Pulmonary Infection
Pneumonia and other pulmonary infections, defined as those involving the lower respiratory tract below the glottis, continue to be the most common cause of illness in children, affecting over 150 million children under the age of 5 years per year worldwide, and are implicated in 20 million hospitalizations annually in the United States.1–5 Since clinical signs and symptoms are poor predictors of pediatric pulmonary infections, and the value of microbial studies is limited, chest radiography with the use of standardized reporting criteria continues to be the best available diagnostic standard.1,3,6,7 The value of lateral radiograph in diagnosis continues to be debated, but the hyperinflation that is a radiographic hallmark of pulmonary infections in young children is more reliably detected on the lateral radiograph than on the frontal radiograph.1,8,9
In the ambulatory care setting, performing routine chest radiography has not been shown to improve the outcomes of pulmonary infections in young children, and it is not indicated in first-time wheezing episodes presumed to be viral or reactive in etiology.10–12 The yield of radiography is greater in the presence of a high temperature and in the absence of a family history of asthma.13 Radiography is most helpful when an inconsistency exists among the data from history, physical examination, and observation.14 Negative chest radiography provides justification to withhold antibiotics in symptomatic children.1 Valid indications for chest radiography, therefore, are severe disease, confirmation or exclusion of diagnosis in the presence of an atypical presentation, assessment of complications, and exclusion of other causes of respiratory distress.10
Maternal antibodies protect newborns against viral pulmonary infections, and bacterial infections are most common in this age group, usually caused by pathogens acquired during labor and delivery. With dropping maternal antibodies, viral infections become more prevalent between ages 2 months and 2 years. After this, bacterial infections again become more common.3 Tuberculosis, fungal infections, and parasitic infestations continue to add substantially to the disease burden of children who are immunocompromised or live in endemic areas.2
The spectrum of pulmonary infections in childhood is categorized in Table 54-1.2,15 However, the distinctions between these categories are arbitrary, since considerable overlap exists both at presentation and during evolution of disease. In young children, the lungs can only respond to insult in a limited number of ways, and this response is more age specific than antigen dependent.16–20 Viral and bacterial infections frequently coexist, and radiographic criteria alone do not reliably distinguish between them.21–23 This is compounded by a reported high interobserver variability for interpretation of chest radiographs.24–27 Use of inexact terminology may hamper communication between radiologists and referring physicians, who agree with radiologists’ interpretations in only 78% of cases, and antibiotics are frequently prescribed even when no bacterial agent can be proven.6,28,29
Table 54-1
Classification of Pulmonary Infections in Childhood
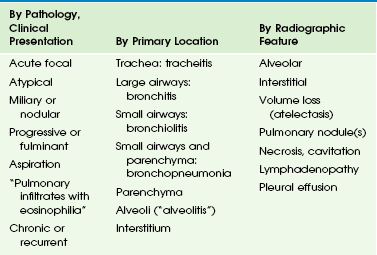
From Eslamy HK, Newman B. Pneumonia in normal and immunocompromised children: an overview and update. Radiol Clin North Am. 2011;49:895-920.
Pulmonary Infections Caused by Viruses
Viral pulmonary infections usually occur after the inhalation of infected air droplets.2 The clinical presentation depends on the infectious agent, patient age, and immune response (mainly cellular, T-cell–mediated immunity). In young children, degrees of mucosal swelling within the small-caliber terminal airways, which would not compromise air exchange in older individuals with relatively larger-caliber airways, lead to diffuse alveolar air trapping. This, in combination with lack of development of collateral pathways of ventilation via the pores of Kohn and canals of Lambert, leads to fixed hyperinflation of the lungs (Fig. 54-1). In addition, more hypersecretion occurs in the inflamed airways in young children, contributing to mucous plugging of the airways; this leads to (sub)segmental atelectasis mimicking alveolar consolidations, which are frequently misinterpreted to represent bacterial pneumonia.1,29–31
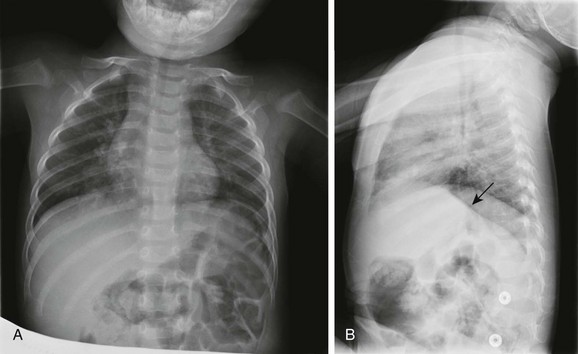
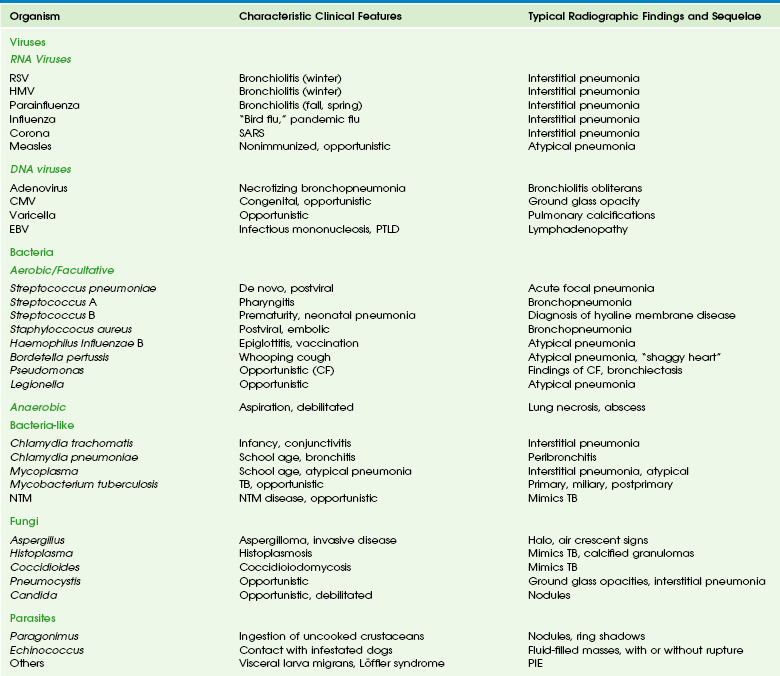
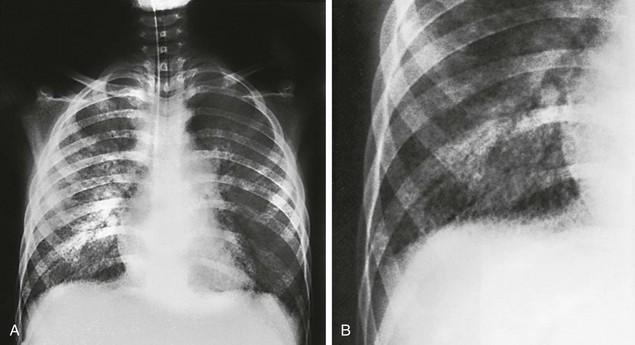
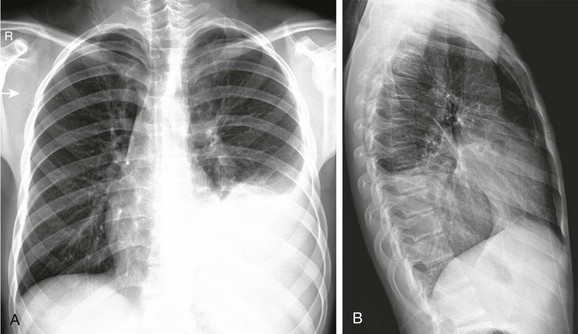
Figure 54-1 Respiratory syncytial virus pneumonia in a 1.5-year-old boy.
A, The frontal chest radiograph shows perihilar streaky lung opacities and peribronchial thickening, typical of viral infections, with more focal opacity medially in the right lung base, from superimposed atelectasis. This was mistaken for alveolar consolidation indicative of bacterial pneumonia, and antibiotic treatment was given unnecessarily. B, The lateral chest radiograph better demonstrates air trapping in the right lung base, with flattening of the right hemidiaphragm (arrow).
Etiology: The most common viral agents causing pulmonary infections in childhood are listed in Table 54-2.
RNA Viruses: Respiratory syncytial virus (RSV) consists of 10 genes encoding 11 proteins that are associated with inhibiting type 1 interferon activity. It is the most common cause of pulmonary infections in infants and young children. The disease can be virulent and is fatal in up to 1% of healthy infants, but those with chronic lung disease from prematurity and cardiovascular disease are at much higher risk.32 Clinical signs range from mild coryza to severe respiratory distress with wheezing, tachypnea, cyanosis, dyspnea, and retractions. Hypoxemia, possibly caused by a ventilation–perfusion imbalance, may be profound and last for several weeks.33 The diagnosis of RSV infection is made by examining the nasoepithelial cells by using direct fluorescent antibody detection.
Human metapneumovirus (HMV) is a negative single-stranded ribonucleic acid (RNA) virus of the family Paramyxoviridae, and the second most common cause of viral pulmonary infections in young children after RSV.34 It affects children who are slightly older than those infected by RSV, and the disease is less severe, except when seen as a coinfection. HMV has a worldwide distribution and causes a disease spectrum indistinguishable from influenza and RSV infection, with the same seasonal variation.
The various subtypes of the influenza virus are common causes of pneumonia requiring hospitalization in young and school-age children, ranking behind RSV and parainfluenza. Influenza attacks the ciliated respiratory epithelium, and lesions may extend to the distal airways, producing severe pneumonia. The onset is often more abrupt and intense than that of RSV or parainfluenza. The newly isolated avian influenza virus (H1N1), originating in Asia from infected poultry, has spread over many parts of the world from 2003 to 2007.34–36 A novel H1N1 infection resulting from antigenic shift of the virus has been reported since 2009 and has been associated with a higher rate of shock, acute respiratory distress syndrome (ARDS), and neurologic complications in children compared with seasonal (non-H1N1) infections.37
Severe acute respiratory distress syndrome (SARS) is caused by a coronavirus, which was recognized in 2003, and in the following year, it was diagnosed in over 8000 patients, primarily in China, Taiwan, Hong Kong, Vietnam, and Toronto.34,38 After 2004, the outbreak appeared to have been contained; since then, SARS has only been sporadically reported. Like most respiratory viral diseases, it is spread by face-to-face contact. SARS typically has a brief prodrome of fever, with or without constitutional symptoms. This quickly progresses to severe respiratory symptoms by day 6 of the fever.39 Most patients require hospitalization. It has an overall mortality of 10%, but children have constituted only 5% of reported cases, and no pediatric deaths have been reported.34 Children have a mild clinical course and recover with no sequelae.38 Recently, newer coronaviruses (NL63 and HKU1), which cause milder forms of respiratory disease, have been discovered.34
DNA Viruses: Adenovirus pneumonia is responsible for about 5% of respiratory tract disease in infants and children, with a peak age between 6 months and 5 years.31 It is a common cause of viral pneumonia, along with RSV, parainfluenza virus, and influenza virus. Adenovirus has also been associated with a pertussis-like syndrome. Although often relatively benign, adenoviral infection may be severe and even fatal in young infants, particularly when caused by the recently identified serotype Ad14.34
Imaging: Bilateral interstitial opacities with peribronchial thickening and hyperinflation (see Fig. 54-1), thought to represent viral bronchiolitis, are nonspecific and are, in fact, indicative of an acute pulmonary infection of any cause (viral or bacterial) in young children.1,15 Pleural effusions are rare in purely viral lung infections. Radiologic abnormalities often clear slowly and lag behind clinical improvement. Complications are superimposed bacterial infection (often hospital-acquired), and postinfectious bronchiolitis obliterans, bronchiectasis, or both.2 These latter conditions, which frequently follow an adenovirus infection, are characterized by features of chronic air trapping and atelectasis resulting from bronchial dysfunction; mosaic perfusion abnormalities, peribronchial thickening, chronic atelectasis and bronchiectasis, best seen on computed tomography (CT).2 Swyer-James-MacLeod syndrome is characterized by a unilateral small hyperlucent lung, which exhibits hypoperfusion and chronic bronchiectasis (e-Fig. 54-2).
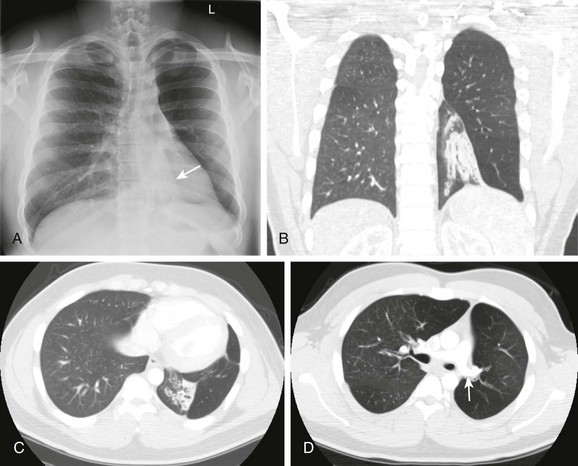
e-Figure 54-2 Swyer-James-MacLeod syndrome in a 20-year-old young man monitored since early childhood; he had chronic left lower lobe collapse and bronchiectasis secondary to bronchiolitis obliterans following an adenovirus infection.
A, The radiograph shows left lower lobe collapse (arrow) and hyperlucency of the left upper lobe. B to D, Computed tomography images demonstrate cylindrical bronchiectasis in the collapsed left lower lobe, and a relatively small left pulmonary artery (arrow in D).
In RSV infection, the lungs are often quite clear, or focal areas of superimposed atelectasis may be noted (see Fig. 54-1); when present, these predict a need for more prolonged mechanical ventilation.40
In mild influenza infection, initial chest radiographs are normal or may demonstrate nonspecific prominence of peribronchial markings and hyperinflation. In children with a more severe clinical course, bilateral symmetric and multifocal areas of consolidation, often associated with ground-glass opacities, are seen (e-Fig. 54-3).41
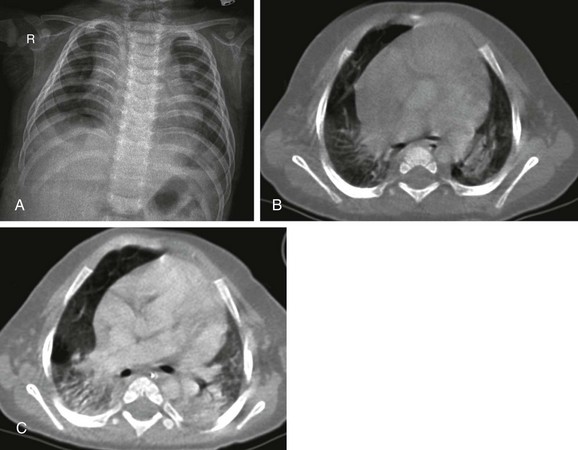
e-Figure 54-3 Lethal H1N1 influenza in an 8-month-old boy who presented with shortness of breath and fever during an outbreak of epidemic flu.
A, The radiograph shows nonspecific increased perivascular markings and somewhat low lung volumes. B, The contrast-enhanced computed tomography (CT) scan demonstrates consolidation in both lower lobes. C, The follow-up CT scan after 1 month showed progression of the consolidations. Soon after this scan was obtained, the baby died from cardiopulmonary failure.
The radiographic manifestations of SARS are typically mild, with interstitial thickening, which may progress to focal consolidation.42,43 In children, unlike in adults, no lymphadenopathy, pleural effusion, or cavitation is reported.44 However, abnormalities may persist on thin-section CT in older children for up to 12 months following infection.45
Measles virus is thought to be the cause of giant cell pneumonia, which produces a diffuse reticulonodular bronchopneumonia-like radiographic pattern (e-Fig. 54-4), with hilar node enlargement and superimposed bacterial infection, usually affecting the lower lobes. Atypical measles pneumonia following vaccination with, or exposure to, live virus was characterized by extensive nonsegmental parenchymal consolidation, with pulmonary nodules, hilar lymph node enlargement, and pleural effusion being common.
e-Figure 54-4 A 12-year-old girl with leukemia and measles pneumonia.
A, Initial radiographic examination showing a diffuse bilateral reticulonodular infiltrate, reflecting the interstitial distribution of the disease. B, Close-up of the right lower lobe showing the reticulonodular pattern to better advantage.
Adenovirus may cause necrotizing bronchopneumonia, bronchitis, or bronchiolitis. Radiographic features are nonspecific but usually include bronchial wall thickening, peribronchiolar densities, air trapping, and patchy or confluent consolidations.30 Adenopathy is more common than in other viral pneumonias. Bronchiectasis or bronchiolitis obliterans may be a permanent sequel (see e-Fig. 54-2).30,31,46
In children infected with CMV, often, a progressive interstitial pneumonitis is seen (e-Fig. 54-5). Gallium-67 scintigraphy may show abnormal uptake in the lungs of patients who have a normal chest CT.47
e-Figure 54-5 Cytomegalic virus infection in a 14-year-old boy with acute lymphoblastic leukemia and immunodeficiency, presenting with dyspnea, fever, cough, and decreased white blood cell counts.
A, The radiograph shows bilateral ill-defined pulmonary vasculature, more prominent in the lower lung zones. B, The contrast-enhanced computed tomography scan demonstrates bilateral ground-glass opacities with relative sparing of the peripheral parenchyma.
Findings in varicella are similar to those of measles pneumonia. Multiple focal calcifications frequently develop after severe chickenpox pneumonia (e-Fig. 54-6).
e-Figure 54-6 Chickenpox pneumonia in a teenager.
A, The detail from a frontal chest radiograph early in the disease reveals diffuse nodules throughout the right lung. B, A computed tomography scan done for clarification reveals nodules ranging from 2 to 4 mm, and many are densely calcified.
In EBV infection, hilar and mediastinal lymph node enlargement may be seen (e-Fig. 54-7). Pulmonary involvement is uncommon but is characterized by bilateral reticular perihilar infitrates.48
Treatment and Follow-up: Effective antiviral therapies have not been established, and treatment of viral pulmonary infections is mainly supportive. Experimental treatments to correct the acquired surfactant deficiency and dysfunction that occurs in critically ill infants with viral pulmonary infections are actively being investigated.37 With a few exceptions, the use of extracorporeal membrane oxygenation to treat severe respiratory failure from ARDS, which frequently complicates severe infections, has not lead to better outcomes than that of optimal less invasive supportive care.37 Certain high-risk infants may qualify for prophylactic injection of monoclonal antibodies against the F-glycoprotein of RSV. Since the most important complication of viral pulmonary infection is a superimposed bacterial infection, this should be actively looked for and treated with antibiotics when confirmed.2
Pulmonary Infections Caused by Bacteria and Bacteria-Like Organisms
Bacterial pulmonary infections are acquired through inhalation, hematogenously, or rarely by direct extension of chest wall or extrathoracic sites. Their course is determined by the balance between the virulence of the organism and the host immune response (mainly humoral, B-cell–mediated immunity, and macrophageal activity). They are characterized by alveolar airspace consolidation, visible as one or more focal lung opacities that exhibit air bronchograms and obliterate normal air–soft tissue interfaces (the silhouette sign). Pleural effusions are common. The most common bacteria and bacteria-like agents causing pulmonary infections in children are listed in Table 54-2.
In young children, the classic segmental or lobar airspace consolidation is rarely present, and it is nearly absent in neonates. This is reflected by the reported low 30% positive predictive value of radiographic criteria for a bacterial cause of pneumonia, accounting for a widespread overprescription of antibiotics and development of antibiotic resistant bacteria.1,21 On the other hand, the high 92% negative predictive value of radiographic criteria for bacterial pneumonia is helpful, allowing clinicians to withhold antibiotics in symptomatic children with a negative chest radiograph and to focus on other potential sources of the fever.1,21 It is, therefore, important not to overcall pediatric chest radiographs for the presence of a pulmonary infection, which is the most common interpretation error made by radiologists unfamiliar with pediatric imaging.49,50
Clinically occult pneumonia may be diagnosed with radiography in up to 19% of children less than 5 years old with fever of unknown origin and leucocytosis, although a more recent study found a lower incidence (5.3%) and a low utility of radiography when cough was not one of the presenting symptoms in this setting.51,52
Aerobic and Facultative Organisms
Etiology: This organism is the most common cause of bacterial pneumonia in children less than 5 years of age. It is a gram-positive diplococcus, which infects healthy patients but also commonly attacks those with underlying illness, including the hospitalized and immunocompromised, and children with sickle cell anemia.53 A strong association with a preceding viral infection, in particular influenza, is seen.54 The virally activated respiratory epithelium has an increased expression of receptors for pneumococcal attachment.55 In the usual case of an infected but otherwise healthy child, the onset is acute with fever, headache, and abdominal or chest pain. The pulse and respirations are rapid. By the second day, cough, expiratory grunts, rales, and pleural friction rub may be heard. Rapid clinical resolution usually occurs within 24 to 48 hours after treatment with antibiotics in patients with uncomplicated infections. However, a rapid increase has been seen in the incidence of partially or fully penicillin-resistant strains of S. pneumoniae.56 Following the institution of conjugate pneumococcal vaccination, the proportion of children younger than 5 years with suspected occult pneumococcal pneumonia confirmed by radiography decreased from 15% to 9%.57
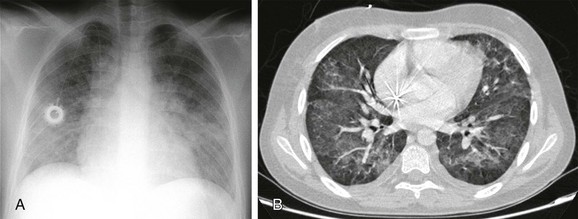
Imaging: Pneumococcal pneumonia is usually confined to one lobe, but only rarely is the entire lobe consolidated. A pattern of homogeneous airspace consolidation is usual but not invariable, especially in the presence of underlying lung disease. This pneumonia may initially have a strikingly round appearance in children younger than 8 years (Fig. 54-8), simulating an intrapulmonary mass or abscess, until it spreads further to reach a normal anatomic boundary such as a fissure.1,58,59 Pleural effusion, empyema (e-Fig. 54-9), and lung necrosis (e-Fig. 54-10) are infrequent complications, seen in less than 30% of patients. Resolution on radiographs is usually complete by 6 to 8 weeks.
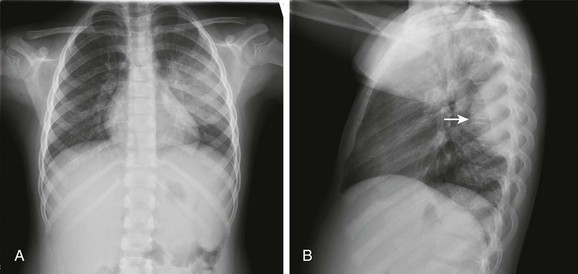
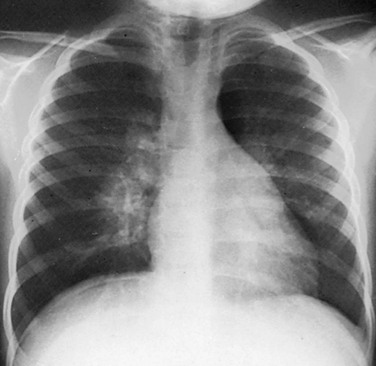
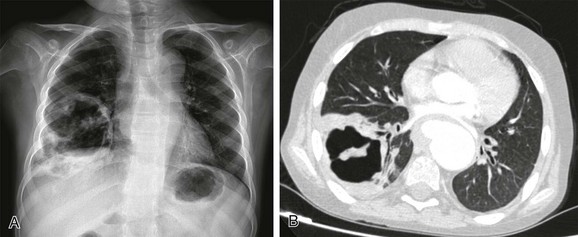
Figure 54-8 Streptococcal round pneumonia in a 5.5-year-old girl presenting with fever and left-sided back and rib pain.
Frontal (A) and lateral (B) radiographs show alveolar consolidation in the superior segment of the left lower lobe with a rounded appearance (arrow in B). Note in A the visibility of the left hilar shadow, which is superimposed on the posteriorly located pulmonary pseudomass (hilar overlay sign).
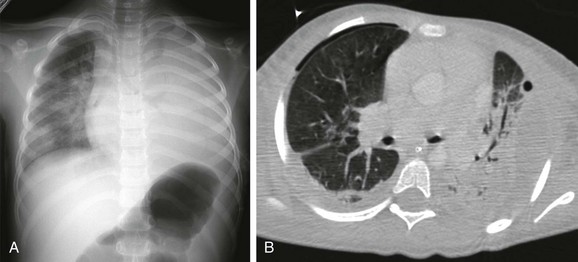
e-Figure 54-9 Streptococcal pneumonia and empyema in a 7-year-old girl who presented with high fever, chest pain, cough, shortness of breath and elevated white blood cell count.
A, The radiograph shows opaque left hemithorax. B, Contrast-enhanced computed tomography demonstrates diffuse consolidation in the left lung and pleural effusion compressing the underlying lung. Air is present in both pleural spaces secondary to intervention. Pleural fluid culture was consistent with Streptococcus pneumoniae type I infection.
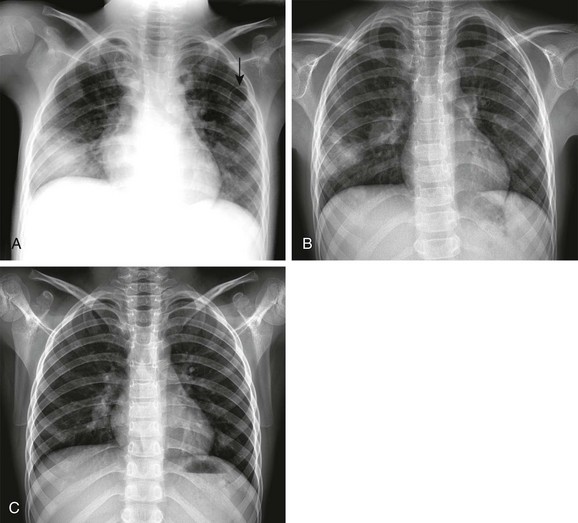
e-Figure 54-10 Necrotizing pneumococcal pneumonia in a 2-year-old boy who presented with cough and fever.
A, The initial radiograph shows lobar consolidation with expansion of the right upper zone. B, On follow-up radiograph in the first week, the consolidation is filled with air cavities, consistent with necrosis. C, Contrast-enhanced computed tomography demonstrates air bronchograms and pneumatoceles in the right upper lobe. Blood culture yielded Streptococcus pneumoniae.
Streptococcus Pyogenes (Group A Streptococci) and Agalactiae (Group B Streptococci)
Etiology: Group A streptococcus usually produces tonsillitis or pharyngitis. In the 1990s, S. pyogenes pneumonia, often associated with the toxic shock syndrome, was increasingly reported in childhood. It may occur de novo in a healthy child or follow a viral infection. In neonates, group B streptococcus is a leading cause of sepsis, including pneumonia and meningitis.
Imaging: Group A Streptococcus produces a bronchopneumonia in a segmental configuration with either homogeneous or patchy consolidation, which frequently affects a lower lobe. It may be bilateral. Pleural effusion and empyema are common in untreated cases. Lung abscess may be a complication. Clinically and on imaging studies, this pneumonia is very similar to staphylococcal pneumonia, although pneumatoceles are less commonly seen. Group B Streptococcus may accompany or mimic hyaline membrane disease in neonates, and is radiographically difficult to distinguish from it, although the presence of pleural effusion favors (concomitant) infection.
Staphylococcus aureus
Etiology: This gram-positive, catalase-positive coccus primarily affects infants under the age of 1 year (70%). In debilitated patients, it occurs as a superinfection, particularly in the hospital. The incidence of “primary” staphylococcal pneumonia has decreased markedly since the early 1950s. However, staphylococcal pneumonia secondary to septicemia rather than inhalation of organisms is increasing and occurs in older children. This form of “embolic” disease may present with multiple nodular masses or abscesses (e-Fig. 54-11). This evolving pulmonary pattern in a child with sepsis should initiate a search for a distant source of infection, often in bones, joints, or skin.60 Despite extensive pulmonary disease, if recovery occurs it is usually without sequelae. In comparison with methicillin-sensitive strains, methicillin-resistant strains of community-acquired S. aureus cause more serious pneumonias in younger children.61,62
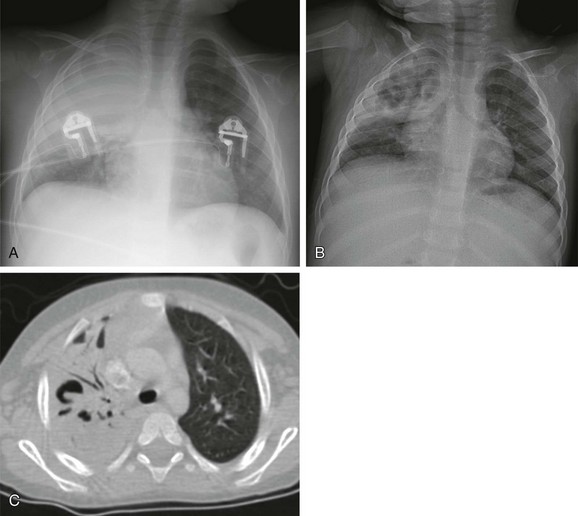
e-Figure 54-11 Embolic form of staphylococcal pneumonia in a 9-year-old boy who presented with a large abscess in the soft tissues of the pelvis.
A, The initial radiograph demonstrates a round consolidation in the right lower lung zone and multiple patchy and nodular opacities throughout the lungs. Pneumatoceles are present in both upper lung zones (arrow). B, The follow-up radiograph after 6 days shows marked resolution of the consolidations and pneumatoceles. C, The radiograph on the 30th day shows almost complete resolution of the lesions. Abscess and blood culture yielded Staphylococcus aureus.
Imaging: In contrast to pneumococcal pneumonia, staphylococcal pneumonia is a lobular or bronchopneumonia, which begins in the airways rather than in the alveoli. Consolidation of peribronchiolar acinar units occurs initially in a segmental distribution. This infectious agent is very virulent, and severe hemorrhagic pulmonary edema may develop rapidly. Pneumatoceles are more common than in any other type (also reported in S. pneumoniae, H. influenzae, and Escherichia coli pneumonia) and occur in 40% to 60% of patients.61,63,64 They usually appear during the first week of the pneumonia and resolve within 3 months. Pneumatoceles often appear as the child is getting better, and their presence does not have prognostic significance. Ten percent of children with staphylococcal pneumonia have a pneumothorax, which may result from the rupture of a pneumatocele.61,63,64 Pleural effusion and empyema are also very frequent, occurring in more than 90% of children (Fig. 54-12).64
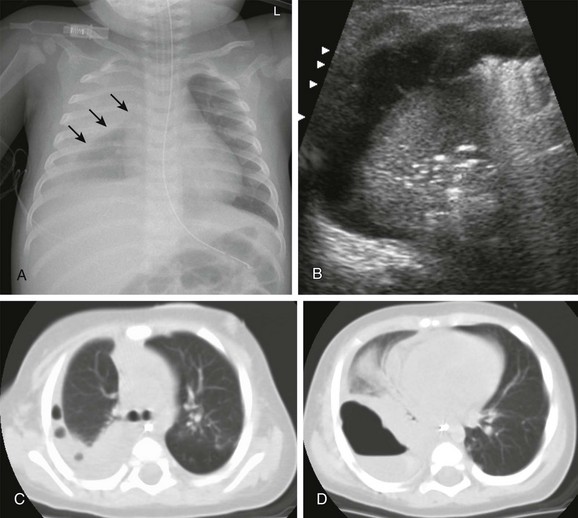
Figure 54-12 Role of cross-sectional imaging in a 7-month-old girl with staphylococcal pneumonia and empyema.
A, The radiograph demonstrates right pleural thickening, which has a convex margin toward the lung (arrows). B, The transverse ultrasound image of the right chest shows a complex pleural effusion, containing thin septations. C and D, Computed tomography confirmed the presence of loculated pleural fluid collections, containing trapped gas bubbles (C) and, more inferiorly, an air-fluid level (D). (Reprinted with permission granted by Springer-Verlag of Westra SJ, Choy G. What imaging should be performed for the diagnosis and management of pulmonary infections?. Pediatr Radiol. 2009;39[suppl 2]:S178-S183 [Figure 1, page S179]).
Haemophilus Influenzae Type B
Etiology: Haemophilus influenzae is a gram-negative, rod-shaped bacterium, which was first discovered in 1892 during an influenza pandemic. In infants and young children, it causes bacteremia, pneumonia, cellulitis, epiglottitis, and meningitis. Since 1990, vaccination has markedly reduced the incidence of this pneumonia (<95%).
Imaging: The radiographic appearance is nonspecific: pulmonary opacities that often begin as a segmental, interstitial-appearing process progresses to airspace consolidation.65 Approximately two thirds of cases have unilateral involvement, but more than one lobe is involved 25% of the time.65 Empyema is a common complication, occurring in about 40%.65
Bordetella pertussis
Etiology: Bordetella pertussis is a gram-negative, aerobic, capsulated coccobacillus. The incidence of pertussis (whooping cough) has decreased significantly with immunization, but it is still seen in young infants, particularly in the unimmunized. This agent is spread by airborne droplets. A characteristic clinical sign is the paroxysmal cough (whoop). Even after convalescence, the patient’s cough may persist for weeks or months. In China, the disease has been termed “the cough of 100 days.”
Imaging: Abnormal but nonspecific findings are present in most patients with pertussis. Because this is an airways-centered disease, it may mimic viral airways disease and pneumonia. The classic appearance is that of the “shaggy heart” (e-Fig. 54-13). However, nonspecific findings such as hyperaeration, atelectasis, segmental consolidations, and hilar lymphadenopathy are seen more commonly. Radiographic changes may persist for several weeks. Bronchiectasis may be a long-term complication.
Pseudomonas aeruginosa
Etiology: Pseudomonas aeruginosa is a gram-negative, aerobic, rod-shaped bacterium, which is an opportunistic pathogen in humans. Lungs, urinary tract, and kidneys are the common sites of infection. P. aeruginosa usually occurs as a nosocomial infection and is a major problem for patients with cystic fibrosis, in which thick layers of lung mucus and alginate produced by the bacteria may limit the diffusion of oxygen. Diagnosis of this infection depends on the Gram stain of sputum or other bacteriologic specimens (e.g., lung tissue, bronchoscopic aspiration, and bronchoalveolar lavage fluid).
Imaging: When the patient is infected by airway contamination, the process tends to involve both lung bases, with extensive bilateral parenchymal consolidation, patchy areas of disease with small abscess formation, or small regions of lobular emphysema. In the bacteremic form, widespread patchy or nodular shadows may be found throughout both lungs. Lung necrosis may also occur.
Anaerobic Organisms
Etiology: Anaerobic bacteria are uncommonly responsible for pneumonia and lung abscess.68 Aspiration is the most common route of exposure, and Fusobacterium species, Bacteroides species, Peptococcus, and Peptostreptococcus may be cultured from the abscess or the pleural fluid.
Imaging: Usually, consolidation occurs in the lower respiratory tract, and clinical course is slowly progressive (e-Fig. 54-14). Lung abscess (e-Fig. 54-15) and fulminant necrotizing pneumonia may eventually develop.
e-Figure 54-14 Anaerobic pulmonary infection in a 15-year-old boy who presented with chronic cough and chest pain without fever.
Frontal (A) and lateral (B) radiographs show a large opacity in the left lower thorax that obscures the diaphragm, consistent with consolidation with pleural fluid. Polymerase chain reaction on the drainage fluid from the pleural cavity was consistent with Fusobacterium infection.
e-Figure 54-15 Anaerobic pulmonary infection in a 12-year-old boy with Wiskott-Aldrich syndrome presenting with multiple cerebral abscesses, septicemia, cough, and fever.
A, The radiograph shows a thick-walled air cavity in the right lower lung zone. B, Contrast-enhanced computed tomography depicts the pulmonary abscess. There is also a pseudoaneurysm in the descending thoracic aorta. Polymerase chain reaction test of the cerebral abscess was consistent with Peptostreptococcus infection.
Bacteria-Like Organisms
Chlamydophila trachomatis and pneumoniae
Etiology: Chlamydia trachomatis is an intracellular bacterium commonly found in the genital tract, where it causes urethritis in men and cervicitis in women. In neonates, it causes conjunctivitis, which is contracted during passage through an infected birth canal. Chlamydia is a common cause of pneumonia in infants between 2 and 14 weeks of age.69 The infant is affected by a staccato-like cough and may have conjunctivitis and eosinophilia, although these are not invariable findings. Usually, the patient is afebrile, with a radiographic appearance suggesting an illness more severe than the clinical findings indicate. Recently, C. pneumoniae has been recognized as a common agent causing community-acquired bronchitis and mild atypical pneumonia in school-age children.3 The clinical response to appropriate therapy is usually rapid.
Imaging: The radiographic findings of C. trachomatis infections are nonspecific (e-Fig. 54-16), but when they are analyzed in conjunction with clinical findings, the disease may be suspected. Bilateral involvement is usual.70 The lungs are usually hyperaerated with increased linear density and patchy areas of consolidation, probably representing subsegmental atelectasis. Lobar consolidation is rare.70
Mycoplasma pneumoniae
Etiology: This organism is one of the most common causes of pulmonary infection in childhood, accounting for 10% to 30% of pediatric pneumonias.71 It is most often seen in school-age children and is uncommon in children less than 3 years of age. It is acquired by droplet inhalation, and it is most commonly noted in families, in schools, and among military recruits. The disease is usually mild, with low-grade malaise, headache, fever, and cough. Stevens-Johnson syndrome (erythema multiforme) may complicate the disease. The diagnosis is established by culturing the organisms from sputum or by demonstration of rising specific Mycoplasma titers. A rise in the titer of cold agglutinins is seen in about 50% of cases but is nonspecific; of greater diagnostic value are immunofluorescent and complement fixation tests for specific antibodies and the recently developed molecular probes.72
Imaging: This classic “atypical pneumonia” has a radiographic appearance that frequently mimics viral pulmonary infection (Fig. 54-17).1 In early cases, a fine reticular pattern suggestive of interstitial inflammation may be seen.73 This tends to be in a segmental distribution and in some cases progresses to airspace consolidation suggestive of bacterial pulmonary infection.74,75 Small pleural effusions are seen in up to 20% of patients. Hilar adenopathy is common. Bilateral involvement occurs in approximately one third of cases. The radiographic changes are frequently more severe compared with the patient’s clinical condition. Resolution is often slow and lags behind clinical improvement.
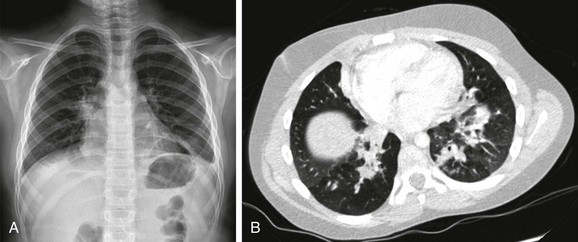
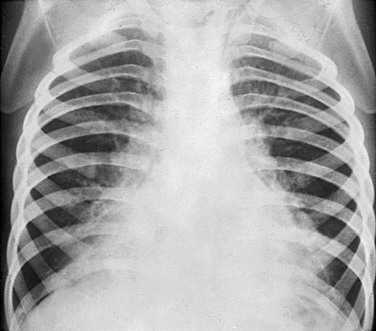
Figure 54-17 Mycoplasma atypical pneumonia in an 8-year-old girl who presented with fatigue, low-grade fever, and cough.
A, The chest radiograph shows irregularity of the right hilum and bibasillar infiltration. B, Contrast-enhanced computed tomography demonstrates bilateral bronchopneumonia in the lower lobes. A direct fluorescent antibody test from nasopharyngeal aspirate confirmed the diagnosis.
Treatment and Follow-up: Treatment of bacterial pulmonary infections is with antibiotics and other chemotherapeutic agents, focused on the causative agent, either presumed (empirical treatment) or preferably directed by the results of culture or immunoassay. Complications of bacterial pulmonary infections are lung necrosis, abscess, empyema, and bronchopleural fistula.2 Ultrasonography is the most effective initial cross-sectional modality when pleural complications are suspected, whereas CT is the preferred modality to diagnose parenchymal complications.76–82 A review of the diagnostic performance of CT versus radiography confirms the expected higher sensitivity of CT.78,83–90 CT is more sensitive than radiography to determine the cause of a delayed response to medical and percutaneous treatment of pediatric chest infections, but it is unclear whether this affects the eventual outcomes of treatment.78,89 Cavitary lung necrosis is demonstrated well by CT.90 However, most of the affected children recover without permanent sequelae on clinical and imaging follow-up.1 For this reason, documentation of complete resolution of pneumonia in children who are otherwise healthy is not indicated.1,22,91 CT and, potentially, magnetic resonance imaging (MRI) are helpful, however, to fully investigate any underlying focal anatomic cause such as anomalous bronchi, bronchogenic cysts, sequestrations, or bronchial atresia (Fig. 54-18), which may underlie recurrent or chronic pneumonia.1,92–94 Conversely, the value of CT to monitor diffuse processes that predispose to repeated pulmonary infections such as chronic granulomatous disease of childhood and cystic fibrosis remains controversial.95,96 Children with these chronic conditions will accumulate a substantial radiation burden from repeated examinations during their lifetime, and the theoretical risks of these procedures with regard to the induction of cancer will have to be balanced against the immediate clinical benefits, which remain unproven.97 MRI has promise as a nonradiating cross-sectional imaging alternative for examination of these conditions.92
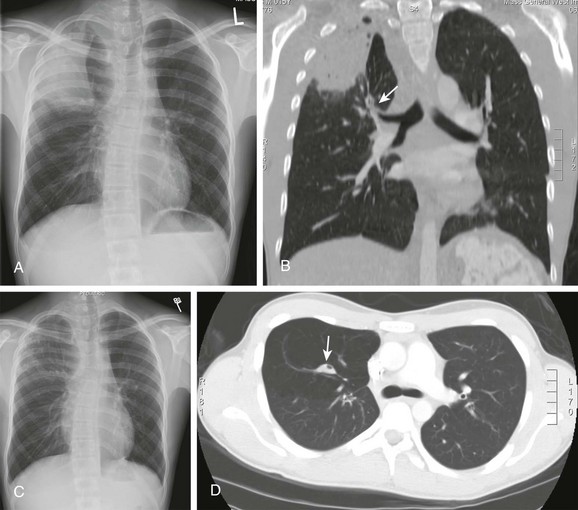
Figure 54-18 Role of computed tomography (CT) in recurrent pneumonia in a 15-year-old boy with bronchogenic cyst and bronchial atresia.
A, The chest radiograph shows consolidation in the right upper lobe and right upper mediastinal widening. B, The CT scan shows atresia in the apical bronchus of the right upper lobe (arrow) and right mediastinal widening caused by a bronchogenic cyst. C and D, The follow-up chest radiograph (C) and CT scan (D) show near-complete resolution of the pneumonia, with persistent architectural distortion of the right upper lobe. The atretic bronchus contains inspissated mucus (arrow in D). Right upper lobectomy and resection of the bronchogenic cyst was performed.
Mycobacterial Infection—Pulmonary Tuberculosis
Etiology: TB is caused by Mycobacterium tuberculosis, an aerobic, nonmotile, non–spore-forming rod. Although the resurgence of TB noted in the United States in the 1980s and 1990s has declined, in 2003, 15,000 new cases were reported, and the rate of decline appears to be slowing.98 The incidence continues to increase worldwide.99–101 Of all new cases of TB, 5% to 6% are in children less than 15 years of age, and approximately 60% of new childhood infections occur in children less than 5 years of age.99 A significant number of new cases occur in immunocompromised patients, particularly in those with HIV infection.102–104 The incidence of TB has decreased in the general population but has increased in the foreign-born population, accounting for 50% of new cases in the United States.
The length of the incubation period of TB depends on the size of the initial inhaled inoculum and varies from 2 to 10 weeks; it ends when the patient becomes sensitized (i.e., has a positive skin test). Annual skin testing in high-risk groups is recommended. In the low-risk child, skin testing at 12 to 15 months, at 4 to 6 years, and in adolescence is a reasonable approach.105 In asymptomatic primary tuberculosis, the disease is detected because of a routine skin test, and chest radiography is most often normal. In endemic areas, radiographs may demonstrate hilar lymphadenopathy only.106–108 Because children produce little sputum, they do not transmit the disease to one another, and a search for the infected adult must be undertaken. The index child is always treated with antibiotics. Following this treatment, no evidence suggests that the few children with a positive skin test and abnormal findings on chest radiography have a clinical course or prognosis different from that of the majority of children with a positive skin test and normal chest radiographs.
If radiographs are positive, usually, a single small primary focus is present (70%), located in the subpleural region. From there, the bacilli spread through the lymphatics to regional hilar and mediastinal lymph nodes (primary complex, e-Fig. 54-19). In postobstructive tuberculosis, large lymph nodes may cause extrinsic bronchial compression, and granuloma formation may cause endobronchial obstruction, leading to segmental air trapping, atelectasis (e-Fig. 54-20), or both. The presence of airway obstruction in a child who does not appear to be sick may spuriously suggest the possibility of an inhaled foreign body.109 For a few patients, bronchoscopy is required to diagnose the initial endobronchial lesion.110 Chronic obstruction may lead to bronchiectasis. Calcification occurs after caseation of the primary lesion and is seen earlier in infants (6 months after infection, e-Fig. 54-21) than in older children (2 to 3 years after infection, e-Fig. 54-22).
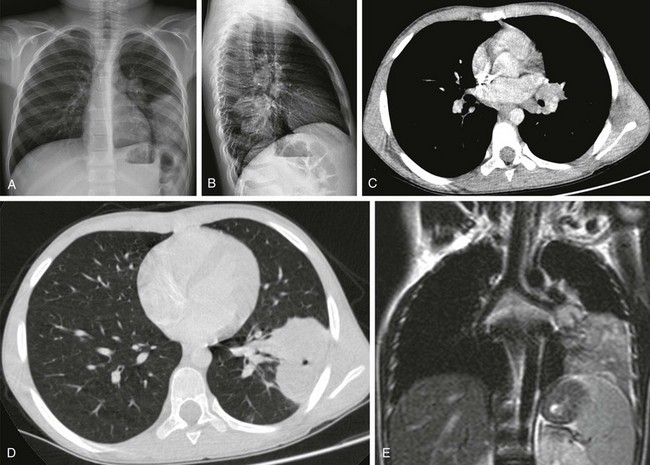
e-Figure 54-19 Primary tuberculosis (TB) in an 8-year-old boy who presented with cough, abdominal pain, and fatigue.
A and B, Radiographs show nodular consolidation in the left lower lung zone and an enlarged left hilum. C and D, Contrast-enhanced computed tomography demonstrates a peripheral consolidation in the left lower lobe, associated with enlarged left hilar lymph nodes. E, On coronal T2-weighted magnetic resonance imaging, the consolidation and lymph nodes exhibit high signal intensity. The parenchymal consolidation and associated ipsilateral lymph nodes constitute the primary Ghon complex in TB. Culture of bronchial lavage fluid confirmed the diagnosis.
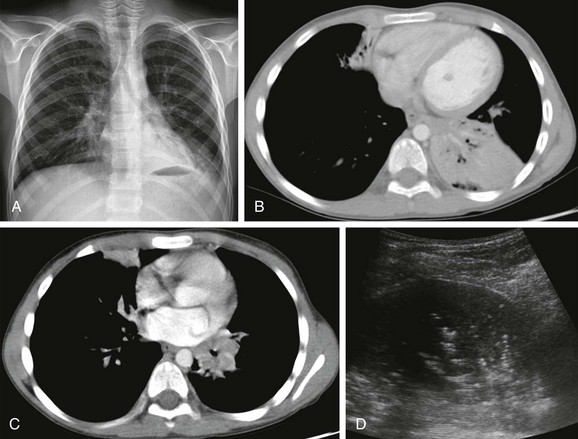
e-Figure 54-20 Consolidation with atelectasis from pulmonary tuberculosis (TB) in an 8-year-old boy with a history of recurrent lung infections presenting with cough.
A, The radiograph shows an enlarged left hilum and consolidation in the left retrocardiac area. B and C, Contrast-enhanced computed tomography demonstrates consolidation with volume loss in the left lower lobe and right middle lobe, with adenopathy in the left pulmonary hilum leading to bronchial compression. D, Ultrasonography scan shows sonographic air bronchograms within the consolidated left lower lobe. The diagnosis of TB was made on the basis of clinical and radiologic findings, although the organism could not be cultured. The patient responded well to anti-TB treatment.
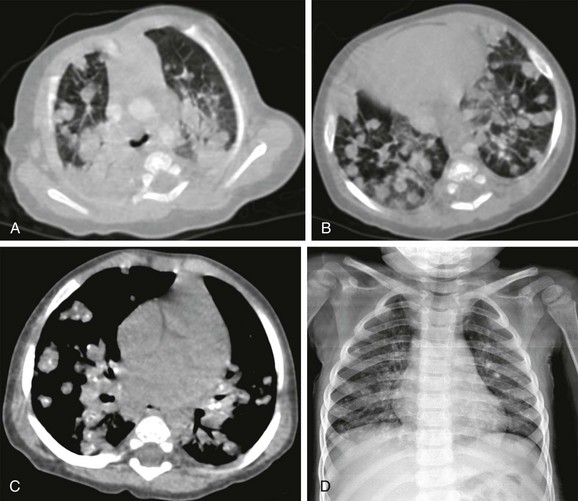
e-Figure 54-21 Disseminated tuberculosis (TB) in infant who presented with fever, cough, tachypnea, and intercostal retractions. Her father had TB.
A and B, Initial contrast-enhanced computed tomography shows multiple bilateral pulmonary nodules (granulomas). Enlarged lymph nodes are present in the mediastinum and hila. C, After 6 months of anti-TB treatment, the nodules and lymph nodes have decreased in number and size, and several show central calcification. D, On 2-year follow-up, the nodules and lymph nodes have resolved, leaving calcified foci in the lung, mediastinum, and hila. The diagnosis of TB was made on the basis of clinical and radiological findings; the microorganism could not be cultured.
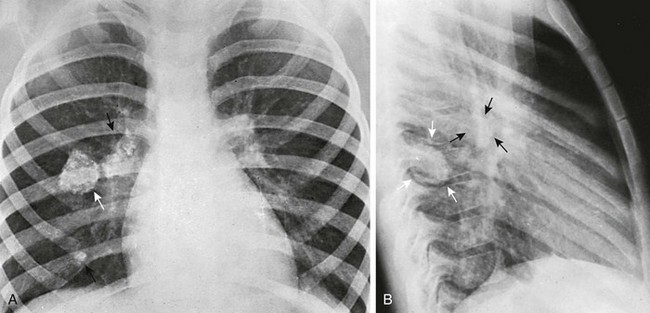
e-Figure 54-22 Calcified lesions of tuberculosis.
A, A large calcified primary tuberculous focus in the right lower lobe that dwarfs the rest of the complex in an asymptomatic 6-year-old girl. The healing and calcification of this huge necrotic focus (white arrow) without manifest clinical disease at any time shows the often benign clinical aspects of severe structural tuberculous disease. The black arrow shows calcified hilar adenopathy. In the frontal projection, the large calcifying focus appears to be perihilar. B, In the lateral projection, this focus lies posterior to right hilar nodes (black arrows) in the apical segment of the right lower lobe (white arrows) near the dorsal pleura.
Progressive primary pulmonary tuberculosis is a serious but rare complication.111 Progressive enlargement of the primary complex occurs, with caseation of the lesion followed by liquefaction (Fig. 54-23). The lesion may rupture into a bronchus, establishing new foci of disease (Fig. 54-24). Affected children are usually quite ill, with weight loss, dyspnea, anorexia, and failure to thrive. Pleural involvement in TB (e-Fig. 54-25) is usually noted in children older than 2 years, who present with fever, chest pain, and symptoms of pneumonia.112 The pleural fluid usually has few organisms, many white cells, a high protein content, and a low glucose content.
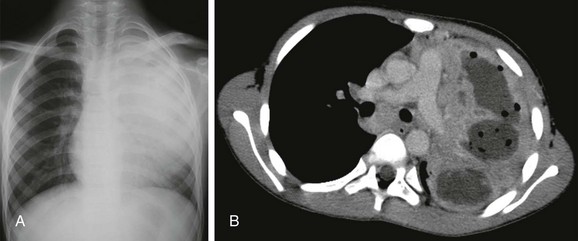
Figure 54-23 Progressive primary tuberculosis in an older child.
A, The radiograph shows dense consolidation with expansion in the left upper lobe, which contains an air-filled cavity. B, Contrast-enhanced computed tomography demonstrates extensive liquefactive parenchymal disease (Courtesy of Bernard Laya, M.D.).
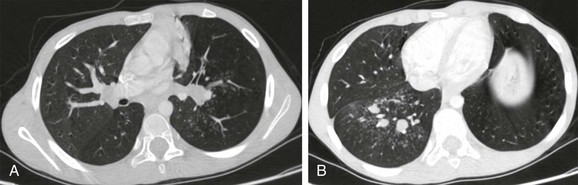
Figure 54-24 Active pulmonary tuberculosis in a 9-year-old boy who presented with cough and fever.
A and B, Contrast-enhanced computed tomography demonstrates centrilobular nodules and branching linear opacities (“tree-in-bud” pattern) in both lower lobes. Dilated fluid-filled bronchi are adjacent to areas of parenchymal abnormality. Atelectasis is also present in the paramediastinal parenchyma of the left lung. Culture of bronchial lavage fluid confirmed the diagnosis.
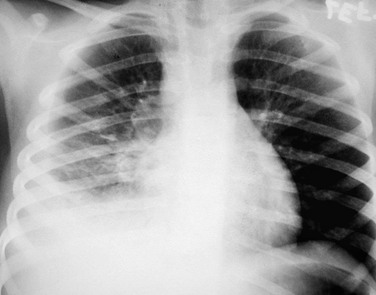
e-Figure 54-25 A 5-year-old girl who presented with a low-grade fever.
The frontal chest radiograph shows right pleural effusion and right hilar and peritracheal adenopathy. The tuberculin skin test was positive.
The host response to lymphohematogenous spread is varied, with immunosuppressed patients being at greatest risk. A few children present with high spiking fevers, hepatomegaly, and positive blood culture (chronic tuberculous bacteremia, e-Fig. 54-26). Miliary tuberculosis usually occurs within 6 months of the primary infection and results from lymphohematogenous dissemination of M. tuberculosis from the primary complex (e-Fig. 54-27).
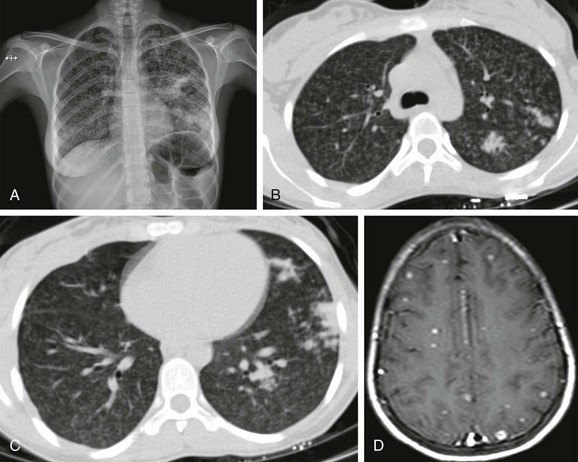
e-Figure 54-26 Progressive tuberculosis (TB) with tuberculous bacteremia in a 14-year-old girl, who presented with a convulsion while on treatment.
A, The radiograph shows multiple patchy nodules in both lungs and parenchymal consolidations in the left middle and lower lung zones. B and C, Contrast-enhanced computed tomography shows multiple nodules with a diameter of 1 to 3 mm throughout both lungs. D, Contrast-enhanced T1-weighted cranial magnetic resonance imaging depicts enhancing lesions consistent with TB granulomas. The diagnosis was made on the basis of clinical and radiological findings, although the organism could not be cultured. The patient responded well to tuberculostatic drug treatment.
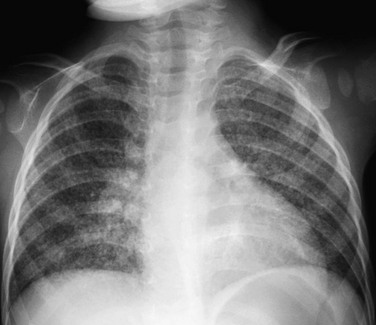
e-Figure 54-27 Miliary tuberculosis in a 6-month-old infant.
She had been adopted from Asia and had not been skin tested for tuberculosis (TB) as she had received Bacille Calmette-Guérin vaccination. The frontal radiograph reveals right hilar lymphadenopathy. A diffuse nodular pattern throughout the lungs is demonstrated. This child presented with dementia and was proven to have TB meningitis, a frequent consequence of miliary TB.
Reactivation or postprimary TB infection is the classic adult or adolescent form of the disease. It is the result of growth of previously dormant bacilli in the apices of the lung. The reactivated lesions in the apical and posterior segments of the upper lobes are composed of foci of caseous necrosis with surrounding edema, hemorrhage, and mononuclear cells. These lesions may liquefy and rupture into a bronchus, spreading the bacilli (e-Fig. 54-28). Cavitation with scarring occurs (Fig. 54-29). Reactivation of tuberculosis is rare in children who were infected with primary tuberculosis before the age of 2 years. It is much more frequent in children whose primary infection occurred after the age of 7 years, particularly if they were initially infected near puberty.98
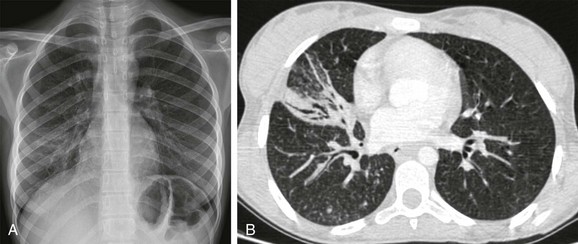
e-Figure 54-28 Postprimary tuberculosis (TB) with peribronchial spread in a 15-year-old girl who presented with a subcutaneous abscess in the neck, fever, productive cough, and elevated white blood cell count, erythrocyte sedimentation rate, and cross-reactive protein. She had a history of proven TB.
A, the chest radiograph shows right paracardiac pulmonary opacities. B, Contrast-enhanced computed tomography demonstrates consolidation the right middle lobe and diffuse centrilobular nodules and branching linear opacities (“tree-in-bud” pattern) in the right lung. Culture from the neck abscess and bronchial lavage fluid both yielded Mycobacterium tuberculosis.
Imaging: Primary TB infection of the lung falls under the subcategory of subacute dense focal (atypical) pneumonia (see e-Fig. 54-19).15,113–115 Chest radiography is insensitive to primary infection.116,117 In children with a positive skin test, screening with only a frontal radiograph is sufficient in nonendemic areas, whereas in endemic areas the addition of the lateral radiograph is beneficial for improved detection of hilar lymphadenopathy.106,118 Almost half (43%) of children diagnosed with tuberculous meningitis have normal chest radiographs.119 CT typically shows a subtle parenchymal opacity with associated lymph node enlargement. In a large study of children with TB, when initial chest radiography was positive, hilar and peritracheal lymphadenopathy was the most common finding, present in 92% of the cases.102,104 Studies using CT confirm that infected patients frequently have abnormal lymph nodes (see e-Figs. 54-19 through 54-21), but 50% may not have nodes larger than 1 cm.120 Affected nodes typically are large and of low density with rim enhancement and calcify later in the course of the disease. The adenopathy may be contralateral to the parenchymal disease in one third of patients. Chest radiography is most useful for following the course of disease in children with recent conversion to tuberculin sensitivity and for the early detection of miliary TB infection (see e-Fig. 54-27), which produces a “snowstorm” pattern in the lung, liver, and spleen.121 Occasionally, this may be present before there is overt clinical manifestation of the disease; as expected, CT is more sensitive than chest radiography.99,100 Regression of abnormalities is slow and may require from 6 months to 2 years to resolve on radiography and up to 15 months on CT evaluation (see e-Fig. 54-21 and Fig. 54-29).104,122
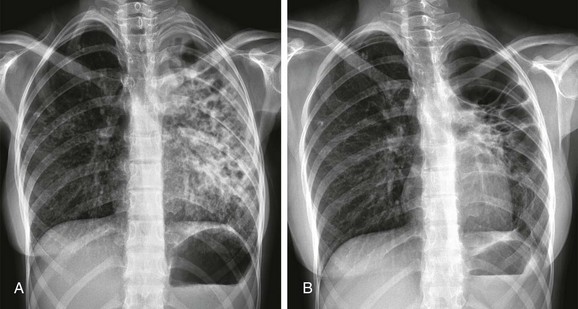
Figure 54-29 Postprimary tuberculosis (TB) with scarring and cavitation in a 13-year-old girl who presented with weight loss and productive cough, and whose mother had a history of TB.
A, The initial radiograph shows diffuse opacities in the left lung associated with bronchiectasis. Sputum culture indicated Mycobacterium tuberculosis infection. B, After 1 year of appropriate anti-TB therapy, the abnormalities had markedly improved, with extensive scarring in the left lung. The disease was no longer clinically active.
Treatment and Follow-up: TB is treated with a combination of antibiotics and chemotherapeutic agents to which the organism is sensitive.123 Multidrug resistance to the regimens is an increasing problem worldwide, but strategies for effective treatment and reinforcing patient adherence have proven successful, even in the developing world.123 Because of the limited sensitivity of radiography, cross-sectional imaging with CT, when locally available, is frequently helpful for patient management (see e-Figs. 54-19 and 21, Figs 54-23, and 54-24; and e-Figs. 54-26 and 54-28).100,103,110–112,121
Nontuberculous Mycobacterial Pulmonary Infection
Etiology: Nontuberculous mycobacteria (NTM) are widely distributed in the environment and may produce infection in patients with normal immunity, although infection is significantly more frequent in patients with immunodeficiencies (particularly HIV infection) or with altered local defenses (e.g., in cystic fibrosis or ciliary dyskinesia). Although the signs and symptoms of NTM pulmonary infection are variable and nonspecific, affected pediatric patients usually present with chronic cough, fever, chills, night sweats, dyspnea on exertion, and weight loss. The diagnosis is based on clinical, radiographic, and bacteriologic criteria.
Imaging: Although the radiologic findings of pulmonary NTM disease are variable, depending on the presence or absence of underlying disease, typical findings include multiple nodules, consolidation, and cavitation. Mediastinal or hilar adenopathy similar to that of TB may be present (e-Fig. 54-30). NTM may also complicate or cause bronchiectasis.
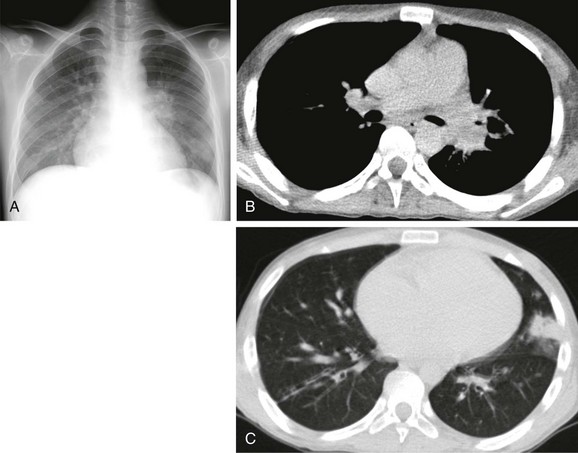
e-Figure 54-30 Nontuberculous mycobacterial infection in a 15-year-old boy with aplastic anemia, who was hospitalized for bone marrow transplantation.
A, The radiograph shows consolidation in the left lower zone and left hilar lymphadenopathy. B and C, Noncontrast computed tomography demonstrates consolidation in the inferior lingular segment and lymphadenopathy in the left hilum. Culture of fluid from the pericardial cavity was consistent with nontuberculous mycobacterial infection.
Pulmonary Infections Caused by Fungi
Fungal infections of the lung predominantly affect children with a suppressed immune system, mainly those with defective T-cell immunity and granulocyte function, either congenital (DiGeorge syndrome, chronic granulomatous disease of childhood) or acquired (AIDS, those undergoing chemotherapy for malignancies). In immunocompromised children, CT may add both sensitivity and specificity to radiography and should be performed with a low threshold.84,88 The imaging appearance of fungal infections is typically within the miliary or nodular pneumonia category.15 The nodules may be surrounded in the acute phase by a ground-glass halo, indicating the angioinvasiveness of the organism, and may eventually calcify.2,15 Hilar and mediastinal lymphadenopathy is frequently seen, and the lymph nodes often calcify, as in TB. The most common fungal agents causing pulmonary infections in children are listed in Table 54-2.
Aspergillosis
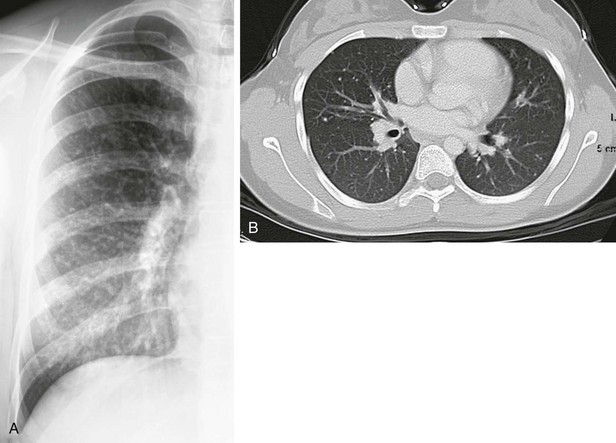
Etiology: Aspergillus species are a group of ubiquitous molds within the environment that may be cultured from a wide variety of soils, water sources, and decaying organic matter. Pulmonary infection is most often caused by Aspergillus fumigatus. Although pulmonary infection with Aspergillus species may take a variety of clinical courses and imaging appearances mainly depend on the affected patients’ immune status, it can be grouped into three main categories: (1) fungal ball, (2) allergic bronchopulmonary aspergillosis (ABPA), and (3) invasive aspergillosis.124 A fungal ball or mycetoma typically grows saprophytically in immunocompetent children with a preexisting cavity often related to cystic fibrosis or postprimary TB. ABPA, characterized by a hypersensitivity response to Aspergillus antigens, typically develops in children with cystic fibrosis or asthma.125 Invasive aspergillosis almost exclusively occurs in immunocompromised children with underlying neutropenia related to bone marrow or solid organ transplantations and others receiving systemic chemotherapy.124 The organism may produce a bronchocentric or angiocentric lesion and is associated with a high morbidity and mortality.126 A semi-invasive, less aggressive form of Aspergillus infection may be seen in patients with underlying lung disease or in mildly immunocompromised patients.127
Imaging: A fungal ball typically appears on chest radiographs as an intracavitary mass within a thick-walled cavity usually located in an upper lobe, especially the apical segment (e-Fig. 54-31). CT shows a round or oval-shaped cavity containing a freely mobile soft tissue mass surrounded by a crescent of air (Monad sign).128 The most common features of ABPA are mucoid impaction within bronchiectasis and segmental and lobar atelectasis. Such dilated bronchi filled with inspissated mucus often cause a homogeneous branching opacity referred to as the finger-in-glove sign. Invasive aspergillosis characteristically appears as one or a few nodular opacities randomly distributed throughout the lungs. Distinctive but nonspecific characteristics include the halo sign and the air crescent sign. The halo sign refers to an irregular ground-glass opacity surrounding a nodule of infection (Fig. 54-32). The indistinct halo probably represents hemorrhage in lung adjacent to the nodule, resulting from vascular invasion. The air crescent sign is an air collection that partially outlines a masslike lesion that represents a contracting nodule.128 This sign is noted in well-established Aspergillus infections when the host’s defense mechanisms have partially recovered.
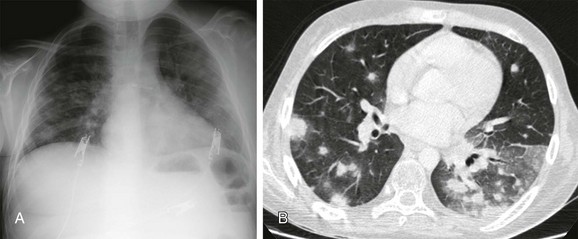
Figure 54-32 Invasive pulmonary aspergillosis in a 14-year-old boy with leukemia, who presented with neutropenia, fever, and a positive serum galactomannan enzyme-linked immunosorbent assay test.
A, The radiograph shows bilateral ill-defined nodular opacities. B, Contrast-enhanced computed tomography demonstrates multiple nodules with, with ground-glass opacity around some of these nodules (the “halo” sign). The diagnosis of invasive pulmonary aspergillosis was made based on the clinical, laboratory, and radiologic findings.
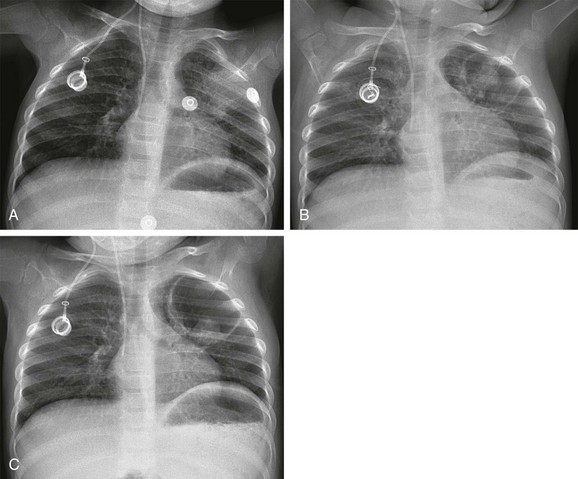
e-Figure 54-31 Invasive pulmonary aspergillosis in a 30-month-old girl with acute leukemia who presented with neutropenia and fever.
A, The initial radiograph shows a round consolidation in the left upper lung zone. B, The follow-up radiograph on the day 9 demonstrates cavitation of the consolidation, with an eccentric “fungus ball” attached to the left cavity wall. C, The radiograph on day 17 shows expansion in the cavity and downward displacement of the “fungus ball,” indicative of its free movement.
Histoplasmosis
Etiology: Histoplasmosis, caused by Histoplasma capsulatum, is common in many parts of the United States. Initial infection is similar to that of the primary tuberculous complex except that in histoplasmosis, large numbers of pulmonary foci are the rule. Histoplasmosis may be categorized into three phases: (1) acute infection, characterized by nonspecific respiratory symptoms that usually develop 12 to 14 days after initial exposure; (2) chronic infection, which may resemble TB; and (3) disseminated infection. The diagnosis of pulmonary histoplasmosis rests on the identification of antigen and the demonstration of Histoplasma-specific antibodies. Rapid diagnosis may be obtained from biopsy of the granulomas with special fungal stains. Histoplasma may be cultured from lavage or blood specimens, but cultures take a long time to grow and have a low sensitivity.
Imaging: Radiographic findings are similar to TB in all phases of these diseases, and in some cases, they resemble those in coccidioidomycosis (e-Fig. 54-33).129 Approximately 95% of patients are asymptomatic during the initial infection, and thus the initial exudative phase of the disease is much less obvious radiographically compared with the sequelae of infection.129 The first phase of the infection is characterized by single or multiple pulmonary nodules that are 1 to 3 cm in size, as well as mediastinal and hilar lymphadenopathy. Pulmonary nodules that have been present for more than a few months usually show central or dystrophic calcification. Calcified mediastinal and hilar lymph nodes are often seen in patients with calcified pulmonary nodules (e-Fig. 54-34). Sarcoidosis may exhibit similar adenopathy. Because treatment of sarcoidosis involves immunosuppression, histoplasmosis must be excluded, particularly in endemic areas. In children with chronic histoplasmosis, upper lobe consolidations simulating TB infection, sometimes associated with cavitation, may be present. Disseminated histoplasmosis has been observed in infancy, and the imaging findings are similar to those in miliary tuberculosis. Massive hepatosplenomegaly is common in disseminated cases. Granulomatous or calcified foci in the spleen are a frequent incidental finding in endemic regions and are almost diagnostic of disease exposure in an otherwise healthy patient. A rare but devastating result of chronic pulmonary histoplasmosis is mediastinal fibrosis, which may lead to pulmonary artery compression (see e-Fig. 54-33) and terminal pulmonary artery hypertension. It may also result in compression of the superior vena cava, esophagus, trachea, and bronchi.
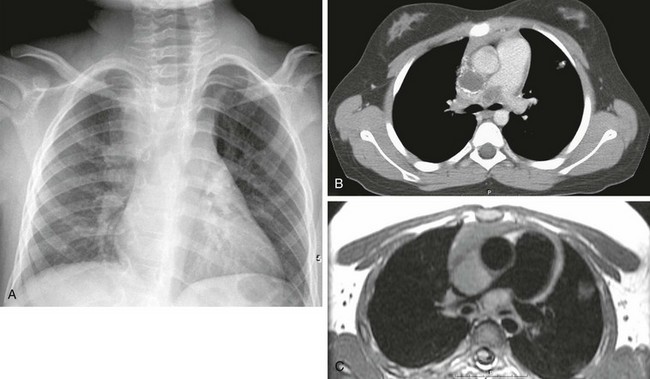
e-Figure 54-33 Histoplasmosis in a 9-year-old girl, who presented with shortness of breath.
A, The radiograph shows mediastinal adenopathy, particularly in the right paratracheal and right hilar regions. Note the shift of the trachea to the left side. B, Computed tomography shows a small calcified nodule in the left upper lobe with extensive adenopathy. The mediastinal nodes are centrally low in density, as would be seen in tuberculosis, and are calcifying peripherally. C, A gated magnetic resonance image shows the marked compression of the right pulmonary artery.
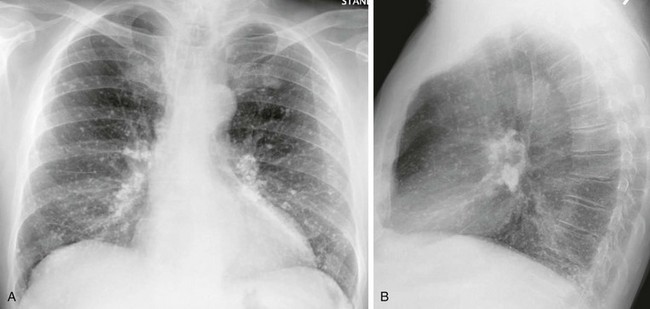
e-Figure 54-34 The lesions in this patient with disseminated healed histoplasmosis were incidental and seen on a radiograph obtained for chest pain.
A, Note the diffuse calcified granulomata, which is not the typical pattern. Most patients will have only a few granulomata. B, On the lateral radiograph, note the densely calcified lymph nodes, as might be found with tuberculosis.
Coccidioidomycosis
Etiology: Coccidioidomycosis, caused by Coccidioides immitis or C. posadasi, is acquired by inhalation. Endemic areas in the United States include the semi-arid regions of California, Arizona, New Mexico, and western Texas. The infection is usually self-limiting, and affected children present with mild flulike illness, with fever, cough, headaches, rash, and myalgia. However, severe pulmonary infection may develop in immunocompromised children (particularly those with HIV infection). The diagnosis may be made by positive reaction to the coccidioidin skin test or by positive culture of the sputum or gastric washings.
Imaging: Radiographic examination during the early stages of disease usually shows lobar or segmental airspace consolidation and enlargement of the regional lymph nodes.130 The pulmonary opacity of primary coccidioidomycosis is similar to that in primary tuberculosis (e-Fig. 54-35). It usually clears after a few weeks—its duration is usually much shorter than in tuberculosis—and residual calcified foci appear later within both the lung and lymph nodes. Small localized or large free pleural effusions may be visualized early.131 Although small, sharply defined, thin-walled pulmonary cavities are commonly noted in adults, they are uncommon in children.
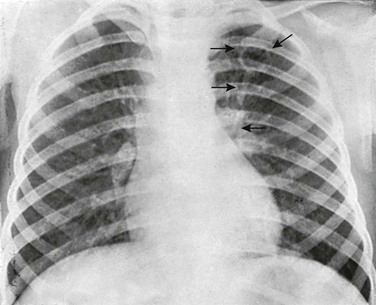
e-Figure 54-35 Coccidioidomycosis of the lung.
A primary complex in a 4-year-old girl whose skin reaction was positive to coccidioidin. The primary pulmonary focus is excavated into a thin-walled cavity—one of the most distinctive radiologic changes in this disease (upper arrows). The strands of increased density between the pulmonary focus and the hilar nodes represent lymphangitis (lower arrows). The regional hilar nodes are enlarged. (Courtesy Dr. Hugh C. Thompson, Tucson, AZ.).
Pneumocystis jiroveci
Etiology: Pneumocystis carinii, a unicellular organism, that infects humans, was recently renamed Pneumocystis jiroveci. Originally considered a parasite, it is currently categorized as a fungus on the basis of molecular similarities to fungal RNA. This potentially life-threatening opportunistic infection most often affects immunodeficient children, particularly those who are infected with HIV and have CD4 counts of less than 100 cells/mm3. The clinical onset is variable but often abrupt, with tachypnea, cough, and cyanosis. A marked decrease in the arterial oxygen saturation is seen, with a normal carbon dioxide level.
Imaging: The characteristic radiographic findings of P. jiroveci pneumonia are symmetric bilateral ground-glass opacities (Fig. 54-36). Such opacities may be diffuse but tend to involve predominately the perihilar regions or middle and lower lung zones.132,133 Disease progression may result in more confluent, mainly perihilar or diffuse, bilateral airspace consolidation. The underlying pathophysiologic process is alveolar filling by a foamy exudate consisting of surfactant, fibrin, and cellular debris.134–137 Associated interstitial edema or cellular infiltration may present on CT as septal thickening, intralobular thickening, or both.138 The combination of such interstitial thickening and ground-glass opacities may produce a CT pattern known as “crazy paving”.139 Terminally, massive consolidation may occur, often complicated by pneumothorax and pneumomediastinum.140 Pleural effusions are rare.141
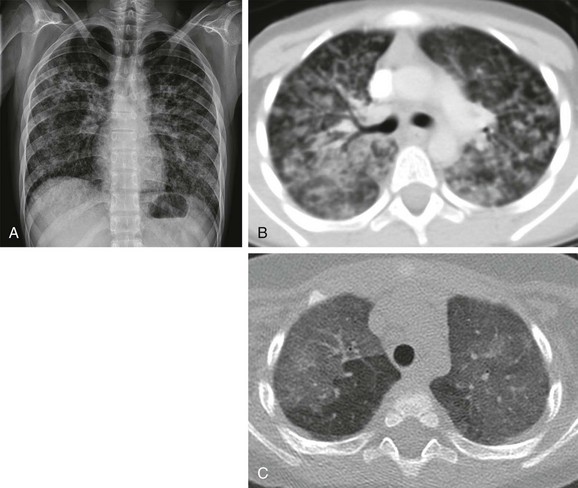
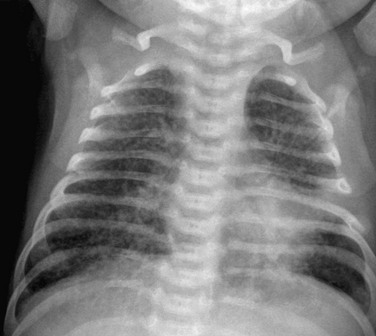
Figure 54-36 Pneumocystis jiroveci infection in a 17-year-old boy with acute lymphoblastic leukemia and immunodeficiency, who presented with dyspnea, fever, nonproductive cough, and decreased white blood cell counts.
A, The radiograph shows diffuse bilateral interstitial opacity throughout the lungs. B, Contrast-enhanced computed tomography (CT) confirms the bilateral patchy and ground-glass opacities in both lungs. The diagnosis was confirmed by a positive polymerase chain reaction test from bronchial lavage fluid. C, CT in a different patient demonstrates a typical “crazy paving” pattern in both upper lobes.
In 10% to 30% of patient with AIDS who received prophylaxis with aerosolized pentamidine and trimethoprim-sulfamethoxazole, a cystic form of the disease may evolve, characterized by either unilateral or bilateral upper lobe predominant thin-walled cysts.138 Such pulmonary cysts are associated with pneumothoraces.142 In recent years, in spite of the advances in the prevention and treatment of P. jiroveci infection, several atypical presentations have emerged, including multiple pulmonary nodules, mass lesions, pleural effusion, and lymph node enlargement.143–146
Treatment and Follow-up: Although imaging findings are not specific for P. jiroveci infection, the presence of bilateral ground-glass opacities in immunocompromised children should lead to this diagnosis.147 Tracheal washings or lung biopsy, when performed, may show the organisms on microscopic examination after silver methenamine staining.
Candidiasis
Etiology: Pulmonary candidiasis is almost always encountered in immunocompromised children, those with indwelling catheters, those who are undergoing prolonged antibiotic therapy, or those who are on steroids. Candida albicans is the responsible organism in the majority of candidal infections.148 The organism regularly colonizes the upper respiratory tract and then spreads to the lungs. Pulmonary candidiasis may be either a primary infection (limited to the lungs) or a secondary infection (caused by hematogenous dissemination from other sites of infection).
Imaging: Although radiographs are often normal in the earliest stages of infection, nonspecific features of bronchopneumonia and lung nodules may be noted (e-Fig. 54-37).148,149 Those nodules may cavitate, and unilateral or bilateral lobar or segmental opacities may also be seen.
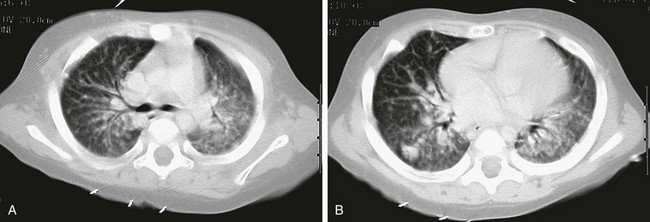
e-Figure 54-37 Candidiasis in a 10-month-old with acute myelogenous leukemia and fever and hazy opacities in left lung on chest radiograph (not shown).
A and B, Helical computed tomography (CT) sections at the middle (A) and lower (B) zones show a bilateral pattern of ill-defined nodules and more confluent opacity in the left lung posteriorly.
Pulmonary Manifestations of Parasitic Infestations
Pulmonary manifestations of parasitic infestations are predominantly encountered in the developing world, in those who have recently traveled to or from these regions, or in immunocompromised patients. A variety of imaging findings are seen, predominantly in the multinodular pneumonia category, as in paragonimiasis and echinococcus (hydatid) disease. However, several types of parasitic infestation may produce clinical and radiographic signs as a consequence of the passage of larval forms through the lung in the course of the parasite’s life cycle. These include Ascaris lumbricoides, Necator americanus, Ancylostoma duodenale (hookworms), Strongyloides stercoralis, and Toxocara cati and canis.150 T. cati and T. canis may produce a localized granulomatous reaction, termed visceral larva migrans, with resultant opacities in the lungs. The first four mentioned species as well as a number of rarer parasites may be associated with Löffler syndrome (acute allergic eosinophilic pneumonia).2 The most common parasitic agents with pulmonary involvement are listed in Table 54-2.
Pulmonary Paragonimiasis
Etiology: The lung fluke Paragonimus westermani is endemic in parts of Southeast Asia, South America, and Western Africa. In the United States, pulmonary paragonimiasis has affected refugee Indochinese children and Latin-American immigrants.151,152 Children are usually infected after ingestion of undercooked crustaceans, including crabs or crayfish, or by drinking contaminated water. In Southeast Asia, infestation of the lungs by P. westermani is said to be a common cause of pediatric lung necrosis and calcification, but it is difficult to differentiate from TB and fungal diseases on the basis of radiographic criteria alone (e-Fig. 54-38). Affected children usually present with fever, pleuritic chest pain, and respiratory symptoms such as chronic cough or hemoptysis. The diagnosis depends on a combination of findings, including history, peripheral blood eosinophilia, identification of ova in the stool or the sputum, and a positive ELISA (enzyme-linked immunosorbent assay) test result.
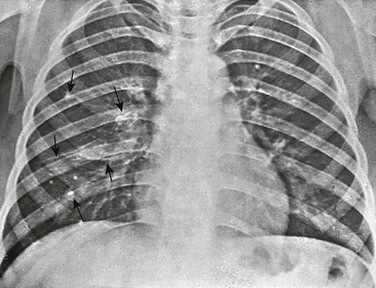
e-Figure 54-38 Calcifying paragonimiasis in the lungs of a 12-year-old Korean boy, whose sputum had contained Paragonimus westermani for 4 years.
The multiple calciferous foci in the right lung and hilum (arrows) are identical to the findings in some cases of primary tuberculosis. The clinician found radiographic signs of calcifying paragonimiasis in 60 of 1000 Korean refugee children examined by him in Poland. (Courtesy Dr. Roman Marciniak, Warsaw, Poland.)
Imaging: Imaging findings depend on the stage of the disease. The early stage is characterized by (1) linear opacities, which are 2 to 4 mm thick and 3 to 7 cm long, extending inward from the pleura representing the migration of juvenile worms; (2) focal airspace consolidation, representing exudative or hemorrhagic pneumonia caused by the migrating worm; (3) pneumothorax; or (4) hydropneumothorax. Features of the late stage of infection include nodules, thin-walled crescent-shaped worm cysts (the “signet ring” sign), dense masslike consolidation, or bronchiectasis. As the flukes move from the abdomen to the chest, they penetrate the diaphragm and pleural layers, and thus pleural effusions and thickening are commonly noted.
Echinococcus Disease of the Lung
Etiology: Echinococcosis or hydatid disease in humans is caused by infestation with Echinococcus granulosus (dog tapeworm). Humans are infected by direct contact with definite hosts (e.g., dogs) or by ingestion of eggs present in infected water, food, or soil. The eggs of E. granulosus hatch into larvae in the duodenum and are typically trapped within the liver (~75%) or lungs (~5% to 15%). They pass through the portal system in the liver or pulmonary alveolar capillaries, respectively. These larvae eventually develop into round or oval-shaped cysts within the liver or lungs (e-Fig. 54-39). Although most children with pulmonary involvement by hydatid disease are asymptomatic, they may present with fever, shortness of breath, cough, chest pain, which is usually a sign of cyst rupture. Diagnosis of hydatid disease depends on the combination of imaging and serology.
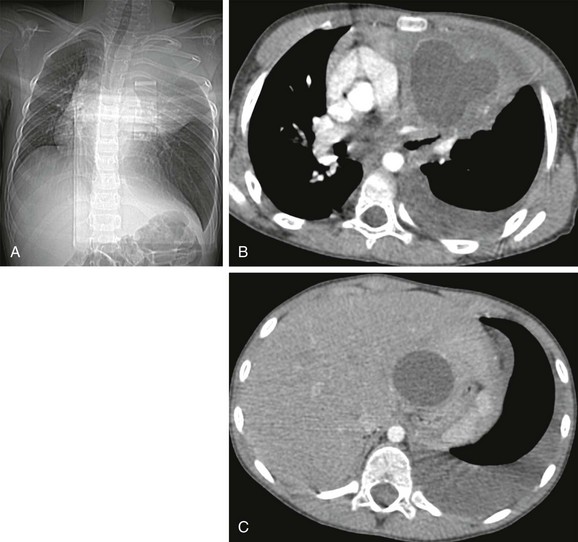
e-Figure 54-39 Hydatid disease in a 7-year-old boy, who presented with left-sided chest pain, fever, and dyspnea.
A, The computed tomography (CT) scanogram shows a large opacity in the left upper lobe that displaces the trachea to the right. B and C, Contrast-enhanced CT demonstrates a round fluid-filled mass with a hyperdense wall in the left apex, as well as a cyst in the left liver lobe. Histopathologic diagnosis after surgical resection was consistent with hydatid infection.
Imaging: The radiologic findings are characterized by single or multiple (~25%), round or oval-shaped, cystic nodules or masses (1-20 cm in diameter) with well-defined walls, surrounded by normal lung parenchyma (see e-Fig. 54-39). Other findings include an air-crescent sign, when a cyst communicates with a bronchus, or the water-lily sign (Fig. 54-40), when a cyst membrane floats in residual fluid after cyst rupture.140,141 Pericystic emphysema may be a sign of impending rupture. After rupture of the cysts, cavitation or abscess formation and bronchiectasis may occur.
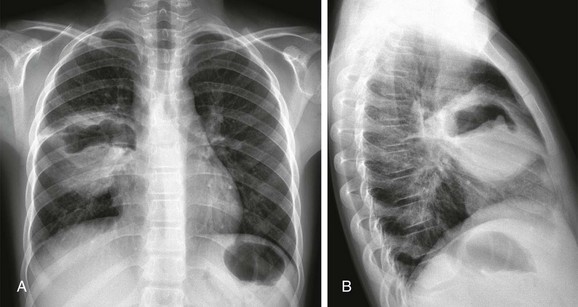
Figure 54-40 Hydatid disease in a 12-year-old boy, who presented with cough.
A and B, Radiographs show a cavitary lesion with an irregular air-fluid level in the right middle lobe. The floating germinative membranes account for the classic “water lily” sign. Histopathologic diagnosis after surgical resection was consistent with hydatid infection.
Treatment and Follow-up: Traditionally, surgical removal of the pulmonary cysts, often with supplemental medication such as benzimidazole, has been performed. Despite the risk of anaphylactic reactions following cyst aspiration, careful image-guided percutaneous techniques appear to be sufficiently safe.153
Bradley, JS, Byington, CL, Shah, SS, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: Clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011;53(7):e25–e76.
Daltro, P, Santos, EN, Gasparetto, TD, et al. Pulmonary infections. Pediatr Radiol. 2011;41(Suppl 1):S69–S82.
Eslamy, HK, Newman, B. Pneumonia in normal and immunocompromised children: An overview and update. Radiol Clin North Am. 2011;49(5):895–920.
McIntosh, K. Community-acquired pneumonia in children. N Engl J Med. 2002;346:429–437.
Ventre, KM, Wolf, GK, Arnold, JH. Pediatric respiratory diseases: 2011 update for the Rogers’ textbook of pediatric intensive care. Pediatr Crit Care Med. 2011;12(3):325–338.
Westra, SJ, Choy, G. What imaging should we perform for the diagnosis and management of pulmonary infections? Pediatr Radiol. 2009;39(suppl 2):S178–S183.
References
1. Donnelly, LF. Practical issues concerning imaging of pulmonary infection in children. J Thorac Imaging. 2001;16:238–250.
2. Daltro, P, Santos, EN, Gasparetto, TD, et al. Pulmonary infections. Pediatr Radiol. 2011;41(suppl 1):S69–S82.
3. McIntosh, K. Community-acquired pneumonia in children. N Engl J Med. 2002;346:429–437.
4. Selwyn, BJ. The epidemiology of acute respiratory tract infection in young children: Comparison of findings from several developing countries. Coordinated Data Group of BOSTID Researchers. Rev Infect Dis. 1990;12(suppl 8):S870–S888.
5. Rudan, I, Boschi-Pinto, C, Biloglav, Z, et al. Epidemiology and etiology of childhood pneumonia. Bull World Health Organ. 2008;86:408–416.
6. Grossman, LK, Caplan, SE. Clinical, laboratory, and radiological information in the diagnosis of pneumonia in children. Ann Emerg Med. 1988;17:43–46.
7. Cherian, T, Mulholland, EK, Carlin, JB, et al. Standardized interpretation of paediatric chest radiographs for the diagnosis of pneumonia in epidemiological studies. Bull World Health Organ. 2005;83:353–359.
8. Lynch, T, Gouin, S, Larson, C, et al. Should the lateral chest radiograph be routine in the diagnosis of pneumonia in children? A review of the literature. Paediatr Child Health. 2003;8:566–568.
9. Manson, D. The lateral chest radiograph. Paediatr Child Health. 2003;8:564–565.
10. Swingler, GH, Hussey, GD, Zwarenstein, M. Randomised controlled trial of clinical outcome after chest radiograph in ambulatory acute lower-respiratory infection in children. Lancet. 1998;351:404–408.
11. Swingler, GH, Zwarenstein, M. Chest radiograph in acute respiratory infections. Cochrane Database Syst Rev. 2008. [CD001268].
12. Mathews, B, Shah, S, Cleveland, RH, et al. Clinical predictors of pneumonia among children with wheezing. Pediatrics. 2009;124:e29–e36.
13. Roback, MG, Dreitlein, DA. Chest radiograph in the evaluation of first time wheezing episodes: review of current clinical practice and efficacy. Pediatr Emerg Care. 1998;14:181–184.
14. Alario, AJ, McCarthy, PL, Markowitz, R, et al. Usefulness of chest radiographs in children with acute lower respiratory tract disease. J Pediatr. 1987;111:187–193.
15. Eslamy, HK, Newman, B. Pneumonia in normal and immunocompromised children: an overview and update. Radiol Clin North Am. 2011;49:895–920.
16. Korppi, M, Don, M, Valent, F, et al. The value of clinical features in differentiating between viral, pneumococcal and atypical bacterial pneumonia in children. Acta Paediatr. 2008;97:943–947.
17. Korppi, M, Kiekara, O, Heiskanen-Kosma, T, et al. Comparison of radiological findings and microbial aetiology of childhood pneumonia. Acta Paediatr. 1993;82:360–363.
18. Virkki, R, Juven, T, Rikalainen, H, et al. Differentiation of bacterial and viral pneumonia in children. Thorax. 2002;57:438–441.
19. Wahlgren, H, Mortensson, W, Eriksson, M, et al. Radiographic patterns and viral studies in childhood pneumonia at various ages. Pediatr Radiol. 1995;25:627–630.
20. Wahlgren, H, Mortensson, W, Eriksson, M, et al. Radiological findings in children with acute pneumonia: age more important than infectious agent. Acta Radiol. 2005;46:431–436.
21. Bettenay, FA, de Campo, JF, McCrossin, DB. Differentiating bacterial from viral pneumonias in children. Pediatr Radiol. 1988;18:453–454.
22. Bradley, JS, Byington, CL, Shah, SS, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011;53:e25–e76.
23. Miller, WT, Jr., Mickus, TJ, Barbosa, E, Jr., et al. CT of viral lower respiratory tract infections in adults: Comparison among viral organisms and between viral and bacterial infections. AJR Am J Roentgenol. 2011;197:1088–1095.
24. Bloomfield, FH, Teele, RL, Voss, M, et al. Inter- and intra-observer variability in the assessment of atelectasis and consolidation in neonatal chest radiographs. Pediatr Radiol. 1999;29:459–462.
25. Johnson, J, Kline, JA. Intraobserver and interobserver agreement of the interpretation of pediatric chest radiographs. Emerg Radiol. 2010;17:285–290.
26. Stickler, GB, Hoffman, AD, Taylor, WF. Problems in the clinical and roentgenographic diagnosis of pneumonia in young children. Clin Pediatr (Phila). 1984;23:398–399.
27. Davies, HD, Wang, EE, Manson, D, et al. Reliability of the chest radiograph in the diagnosis of lower respiratory infections in young children. Pediatr Infect Dis J. 1996;15:600–604.
28. Spottswood, SE, Liaw, K, Hernanz-Schulman, M, et al. The clinical impact of the radiology report in wheezing and nonwheezing febrile children: a survey of clinicians. Pediatr Radiol. 2009;39:348–353.
29. Shiley, KT, Lautenbach, E, Lee, I. The use of antimicrobial agents after diagnosis of viral respiratory tract infections in hospitalized adults: antibiotics or anxiolytics? Infect Control Hosp Epidemiol. 2010;31:1177–1183.
30. Han, BK, Son, JA, Yoon, HK, et al. Epidemic adenoviral lower respiratory tract infection in pediatric patients: radiographic and clinical characteristics. AJR Am J Roentgenol. 1998;170:1077–1080.
31. Osborne, D, White, P. Radiology of epidemic adenovirus 21 infection of the lower respiratory tract in infants and young children. AJR Am J Roentgenol. 1979;133:397–400.
32. Welliver, RC, Sr., Checchia, PA, Bauman, JH, et al. Fatality rates in published reports of RSV hospitalizations among high-risk and otherwise healthy children. Curr Med Res Opin. 2010;26:2175–2181.
33. Hall, CB, Hall, WJ, Speers, DM. Clinical and physiological manifestations of bronchiolitis and pneumonia. Outcome of respiratory syncytial virus. Am J Dis Child. 1979;133:798–802.
34. Brodzinski, H, Ruddy, RM. Review of new and newly discovered respiratory tract viruses in children. Pediatr Emerg Care. 2009;25:352–360.
35. Qureshi, NR, Hien, TT, Farrar, J, et al. The radiologic manifestations of H5N1 avian influenza. J Thorac Imaging. 2006;21:259–264.
36. Jartti, A, Rauvala, E, Kauma, H, et al. Chest imaging findings in hospitalized patients with H1N1 influenza. Acta Radiol. 2011;52:297–304.
37. Ventre, KM, Wolf, GK, Arnold, JH. Pediatric respiratory diseases: 2011 update for the Rogers’ Textbook of Pediatric Intensive Care. Pediatr Crit Care Med. 2011;12:325–338.
38. Ng, DK, Lau, WF, Chan, KK, et al. Severe acute respiratory syndrome coronavirus infection in children. Pediatr Int. 2005;47:452–455.
39. Antonio, GE, Wong, KT, Tsui, EL, et al. Chest radiograph scores as potential prognostic indicators in severe acute respiratory syndrome (SARS). AJR Am J Roentgenol. 2005;184:734–741.
40. Prodhan, P, Westra, SJ, Lin, J, et al. Chest radiological patterns predict the duration of mechanical ventilation in children with RSV infection. Pediatr Radiol. 2009;39:117–123.
41. Lee, EY, McAdam, AJ, Chaudry, G, et al. Swine-origin influenza a (H1N1) viral infection in children: initial chest radiographic findings. Radiology. 2010;254:934–941.
42. Li, AM, So, HK, Chu, W, et al. Radiological and pulmonary function outcomes of children with SARS. Pediatr Pulmonol. 2004;38:427–433.
43. Antonio, GE, Wong, KT, Chu, WC, et al. Imaging in severe acute respiratory syndrome (SARS). Clin Radiol. 2003;58:825–832.
44. Babyn, PS, Chu, WC, Tsou, IY, et al. Severe acute respiratory syndrome (SARS): chest radiographic features in children. Pediatr Radiol. 2004;34:47–58.
45. Chu, WC, Li, AM, Ng, AW, et al. Thin-section CT 12 months after the diagnosis of severe acute respiratory syndrome in pediatric patients. AJR Am J Roentgenol. 2006;186:1707–1714.
46. Gold, R, Wilt, JC, Adhikari, PK, et al. Adenoviral pneumonia and its complications in infancy and childhood. J Can Assoc Radiol. 1969;20:218–224.
47. Chamroonrat, W, Cheng, G, Servaes, S, et al. Cytomegalovirus pneumonitis detected by gallium-67 scintigraphy with a negative diagnostic chest computed tomography. Clin Nucl Med. 2010;35:542–544.
48. Pfleger, A, Eber, E, Popper, H, et al. Chronic interstitial lung disease due to Epstein-Barr virus infection in two infants. Eur Respir J. 2000;15:803–806.
49. Riggs, W. Why radiologists tend to overcall pediatric chest radiographs. Appl Radiol. 1996:38–39.
50. Donnelly, LF. Diagnostic imaging: pediatrics. Salt Lake City, UT: Amirsys; 2005.
51. Bachur, R, Perry, H, Harper, MB. Occult pneumonias: empiric chest radiographs in febrile children with leukocytosis. Ann Emerg Med. 1999;33:166–173.
52. Murphy, CG, van de Pol, AC, Harper, MB, et al. Clinical predictors of occult pneumonia in the febrile child. Acad Emerg Med. 2007;14:243–249.
53. Shah, RM, Gupta, S, Angeid-Backman, E, et al. Pneumococcal pneumonia in patients requiring hospitalization: effects of bacteremia and HIV seropositivity on radiographic appearance. AJR Am J Roentgenol. 2000;175:1533–1536.
54. O’Brien, KL, Walters, MI, Sellman, J, et al. Severe pneumococcal pneumonia in previously healthy children: the role of preceding influenza infection. Clin Infect Dis. 2000;30:784–789.
55. Tuomanen, EI, Austrian, R, Masure, HR. Pathogenesis of pneumococcal infection. N Engl J Med. 1995;332:1280–1284.
56. Minton, EJ, Macfarlane, JT. Antibiotic resistant Streptococcus pneumoniae. Thorax. 1996;51(suppl 2):S45–S50.
57. Rutman, MS, Bachur, R, Harper, MB. Radiographic pneumonia in young, highly febrile children with leukocytosis before and after universal conjugate pneumococcal vaccination. Pediatr Emerg Care. 2009;25:1–7.
58. Kim, YW, Donnelly, LF. Round pneumonia: imaging findings in a large series of children. Pediatr Radiol. 2007;37:1235–1240.
59. Restrepo, R, Palani, R, Matapathi, UM, et al. Imaging of round pneumonia and mimics in children. Pediatr Radiol. 2010;40:1931–1940.
60. Felman, AH, Shulman, ST. Staphylococcal osteomyelitis, sepsis, and pulmonary disease. Observations of 10 patients with combined osseous and pulmonary infections. Radiology. 1975;117:649–655.
61. Erdem, G, Bergert, L, Len, K, et al. Radiological findings of community-acquired methicillin-resistant and methicillin-susceptible Staphylococcus aureus pediatric pneumonia in Hawaii. Pediatr Radiol. 2010;40:1768–1773.
62. Kefala-Agoropoulou, K, Protonotariou, E, Vitti, D, et al. Life-threatening infection due to community-acquired methicillin-resistant Staphylococcus aureus: case report and review. Eur J Pediatr. 2010;169:47–53.
63. Macfarlane, J, Rose, D. Radiographic features of staphylococcal pneumonia in adults and children. Thorax. 1996;51:539–540.
64. Joosten, KF, Hazelzet, JA, Tiddens, HA, et al. Staphylococcal pneumonia in childhood: will early surgical intervention lower mortality? Pediatr Pulmonol. 1995;20:83–88.
65. Asmar, BI, Slovis, TL, Reed, JO, et al. Hemophilus influenzae type B pneumonia in 43 children. J Pediatr. 1978;93:389–393.
66. Campins, M, Ferrer, A, Callis, L, et al. Nosocomial legionnaire’s disease in a children’s hospital. Pediatr Infect Dis J. 2000;19:228–234.
67. Quagliano, PV, Das Narla, L. Legionella pneumonia causing multiple cavitating pulmonary nodules in a 7-month-old infant. AJR Am J Roentgenol. 1993;161:367–368.
68. Brook, I. Anaerobic infections in children. Microbes Infect. 2002;4:1271–1280.
69. Lumicao, GG, Heggie, AD. Chlamydial infections. Pediatr Clin North Am. 1979;26:269–282.
70. Radkowski, MA, Kranzler, JK, Beem, MO, et al. Chlamydia pneumonia in infants: radiography in 125 cases. AJR Am J Roentgenol. 1981;137:703–706.
71. Guckel, C, Benz-Bohm, G, Widemann, B. Mycoplasmal pneumonias in childhood. Roentgen features, differential diagnosis and review of literature. Pediatr Radiol. 1989;19:499–503.
72. Waites, KB. New concepts of Mycoplasma pneumoniae infections in children. Pediatr Pulmonol. 2003;36:267–278.
73. Reittner, P, Muller, NL, Heyneman, L, et al. Mycoplasma pneumoniae pneumonia: radiographic and high-resolution CT features in 28 patients. AJR Am J Roentgenol. 2000;174:37–41.
74. John, SD, Ramanathan, J, Swischuk, LE. Spectrum of clinical and radiographic findings in pediatric mycoplasma pneumonia. Radiographics. 2001;21:121–131.
75. Yang, E, Altes, T, Anupindi, SA. Early Mycoplasma pneumoniae infection presenting as multiple pulmonary masses: an unusual presentation in a child. Pediatr Radiol. 2008;38:477–480.
76. Ramnath, RR, Heller, RM, Ben-Ami, T, et al. Implications of early sonographic evaluation of parapneumonic effusions in children with pneumonia. Pediatrics. 1998;101:68–71.
77. King, S, Thomson, A. Radiological perspectives in empyema. Br Med Bull. 2002;61:203–214.
78. Donnelly, LF, Klosterman, LA. CT appearance of parapneumonic effusions in children: findings are not specific for empyema. AJR Am J Roentgenol. 1997;169:179–182.
79. Tan Kendrick, AP, Ling, H, Subramaniam, R, et al. The value of early CT in complicated childhood pneumonia. Pediatr Radiol. 2002;32:16–21.
80. Donnelly, LF. Imaging in immunocompetent children who have pneumonia. Radiol Clin North Am. 2005;43:253–265.
81. Kearney, SE, Davies, CW, Davies, RJ, et al. Computed tomography and ultrasound in parapneumonic effusions and empyema. Clin Radiol. 2000;55:542–547.
82. Tu, CY, Hsu, WH, Hsia, TC, et al. Pleural effusions in febrile medical ICU patients: chest ultrasound study. Chest. 2004;126:1274–1280.
83. Westra, SJ, Choy, G. What imaging should we perform for the diagnosis and management of pulmonary infections? Pediatr Radiol. 2009;39(suppl 2):S178–S183.
84. Heussel, CP, Kauczor, HU, Heussel, G, et al. Early detection of pneumonia in febrile neutropenic patients: use of thin-section CT. AJR Am J Roentgenol. 1997;169:1347–1353.
85. Winer-Muram, HT, Arheart, KL, Jennings, SG, et al. Pulmonary complications in children with hematologic malignancies: accuracy of diagnosis with chest radiography and CT. Radiology. 1997;204:643–649.
86. Mori, M, Galvin, JR, Barloon, TJ, et al. Fungal pulmonary infections after bone marrow transplantation: evaluation with radiography and CT. Radiology. 1991;178:721–726.
87. Janzen, DL, Padley, SP, Adler, BD, et al. Acute pulmonary complications in immunocompromised non-AIDS patients: comparison of diagnostic accuracy of CT and chest radiography. Clin Radiol. 1993;47:159–165.
88. Winer-Muram, HT, Rubin, SA, Fletcher, BD, et al. Childhood leukemia: diagnostic accuracy of bedside chest radiography for severe pulmonary complications. Radiology. 1994;193:127–133.
89. Donnelly, LF, Klosterman, LA. The yield of CT of children who have complicated pneumonia and noncontributory chest radiography. AJR Am J Roentgenol. 1998;170:1627–1631.
90. Donnelly, LF, Klosterman, LA. Cavitary necrosis complicating pneumonia in children: sequential findings on chest radiography. AJR Am J Roentgenol. 1998;171:253–256.
91. Virkki, R, Juven, T, Mertsola, J, et al. Radiographic follow-up of pneumonia in children. Pediatr Pulmonol. 2005;40:223–227.
92. Montella, S, Santamaria, F, Salvatore, M, et al. Assessment of chest high-field magnetic resonance imaging in children and young adults with noncystic fibrosis chronic lung disease: comparison to high-resolution computed tomography and correlation with pulmonary function. Invest Radiol. 2009;44:532–538.
93. Peltola, V, Ruuskanen, O, Svedstrom, E. Magnetic resonance imaging of lung infections in children. Pediatr Radiol. 2008;38:1225–1231.
94. Yikilmaz, A, Koc, A, Coskun, A, et al. Evaluation of pneumonia in children: comparison of MRI with fast imaging sequences at 1.5T with chest radiographs. Acta Radiol. 2011;52:914–919.
95. Yin, EZ, Frush, DP, Donnelly, LF, et al. Primary immunodeficiency disorders in pediatric patients: clinical features and imaging findings. AJR Am J Roentgenol. 2001;176:1541–1552.
96. Langton Hewer, SC. Is limited computed tomography the future for imaging the lungs of children with cystic fibrosis? Arch Dis Child. 2006;91:377–378.
97. de Jong, PA, Mayo, JR, Golmohammadi, K, et al. Estimation of cancer mortality associated with repetitive computed tomography scanning. Am J Respir Crit Care Med. 2006;173:199–203.
98. Agrons, GA, Markowitz, RI, Kramer, SS. Pulmonary tuberculosis in children. Semin Roentgenol. 1993;28:158–172.
99. Buckner, CB, Leithiser, RE, Walker, CW, et al. The changing epidemiology of tuberculosis and other mycobacterial infections in the United States: implications for the radiologist. AJR Am J Roentgenol. 1991;156:255–264.
100. Kim, WS, Moon, WK, Kim, IO, et al. Pulmonary tuberculosis in children: Evaluation with CT. AJR Am J Roentgenol. 1997;168:1005–1009.
101. Fonseca-Santos, J. Tuberculosis in children. Eur J Radiol. 2005;55:202–208.
102. Bosch-Marcet, J, Serres-Creixams, X, Zuasnabar-Cotro, A, et al. Comparison of ultrasound with plain radiography and CT for the detection of mediastinal lymphadenopathy in children with tuberculosis. Pediatr Radiol. 2004;34:895–900.
103. Andreu, J, Caceres, J, Pallisa, E, et al. Radiological manifestations of pulmonary tuberculosis. Eur J Radiol. 2004;51:139–149.
104. Leung, AN, Muller, NL, Pineda, PR, et al. Primary tuberculosis in childhood: radiographic manifestations. Radiology. 1992;182:87–91.
105. Leung, AN. Pulmonary tuberculosis: the essentials. Radiology. 1999;210:307–322.
106. Smuts, NA, Beyers, N, Gie, RP, et al. Value of the lateral chest radiograph in tuberculosis in children. Pediatr Radiol. 1994;24:478–480.
107. Lighter, J, Rigaud, M. Diagnosing childhood tuberculosis: traditional and innovative modalities. Curr Probl Pediatr Adolesc Health Care. 2009;39:61–88.
108. Theart, AC, Marais, BJ, Gie, RP, et al. Criteria used for the diagnosis of childhood tuberculosis at primary health care level in a high-burden, urban setting. Int J Tuberc Lung Dis. 2005;9:1210–1214.
109. Caglayan, S, Coteli, I, Acar, U, et al. Endobronchial tuberculosis simulating foreign body aspiration. Chest. 1989;95:1164.
110. Choe, KO, Jeong, HJ, Sohn, HY. Tuberculous bronchial stenosis: CT findings in 28 cases. AJR Am J Roentgenol. 1990;155:971–976.
111. Jamieson, DH, Cremin, BJ. High resolution CT of the lungs in acute disseminated tuberculosis and a pediatric radiology perspective of the term “miliary.”. Pediatr Radiol. 1993;23:380–383.
112. Moon, WK, Kim, WS, Kim, IO, et al. Complicated pleural tuberculosis in children: CT evaluation. Pediatr Radiol. 1999;29:153–157.
113. Curvo-Semedo, L, Teixeira, L, Caseiro-Alves, F. Tuberculosis of the chest. Eur J Radiol. 2005;55:158–172.
114. Lamont, AC, Cremin, BJ, Pelteret, RM. Radiological patterns of pulmonary tuberculosis in the paediatric age group. Pediatr Radiol. 1986;16:2–7.
115. Van Dyck, P, Vanhoenacker, FM, Van den Brande, P, et al. Imaging of pulmonary tuberculosis. Eur Radiol. 2003;13:1771–1785.
116. Andronikou, S, Wieselthaler, N. Modern imaging of tuberculosis in children: thoracic, central nervous system and abdominal tuberculosis. Pediatr Radiol. 2004;34:861–875.
117. Swingler, GH, du Toit, G, Andronikou, S, et al. Diagnostic accuracy of chest radiography in detecting mediastinal lymphadenopathy in suspected pulmonary tuberculosis. Arch Dis Child. 2005;90:1153–1156.
118. Lee, EY, Tracy, DA, Eisenberg, RL, et al. Screening of asymptomatic children for tuberculosis is a lateral chest radiograph routinely indicated? Acad Radiol. 2011;18:184–190.
119. Zarabi, M, Sane, S, Girdany, BR. The chest roentgenogram in the early diagnosis of tuberculous meningitis in children. Am J Dis Child. 1971;121:389–392.
120. Delacourt, C, Mani, TM, Bonnerot, V, et al. Computed tomography with normal chest radiograph in tuberculous infection. Arch Dis Child. 1993;69:430–432.
121. McGuinness, G, Naidich, DP, Jagirdar, J, et al. High resolution CT findings in miliary lung disease. J Comput Assist Tomogr. 1992;16:384–390.
122. Im, JG, Itoh, H, Shim, YS, et al. Pulmonary tuberculosis: CT findings—early active disease and sequential change with antituberculous therapy. Radiology. 1993;186:653–660.
123. Vijayasekaran, D. Treatment of childhood tuberculosis. Indian J Pediatr. 2011;78:443–448.
124. Aquino, SL, Kee, ST, Warnock, ML, et al. Pulmonary aspergillosis: imaging findings with pathologic correlation. AJR Am J Roentgenol. 1994;163:811–815.
125. Mintzer, RA, Rogers, LF, Kruglik, GD, et al. The spectrum of radiologic findings in allergic bronchopulmonary aspergillosis. Radiology. 1978;127:301–307.
126. Greif, Z, Moscuna, M, Suprun, H, et al. Fatal childhood pulmonary aspergillosis from contact with pigeons. Clin Pediatr (Phila). 1981;20:357–359.
127. Gefter, WB, Weingrad, TR, Epstein, DM, et al. “Semi-invasive” pulmonary aspergillosis: a new look at the spectrum of Aspergillus infections of the lung. Radiology. 1981;140:313–321.
128. Abramson, S. The air crescent sign. Radiology. 2001;218:230–232.
129. Gurney, JW, Conces, DJ. Pulmonary histoplasmosis. Radiology. 1996;199:297–306.
130. Greendyke, WH, Resnick, DL, Harvey, WC. The varied roentgen manifestations of primary coccidioidomycosis. Am J Roentgenol Radium Ther Nucl Med. 1970;109:491–499.
131. Pinckney, L, Parker, BR. Primary coccidioidomycosis in children presenting with massive pleural effusion. AJR Am J Roentgenol. 1978;130:247–249.
132. Crans, CA, Jr., Boiselle, PM. Imaging features of Pneumocystis carinii pneumonia. Crit Rev Diagn Imaging. 1999;40:251–284.
133. Boiselle, PM, Tocino, I, Hooley, RJ, et al. Chest radiograph interpretation of Pneumocystis carinii pneumonia, bacterial pneumonia, and pulmonary tuberculosis in HIV-positive patients: accuracy, distinguishing features, and mimics. J Thorac Imaging. 1997;12:47–53.
134. Bankier, AA, Stauffer, F, Fleischmann, D, et al. Radiographic findings in patients with acquired immunodeficiency syndrome, pulmonary infection, and microbiologic evidence of Mycobacterium xenopi. J Thorac Imaging. 1998;13:282–288.
135. Barry, SM, Lipman, MC, Johnson, MA, et al. Respiratory infections in immunocompromised patients. Curr Opin Pulm Med. 1999;5:168–173.
136. Boiselle, PM, Aviram, G, Fishman, JE. Update on lung disease in AIDS. Semin Roentgenol. 2002;37:54–71.
137. Hidalgo, A, Falco, V, Mauleon, S, et al. Accuracy of high-resolution CT in distinguishing between Pneumocystis carinii pneumonia and non- Pneumocystis carinii pneumonia in AIDS patients. Eur Radiol. 2003;13:1179–1184.
138. Boiselle, PM, Crans, CA, Jr., Kaplan, MA. The changing face of Pneumocystis carinii pneumonia in AIDS patients. AJR Am J Roentgenol. 1999;172:1301–1309.
139. Rossi, SE, Erasmus, JJ, Volpacchio, M, et al. “Crazy-paving” pattern at thin-section CT of the lungs: radiologic-pathologic overview. Radiographics. 2003;23:1509–1519.
140. Chow, C, Templeton, PA, White, CS. Lung cysts associated with Pneumocystis carinii pneumonia: Radiographic characteristics, natural history, and complications. AJR Am J Roentgenol. 1993;161:527–531.
141. Kuhlman, JE. Pneumocystic infections: the radiologist’s perspective. Radiology. 1996;198:623–635.
142. McGuinness, G. Changing trends in the pulmonary manifestations of AIDS. Radiol Clin North Am. 1997;35:1029–1082.
143. Padley, SP, King, LJ. Computed tomography of the thorax in HIV disease. Eur Radiol. 1999;9:1556–1569.
144. Primack, SL, Muller, NL. High-resolution computed tomography in acute diffuse lung disease in the immunocompromised patient. Radiol Clin North Am. 1994;32:731–744.
145. Hartman, TE, Primack, SL, Muller, NL, et al. Diagnosis of thoracic complications in AIDS: accuracy of CT. AJR Am J Roentgenol. 1994;162:547–553.
146. Logan, PM, Finnegan, MM. Pulmonary complications in AIDS: CT appearances. Clin Radiol. 1998;53:567–573.
147. Gruden, JF, Huang, L, Turner, J, et al. High-resolution CT in the evaluation of clinically suspected Pneumocystis carinii pneumonia in AIDS patients with normal, equivocal, or nonspecific radiographic findings. AJR Am J Roentgenol. 1997;169:967–975.
148. Kassner, EG, Kauffman, SL, Yoon, JJ, et al. Pulmonary candidiasis in infants: clinical, radiologic, and pathologic features. AJR Am J Roentgenol. 1981;137:707–716.
149. Buff, SJ, McLelland, R, Gallis, HA, et al. Candida albicans pneumonia: radiographic appearance. AJR Am J Roentgenol. 1982;138:645–648.
150. Woodring, JH, Halfhill, H, 2nd., Reed, JC. Pulmonary strongyloidiasis: clinical and imaging features. AJR Am J Roentgenol. 1994;162:537–542.
151. Burton, K, Yogev, R, London, N, et al. Pulmonary paragonimiasis in Laotian refugee children. Pediatrics. 1982;70:246–248.
152. Wall, MA, McGhee, G. Paragonimiasis. Atypical appearances in two adolescent Asian refugees. Am J Dis Child. 1982;136:828–830.
153. Neumayr, A, Troia, G, de Bernardis, C, et al. Justified concern or exaggerated fear: the risk of anaphylaxis in percutaneous treatment of cystic echinococcosis—a systematic literature review. PLoS Negl Trop Dis. 2011;5:e1154.