD. Thorax. 1997;52(11):981-986.
Barr RG, Bourbeau J, Camargo CA, et al. Tiotropium for stable chronic obstructive pulmonary disease: a metaanalysis. Thorax. 2006;61(10):854-862.
Belman MJ, Kendregan BA. Exercise training fails to increase skeletal muscle enzymes in patients with chronic obstructive pulmonary disease. American Review of Respiratory Disease. 1981;123:256-261.
Belman MJ, Shadmehr R. Targeted resistive muscle training in COPD. Journal of Applied Physiology. 1988;65:2726-2735.
Belman MJ, Thomas S, Lewis M. Resistive breathing training in patients with chronic obstructive pulmonary disease. Chest. 1986;90:662-669.
Belman MJ, Botnick WC, Nathan SD, et al. Ventilatory load characteristics during ventilatory muscle training. American Journal of Respiratory and Critical Care Medicine. 1994;149(4):925-929.
Bendstrup KE, Ingemann JJ, Holm S, et al. Out-patient rehabilitation improves activities of daily living, quality of life and exercise tolerance in COPD. European Respiratory Journal. 1997;10:2801-2806.
Bernard S, Whittom F, Leblanc P, et al. Aerobic and strength training in patients with chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine. 1999;159:896-901.
Berry M, Norman A, Sevensky S, et al. Inspiratory muscle training and whole body reconditioning in COPD. American Journal of Respiratory and Critical Care Medicine. 1996;153:1812-1816.
Berry MJ, Rejeski WJ, Adair NE, et al. Exercise rehabilitation and COPD stage. American Journal of Respiratory and Critical Care Medicine. 1999;160:1248-1253.
Berry MJ, Rejeski WJ, Adair NE, et al. A randomized controlled trial comparing long-term and short-term exercise in patients with COPD. Journal of Cardiopulmonary Rehabilitation. 2003;23:60-68.
Bestall JC, Paul EA, Garrod R, et al. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with COPD. Thorax. 1999;54:581-586.
Borg C. Psychophysical basis of perceived exertion. Medicine and Science in Sports and Exercise. 1982;14:377-381.
Bott J. Physiotherapy. In: Singh SJ, Morgan MDL, editors. Practical pulmonary rehabilitation. London: Chapman & Hall; 1997:156-176.
Bourbeau J, Collet JP, Schwartzman K, et al. Economic benefits of self-management education in COPD. Chest. 2006;130(6):1704-1711.
Bourbeau J, Julien M, Maltais F, et al. Reduction of hospital utilization in patients with chronic obstructive pulmonary disease: a disease-specific self-management intervention. Archives of Internal Medicine. 2003;163(5):585-591. 10
Bourjeily-Habr G, Rochester C, Palermo F, et al. Randomized controlled trial of transcutaneous electrical muscle stimulation of the lower extremities in patients with chronic obstructive pulmonary disease. Thorax. 2002;57:1045-1049.
British Lung Foundation/British Thoracic Society Pulmonary Rehabilitation Survey, 2002 www.lunguk.org/downloads/BLF_pul_rehab_survey.pdf (Accessed 16 July 2007)
Broekhuizen R, Wouters EF, Creutzberg EC, Schols AM. Raised CRP levels mark metabolic and functional impairment in advanced COPD. Thorax. 2006;61(1):17-22.
Brolin SE, Cecins NM, Jenkins SC. Questioning the use of heart rate and dyspnea in the prescription of exercise in subjects with chronic obstructive pulmonary disease. Journal of Cardiopulmonary Rehabilitation. 2003;23(3):228-234.
Brooks D, Krip B, Mangovski-Alzamora S, et al. The effect of post-rehabilitation programmes among individuals with chronic obstructive pulmonary disease. European Respiratory Journal. 2002;20(1):20-29.
Burdet L, Muralt B, Schutz Y, et al. Administration of growth hormone to underweight patients with chronic obstructive pulmonary disease. A prospective randomized, controlled study. American Journal of Respiratory and Critical Care Medicine. 1997;156:1800-1806.
Cambach W, Chadwick-Straver RVM, Wagenaar RC, et al. The effects of a community based pulmonary rehabilitation programme on exercise tolerance and quality of life: a randomized controlled trial. European Respiratory Journal. 1997;10:104-113.
Canadian Occupational Performance Measure, 2005 www.caot.ca/copm/index.htm (Accessed 17 July 2007)
Carrieri-Kohlman V, Nguyen HQ, Doneski-Cuenco D, et al. Impact of brief or extended exercise training on the benefit of a dyspnea self-management program in COPD. Journal of Cardiopulmonary Rehabilitation. 2005;25(5):275-284.
Carter R, Holiday DB, Nwasuruba C, et al. 6-minute walk work for assessment of functional capacity in patients with COPD. Chest. 2003;123(5):1408-1415.
Casaburi R, Bhasin S, Cosentino L, et al. Effects of testosterone replacement and strength training in men with COPD. American Journal of Respiratory and Critical Care Medicine. 2004;170:870-878.
Casaburi R, Kukafka D, Cooper CB, et al. Improvement in exercise tolerance with the combination of tiotropium and pulmonary rehabilitation in patients with COPD. Chest. 2005;127(3):809-817.
Casaburi R, Patessio A, Ioli F, et al. Reductions in exercise lactic acidosis and ventilation as a result of exercise training in patients with obstructive lung disease. American Review of Respiratory Disease. 1991;143:9-18.
Casaburi R, Porszasz J, Burns MR, et al. Physiologic benefits of exercise training in rehabilitation of patients with severe COPD. American Journal of Respiratory and Critical Care Medicine. 1997;155:1541-1551.
Celli BR, Cote CG, Marin JM, et al. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. New England Journal of Medicine. 2004;350(10):1005-1012.
Chen H, Dukes R, Martin B. Inspiratory resistance training in patients with COPD. American Review of Respiratory Disease. 1985;131:251-255.
Clark CJ, Cochrane L, Mackay E. Low intensity peripheral muscle conditioning improves exercise tolerance and breathlessness in COPD. European Respiratory Journal. 1996;9(12):2590-2596.
Cockcroft AE, Saunders MJ, Berry G. Randomized controlled trial of rehabilitation in chronic respiratory disability. Thorax. 1981;36:200-203.
Coppoolse R, Schols A, Baarends E, et al. Interval versus continuous training in patients with severe COPD: a randomized controlled trial. European Respiratory Journal. 1999;14:258-263.
Coronado M, Janssens JP, de Muralt B, et al. Walking activity measured by accelerometry during respiratory rehabilitation. Journal of Cardiopulmonary Rehabilitation. 2003;23:357-364.
Coronell C, Orozco-Levi M, Mendez R, et al. Relevance of assessing quadriceps endurance in patients with COPD. European Respiratory Journal. 2004;24(1):129-136.
Creutzberg EC, Schols AM, Weling-Scheepers CA, et al. Characterization of nonresponse to high caloric oral nutritional therapy in depleted patients with COPD. American Journal of Respiratory and Critical Care Medicine. 2000;161(3 Pt 1):745-752.
Creutzberg EC, Wouters EF, Mostert R, et al. A role for anabolic steroids in the rehabilitation of patients with COPD? A double-blind, placebo-controlled, randomized trial. Chest. 2003;124(5):1733-1742.
Dal Corso S, Nápolis L, Malaguti C, et al. Skeletal muscle structure and function in response to electrical stimulation in moderately impaired COPD patients. Respiratory Medicine. 2007;101(6):1236-1243.
De Torres JP, Cordoba-Lanus E, Lopez-Aguilar C, et al. C-reactive protein levels and clinically important predictive outcomes in stable COPD patients. European Respiratory Journal. 2006;27:902-907.
De Torres JP, Pinto-Plata V, Ingenito E, et al. Power of outcome measurements to detect clinically significant changes in pulmonary rehabilitation of patients with COPD. Chest. 2002;121(4):1092-1098.
Debigare R, Cote CH, Maltais F. Peripheral muscle wasting in chronic obstructive pulmonary disease: clinical relevance and mechanisms. American Journal of Respiratory and Critical Care Medicine. 2001;164:1712-1717.
Debigare R, Maltais F, Mallet M, et al. Influence of work rate incremental rate on the exercise responses in patients with COPD. Medicine and Science in Sports and Exercise. 2000;32:1365-1368.
Devine E, Pearcy J. Meta-analysis of the effect of psycho-educational care in adults with COPD. Patient Education and Counselling. 1996;29(2):167-178.
Donaldson GC, Wilkinson TM, Hurst JR, et al. Exacerbations and time spent outdoors in chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine. 2005;171:446-452.
Dourado VZ, Antunes LC, Tanni SE, et al. Relationship of upper-limb and thoracic muscle strength to 6-min walk distance in COPD patients. Chest. 2006;129(3):551-557.
Dyer CA, Singh SJ, Stockley RA, et al. The incremental shuttle walking test in elderly people with chronic airflow limitation. Thorax. 2002;57:34-38.
Eakin E, Resnikoff P, Prewitt L, et al. Validation of a new dyspnoea measure: the UCSD shortness of breath questionnaire. Chest. 1998;113:619-624.
Eaton T, Young P, Nicol K, et al. The endurance shuttle walking test: a responsive measure in pulmonary rehabilitation for COPD patients. Chronic Respiratory Disease. 2006;3:3-9.
Eiser N, West C, Evans S, et al. Effects of psychotherapy in moderately severe COPD: a pilot study. European Respiratory Journal. 1997;10(7):1581-1584.
Emtner M, Porszasz J, Burns M, et al. Benefits of supplemental oxygen in exercise training in nonhypoxemic chronic obstructive pulmonary disease patients. American Journal of Respiratory and Critical Care Medicine. 2003;168:1034-1042.
Fabbri LM, Hurd SS, GOLD Scientific Committee. Global strategy for the diagnosis, management and prevention of COPD: 2003 update. European Respiratory Journal. 2003;22(1):1-2.
Ferreira IM, Brooks D, Lacasse Y, et al. Nutritional supplementation for stable chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2002. (1):2005. CD000998
Fletcher CM. Standardised questionnaire on respiratory symptoms: a statement prepared and approved by the MRC committee on the aetiology of chronic bronchitis (MRC Breathlessness Score). British Medical Journal. 1960;2:1665.
Foglio K, Bianchi L, Ambrosino N. Is it really useful to repeat outpatient pulmonary rehabilitation programs in patients with chronic airway obstruction? A 2-year controlled study. Chest. 2001;119:1696-1704.
Foglio K, Bianchi L, Bruletti G, et al. Long-term effectiveness of pulmonary rehabilitation in patients with COPD. European Respiratory Journal. 1999;13:125-132.
Folgering H, Rooyakkers J, Herwaarden C. Education and cost benefit ratios in pulmonary patients. Monaldi Archives of Chest Disease. 1994;49(2):166-168.
Franssen FM, Broekhuizen R, Janssen PP, et al. Limb muscle dysfunction in COPD: effects of muscle wasting and exercise training. Medicine and Science in Sports and Exercise. 2005;37(1):2-9.
Franssen FM, Wouters EF, Baarends EM, et al. Arm mechanical efficiency and arm exercise capacity are relatively preserved in chronic obstructive pulmonary disease. Medicine and Science in Sports and Exercise. 2002;34(10):1570-1576.
Fuchs-Climent D, Le Gallais D, Varray A, et al. Quality of life and exercise tolerance in COPD: effects of a short and intensive inpatient rehabilitation program. American Journal of Physical Medicine and Rehabilitation. 1999;78:330-335.
Gallefoss F, Bakke PS, Kjaersgaard P. Quality of life assessment after patient education in a randomized controlled study on asthma and chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine. 1999;159:812-817.
Gan WQ, Man SF, Senthilselvan A, et al. The association between chronic obstructive pulmonary disease and systemic inflammation: a systematic review and a meta-analysis. Thorax. 2004;59:574-580.
Garcia-Aymerich J, Farrero E, Felez MA, et al. Risk factors of readmission to hospital for a COPD exacerbation: a prospective study. Thorax. 2003;58:100-105.
Garrod R. Pulmonary rehabilitation: the pros and cons of rehabilitation at home. Physiotherapy. 1998;84(12):603-607.
Garrod R, Backley J. Community-based pulmonary rehabilitation: meeting demand in chronic obstructive pulmonary disease. Physical Therapy Reviews. 2006;11:57-61.
Garrod R, Paul EA, Wedzicha JA. Supplemental oxygen during pulmonary rehabilitation in patients with COPD and exercise hypoxaemia. Thorax. 2000;55:539-543.
Garrod R, Mikelsons C, Paul EA, Wedzicha JA. Randomized controlled trial of domiciliary noninvasive positive pressure ventilation and physical training in severe chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine. 2000;162(41):1335-1341.
Garrod R, Bestall J, Wedzicha JA, et al. Development and validation of a standardised measure of activity of daily living in patients with COPD: the London Chest Activity of Daily Living Scale (LCADL). Respiratory Medicine. 2000;94(6):589-596.
Garrod R, Paul EA, Wedzicha JA. An evaluation of the reliability and sensitivity of the London Chest Activity of Daily Living Scale (LCADL). Respiratory Medicine. 2002;96:725-730.
Garrod R, Dallimore K, Cook J, et al. An evaluation of the acute impact of pursed lips breathing on walking distance in nonspontaneous pursed lips breathing chronic obstructive pulmonary disease patients. Chronic Respiratory Disease. 2005;2:67-72.
Garrod R, Marshall J, Barley E, et al. Predictors of success and failure in pulmonary rehabilitation. European Respiratory Journal. 2006;27:788-794.
Garrod R, Marshall J, Fredericks S, et al. CRP as a marker of impairment and disability in chronic obstructive pulmonary disease (COPD). Proceedings of The American Thoracic Society. 2005;2:A639.
Gigliotti F, Colli C, Bianchi R, et al. Arm exercise and hyperinflation in patients with COPD: effect of arm training. Chest. 2005;128(3):1225-1232.
Gimenez M, Serverra E, Vergara P, et al. Endurance training in patients with COPD: a comparison of high versus moderate intensity. Archives of Physical Medicine and Rehabilitation. 2000;81:102-109.
Global Initiative for Chronic Obstructive Lung Disease (GOLD), 2001. Global Strategy for the diagnosis, management and prevention of COPD. NHLBI/WHO workshop report. Bethesda, National Heart, Lung and Blood Institute, 2001. www.goldcopd.org/Guidelineitem.asp?l1=2&l2=1&intId=989. Date last updated: November 2006, accessed 16 July 2007
Goldstein MF, Fallon JJJr., Harning R. Chronic glucocorticoid therapy-induced osteoporosis in patients with obstructive lung disease. Chest. 1999;116(6):1733-1749.
Goldstein R. Ventilatory muscle training. Thorax. 1993;48:1025-1033.
Goldstein RS, Gort EH, Stubbing D, et al. Randomized controlled trial of respiratory rehabilitation. Lancet. 1994;344:1394-1397.
Gosker HR, Lencer NH, Franssen FM, et al. Striking similarities in systemic factors contributing to decreased exercise capacity in patients with severe chronic heart failure or COPD. Chest. 2003;123(5):1416-1424.
Gosker HR, van Mameren H, van Dijk PJ, et al. Skeletal muscle fibre-type shifting and metabolic profile inpatients with COPD. European Respiratory Journal. 2002;19:617-625.
Gosselink R. Controlled breathing and dyspnea in patients with chronic obstructive pulmonary disease (COPD). Journal of Rehabilitation Research and Development. 2003;40(5 Suppl 2):25-33.
Gosselink R, Troosters T, Decramer M. Peripheral muscle weakness contributes to exercise limitation in COPD. American Journal of Respiratory and Critical Care Medicine. 1996;153(3):976-980.
Gosselink R, Troosters T, Decramer M. Distribution of muscle weakness in stable patients with COPD. Journal of Cardiopulmonary Rehabilitation. 2000;20:353-360.
Gosselink RA, Wagenaar RC, Sargeant AJ, et al. Diaphragmatic breathing reduces efficiency of breathing in COPD. American Journal of Respiratory and Critical Care Medicine. 1995;151:1136-1142.
Green RH, Singh SJ, Williams J, et al. A randomized controlled trial of 4 weeks versus 7 weeks of pulmonary rehabilitation in COPD. Thorax. 2001;56:143-145.
Griffiths TL, Burr ML, Campbell IA, et al. Results at 1 year of out-patient multidisciplinary pulmonary rehabilitation: a randomized controlled trial. Lancet. 2000;355:362-368.
Guell R, Casan P, Belda J, et al. Long-term effects of outpatient rehabilitation of COPD: a randomized trial. Chest. 2000;117:976-983.
Gupta RB, Brooks D, Lacasse Y, et al. Effect of rollator use on health-related quality of life in individuals with COPD. Chest. 2006;130:1089-1095.
Guyatt GH, Feeny D, Patrick D. Issues in quality of life measurement in clinical trials. Controlled Clinical Trials. 1991:81S-90S.
Guyatt GH, Keller J, Singer J, et al. Controlled trial of respiratory muscle training in chronic airflow limitation. Thorax. 1992;47:598-602.
Guyatt GH, Townsend M, Berman L, et al. A measure of quality of life for clinical trials in chronic lung disease. Thorax. 1987;42:773-778.
Harver A, Mahler D, Daubenspeck J. Targeted inspiratory muscle training improves respiratory muscle function and dyspnoea in patients with COPD. Annals of Internal Medicine. 1989;111(2):117-124.
Heppner PS, Morgan C, Kaplan RM, et al. Regular walking and long-term maintenance of outcomes after pulmonary rehabilitation. Journal of Cardiopulmonary Rehabilitation. 2006;26(1):44-53.
Hill K, Jenkins SC, Philippe DL, et al. High-intensity inspiratory muscle training in COPD. European Respiratory Journal. 2006;27(6):1119-1128.
Hoo GW. Non-pharmacologic adjuncts to training during pulmonary rehabilitation: the role of supplemental oxygen and non-invasive ventilation. Journal of Rehabilitation Research and Development. 2003;40(5):S2. 81-98
Horowitz MB, Littenberg B, Mahler DA. Dyspnoea ratings for prescribing exercise intensity in patients with COPD. Chest. 1996;109(5):1169-1175.
Hunter S, Hall S. The effect of an educational support programme on dyspnoea and the emotional status of COPD clients. Rehabilitation Nursing Journal. 1989;14(4):200-202.
Jaeshchke R, Singer J, Guyatt GH. Measurement of health status. Ascertaining the minimal clinically important difference. Controlled Clinical Trials. 1989;10(4):407-415.
Janaudis-Ferreira T, Wadell K, Sundelin G, et al. Thigh muscle strength and endurance in patients with COPD compared with healthy controls. Respiratory Medicine. 2006;100(8):1451-1457.
Jones AY, Dean E, Chow CC. Comparison of the oxygen cost of breathing exercises and spontaneous breathing in patients with stable chronic obstructive pulmonary disease. Physical Therapy. 2003;83:424-431.
Jones PW. Measurement of health in asthma and chronic obstructive airways disease. Pharmaceutical Medicine. 1992;6:13-22.
Jones PW. St. George’s Respiratory Questionnaire: MCID. COPD. 2005;2(1):75-79.
Jones PW, Quirk FH, Baveystock CM. The St George’s Respiratory Questionnaire (SGRQ). Respiratory Medicine. 1991;85(Suppl B):25-31.
Kawane H. Smoking cessation in comprehensive pulmonary rehabilitation. Lancet. 1997;349:285.
Killian KJ, Leblanc P, Martin DH, et al. Exercise capacity and ventilatory, circulatory, and symptom limitation in patients with chronic airflow limitation. American Review of Respiratory Disease. 1992;146:935-940.
Koppers RJ, Vos PJ, Boot CR. Exercise performance improves in patients with COPD due to respiratory muscle endurance training. Chest. 2006;129(4):886-892.
Lacasse Y, Brosseau L, Milne S, et al. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews No 3. 2002. CD003793
Lacasse Y, Goldstein R, Lasserson TJ et al. 2006 Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database of Systemic Reviews, Issue 4, Art. No.: CD0003793
Lacasse Y, Guyatt GH, Goldstein RS. The components of a respiratory rehabilitation programme. Chest. 1997;111:1077-1088.
Lake F, Henderson K, Briffa T, et al. Upper limb and lower limb exercise training in patients with chronic airflow obstruction. Chest. 1990;97:1077-1082.
Landbo C, Prescott E, Lange P, et al. Prognostic value of nutritional status in COPD. American Journal of Respiratory and Critical Care Medicine. 1999;160(6):1856-1861.
Lareau SC, Carrieri-Kohlmon V, Janson-Bjerklie S, et al. Development of the Pulmonary Functional Status and Dyspnea Questionnaire (PFSDQ). Heart and Lung. 1994;23(3):242-250.
Lareau SC, Meek PM, Roos PJ. Development and testing of the modified version of the Pulmonary Functional Status and Dyspnea Questionnaire (PFSDQ-M). Heart and Lung. 1998;27(3):159-168.
Larson J, Kim J, Sharp T, et al. Inspiratory muscle training with a pressure threshold breathing device in patients with COPD. American Review of Respiratory Disease. 1999;138:689-696.
Locke E, Shaw K, Sari L, et al. Goal setting and task performance 1969-1980. Psychological Bulletin. 1981;90(1):125-152.
Lorenzi CM, Cilione C, Rizzardi R, et al. Occupational therapy and pulmonary rehabilitation of disabled COPD patients. Respiration. 2004;71(3):246-251.
Lotters F, van Tol B, Kwakkel G, et al. Effects of controlled inspiratory muscle training in patients with COPD: a meta-analysis. European Respiratory Journal. 2002;20(3):570-576.
Maa SH, Gauthier D, Turner M. Acupressure as an adjunct to a pulmonary rehabilitation programme. Journal of Cardiopulmonary Rehabilitation. 1997;17(4):268-276.
MacIntyre NR. Corticosteroid therapy and chronic obstructive pulmonary disease. Respiratory Care. 2006;51(3):289-296.
Mackay L. Health education and COPD rehabilitation: a study. Nursing Standard. 1996;10(40):34-39.
Mador MJ, Deniz O, Aggarwal A, et al. Quadriceps fatigability after single muscle exercise in patients with chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine. 2003;168(1):102-108.
Mahler DA, Ward J, Mejia-Alfaro R. Stability of dyspnea ratings after exercise training in patients with COPD. Medicine and Science in Sports and Exercise. 2003;35(7):1083-1087.
Mahler DA, Weinberg DH, Wells CK, et al. The measurement of dyspnea; contents, inter-observer agreement and physiologic correlates of two new clinical indexes. Chest. 1984;85:751-758.
Maltais F, Leblanc P, Simard C, et al. Skeletal muscle adaptation to endurance training in patients with COPD. American Journal of Critical Care Medicine. 1996;154:442-447.
Maltais F, Leblanc P, Jobin J, et al. Intensity of training and physiologic adaptation with COPD. American Journal of Respiratory and Critical Care Medicine. 1997;155:555-561.
Maltais F, Sullivan MJ, LeBlanc P, et al. Altered expression of myosin heavy chain in the vastus lateralis muscle in patients with COPD. European Respiratory Journal. 1999;13:850-854.
Man WD, Polkey MI, Donaldson N, et al. Community pulmonary rehabilitation after hospitalisation for acute exacerbations of chronic obstructive pulmonary disease: randomized controlled study. British Medical Journal. 2004;329:1209.
Man WD, Soliman MG, Gearing J, et al. Symptoms and quadriceps fatigability after walking and cycling in chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine. 2003;168(5):562-567.
Marlow SP, Stoller JK. Smoking cessation. Respiratory Care. 2003;48(12):1238-1254.
Mazzuca S. Does patient education in chronic disease have therapeutic value? Journal of Chronic Diseases. 1982;35(7):521-529.
McGavin CR, Gupta SP, Lloyd EL, et al. Physical rehabilitation for the chronic bronchitic: results of a controlled trial of exercises in the home. Thorax. 1977;32:307-311.
McKeough ZJ, Alison JA, Bye PT. Arm exercise capacity and dyspnea ratings in subjects with chronic obstructive pulmonary disease. Journal of Cardiopulmonary Rehabilitation. 2003;23(3):218-225.
Mercken EM, Hageman GJ, Schols AM, et al. Rehabilitation decreases exercise-induced oxidative stress in COPD. American Journal of Respiratory and Critical Care Medicine. 2005;172:994-1001.
Midorikawa J, Hida W, Taguchi O, et al. Lack of ventilatory threshold in patients with COPD. Respiration. 1997;64:76-80.
Monninkhof EM, van der Valk PDLPM, van der Palen J, et al. Self-management education for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews No.1. 2003. CD002990
Morgan MD, Calverley PM, Clark C, et al. British Thoracic Society Statement on Pulmonary Rehabilitation. Thorax. 2001;56:827-834.
Mota S, Güell R, Barreiro E, et al. Clinical outcomes of expiratory muscle training in severe COPD patients. Respiratory Medicine. 2007;101(3):516-524.
Mueller RE, Petty TL, Filley GF. Ventilation and arterial blood gas changes induced by pursed lips breathing. Journal of Applied Physiology. 1970;28(6):784-789.
National Institutes of Health. Pulmonary rehabilitation research. National Institutes of Health workshop summary. American Review of Respiratory Disease. 1994;149:825-893.
Neder JA, Jones PW, Nery LE, et al. Determinants of the exercise endurance capacity in patients with COPD: the power-duration relationship. American Journal of Respiratory and Critical Care Medicine. 2000;162:497-504.
Neder JA, Sword D, Ward SA, et al. Home based neuromuscular electrical stimulation as a new rehabilitative strategy for severely disabled patients with chronic obstructive pulmonary disease (COPD). Thorax. 2002;57(4):333-337.
Nguyen HQ, Carrieri-Kohlman V. Dyspnea self-management in patients with chronic obstructive pulmonary disease: moderating effects of depressed mood. Psychosomatics. 2005;46(5):402-410.
Nici L, Donner C, Wouters E, et al. American Thoracic Society/European Respiratory Society Statement on Pulmonary Rehabilitation. American Journal of Respiratory and Critical Care Medicine. 2006;173:1390-1413.
Normandin EA, McCusker C, Connors M, et al. An evaluation of two approaches to exercise conditioning in pulmonary rehabilitation. Chest. 2002;121(4):1085-1091.
Norweg AM, Whiteson J, Malgady R, et al. The effectiveness of different combinations of pulmonary rehabilitation program components: a randomized controlled trial. Chest. 2005;128(2):663-672.
O’Brien Cousins S, Keating N. Life cycle patterns of physical activity among sedentary and older women. Journal of Ageing and Physical Activity. 1995;3:340-359.
O’Donnell D. Breathlessness in patients with chronic airflow limitation. Chest. 1994;106:905-912.
O’Donnell DE, D’Arsigny C, Webb KA. Effects of hyperoxia on ventilatory limitation in advanced COPD. American Journal of Respiratory and Critical Care Medicine. 2001;163:892-898.
O’Donnell DE, Sciurba F, Celli B, et al. Effect of fluticasone propionate/salmeterol on lung hyperinflation and exercise endurance in COPD. Chest. 2006;130(3):647-656.
O’Neill S, McCarthy DS. Postural relief of dyspnoea in severe chronic airflow limitation. Thorax. 1983;38:595-600.
Ong KC, Chong WF, Soh C, et al. Comparison of different exercise tests in assessing outcomes of pulmonary rehabilitation. Respiratory Care. 2004;49(12):1498-1503.
O’Shea SD, Taylor NF, Paratz J. Peripheral muscle strength training in COPD: a systematic review. Chest. 2004;126:903-914.
Palange P, Carlone S, Forte S, et al. Cardiopulmonary exercise testing in the evaluation of patients with ventilatory vs circulatory causes of reduced exercise tolerance. Chest. 1994;105:1122-1126.
Partridge C, Pryor J, Webber B. Characteristics of the forced expiration technique. Physiotherapy. 1989;73(3):193-194.
Petty TL. Pulmonary rehabilitation in chronic respiratory insufficiency. 1. Pulmonary rehabilitation in perspective: historical roots, present status, and future projections. Thorax. 1993;48(8):855-862.
Pinto-Plata CM, Cote C, Cabral H, et al. The 6-min walk distance: change over time and value as a predictor of survival in severe COPD. European Respiratory Journal. 2004;23(1):28-33.
Pitta FO, Brunetto AF, Padovani CR, et al. Effects of isolated cycle ergometer training on patients with moderate-to-severe chronic obstructive pulmonary disease. Respiration. 2004;71(5):477-483.
Pitta F, Troosters T, Probst VS, et al. Physical activity and hospitalization for exacerbation of COPD. Chest. 2006;129:536-544.
Pitta F, Troosters T, Probst VS, et al. Quantifying physical activity in daily life with questionnaires and motion sensors in COPD. European Respiratory Journal. 2006;27:1040-1055.
Pitta F, Troosters T, Spruit MA, et al. Characteristics of physical activities in daily life in chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine. 2005;171:972-977.
Pitta F, Troosters T, Spruit MA, et al. Activity monitoring for assessment of physical activities in daily life in patients with chronic obstructive pulmonary disease. Archives of Physical Medicine and Rehabilitation. 2005;86(10):1979-1985.
Probst VS, Troosters T, Celis G, et al. Effects of resistance exercise training during hospitalization due to acute exacerbation of COPD – preliminary results [abstract]. European Respiratory Journal. 2005;26(Suppl 49):432S.
Probst VS, Troosters T, Coosemans I, et al. Mechanisms of improvement in exercise capacity using a rollator in patients with COPD. Chest. 2004;126:1102-1107.
Probst VS, Troosters T, Pitta F, et al. Cardiopulmonary stress during exercise training in patients with COPD. European Respiratory Journal. 2006;27(6):1110-1118.
Puente-Maestu L, Sanz M, Sanz P, et al. Comparison of effects of supervised versus self-monitored training programmes in patients with chronic obstructive pulmonary disease. European Respiratory Journal. 2000;15(3):517-525.
Puhan MA, Guyatt GH, Goldstein R, et al. Relative responsiveness of the Chronic Respiratory Questionnaire, St. Georges Respiratory Questionnaire and four other health-related quality of life instruments for patients with chronic lung disease. Respiratory Medicine. 2007;101(2):308-316.
Puhan MA, Schunemann HJ, Frey M, et al. How should COPD patients exercise during respiratory rehabilitation? Comparison of exercise modalities and intensities to treat skeletal muscle dysfunction. Thorax. 2005;60(5):367-375.
Punzal PA, Ries AL, Kaplan RM, et al. Maximum intensity training in patients with COPD. Chest. 1991;100:618-623.
Reardon J, Awad E, Normandin E, et al. The effect of comprehensive outpatient pulmonary rehabilitation on dyspnea. Chest. 1994;105:1046-1052.
Redelmeier D, Bayoumi A, Goldstein R, et al. Interpreting small differences in functional status: the Six Minute Walk test in chronic lung disease patients. American Journal of Respiratory and Critical Care Medicine. 1997;155(4):1278-1282.
Reid W, Samrai B. Respiratory muscle training for patients with COPD. Physical Therapy. 1995;75(11):70-79.
Renfroe K. Effect of progressive relaxation on dyspnoea and state anxiety in patients with COPD. Heart and Lung. 1988;17:408-413.
Restrick LJ, Paul EA, Braid GM, et al. Assessment and follow-up of patients prescribed long-term oxygen treatment. Thorax. 1993;48:708-713.
Revill SM, Morgan MDL, Singh SJ, et al. The endurance shuttle walk: a new field test for the assessment of endurance capacity in chronic obstructive pulmonary disease. Thorax. 1999;54:213-220.
Richardson RS, Leek BT, Gavin TP, et al. Reduced mechanical efficiency in chronic obstructive pulmonary disease but normal peak VO2 with small muscle mass exercise. American Journal of Respiratory and Critical Care Medicine. 2004;169(1):89-96.
Ries AL, Kaplan RM, Limberg TM, et al. Effects of pulmonary rehabilitation on physiologic and psychosocial outcomes in COPD. Annals of Internal Medicine. 1995;122(11):823-831.
Ries AL, Kaplan RM, Myers R, et al. Maintenance after pulmonary rehabilitation in chronic lung disease: a randomized trial. American Journal of Respiratory and Critical Care Medicine. 2003;167(6):880-888.
Ringbaek TJ, Broendum E, Hemmingsen L, et al. Rehabilitation of patients with chronic obstructive pulmonary disease: exercise twice a week is not sufficient. Respiratory Medicine. 2000;94:150-154.
Romagnoli M, Dell’Orso D, Lorenzi C, et al. Repeated pulmonary rehabilitation in severe and disabled COPD patients. Respiration. 2006;73(6):769-776.
Roomi J, Johnson MM, Waters K, et al. Respiratory rehabilitation, exercise capacity and quality of life in chronic airways disease in old age. Age and Ageing. 1996;25:12-16.
Rooyakers JM, Dekhuijzen PNR, Van Herwaarden CLA, et al. Training with supplemental oxygen in patients with COPD and hypoxaemia at peak exercise. European Respiratory Journal. 1997;10:1278-1284.
Rossi G, Florini F, Romagnoli M, et al. Length and clinical effectiveness of pulmonary rehabilitation in outpatients with chronic airway obstruction. Chest. 2005;127:105-109.
Rutten-van Mölken M, Roos B, Van Noord JA. An empirical comparison of the St George’s Respiratory Questionnaire (SGRQ) and the Chronic Respiratory Disease Questionnaire (CRQ) in a clinical trial setting. Thorax. 1999;54(11):995-1003.
Sabapathy S, Kingsley RA, Schneider DA, et al. Continuous and intermittent exercise responses in individuals with chronic obstructive pulmonary disease. Thorax. 2004;59(12):1026-1031.
Sackner MA, Gonzales HF, Jenouri G, et al. Effects of abdominal and thoracic breathing on breathing pattern components in normal subjects and in patients with COPD. American Review of Respiratory Disease. 1984;130:584-587.
Salman GF, Mosier MC, Beasley BW, et al. Rehabilitation for patients with chronic obstructive pulmonary disease. Journal of General Internal Medicine. 2003;18:213-221.
Sandland CJ, Singh SJ, Curcio A, et al. A profile of daily activity in chronic obstructive pulmonary disease. Journal of Cardiopulmonary Rehabilitation. 2005;25(3):181-183.
Sassi-Dambron DE, Eakin EG, Ries AL, et al. Treatment of dyspnea in COPD. A controlled clinical trial of dyspnea management strategies. Chest. 1995;107:724-729.
Schenkel NS, Muralt BB, Fitting JW. Oxygen saturation during daily activities in chronic obstructive pulmonary disease. European Respiratory Journal. 1996;9:2584-2589.
Schols AM, Buurman WA, Staal van den Brekel AJ, et al. Evidence for a relation between metabolic derangements and increased levels of inflammatory mediators in a subgroup of patients with COPD. Thorax. 1996;51:819-824.
Schols A, Mostert R, Soeters P, et al. Body composition and exercise performance in patients with chronic obstructive pulmonary disease. Thorax. 1991;46(10):695-699.
Schols AM, Soeters PB, Mostert R, et al. Physiologic effects of nutritional support and anabolic steroids in patients with chronic obstructive pulmonary disease. A placebo-controlled randomized trial. American Journal of Respiratory and Critical Care Medicine. 1995;152(4 Pt 1):1268-1274.
Schunemann HJ, Guyatt GH, Griffith L, et al. A randomized controlled trial to evaluate the effect of informing patients about their pretreatment responses to two respiratory questionnaires. Chest. 2002;122(5):1701-1708.
Sewell L, Singh SJ, Williams JE, et al. Can individualized rehabilitation improve functional independence in elderly patients with COPD? Chest. 2005;128(3):1194-1200.
Sharp JT, Drutz WS, Moisan T, et al. Postural relief of dyspnoea in severe COPD. American Review of Respiratory Disease. 1980;122:201-211.
Simpson K, Killian K, McCartney N, et al. Randomized controlled trial of weightlifting exercise in patients with chronic airflow limitation. Thorax. 1992;47:70-75.
Sinclair DJM, Ingram CG. Controlled trial of supervised exercise training in chronic bronchitis. British Medical Journal. 1980;280:519-521.
Singh SJ, Morgan MDL, Hardman AE, et al. Comparison of oxygen uptake during a conventional treadmill test and the shuttle walk test in chronic airflow limitation. European Respiratory Journal. 1994;7:2016-2020.
Singh SJ, Morgan MDL, Scott S, et al. Development of a shuttle walking test of disability in patients with chronic airways obstruction. Thorax. 1992;47:1019-1024.
Singh SJ, Sodergren SC, Hyland ME, et al. A comparison of three disease-specific and two generic health-status measures to evaluate the outcome of pulmonary rehabilitation in COPD. Respiratory Medicine. 2001;95(1):71-77.
Sivori M, Rhodius E, Kaplan P, et al. Exercise training in chronic obstructive pulmonary disease. Comparative study of aerobic training of lower limbs vs combination with upper limbs. Medicina. 1998;58(6):712-727.
Solway S, Brooks D, Lau L, et al. The short-term effect of a rollator on functional exercise capacity among individuals with severe COPD. Chest. 2002;122:56-65.
Somfay A, Porszasz J, Lee SM, et al. Dose-response effect of oxygen on hyperinflation and exercise endurance in nonhypoxaemic COPD patients. European Respiratory Journal. 2001;18:77-84.
Spahija J, de Marchie M, Grassino A. Effects of imposed pursed-lips breathing on respiratory mechanics and dyspnea at rest and during exercise in COPD. Chest. 2005;128(2):640-650.
Spruit MA, Gosselink R, Troosters T, et al. Resistance versus endurance training in patients with COPD and peripheral muscle weakness. European Respiratory Journal. 2002;19:1072-1078.
Spruit MA, Gosselink R, Troosters T, et al. Muscle force during an acute exacerbation in hospitalised COPD patients and its relationship with CXCL8 and IGF1. Thorax. 2003;58:752-756.
Spruit MA, Troosters T, Gosselink R, et al. Low-grade systemic inflammation and the response to exercise training in patients with advanced COPD. Chest. 2005;128(5):3183-3190.
Steele BG, Belza B, Hunziker J, et al. Monitoring daily activity during pulmonary rehabilitation using a triaxial accelerometer. Journal of Cardiopulmonary Rehabilitation. 2003;23:139-142.
Steiner MC, Singh SJ, Morgan MD. The contribution of peripheral muscle function to shuttle walking performance in patients with chronic obstructive pulmonary disease. Journal of Cardiopulmonary Rehabilitation. 2005;25(1):43-49.
Strijbos JH, Postma DS, Van Altena R, et al. A comparison between an outpatient hospital based pulmonary rehabilitation program and a home care pulmonary rehabilitation program in patients with COPD. Chest. 1996;109:366-372.
Takabatake N, Nakamura H, Abe S, et al. The relationship between chronic hypoxemia and activation of the tumor necrosis factor-alpha system in patients with chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine. 2000;161:1179-1184.
Trappenburg JC, Troosters T, Spruit MA, et al. Psychosocial conditions do not affect short-term outcome of multidisciplinary rehabilitation in chronic obstructive pulmonary disease. Archives of Physical Medicine and Rehabilitation. 2005;86(9):1788-1792.
Troosters T, Casaburi R, Gosselink R, et al. Pulmonary rehabilitation in chronic obstructive pulmonary disease (state of the art). American Journal of Respiratory and Critical Care Medicine. 2005;172:19-38.
Troosters T, Gosselink R, Decramer M. Six minute walking distance in healthy elderly subjects. European Respiratory Journal. 1999;14(2):270-274.
Troosters T, Gosselink R, Decramer M. Short and long-term effects of outpatient rehabilitation in patients with COPD: a randomized trial. American Journal of Medicine. 2000;109:207-212.
Troosters T, Gosselink R, Decramer M. Exercise training in COPD: how to distinguish responders from non-responder. Journal of Cardiopulmonary Rehabilitation. 2001;21:10-17.
Troosters T, Vilaro J, Rabinovich R, et al. Physiological responses to the 6-min walk test in patients with chronic obstructive pulmonary disease. European Respiratory Journal. 2002;20(3):564-569.
Turner SE, Eastwood PR, Cecins NM, et al. Physiologic responses to incremental and self-paced exercise in COPD: a comparison of three tests. Chest. 2004;126(3):766-773.
Vale F, Reardon J, ZuWallack R. The long-term benefits of outpatient pulmonary rehabilitation on exercise endurance and quality of life. Chest. 1993;103:42-45.
van der Schans CP, De Jong W, Kort E, et al. Mouth pressures during pursed lip breathing. Physiotherapy Theory and Practice. 1995;11:29-34.
van Manen JG, Bindels PH, Dekker FW, et al. Risk of depression in patients with COPD and its determinants. Thorax. 2002;57:412-416.
van’t Hul A, Kwakkel G, Gosselink R. The acute effects of noninvasive ventilatory support during exercise on exercise endurance and dyspnea in patients with chronic obstructive pulmonary disease. Journal of Cardiopulmonary Rehabilitation. 2002;22:290-297.
Velloso M, Jardim JR. Study of energy expenditure during activities of daily living using and not using body position recommended by energy conservation techniques in patients with COPD. Chest. 2006;130(1):126-132.
Vitacca M, Clini E, Bianchi L, et al. Acute effects of deep diaphragmatic breathing in COPD patients with chronic respiratory insufficiency. European Respiratory Journal. 1998;11:408-415.
Vivodtzev I, Pepin JL, Vottero G, et al. Improvement in quadriceps strength and dyspnea in daily tasks after 1 month of electrical stimulation in severely deconditioned and malnourished COPD. Chest. 2006;129(6):1540-1548.
Vogiatzis I, Nanas S, Kastanakis E, et al. Dynamic hyperinflation and tolerance to interval exercise in patients with advanced COPD. European Respiratory Journal. 2004;24:385-390.
Vogiatzis I, Terzis G, Nanas S, et al. Skeletal muscle adaptations to interval training in patients with advanced COPD. Chest. 2005;128:3838-3845.
Wadell K, Henriksson-Larsen K, Lundgren R. Physical training with and without oxygen in patients with chronic obstructive pulmonary disease and exercise-induced hypoxaemia. Journal of Rehabilitation Medicine. 2001;33(5):200-205.
Wadell K, Henriksson-Larsen K, Lundgren R, et al. Group training in patients with COPD – long-term effects after decreased training frequency. Disability and Rehabilitation. 2005;27(10):571-581.
Wanke TH, Formanek D, Lahrmann H, et al. Effects of combined inspiratory muscle and cycle ergometer training on exercise performance in patient with COPD. European Respiratory Journal. 1994;7:2205-2211.
Wedzicha JA, Bestall JC, Garrod R, et al. Randomized controlled trial of pulmonary rehabilitation in severe chronic obstructive pulmonary disease patients, stratified with the MRC scale. European Respiratory Journal. 1998;12:363-369.
Weiner P, Magadle R, Beckerman M, et al. Specific expiratory muscle training in COPD. Chest. 2003;124(2):468-473.
Weiner P, Magadle R, Beckerman M, et al. Comparison of specific expiratory, inspiratory, and combined muscle training programs in COPD. Chest. 2003;124(4):1357-1364.
Whittom F, Jobin J, Simard PM, et al. Histochemical and morphological characteristics of the vastus lateralis muscle in patients with chronic obstructive pulmonary disease. Medicine and Science in Sports and Exercise. 1998;30(10):1467-1474.
Wijkstra PJ, Mark TW, Kraan J, et al. Long-term effects of home rehabilitation on physical performance in chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine. 1996;153:1234-1241.
Wijkstra PJ, Ten Vergert EM, Van Altena R, et al. Long-term effects of rehabilitation at home on quality of life and exercise tolerance in patients with chronic obstructive pulmonary disease. Thorax. 1995;50:824-828.
Wijkstra PJ, Van Altena R, Kraan J, et al. Quality of life in patients with COPD improves after rehabilitation at home. European Respiratory Journal. 1994;7:269-273.
Willeput R, Vachaudez JP, Lenders D, et al. Thoracoabdominal motion during chest physiotherapy in patients affected by chronic obstructive lung disease. Respiration. 1983;44:204-214.
Withers NJ, Rudkin ST, White RJ. Anxiety and depression in severe COPD: the effects of pulmonary rehabilitation. Journal of Cardiopulmonary Rehabilitation. 1999;19:362-365.
Yende S, Waterer GW, Tolley EA, et al. Inflammatory markers are associated with ventilatory limitation and muscle dysfunction in obstructive lung disease in well functioning elderly subjects. Thorax. 2006;61(1):10-16.
Yohannes AM, Roomi J, Winn S, et al. The Manchester Respiratory Activities of Daily Living questionnaire: development, reliability, validity, and responsiveness to pulmonary rehabilitation. Journal of the American Geriatric Society. 2000;48(11):1496-1500.
Young P, Dewse M, Fergusson W, et al. Respiratory rehabilitation in COPD: predictors of non-adherence. European Respiratory Journal. 1999;13:855-859.
Zacarias EC, Neder JA, Cendom SP, et al. Heart rate at the estimated lactate threshold in patients with COPD: effects on the target intensity for dynamic exercise training. Journal of Cardiopulmonary Rehabilitation. 2000;20:369-376.
Zack MB, Palange AV. Oxygen-supplemented exercise of ventilatory and non-ventilatory muscles in pulmonary rehabilitation. Chest. 1985;88(5):669-675.
Zanotti E, Felicetti G, Maini M, et al. Peripheral muscle strength training in bedbound patients with COPD receiving mechanical ventilation: effect of electrical stimulation. Chest. 2003;124:292-296.
ZuWallack RL, Patel K, Reardon J, et al. Predictors of improvement in the 12-minute walking distance following a 6-week outpatient pulmonary rehabilitation programme. Chest. 1991;99(4):805-808.