Chapter 401 Pulmonary Embolism, Infarction, and Hemorrhage
401.1 Pulmonary Embolus and Infarction
Venous thromboembolic disease (VTE) is well described in children and adolescents with or without risk factors (Table 401-1). Improvements in therapeutics for childhood illnesses and increased survival with chronic illness may contribute to the larger number of children presenting with thromboembolic events, which can be a significant source of morbidity and mortality.
Table 401-1 RISK FACTORS FOR PULMONARY EMBOLISM
ENVIRONMENTAL
WOMEN’S HEALTH
MEDICAL ILLNESS
SURGICAL
THROMBOPHILIA
NONTHROMBOTIC
Modified from Goldhaber SZ: Pulmonary embolism, Lancet 363:1295–1305, 2004.
Etiology
Commonly appreciated risk factors for thromboembolic disease in adults include immobility, malignancy, pregnancy, infection, and hypercoagulability; up to 20% of adults with this disorder may have no identifiable risk factor (see Table 401-1). Children with deep venous thrombosis (DVT) and pulmonary embolism (PE) are much more likely to have 1 or more identifiable conditions or circumstances placing them at risk. In a large Canadian registry, 96% of pediatric patients were found to have 1 risk factor and 90% had 2 or more risk factors.
Prothrombotic disease can also manifest in older infants and children. Disease can be congenital or acquired; DVT/PE may be the initial presentation. Factor V Leiden mutation (Chapter 472), hyperhomocysteinemia (Chapter 79.3), prothrombin 20210A mutation (Chapter 472), anticardiolipin antibody, and elevated values of lipoprotein A have all been linked to thromboembolic disease. Children with sickle cell disease are also at high risk for pulmonary embolus and infarction. Acquired prothrombotic disease is represented by nephrotic syndrome (Chapter 521) and antiphospholipid antibody syndrome. From one quarter to one half of children with systemic lupus erythematosus (Chapter 152) have thromboembolic disease.
Epidemiology
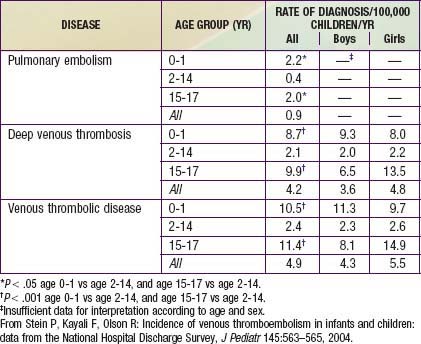
Younger age appears to be somewhat protective in thromboembolic disease. The DVT incidence in 1 study of hospitalized children was 5.3/10,000 admissions. A study that analyzed data from 1979 through 2001 found 0.9 cases of PE per 100,000 children per yr, 4.2 cases of DVT per 100,000 children per yr, and 4.9 cases of VTE per 100,000 children per yr (Table 401-2).
Askegard-Giesmann JR, Caniano DA, Kenney BD. Rare but serious complications of central line insertion. Semin Pediatr Surg. 2009;18:73-83.
Baird JS, Killinger JS, Kalkbrenner KJ, et al. Massive pulmonary embolism in children. J Pediatr. 2010;156:148-151.
Bonduel M, Hepner M, Sciuccati G, et al. Prothrombotic abnormalities in children with venous thromboembolism. J Pediatr Hematol Oncol. 2000;22:66-72.
Chan AK, Deveber G, Monagle P, et al. Venous thrombosis in children. J Thromb Haemost. 2003;1:1443-1455.
Holzer R, Peart I, Ciotti G, et al. Successful treatment with rTPA. Pediatr Cardiol. 2002;23:548-552.
Hull RD. Diagnosing pulmonary embolism with improved certainty and simplicity. JAMA. 2006;295:213-215.
Johnson AS, Bolte RG. Pulmonary embolism in the pediatric patient. Pediatr Emerg Care. 2004;20:555-560.
Kyrle PA, Eichinger S. New diagnostic strategies for pulmonary embolism. Lancet. 2008;371:1312-1314.
Manco-Johnson MJ, Nuss R, Hays T, et al. Combined thrombolytic and anticoagulant therapy for venous thrombosis in children. J Pediatr. 2000;136:446-453.
Nowak-Gottl U, Bidlingmaier C, et al. Pharmacokinetics, efficacy, and safety of LMWHs in venous thrombosis and stroke in neonates, infants and children. Br J Pharmacol. 2008;153:1120-1127.
Perrier A, Roy PM, Sanchez O, et al. Multidetector-row computed tomography in suspected pulmonary embolism. N Engl J Med. 2005;352:1760-1768.
Stein P, Kayali F, Olson R. Incidence of venous thromboembolism in infants and children: data from the National Hospital Discharge Survey. J Pediatr. 2004;145:563-565.
Stein PD, Fowler SE, Goodman LR. Multidetector computed tomography for acute pulmonary embolism. N Engl J Med. 2006;354:2317-2326.
Tapson VF. Acute pulmonary embolism. N Engl J Med. 2008;358:1037-1052.
van Belle A, Buller HR, Huisman MV, et al. Effectiveness of managing suspected pulmonary embolism using an algorithm combining clinical probability, D-Dimer testing, and computed tomography. Christopher Study Investigators. JAMA. 2006;295:172-179.
Wong KS, Lin TY, Huang YC, et al. Clinical and radiographic spectrum of septic pulmonary embolism. Arch Dis Child. 2002;87:312-315.
401.2 Pulmonary Hemorrhage and Hemoptysis
Etiology
Conditions that can manifest as pulmonary hemorrhage or hemoptysis in children are found in Table 401-3. The chronic (opposed to an acute) presence of a foreign body can lead to inflammation and/or infection, thereby inducing hemorrhage. Hemorrhage most commonly reflects chronic inflammation and infection such as that seen in cystic fibrosis with bronchiectasis or in tuberculosis with cavitary disease, although it may also reflect an acute condition such as bronchitis and bronchopneumonia. Other relatively common etiologies are congenital heart disease and trauma. Traumatic irritation or damage of the airway is often accidental in nature. Bleeding can also be related to instrumentation of the airway as is commonly seen in a child with a tracheostomy. It is important to note, however, that children who have been victims of nonaccidental trauma or deliberate suffocation can also be found to have blood in the mouth or airway. Less commonly, syndromes associated with vasculitic, autoimmune, and idiopathic disorders can be associated with diffuse alveolar hemorrhage (DAH). The mechanisms of DAH are multiple and are discussed further in Chapter 400.
Table 401-3 ETIOLOGY OF PULMONARY HEMORRHAGE (HEMOPTYSIS)
FOCAL HEMORRHAGE
DIFFUSE HEMORRHAGE
Acute idiopathic pulmonary hemorrhage (AIPH) occurs in young infants as a distinct entity, but the disorders in Table 401-3 must also be considered.
Laboratory Findings and Diagnosis
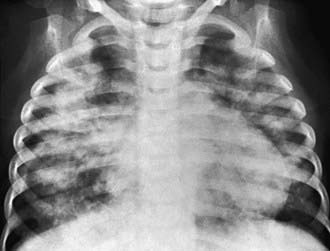
Every patient with suspected hemorrhage should have a laboratory evaluation with CBC and coagulation studies. The CBC result may demonstrate a microcytic, hypochromic anemia. Other laboratory findings are highly dependent on the underlying diagnosis. A urinalysis may show evidence of nephritis in patients with concomitant pulmonary and renal diseases. The classic finding, which defines pulmonary hemorrhage, is that of hemosiderin-laden macrophages in pulmonary secretions. These can be detected by sputum analysis with Prussian blue staining. Chest radiographs may demonstrate fluffy bilateral densities, as seen in AIPH of infancy (Fig. 401-1) or the patchy consolidation seen in idiopathic pulmonary hemosiderosis (Fig. 401-2). Alveolar infiltrates seen on chest radiograph may be regarded as a representation of recent bleeding, but their absence does not rule out a hemorrhage. Infiltrates, when present, are often symmetric and diffuse. CT may be indicated to assess for underlying disease processes.
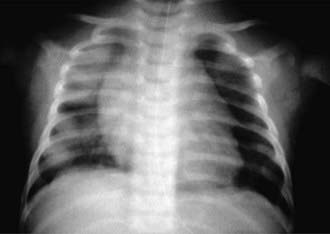
Figure 401-1 Radiographic appearance of acute idiopathic pulmonary hemorrhage in infancy.
(From Brown CM, Redd SC, Damon SA; Centers for Disease Control and Prevention [CDC]: Acute idiopathic pulmonary hemorrhage among infants: recommendations from the Working Group for Investigation and Surveillance, MMWR Recomm Rep 53:1–12, 2004.)
Bartyik K, Bede O, Tiszlavicz L, et al. Pulmonary capillary haemangiomatosis in children and adolescents: report of a new case and a review of the literature. Eur J Pediatr. 2004;163:731-737.
Brown CM, Redd SC, Damon SA, Centers for Disease Control and Prevention (CDC). Acute idiopathic pulmonary hemorrhage among infants: Recommendations from the Working Group for Investigation and Surveillance. MMWR Recomm Rep. 2004;53:1-12.
Centers for Disease Control and Prevention (CDC). Investigation of acute idiopathic pulmonary hemorrhage among infants—Massachusetts, December 2002-June 2003. MMWR Morbid Mortal Wkly Rep. 2004;53:817-820.
Coss-Bu JA, Sachdeva RC, Bricker JT, et al. Hemoptysis: a 10-year retrospective study. Pediatrics. 1997;100:E7.
Cottin V, Chinet T, Lavolé A, et al. Pulmonary arteriovenous malformations in hereditary hemorrhagic telangiectasia: a series of 126 patients. Medicine. 2007;86:1-17.
Godfrey S. Pulmonary hemorrhage/hemoptysis in children. Pediatr Pulmonol. 2004;37:476-484.
Lazor R, Bigay-Game L, Cottin V, et al. Alveolar hemorrhage in anti-basement membrane antibody disease: a series of 28 cases. Medicine. 2007;86:181-193.

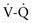 ) radionuclide scan is a noninvasive and potentially sensitive method of pulmonary embolus detection, the interpretation of
) radionuclide scan is a noninvasive and potentially sensitive method of pulmonary embolus detection, the interpretation of 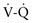 scans can be problematic. Helical or spiral CT with an intravenous contrast agent is valuable and the diagnostic test of choice to detect a PE. Specificity exceeds 90%. CT studies detect emboli in lobar and segmental vessels with acceptable sensitivities. Poorer sensitivities may be encountered in the evaluation of the subsegmental pulmonary vasculature. Pulmonary angiography is the gold standard for diagnosis of PE, but with current availability of multidetector spiral CT angiography, it is not necessary except in unusual cases.
scans can be problematic. Helical or spiral CT with an intravenous contrast agent is valuable and the diagnostic test of choice to detect a PE. Specificity exceeds 90%. CT studies detect emboli in lobar and segmental vessels with acceptable sensitivities. Poorer sensitivities may be encountered in the evaluation of the subsegmental pulmonary vasculature. Pulmonary angiography is the gold standard for diagnosis of PE, but with current availability of multidetector spiral CT angiography, it is not necessary except in unusual cases.