Pulmonary Edema
After reading this chapter, you will be able to:
• List the anatomic alterations of the lungs associated with pulmonary edema.
• Describe the causes of pulmonary edema.
• List the cardiopulmonary clinical manifestations associated with pulmonary edema.
• Describe the general management of pulmonary edema.
• Describe the clinical strategies and rationales of the SOAPs presented in the case study.
• Define key terms and complete self-assessment questions at the end of the chapter and on Evolve.
Anatomic Alterations of the Lungs
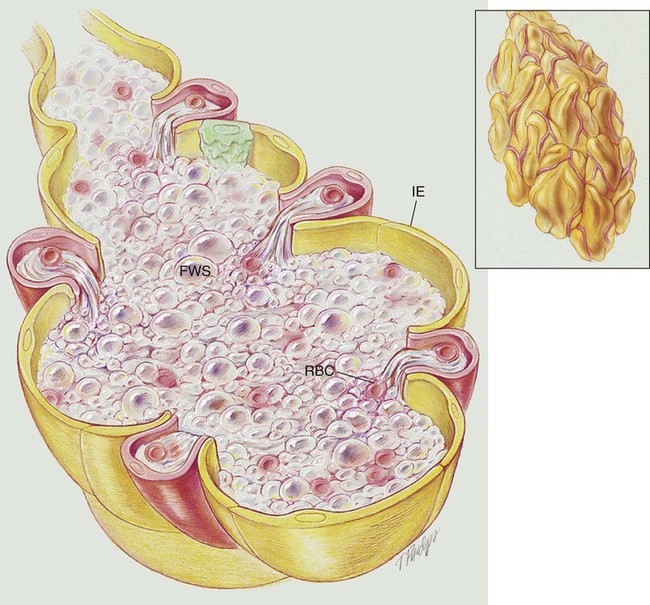
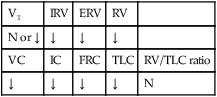
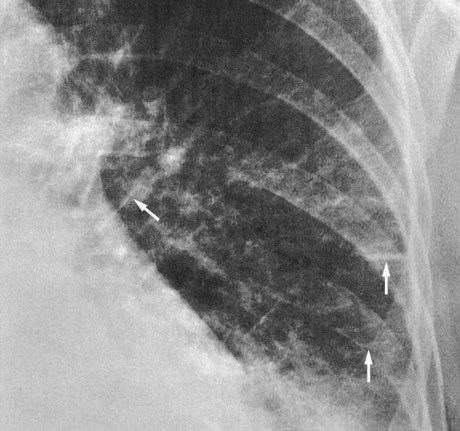
Pulmonary edema results from excessive movement of fluid from the pulmonary vascular system to the extravascular system and air spaces of the lungs. Fluid first seeps into the perivascular and peribronchial interstitial spaces; depending on the degree of severity, fluid may progressively move into the alveoli, bronchioles, and bronchi (see Figure 19-1).
Etiology and Epidemiology
Cardiogenic Pulmonary Edema
Movement of fluid in and out of the capillaries is expressed by Starling’s equation:
< ?xml:namespace prefix = "mml" />
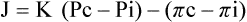
where J is the net fluid movement out of the capillary, K is the capillary permeability factor, Pc and Pi are the hydrostatic pressures in the capillary and interstitial space, and πc and πi are the oncotic pressures in the capillary and interstitial space.
When the hydrostatic pressure within the pulmonary vascular system rises to more than 25 to 30 mm Hg, the oncotic pressure loses its holding force over the fluid within the pulmonary capillaries. Consequently fluid starts to spill into the interstitial and air spaces of the lungs (see Figure 19-1).
Clinically, the patient with left ventricular failure often has anxiety, delirium, dyspnea, orthopnea, paroxysmal nocturnal dyspnea, cough, fatigue, and adventitious breath sounds. Because of poor peripheral circulation, such patients often have cool skin, diaphoresis, cyanosis of the digits, and peripheral pallor. Increased pulmonary capillary hydrostatic pressure is the most common cause of pulmonary edema. Box 19-1 provides common causes of cardiogenic pulmonary edema. Box 19-2 provides common risk factors for coronary heart disease (CHD).
Noncardiogenic Pulmonary Edema
Increased Capillary Permeability
General Management of Pulmonary Edema
Medications and Procedures Commonly Prescribed by the Physician
Positive Inotropic Agents (Improve Cardiac Output)
When left-sided heart failure is present, positive inotropic agents (e.g., digitalis, dopamine, dobutamine, and amiodarone) are commonly administered to increase cardiac output. Digitalis is the most frequently prescribed inotropic agent for heart failure and is the drug of choice (see Appendix II, Positive Inotropic Agents).
Respiratory Care Treatment Protocols
Oxygen Therapy Protocol
Oxygen therapy is used to treat hypoxemia, decrease the work of breathing, and decrease myocardial work. The hypoxemia that develops in pulmonary edema is most commonly caused by the interstitial and alveolar fluid, atelectasis, and capillary shunting associated with the disorder. Hypoxemia caused by capillary shunting is at least partially refractory to oxygen therapy (see Oxygen Therapy Protocol, Protocol 9-1).
Lung Expansion Therapy Protocol
Lung expansion therapy is commonly prescribed to offset the fluid accumulation and alveolar shrinkage associated with pulmonary edema. For example, high-flow mask continuous positive airway pressure (CPAP) has been shown to produce a significant and rapid improvement in oxygenation and ventilatory status in patients with pulmonary edema. Mask CPAP improves decreased lung compliance, decreases the work of breathing, enhances gas exchange, and decreases vascular congestion in patients with pulmonary edema. In fact, mask CPAP is prescribed (at least for a trial period) for patients with pulmonary edema who have arterial blood gas values that reveal impending or acute ventilatory failure—the hallmark clinical manifestation for mechanical ventilation. Often, mask CPAP dramatically improves oxygenation and ventilatory status in these patients and eliminates the need for mechanical ventilation (see Lung Expansion Therapy Protocol, Protocol 9-3).
Respiratory Assessment and Plan
S Patient states “a feeling of suffocation.”
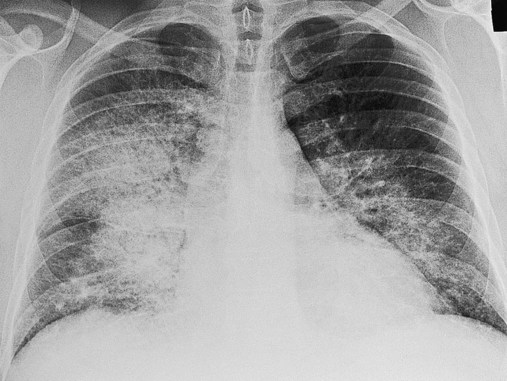
O Cyanosis, disorientation. Distended neck veins and mottled extremities. BP 105/50, HR 124, RR 28. ECG: sinus tach and occasional PVCs. Distended neck veins, mottled extremities, coarse rhonchi and crackles bilaterally. Frothy pink sputum. CXR: Bilateral fluffy infiltrates and an enlarged heart. ABG: pH 7.11, Paco2 72, 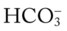 25, and Pao2 56 (Fio2 0.30). Spo2 87%.
25, and Pao2 56 (Fio2 0.30). Spo2 87%.
P Oxygen Therapy Protocol: Increase Fio2 to 0.60 via continuous CPAP mask at 25 cm H2O per Lung Expansion Therapy Protocol. Remain on standby for emergency endotracheal intubation and ventilator support. Continue ECG and oximetry monitoring, and repeat ABG in 30 minutes.
Respiratory Assessment and Plan
S Patient states, “I’m less short of breath. No pain.”
O Not cyanotic. BP 126/70, HR 96, RR 18. ECG: Mild sinus tachycardia without ectopic beats. Fewer crackles; no sputum production; CXR: Improved. ABG: pH 7.35, Paco2 46, 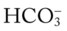 24, Pao2 120 (Fio2 0.50).
24, Pao2 120 (Fio2 0.50).
• Decreased pulmonary edema (overall impression from the data)
• No longer in ventilatory failure (ABG)
• Acceptable acid-base status with excessively corrected hypoxemia (ABG)
P Reduce O2 per Oxygen Therapy Protocol to 2 L/min by nasal cannula. Discontinue CPAP per Lung Expansion Therapy Protocol. Continue ECG and oximetric monitoring. Repeat ABG in 60 minutes.
Discussion
Acute pulmonary edema is a classic finding in CHF. Several clinical manifestations associated with Increased Alveolar-Capillary Membrane Thickness (see Figure 9-10) were present in this case. For example, the patient’s decreased lung compliance was manifested in his tachycardia and tachypnea, whereas his hypoxemia reflected diffusion blockade associated with classic pulmonary edema. His lung compliance was so reduced that he had progressed to acute ventilatory failure—the severe stage of pulmonary edema. Some Atelectasis (see Figure 9-8) was doubtless also present and was the rationale for CPAP therapy. In addition, the clinical scenario associated with Excessive Bronchial Secretions (see Figure 9-12) also was evident initially with frothy blood-tinged sputum and coarse rhonchi and crackles in both lower lung fields. The patient was too ill to allow valid pulmonary function testing, but the suspicion is that a combined obstructive and restrictive pattern may have been present at the time of the first assessment.


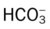

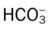
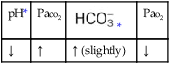
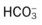 values will be lower than expected for a particular Pa
values will be lower than expected for a particular Pa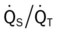
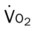
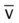 )
)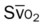

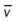 )O2, Arterial-venous oxygen difference; DO2, total oxygen delivery; O2ER, oxygen extraction ratio;
)O2, Arterial-venous oxygen difference; DO2, total oxygen delivery; O2ER, oxygen extraction ratio; 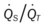 , pulmonary shunt fraction;
, pulmonary shunt fraction; 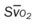 , mixed venous oxygen saturation;
, mixed venous oxygen saturation; 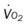 , oxygen consumption.
, oxygen consumption.
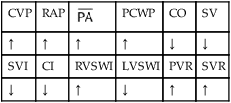
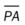 , mean pulmonary artery pressure; PCWP, pulmonary capillary wedge pressure; PVR, pulmonary vascular resistance; RAP, right atrial pressure; RVSWI, right ventricular stroke work index; SV, stroke volume; SVI, stroke volume index; SVR, systemic vascular resistance.
, mean pulmonary artery pressure; PCWP, pulmonary capillary wedge pressure; PVR, pulmonary vascular resistance; RAP, right atrial pressure; RVSWI, right ventricular stroke work index; SV, stroke volume; SVI, stroke volume index; SVR, systemic vascular resistance.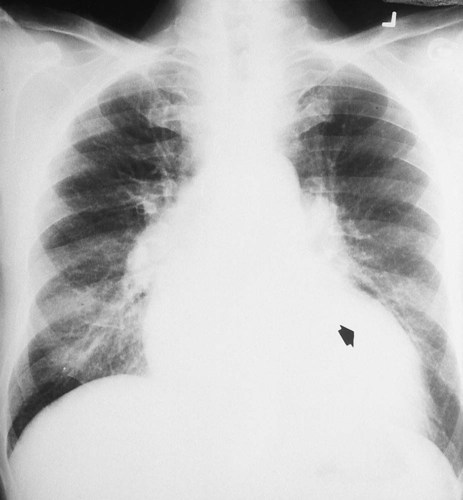
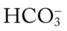 27, and Pa
27, and Pa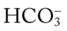 24, and Pa
24, and Pa