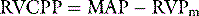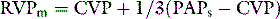Chapter 30 Pulmonary embolism
Pulmonary embolism (PE) is a commonly considered, but relatively uncommonly diagnosed, condition. It is important to have an adequate understanding of the pathophysiology, as well as a rapid and reliable strategy of investigation and management. This is particularly important in critically ill patients where diagnosis can be difficult and PE may be life-threatening.
AETIOLOGY
Predisposing risk factors for VTE involve one or more components of Virchow’s triad: (1) venous stasis; (2) vein wall injury; and (3) hypercoagulability of blood. The main factors are immobility (from any cause), surgery, trauma, malignancy, pregnancy and thrombophilia (Table 30.1).
| Primary hypercoagulable states (thrombophilia) |
| Antithrombin III deficiency |
| Protein C deficiency |
| Protein S deficiency |
| Resistance to activated protein C resistance (inherited factor V Leiden mutation) |
| Hyperhomocysteinaemia |
| Lupus anticoagulant (antiphospholipid antibody) |
| Secondary hypercoagulable states |
| Immobility |
| Surgery |
| Trauma |
| Malignancy |
| Pregnancy and the puerperium |
| Obesity |
| Smoking |
| Oestrogen-containing oral contraception or hormone replacement therapy |
| Indwelling catheters in great veins and the right heart |
| Burns |
| Patients with limb paralysis (e.g. spinal injuries) |
| Heart failure |
| Increasing age |
VTE can be recurrent, which should prompt investigation for thrombophilia, which describes a group of conditions which are inherited and associated with a high incidence of VTE. The most important of these is activated protein C resistance, which is mediated by the factor V Leiden mutation. Up to 50% of patients with recurrent VTE episodes (as well as 20% of patients with a single episode) have this condition; however, its association appears to be greater with DVT than with PE.1 Up to 5% of patients with VTE develop chronic pulmonary hypertension.2
PATHOPHYSIOLOGY
Acute pulmonary hypertension increases right ventricular (RV) afterload and RV wall tension, which leads to RV dilatation and dysfunction, with coronary ischaemia being a major contributing mechanism.3 In massive PE, the combination of coronary ischaemia, RV systolic failure, paradoxical interventricular septal shift and pericardial constraint leads to left ventricular (LV) dysfunction and a ‘low-cardiac-output’ shock state. In patients with underlying cardiorespiratory disease, a small PE can have profound consequences.
CLINICAL PRESENTATION
PE is relatively uncommon in critically ill patients despite the frequent presence of risk factors for VTE. However, when PE does occur, the diagnosis is frequently overlooked or is difficult to confirm because of the presence of coexistent cardiorespiratory disease. Clinical assessment raises the suspicion of PE but is neither sensitive nor specific. A number of clinical prediction systems have been developed, the most widely reported of which are the Wells’ score and the Geneva score.4 The differential diagnosis is listed in Table 30.2.
| Acute myocardial infarction |
| Acute pulmonary oedema |
| Pneumonia |
| Asthma or exacerbation of chronic obstructive pulmonary disease |
| Pericardial tamponade |
| Pleural effusion |
| Fat embolism |
| Pneumothorax |
| Aortic dissection |
| Rib fracture |
| Musculoskeletal pain |
| Anxiety |
SYMPTOMS
Dyspnoea, pleuritic chest pain, and haemoptysis are the classic symptoms of PE. Most patients will have at least one of these symptoms, with dyspnoea being the most common. The combination of pleuritic chest pain and haemoptysis reflects a late presentation where pulmonary infarction has occurred. If syncope occurs there is a high likelihood that there has been a massive PE. A family history of venous thrombosis raises the possibility of inherited thrombophilia.
INVESTIGATIONS
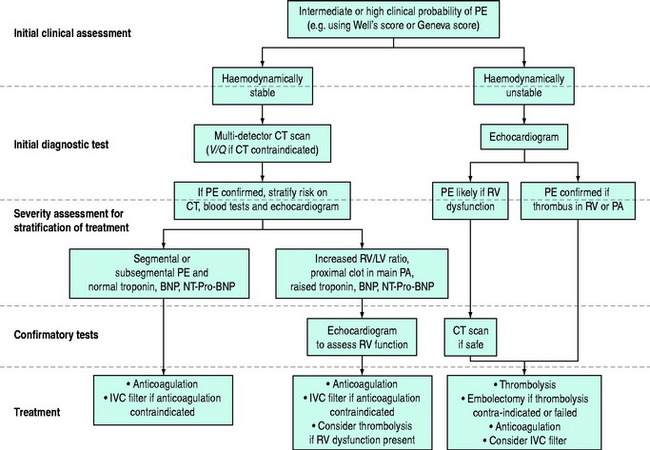
The diagnosis of PE requires a high level of clinical suspicion and the appropriate use of investigations. The aim of these investigations is to confirm or exclude the presence of PE and then to stratify treatment accordingly. The optimal investigation strategy depends upon the individual patient and institution as a number of investigations are available. Pulmonary angiography has traditionally been considered the ‘gold standard’ for the diagnosis of PE. The advent of multidetector row computed tomography (CT) scanning (which compares well with standard pulmonary angiography5) has led to the emergence of this as the ‘first-line’ test in many centres. A suggested investigation algorithm is shown in Figure 30.1.
D-DIMER
The serum D-dimer level is useful for exclusion of VTE, particularly when it is normal and combined with a low-risk clinical assessment.6 A variety of different assays are available. Negative tests, particularly from enzyme-linked immunosorbent assays (ELISA), are highly predictive of the absence of both DVT and PE.7 A high D-dimer concentration is also an independent predictive factor associated with mortality.8
TROPONIN, BRAIN NATRIURETIC PEPTIDE (BNP) AND NT-TERMINAL PRO-BNP
Although of little use for diagnosis, measurement of troponin, BNP or NT-terminal Pro-BNP can add to the risk stratification of patients with known PE. The presence of a raised troponin is associated with haemodynamic instability in patients with non-massive PE independently of clinical, echocardiographic and laboratory findings.9 Raised troponin also predicts a higher mortality.10 Low levels of BNP and NT-terminal Pro-BNP have been shown to correlate with an uneventful course in patients with known PE.11 NT-terminal Pro-BNP appears to be a better predictor of outcome than troponin.12
ARTERIAL BLOOD GASES
A normal arterial blood gas profile does not rule out the diagnosis; however hypoxaemia (with a widened alveolar–arterial oxygen gradient), hypocapnia and an increased end-tidal CO2 gradient13 should raise the suspicion of PE, even though there are many other causes of these findings in critically ill patients.14 Metabolic acidosis may be present if shock from a large PE occurs.
CHEST X-RAY
The chest X-ray is often normal or only slightly abnormal, with non-specific signs such as cardiac enlargement, pleural effusion, elevated hemidiaphragm, atelectasis and localised infiltrates. More specific findings, including focal oligaemia, a peripheral wedge-shaped density above the diaphragm or an enlarged right descending pulmonary artery,15 are uncommon and difficult for non-radiologists to identify. The chest X-ray is also useful in identifying an alternative diagnosis such as pneumothorax, pneumonia, acute pulmonary oedema, rib fracture and pleural effusion.
COMPUTED TOMOGRAPHY
As CT technology has improved, CT angiography (CTA) has emerged as a cost-effective and clinically reliable alternative to the V/Q scan.16 The single-detector row CT has been superseded by multidetector row CT, which allows imaging of the entire chest with high-resolution images in ‘in-plane’ and ‘through-plane’ resolution. High-resolution images to the level of segmental and in some cases subsegmental pulmonary arteries can be obtained in a short time period (often a single breath-hold).
When CTA is compared to conventional angiography it appears reliable, with sensitivity, specificity and accuracy of 100%, 89% and 91% respectively.5 Adding venography of the leg veins to the CTA (CTA-CTV) further increases the diagnostic certainty.17 It is therefore recommended that CTA or CTA-CTV should be the principal radiological test for patients with high and moderate probability of PE.18
Although the ability of many CT scanners to detect PE at the subsegmental level is limited, it is debatable whether this is of clinical significance. Patients who have negative or indeterminate CT scans and in whom anticoagulation is withheld have low subsequent rates of thromboembolic events.19
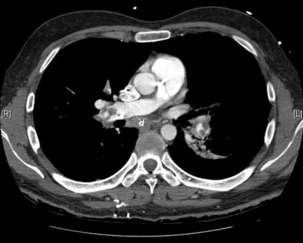
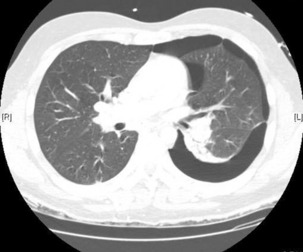
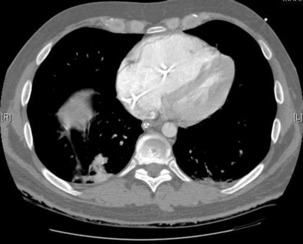
The advantage of CT is that it can not only diagnose PE but can also be used to assess severity of the condition. Increased RV/LV ratio (> 0.9)20 and clot in the proximal branches of the pulmonary artery21 correlate with the clinical severity of PE. Severity stratification is further increased by combining CT with other tests such as troponin9 and BNP or NT-terminal ProBNP.11 CT scanning may also identify the causative DVT in the veins of the legs, pelvis and abdomen or detect alternative or additional diagnoses such as a pulmonary mass, pneumonia, emphysema, pneumothorax, pleural effusion or mediastinal adenopathy (Figure 30.2).
ECHOCARDIOGRAPHY
Because of its portability, echocardiography has become increasingly useful in critically ill patients with possible PE. Many patients with PE have an echocardiographic abnormality, the most common being RV dilatation, RV hypokinesis, paradoxical interventricular septal motion toward the LV, tricuspid regurgitation and pulmonary hypertension.22 The pattern of RV hypokinesis with apical sparing is considered pathognomonic for PE.23 The presence of RV dysfunction correlates with mortality.24 Echocardiography is poor at excluding PE, as a negative echocardiogram can miss up to 50% of PEs.25
Transthoracic echocardiography will also allow estimation of pulmonary arterial pressure, identification of intracardiac thrombi (which usually requires surgical embolectomy) and aids in differential diagnosis by excluding aortic dissection and pericardial tamponade. Transoesophageal echocardiography has the additional benefit of directly identifying embolus in the proximal pulmonary arteries, which is common in patients with haemodynamically significant PE.26
VENTILATION/PERFUSION SCANNING
The lung ventilation/perfusion (V/Q) scan is becoming less commonly used with the advent of multidetector row CT. The perfusion scan identifies defects in perfusion which may be classified as single or multiple, and as subsegmental, segmental or lobar. By combining this with ventilation scanning, these perfusion defects can then be labelled as mismatched (normal ventilation in a zone of perfusion defect) or matched defects (ventilation defect corresponds to perfusion defect). The probability of PE is then classified as high, intermediate or low probability for PE, or normal.27
Despite the increased use of CT, the V/Q scan retains a role when CT is either unavailable or contraindicated (e.g. significant renal impairment, anaphylaxis to intravenous contrast or pregnancy).15V/Q scanning also allows quantification of regional blood flow within the lungs, which may be required in the assessment of chronic pulmonary venous embolism.
SEARCH FOR DEEP VENOUS THROMBOSIS
Leg venography is a more sensitive test; however, it is invasive and now uncommonly performed.
INVESTIGATION STRATEGY IN HAEMODYNAMICALLY STABLE PATIENTS
INVESTIGATION STRATEGY IN HAEMODYNAMICALLY UNSTABLE PATIENTS
MANAGEMENT
MANAGEMENT PRINCIPLES
Unless there is a serious contraindication, nearly all patients should receive anticoagulation with either unfractionated or low-molecular-weight heparin (LMWH) to prevent VTE recurrence. However removal or destruction of the embolus is a key principle in treatment of the more severe cases of PE based on the importance of RV dysfunction to both the pathophysiology and the outcome of PE.
To assist in planning management, it is worth grading the severity of PE as follows:
SUBMASSIVE PULMONARY EMBOLISM (HAEMODYNAMICALLY STABLE WITH EVIDENCE OF RIGHT VENTRICULAR DYSFUNCTION)
Patients with PE and evidence of RV dysfunction have higher mortality and recurrence rates than those with normal RV function.24 They also develop shock and RV thrombi more frequently. These patients require prevention of further embolisation but also warrant strong consideration of removal of embolus using a thrombolytic agent. Thrombolysis appears to improve outcomes,28 although this appears to be at the expense of a higher bleeding risk.29 Echocardiogram is not always required to diagnose RV dysfunction as CT-diagnosed RV dilatation has also been shown to predict poor outcomes.30
PREVENTION OF FURTHER EMBOLISATION
ANTICOAGULATION
Heparin has been known to prevent recurrence and reduce the mortality from PE for over 35 years. LMWHs are as effective and safe as unfractionated heparin31 and may even be better.32 LMWHs offer several advantages over unfractionated heparin, including a longer half-life, increased bioavailability, a more predictable dose–response and less requirement for monitoring and dose adjustments, and can be readily used in the stable patient with PE.
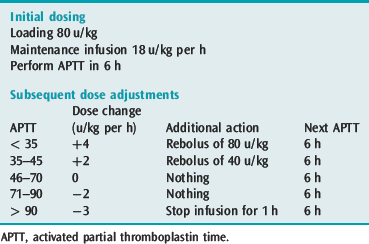
Unfractionated heparin should be used for those patients who have recently had a thrombolytic agent or embolectomy as it can be easily and rapidly reversed. Heparin should be administered by intravenous infusion with a bolus and initial monitoring should be with 6-hourly activated partial thromboplastin time (APTT) testing. Since subtherapeutic levels of anticoagulation increase the risk of recurrence, it is important to achieve therapeutic heparinisation rapidly. Weight-based dosing of heparin should be used as target anticoagulation levels are reached sooner33 (Table 30.3).
INFERIOR VENA CAVA FILTER
IVC filters are another method used to prevent further embolisation. They are indicated for patients in whom anticoagulation is contraindicated, those who experience recurrent PE despite adequate anticoagulation and those undergoing open surgical embolectomy.34 Insertion is usually performed percutaneously in a radiology department, but it can be done at the bedside.35 They lower early recurrence rates but increase long-term DVT recurrence rates,36 although newer retrievable designs may be better.37 A recent analysis of a multicentre register analysis demonstrated a significant reduction in 90-day mortality associated with IVC filters.38
REMOVAL OF EMBOLI
THROMBOLYTIC AGENTS
A large multicentre patient registry found that patients receiving thrombolytic agents (clearly on an ad-hoc basis) had lower rates of mortality and recurrence than patients receiving heparin.39 Despite this, there has been no randomised study comparing a thrombolytic agent with standard anticoagulation which has found a mortality difference, although the studies have likely been under- powered to detect this.
A meta-analysis found that thrombolytic therapy was associated with a non-significant reduction in recurrent PE or death when compared to heparin in patients with PE. However, when only studies that included massive PE were aggregated, there was indeed a significant reduction in mortality in favour of thrombolysis.29
Thrombolytics in patients with submassive PE do not reduce mortality but do significantly reduce clinical deterioration, requiring an escalation of treatment in the ICU.28
Comparative studies between the various thrombolytic agents have been too few to make rational comparison and, although there may be slight differences between thrombolytic agents, the choice of drug is less important than the choice to give a thrombolytic at all. One should be given in the doses recommended in Table 30.4, and this can be administered through either a peripheral or a central venous catheter. In contrast to the use of thrombolytics in acute myocardial infarction, thrombolytics are useful in PE when given up to 14 days after symptoms begin. Once the thrombolytic agent has been ceased, heparin should be commenced.
Table 30.4 Recommended doses of thrombolytic agents for pulmonary embolism
| Urokinase | 4400 u/kg bolus (over 10 min) followed by 4400 u/kg per h for 12 h |
| Streptokinase | 250 000 unit bolus (over 15 min) followed by 100 000 u/h for 24 h |
| Alteplase | 10 mg bolus followed by 90 mg over 2 h |
| Reteplase | 10 unit boluses 30 min apart |
Haemorrhagic complications are not uncommon and can significantly affect patient morbidity; however it is difficult to predict those patients who are at the highest risk for bleeding. Major clinically significant bleeding can occur in up to 10% of patients, although cerebral haemorrhage is fortunately uncommon (0.5%).29 Whilst recent surgery is often considered a contraindication, acceptable safety has been demonstrated in these patients. Clearly individual patients should have the risks and the benefits weighed up: in shocked patients with PE the balance appears to be in favour of thrombolytic agents for most patients.
SURGICAL EMBOLECTOMY
A recent study in an academic centre has documented a perioperative mortality of 6% and a total mortality of 18% when a surgical approach that included rapid diagnosis, prompt surgical intervention and a high frequency of concurrent IVC filter placement was used.40 Embolectomy was performed in more than half of these patients because either there were contraindications to thrombolytics or the thrombolytic treatment had failed.
Therefore, whilst patients with massive PE who have no contraindications to thrombolysis should receive a thrombolytic agent, surgical embolectomy should be strongly considered in centres with access to cardiothoracic surgeons when a contraindication to thrombolysis exists or the therapy fails.
PERCUTANEOUS EMBOLECTOMY
Percutaneous methods include either embolus extraction techniques (pure percutaneous embolectomy) or embolus disruption techniques (including catheter-directed thrombolysis and percutaneous thrombus fragmentation techniques).41 Successful embolectomy does not always occur and mortality is still about 20–30%. Rheolytic thrombectomy42 and methods combining mechanical disruption and thrombolytic therapy43 have emerged as promising interventions. Whilst awaiting well-designed studies of these interventions, they remain limited to specialised centres.
CONCURRENT HAEMODYNAMIC SUPPORT
INTRAVENOUS FLUIDS
In patients with moderate or massive PE, volume loading can improve haemodynamic status,44 although if excessive, fluid therapy worsens RV function which can in turn affect LV function and become detrimental.45 For this reason cautious amounts of intravenous fluid should be given.
INTRAVENOUS VASOACTIVE AGENTS
Beneficial effects have been shown with intra-aortic balloon counterpulsation in animal studies. In patients apparently dying of PE despite thrombolytics and ongoing resuscitative efforts, this appears a reasonable approach as a method of augmenting RVCPP, as long as it is in association with the use of pressor agents. Extracorporeal membrane oxygenation (ECMO) is an extreme but alternative form of mechanical assistance which may be available in more specialised institutions.46 Technological advances appear to have reduced the number of procedure-related complications attributed to ECMO.47
SELECTIVE PULMONARY VASODILATORS
Inhaled nitric oxide has been used in patients with massive PE based on animal studies and case series,48 because it selectively decreases pulmonary arterial pressure without influencing systemic haemodynamics. It may have dramatic haemodynamic effects but should be adjunctive to other resuscitative efforts, including elevation of the systemic blood pressure. Inhaled prostacyclin is an alternative, but has limited supporting evidence.49
OTHER MANAGEMENT ISSUES
If chest pain is prominent, morphine should be given. To guide resuscitation, it is useful to have at least a central venous catheter, particularly before a thrombolytic agent is given. Although not essential, if a patient is severely shocked, a pulmonary artery catheter is recommended to assist in the titration of vasoactive agents and measure the pulmonary artery pressure. The pulmonary artery catheter also assists in the estimation of the RVCPP, either through direct measurement of the mean RV pressure using the 20-cm lumen of a pacing pulmonary artery catheter, or by calculation using the formula above. While there is increased risk of bleeding due to the concurrent administration of thrombolytic agents and/or anticoagulants in these patients, this is probably outweighed by the importance of secure venous access and monitoring of the circulation in patients with haemodynamic compromise due to PE.
PREVENTION
Prophylaxis is the most important management aspect of VTE. All ICU patients should have an adequate assessment as to whether prophylaxis is warranted, although most should receive it.50 Careful attention to the intervention and dose is required, as omission of prophylaxis51 and failure of prophylaxis52 are common. Some groups of surgical patients also appear to benefit from extended out-of-hospital prophylaxis.53
Traditional prophylaxis with fixed low-dose subcutaneous unfractionated heparin (i.e. 5000 units twice or three times daily) is now being challenged by both LMWHs and fondaparinux (a factor Xa inhibitor).50 Both have been shown to be as efficacious as unfractionated heparin in many patient groups and they are associated with less bleeding and less HITTS. Although questions remain about the cost-effectiveness of LMWHs54 and about comparisons between the various LMWH agents and fondaparinux, LMWHs require less monitoring than unfractionated heparin and are being more commonly recommended.50
Aspirin has also been shown to reduce VTE rate and mortality in high-risk patients,55 although it seems less efficacious than other anticoagulants and is not currently recommended.50
Mechanical approaches (including graduated-compression stockings and intermittent pneumatic compression devices56) seem best utilised in low-risk patients, patients with contraindications to anticoagulation, or when used in addition to anticoagulation in high-risk patients.
A recommended approach to prophylaxis is included in Table 30.5.50
| Category of patient | Recommendation |
|---|---|
| All patients with high bleeding risk | IPC or GCS |
| General (and other) surgery | |
| Low-risk | Early mobilisation |
| Moderate-risk | Heparin bd or LMWH |
| High-risk | Heparin tds or LMWH |
| Vascular surgery | |
| No risk factors | Early mobilisation |
| Risk factors | Heparin bd or LMWH |
| Orthopaedic surgery | |
| Hip replacement | LMWH or fondaparinux or warfarin |
| Knee arthroplasty | LMWH or fondaparinux or warfarin or IPC |
| Hip fracture surgery | LMWH or fondaparinux or warfarin or heparin bd |
| Neurosurgery | |
| Intracranial surgery | IPC ± GCS or heparin bd or LMWH |
| High-risk | GCS/IPC + heparin/LMWH |
| Trauma patients | |
| No anticoagulation contraindication | LMWH |
| Anticoagulation contraindication | IPC ± GCS |
| Spinal cord injury | LMWH or IPC + heparin or IPC + LMWH |
| Burns | Heparin or LMWH |
| Medical patients | |
| Risk factors | Heparin or LMWH |
| Anticoagulation contraindication | GCS or IPC |
| Risk definitions | |
| Low-risk surgery | |
| Minor surgery in patients < 40 years old with no risk factors | |
| Moderate-risk surgery | |
| Non-major surgery in patients who are 40–60 years old with no risk factors | |
| Non-major surgery in patients < 60 years old with risk factors | |
| Major surgery in patients < 40 years old with no risk factors | |
| High-risk surgery | |
| Non-major surgery in patients > 60 years old with or without risk factors | |
| Major surgery in patients > 40 years old with no risk factors | |
| Major surgery in patients < 40 years old with risk factors | |
IPC, intermittent pneumatic compression device; GCS, graduated compression stockings; heparin bd, 5000 units subcutaneously twice daily; LMWH, low-molecular-weight heparin; heparin tds, 5000 units subcutaneously three times daily.
(Information from Geerts et al.50)
1 Bounameaux H. Factor V Leiden paradox: risk of deep-vein thrombosis but not of pulmonary embolism. Lancet. 2000;356:182-183.
2 Kearon C. Natural history of venous thromboembolism. Circulation. 2003;107:I22-I30.
3 Kreit JW. The impact of right ventricular dysfunction on the prognosis and therapy of normotensive patients with pulmonary embolism. Chest. 2004;125:1539-1545.
4 Chagnon I, Bounameaux H, Aujesky D, et al. Comparison of two clinical prediction rules and implicit assessment among patients with suspected pulmonary embolism. Am J Med. 2002;113:269-275.
5 Winer-Muram HT, Rydberg J, Johnson MS, et al. Suspected acute pulmonary embolism: evaluation with multi-detector row CT versus digital subtraction pulmonary arteriography. Radiology. 2004;233:806-815.
6 Fancher TL, White RH, Kravitz RL. Combined use of rapid d-dimer testing and estimation of clinical probability in the diagnosis of deep vein thrombosis: systematic review. Br Med J. 2004;329:821.
7 Stein PD, Hull RD, Patel KC, et al. d-dimer for the exclusion of acute venous thrombosis and pulmonary embolism: a systematic review. Ann Intern Med. 2004;140:589-602.
8 Grau E, Tenias JM, Soto MJ, et al. d-dimer levels correlate with mortality in patients with acute pulmonary embolism: findings from the RIETE registry. Crit Care Med. 2007;35:1937-1941.
9 Gallotta G, Palmieri V, Piedimonte V, et al. Increased troponin I predicts in-hospital occurrence of hemodynamic instability in patients with sub-massive or non-massive pulmonary embolism independent to clinical, echocardiographic and laboratory information. Int J Cardiol. 2008;124:351-357.
10 Becattini C, Vedovati MC, Agnelli G. Prognostic value of troponins in acute pulmonary embolism A meta-analysis. Circulation. 2007;116:427-433.
11 Kucher N, Goldhaber SZ. Risk stratification of acute pulmonary embolism. Semin Thromb Hemost. 2006;32:838-847.
12 Maziere F, Birolleau S, Medimagh S, et al. Comparison of troponin I and N-terminal-pro B-type natriuretic peptide for risk stratification in patients with pulmonary embolism. Eur J Emerg Med. 2007;14:207-211.
13 Kline JA, Kubin AK, Patel MM, et al. Alveolar dead space as a predictor of severity of pulmonary embolism. Acad Emerg Med. 2000;7:611-617.
14 Hedenstierna G, Sandhagen B. Assessing dead space. A meaningful variable? Minerva Anestesiol. 2006;72:521-528.
15 Piazza G, Goldhaber SZ. Acute pulmonary embolism: part I: epidemiology and diagnosis. Circulation. 2006;114:e28-e32.
16 Quiroz R, Schoepf UJ. CT pulmonary angiography for acute pulmonary embolism: cost-effectiveness analysis and review of the literature. Semin Roentgenol. 2005;40:20-24.
17 Stein PD, Fowler SE, Goodman LR, et al. Multidetector computed tomography for acute pulmonary embolism. N Engl J Med. 2006;354:2317-2327.
18 Stein PD, Woodard PK, Weg JG, et al. Diagnostic pathways in acute pulmonary embolism: recommendations of the PIOPED II investigators. Radiology. 2007;242:15-21.
19 Quiroz R, Kucher N, Zou KH, et al. Clinical validity of a negative computed tomography scan in patients with suspected pulmonary embolism: a systematic review. JAMA. 2005;293:2012-2017.
20 van der Meer RW, Pattynama PM, van Strijen MJ, et al. Right ventricular dysfunction and pulmonary obstruction index at helical CT: prediction of clinical outcome during 3-month follow-up in patients with acute pulmonary embolism. Radiology. 2005;235:798-803.
21 Ghanima W, Abdelnoor M, Holmen LO, et al. The association between the proximal extension of the clot and the severity of pulmonary embolism (PE): a proposal for a new radiological score for PE. J Intern Med. 2007;261:74-81.
22 Goldhaber SZ. Echocardiography in the management of pulmonary embolism. Ann Intern Med. 2002;136:691-700.
23 McConnell MV, Solomon SD, Rayan ME, et al. Regional right ventricular dysfunction detected by echocardiography in acute pulmonary embolism. Am J Cardiol. 1996;78:469-473.
24 Kucher N, Rossi E, De Rosa M, et al. Prognostic role of echocardiography among patients with acute pulmonary embolism and a systolic arterial pressure of 90 mmHg or higher. Arch Intern Med. 2005;165:1777-1781.
25 Miniati M, Monti S, Pratali L, et al. Value of transthoracic echocardiography in the diagnosis of pulmonary embolism: results of a prospective study in unselected patients. Am J Med. 2001;110:528-535.
26 Pruszczyk P, Torbicki A, Kuch-Wocial A, et al. Diagnostic value of transoesophageal echocardiography in suspected haemodynamically significant pulmonary embolism. Heart. 2001;85:628-634.
27 The PIOPED investigators. Value of the ventilation/perfusion scan in acute pulmonary embolism Results of the prospective investigation of pulmonary embolism diagnosis (PIOPED). JAMA. 1990;263:2753-2759.
28 Konstantinides S, Geibel A, Heusel G, et al. Heparin plus alteplase compared with heparin alone in patients with submassive pulmonary embolism. N Engl J Med. 2002;347:1143-1150.
29 Wan S, Quinlan DJ, Agnelli G, et al. Thrombolysis compared with heparin for the initial treatment of pulmonary embolism: a meta-analysis of the randomized controlled trials. Circulation. 2004;110:744-749.
30 Schoepf UJ, Kucher N, Kipfmueller F, et al. Right ventricular enlargement on chest computed tomography: a predictor of early death in acute pulmonary embolism. Circulation. 2004;110:3276-3280.
31 Segal JB, Streiff MB, Hofmann LV, et al. Management of venous thromboembolism: a systematic review for a practice guideline. Ann Intern Med. 2007;146:211-222.
32 Hull RD, Raskob GE, Brant RF, et al. Low-molecular-weight heparin vs heparin in the treatment of patients with pulmonary embolism. American-Canadian Thrombosis Study Group. Arch Intern Med. 2000;160:229-236.
33 Raschke RA, Reilly BM, Guidry JR, et al. The weight-based heparin dosing nomogram compared with a “standard care” nomogram. A randomized controlled trial. Ann Intern Med. 1993;119:874-881.
34 Piazza G, Goldhaber SZ. Acute pulmonary embolism: part II: treatment and prophylaxis. Circulation. 114, 2006.
35 Sing RF, Jacobs DG, Heniford BT. Bedside insertion of inferior vena cava filters in the intensive care unit. J Am Coll Surg. 2001;192:570-575.
36 Decousus H, Leizorovicz A, Parent F, et al. A clinical trial of vena caval filters in the prevention of pulmonary embolism in patients with proximal deep-vein thrombosis. Prevention du Risque d’Embolie Pulmonaire par Interruption Cave Study Group. N Engl J Med. 1998;338:409-415.
37 Imberti D, Ageno W, Carpenedo M. Retrievable vena cava filters: a review. Curr Opin Hematol. 2006;13:351-356.
38 Kucher N, Rossi E, De Rosa M, et al. Massive pulmonary embolism. Circulation. 2006;113:577-582.
39 Konstantinides S, Geibel A, Olschewski M, et al. Association between thrombolytic treatment and the prognosis of hemodynamically stable patients with major pulmonary embolism: results of a multicenter registry. Circulation. 1997;96:882-888.
40 Leacche M, Unic D, Goldhaber SZ, et al. Modern surgical treatment of massive pulmonary embolism: results in 47 consecutive patients after rapid diagnosis and aggressive surgical approach. J Thorac Cardiovasc Surg. 2005;129:1018-1023.
41 Uflacker R. Interventional therapy for pulmonary embolism. J Vasc Interv Radiol. 2001;12:147-164.
42 Chauhan MS, Kawamura A. Percutaneous rheolytic thrombectomy for large pulmonary embolism: a promising treatment option. Catheter Cardiovasc Interv. 2007;70:123-130.
43 Tajima H, Murata S, Kumazaki T, et al. Hybrid treatment of acute massive pulmonary thromboembolism: mechanical fragmentation with a modified rotating pigtail catheter, local fibrinolytic therapy, and clot aspiration followed by systemic fibrinolytic therapy. Am J Roentgenol. 2004;183:589-595.
44 Mercat A, Diehl JL, Meyer G, et al. Hemodynamic effects of fluid loading in acute massive pulmonary embolism. Crit Care Med. 1999;27:540-544.
45 Wood KE. Major pulmonary embolism: review of a pathophysiologic approach to the golden hour of hemodynamically significant pulmonary embolism. Chest. 2002;121:877-905.
46 Maggio P, Hemmila M, Haft J, et al. Extracorporeal life support for massive pulmonary embolism. J Trauma. 2007;62:570-576.
47 Mielck F, Quintel M. Extracorporeal membrane oxygenation. Curr Opin Crit Care. 2005;11:87-93.
48 Capellier G, Jacques T, Balvay P, et al. Inhaled nitric oxide in patients with pulmonary embolism. Intens Care Med. 1997;23:1089-1092.
49 Webb SA, Stott S, van Heerden PV. The use of inhaled aerosolized prostacyclin (IAP) in the treatment of pulmonary hypertension secondary to pulmonary embolism. Intens Care Med. 1996;22:353-355.
50 Geerts WH, Pineo GF, Heit JA, et al. Prevention of venous thromboembolism: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126:338S-400S.
51 Kahn SR, Panju A, Geerts W, et al. Multicenter evaluation of the use of venous thromboembolism prophylaxis in acutely ill medical patients in Canada. Thromb Res. 2007;119:145-155.
52 Goldhaber SZ, Dunn K, MacDougall RC. New onset of venous thromboembolism among hospitalized patients at Brigham and Women’s Hospital is caused more often by prophylaxis failure than by withholding treatment. Chest. 2000;118:1680-1684.
53 Eikelboom JW, Quinlan DJ, Douketis JD. Extended-duration prophylaxis against venous thromboembolism after total hip or knee replacement: a meta-analysis of the randomised trials. Lancet. 2001;358:9-15.
54 Velmahos GC, Oh Y, McCombs J, et al. An evidence-based cost-effectiveness model on methods of prevention of posttraumatic venous thromboembolism. J Trauma. 2000;49:1059-1064.
55 Pulmonary Embolism Prevention (PEP) Trial Collaborative Group. Prevention of pulmonary embolism and deep vein thrombosis with low dose aspirin: Pulmonary Embolism Prevention (PEP) trial. Lancet. 2000;355:1295-1302.
56 Urbankova J, Quiroz R, Kucher N, et al. Intermittent pneumatic compression and deep vein thrombosis prevention. A meta-analysis in postoperative patients. Thromb Haemost. 2005;94:1181-1185.