Chapter 24 Pulmonary Atresia with Intact Ventricular Septum
Thomas Peacock,1 in his first edition of Malformations of the Human Heart, wrote:
“… the orifice or trunk of the pulmonary artery is entirely impervious. A case of this description was described by John Hunter in 1783.2 The child was born at the eighth month, was very livid … and died in convulsions on the thirteenth day. The pulmonary artery was found entirely impervious. The septum of the ventricles was entire, and the right ventricle had scarcely any cavity left, while the left ventricle was large and powerful. The foramen ovale continued open, and the pulmonary branches received their supply of blood from the aorta, through the medium of the arterial duct.”
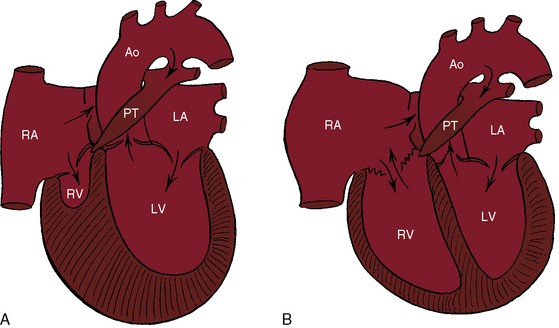
Peacock referred to what is now called pulmonary atresia with intact ventricular septum, a malformation in which an imperforate pulmonary valve completely obstructs forward flow into the pulmonary trunk (Figure 24-1). The ventricular septum is, by definition, intact, and a restrictive interatrial communication is the only exit from the right atrium. The imperforate pulmonary valve consists of three fused but well-formed cusps with triradiate commissural ridges that converge at the center of a sealed orifice that is structurally similar, if not identical, to pinpoint pulmonary valve stenosis (see Chapter 11).3 Less commonly, commissural ridges are confined to the periphery of the valve, which has an imperforate dimple at the center.3 The atresia is valvular (i.e., membranous) in 75% of cases and muscular (i.e., infundibular) in the remainder.4,5 Muscular obliteration within the right ventricle results in a “unipartite, bipartite, or tripartite” ventricular chamber with an obliterated apex.4,5
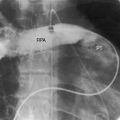
The reported incidence rate of pulmonary atresia with intact ventricular septum is 1 per 22,000 live births6 to 4.2 per 100,000 live births.5,7 A well-developed pulmonary trunk (see Figure 24-1) implies that forward flow once occurred across a pulmonary valve that was stenotic but patent during much, if not most, of fetal life. A normally formed ductus arteriosus implies that intrauterine blood flow was from the right ventricle into the pulmonary trunk through the ductus and into the aorta (see Figure 24-5), in contrast to Fallot’s tetralogy with pulmonary atresia in which a long tortuous ductus functions in utero as a systemic artery that carries blood from the aorta into the pulmonary artery (see Chapter 18).
The normal right ventricle consists of an inlet portion that is part of the diastolic filling mechanism and a trabecular and an infundibular portion that are parts of the systolic pump mechanism. More than three quarters of cases of pulmonary atresia with intact ventricular septum are characterized by a small thick-walled right ventricle (Figures 24-1A and 24-2A, B) that is equipped with all three of these morphologic portions (see Figures 24-1A and 24-2A, B),8,9 together with fiber disarray, capillary disorganization, and endocardial fibroelastosis.10,11 Pinpoint pulmonary valve stenosis in the neonate occasionally exists with a diminutive right ventricular cavity analogous to pulmonary atresia with intact ventricular septum.
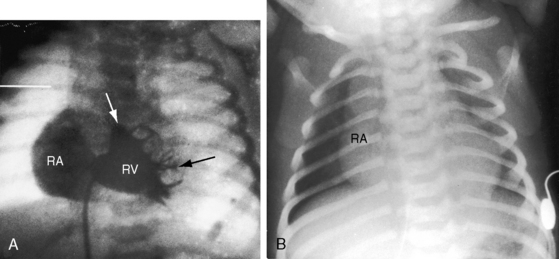
When the right ventricle is small and thick-walled, the tricuspid annulus is also small and houses a small, thickened valve with poorly delineated chordal attachments and hypoplastic papillary muscles. When the right ventricle is dilated and thin-walled, the tricuspid valve is incompetent and either resembles Ebstein’s malformation or is dysplastic with normally attached tricuspid leaflets. Functional pulmonary atresia has been applied when a dilated mechanically inadequate right ventricle with tricuspid regurgitation cannot generate sufficient systolic pressure to open a stenotic but nonatretic pulmonary valve.12 Functional pulmonary atresia also occurs during transient neonatal tricuspid regurgitation.13 In a small subset of patients with florid tricuspid regurgitation, the right ventricle and right atrium are enormous—“wall-to-wall hearts” (see section X-Ray).14 Rarely, a suprasystemic right ventricle causes leftward displacement of the ventricular septum and obstruction to left ventricular outflow.15,16
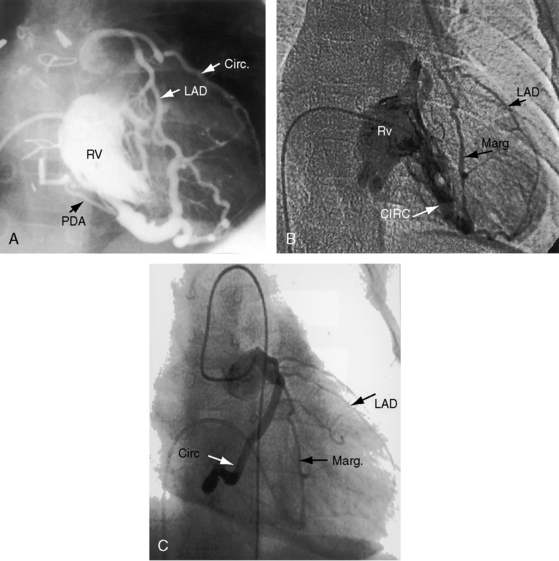
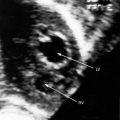
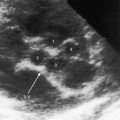
The coronary circulation in pulmonary atresia with intact ventricular septum and small right ventricle has long been the focus of lively interest.11,17–24 Intramyocardial sinusoids were described in 192625 and play important roles in the pathogenesis of right ventricular-to-coronary artery communications (see Figure 24-2).17,18 Morphogenetic studies have shed new light on coronary vascular bed abnormalities in this malformation (see Chapter 32).19,26 The normal coronary circulation develops in an orderly sequence of blood islands, coronary venous connections, and coronary artery-to-aortic connections. The blood islands proliferate and coalesce to form networks of vascular channels that have no connection with other blood islands or with the ventricular cavity. Suprasystemic systolic pressure from the small isovolumetrically contracting right ventricle drives blood through primitive vascular channels that are composed of thickened intima and fibroelastic walls19 and that connect the right ventricular cavity to epicardial coronary arteries (see Figure 24-2A, B).18,19 Abnormalities of the coronary vascular bed occur in 50% of patients with pulmonary atresia, intact ventricular septum, and a small right ventricle and are either secondary to the hemodynamic derangements of the malformation or are coexisting morphologic abnormalities,18,23 such as ostial atresia24,27or fibromuscular dysplasia with focal luminal narrowing.17 Myocardial sinusoids are rarely primary developmental faults.28 Epicardial veins may be prominent and thick-walled.19 Ventriculocoronary arterial connections are located chiefly in the region of the apex of right ventricle and communicate chiefly with the distal left anterior descending coronary arterial system (see Figure 24-2A, B).19 Intramyocardial channels that end blindly punctuate the endocardium of the thick-walled right ventricle, creating the appearance of a highly trabeculated muscular wall (Figures 24-3 and 24-4).19
Ventriculocoronary arterial communications have been found in the inverted left ventricle of congenitally corrected transposition of the great arteries with pulmonary atresia and intact ventricular septum.29,30 Left ventricular–to–coronary arterial connections in aortic atresia with intact ventricular septum differ from right ventricular–to–coronary arterial connections in pulmonary atresia with intact ventricular septum (see Chapters 31 and 32).19,31,32
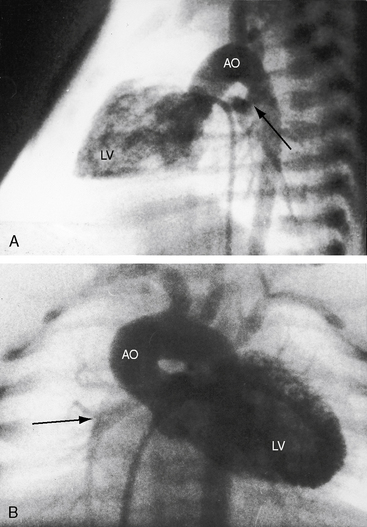
Myocardial ischemia is an important sequel of ventriculocoronary arterial connections.18,19 Large unobstructed connections (see Figure 24-2A, C) function as a fistulous steal because blood from the aortic root flows freely into the right ventricular cavity during diastole. More commonly and more importantly, ischemia and occasionally myocardial infarction result from obstructing luminal abnormalities that range from mild medial and intimal thickening to luminal obliteration and that extend from the origins of the intramyocardial connections to the coronary artery ostia in the aortic sinuses.17–19 Luminal obstructive lesions that originate in the fetus and neonate can evolve into severe stenoses or obliteration.18,19 Proximal discontinuity of a coronary artery is the most egregious form of this prejudicial coronary circulation and is the result of acquired ostial obliteration or congenital atresia of an aortic sinus ostium. Left ventricular aneurysm9 and right ventricular rupture33,34 are consequences of myocardial ischemia and infarction that are largely responsible for the high mortality rate.18 Proximal discontinuity and luminal obstruction set the stage for a right ventricular–dependent coronary circulation in which suprasystemic systolic pressure is required to generate retrograde coronary blood flow on which myocardial perfusion depends.33 The right atrium enlarges only moderately when the right ventricle is diminutive, but when the right ventricle is dilated and tricuspid regurgitation is severe, the right atrium can be aneurysmal (see Figure 24-4). The left atrium enlarges because it receives venous return from both the systemic and the pulmonary circulations. The left ventricle is initially large and powerful as Peacock1 described (Figure 24-5), but its compliance and ejection fraction diminish because it pumps the entire output for both circulations.35 The course of the atrioventricular conduction system is normal.36
The physiologic derangements in pulmonary atresia with intact ventricular septum are implicit consequences of the anatomic features of the malformation. Physiologic classification is based on the tripartite morphology of the right ventricle and on whether the right ventricular hypoplasia is mild, moderate, or severe. Venous blood reaches the systemic circulation via an interatrial communication that is usually a restrictive patent foramen ovale, which is the only exit from the right atrium (see Figure 24-1; see previous). Unoxygenated right atrial blood mixes with oxygenated left atrial blood and flows into the left ventricle and into the aorta, so systemic arterial oxygen saturation is reduced. Blood from the aorta reaches the pulmonary circulation through a tenuously patent ductus arteriosus on which survival depends (see Figures 24-1 and 24-5).37 When the ductus closes, effective pulmonary blood flow ceases.37 Systemic-to-pulmonary collaterals are inadequate for survival.37
These physiologic pathways from the right atrium to the left side of the heart are relatively constant from patient to patient, but the patterns in the right side of the heart vary considerably. When the right ventricle is small, little blood enters or leaves its cavity because the diminutive chamber and the diminutive tricuspid valve obstruct right atrial flow, which is further compromised by a thick ventricular wall and endocardial fibroelastosis. Whatever blood enters the right ventricle during diastole is trapped during systole, except for the small portion that exits through ventriculocoronary arterial connections. When the right ventricle is dilated and thin-walled, and the tricuspid valve is incompetent, right atrial blood copiously enters the ventricular cavity during diastole, only to be returned to the right atrium during the next systole. Systolic pressure is comparatively low when the right ventricle and right atrium communicate freely across an incompetent tricuspid valve. Although right atrial blood readily flows into the right ventricle, no useful purpose is served because forward flow into the pulmonary trunk is impossible. Back-and-forth movement of blood across the tricuspid orifice results in progressive enlargement of the right side of the heart, which becomes massive (see previous).14
History
Pulmonary atresia with intact ventricular septum is equally prevalent in males and females.38 Reports exist of the malformation in siblings,39,40 in first cousins,41 and in monozygotic twins,42 but familial recurrence is rare. More than half of newborns die in the first month of life, a considerable majority die within the first 3 months, and very few live beyond the first year. Survival hinges on tenuous patency of the ductus arteriosus and on the adequacy of the interatrial communication, which is usually restrictive.37 Cyanosis begins immediately after birth, and the degree depends on patency of the ductus arteriosus.37 Pulmonary blood flow diminishes as the ductus involutes, and when the ductus closes, pulmonary blood flow ceases. Despite this ominous outlook, isolated examples of survival have been reported to age 3.5 years43 and 14 years,44 and four patients lived for 20 to 21 years.45–48 The 21-year-old patient had a closed ductus arteriosus at necropsy and had instead a sizable connection between the aortic root and pulmonary trunk and a large atrial septal defect.48 Another 21-year-old woman survived without a patent ductus because the anterior descending branch of the left coronary artery originated from the pulmonary trunk and provided pulmonary blood flow via intercoronary anastomoses.49
Precordial movement and palpation
A right ventricular impulse is necessarily absent when pulmonary atresia with intact ventricular septum occurs with a small right ventricle. The impulse of an enlarged left ventricle occupies the apex (see Figure 24-5). When the right ventricle is dilated and the tricuspid valve is incompetent, a right ventricular impulse is readily palpable. The thrill of tricuspid regurgitation may be present at the lower left sternal edge. A very large right atrium with severe tricuspid regurgitation generates a visible and palpable systolic impulse at the right sternal border and over the liver.
Auscultation
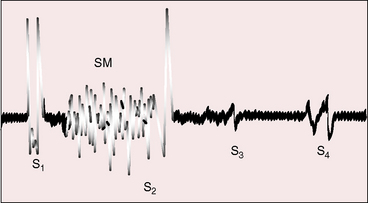
When the right ventricle is small, the hypoplastic tricuspid valve mechanism generates no closure sound, so the first heart sound consists of the single mitral component. When the right ventricle is dilated, audibility of the tricuspid component of the first heart sound depends on mobility of the anterior leaflet, as in Ebstein’s anomaly (Figure 24-6).
A soft transient systolic murmur has been ascribed to flow through the patent ductus before its involution, and a soft midsystolic aorta flow murmur occasionally results from increased left ventricular stroke volume.37 The murmur of tricuspid regurgitation is present at birth when the right ventricle is dilated and the malformed tricuspid valve is incompetent (see Figure 24-6). The murmur is medium frequency and decrescendo because right ventricular systolic pressure is relatively low. Maximal intensity is along the lower left sternal edge toward the apex, topographically coinciding with the displaced tricuspid valve, but the murmur may radiate to the right side of the chest and even to the right axilla when a very large right atrium receives tricuspid regurgitant flow (see Figure 24-4).
Continuous murmurs that originate in the ductus arteriosus are rare because ductal patency is transient. In the 21-year -ld woman referred to previously, a continuous murmur was generated, not by a ductus but by flow through intercoronary anastomoses between the right coronary artery and the anterior descending branch of the left coronary artery.49 In the other 21-year-old woman, a systolic/diastolic murmur originated in a communication between the aortic root and the pulmonary trunk.48 A 2-month-old girl with bidirectional ventriculocoronary arterial flow had a to-and-fro murmur.50
The second heart sound is necessarily single because there is only one functional semilunar valve. Third and fourth heart sounds occur with tricuspid regurgitation because of rapid mid-diastolic filling and an increased force of right atrial contraction transmitted into an unobstructed right ventricle (see Figure 24-6).
Electrocardiogram
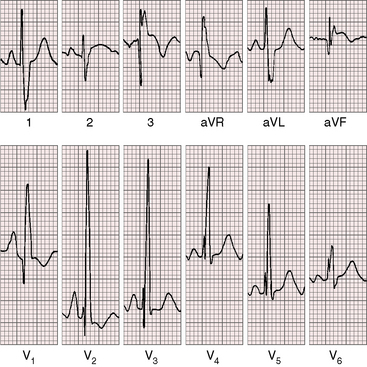
The electrocardiogram is a key to the clinical recognition of pulmonary atresia with intact ventricular septum and a small right ventricle because it calls attention to the combination of cyanosis with a dominant left ventricle. The electrocardiogram also distinguishes this malformation from tricuspid atresia, which it physiologically resembles (see Chapter 25).
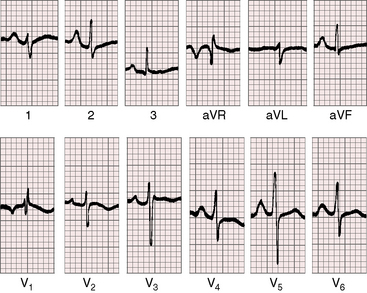
When the right ventricle is small, peaked right atrial P waves appear in limb leads and in right precordial leads (Figures 24-7 and 24-8).51 Biatrial P waves occasionally emerge because the left atrium receives systemic and pulmonary venous return.51 The QRS mean axis is normal or rightward with clockwise deplorization (see Figures 24-7 and 24-8),51 which is a useful means of distinguishing pulmonary atresia with intact ventricular septum and small right ventricle from tricuspid atresia, which is characterized by left axis deviation and counterclockwise depolarization of the QRS.51 Precordial leads display an adult QRS pattern (see Figures 24-7 and 24-8).8,51 Right ventricular hypertrophy is conspicuously absent. The small right ventricle does not generate large precordial QRS amplitudes despite its thick wall because the cavity contains so little blood.51 Similar electrocardiographic patterns can occur in neonates with pinpoint pulmonary stenosis and diminutive right ventricle (see Chapter 11). Left ventricular hypertrophy is manifested by the voltage,51 but ST segment abnormalities reflect left ventricular ischemia rather than hypertrophy.
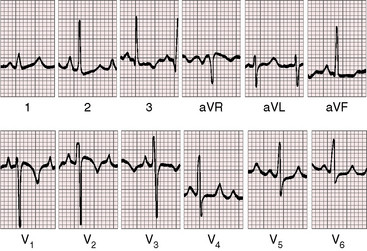
In the first week of life, the electrocardiogram does not reliably distinguish pulmonary atresia with intact ventricular septum and small right ventricle from pulmonary atresia with intact ventricular septum and a large right ventricle. Soon, however, the electrocardiogram associated with a dilated right ventricle and tricuspid regurgitation reveals itself because of a rightward QRS axis and right ventricular hypertrophy in precordial leads (Figure 24-9).8,51,52 Right atrial enlargement is reflected in exceptionally tall P waves and a qR complex in lead V1 (see Figure 24-9).
X-ray
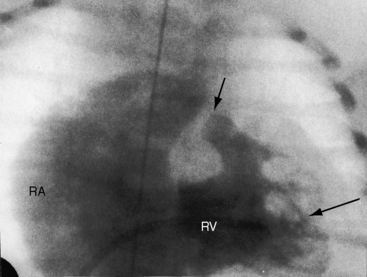
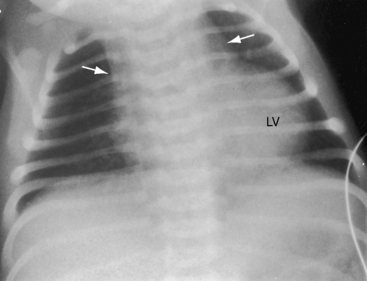
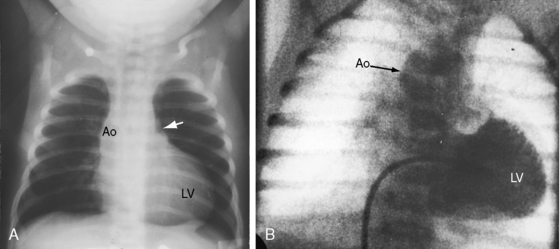
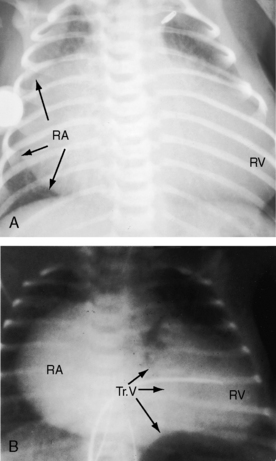
When the right ventricle is small, the cardiac silhouette at birth may be normal or nearly so (Figure 24-10).52 The pulmonary artery segment is normal because the pulmonary trunk develops normally despite pulmonary valve atresia (Figure 24-11). The ascending aorta is enlarged (see Figures 24-5B, and 24-11).43 The right atrial shadow is moderately prominent (see Figure 24-11). A well-formed convex left ventricle occupies the apex (see Figures 24-5, 24-10, and 24-11).43
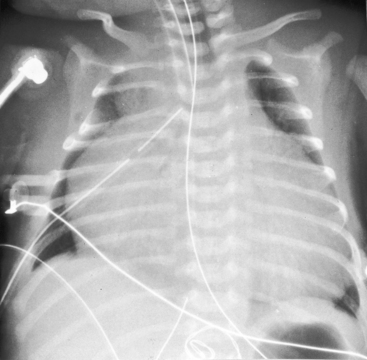
The x-ray differs appreciably when the right ventricle is dilated and the tricuspid valve is incompetent.43 The cardiac silhouette virtually fills the chest—“wall-to-wall”14—because of remarkable enlargement of the right atrium and right ventricle (Figures 24-12 and 24-13).
Echocardiogram
Echocardiography with color flow imaging and Doppler interrogation establishes the diagnosis of pulmonary atresia with intact ventricular septum, identifies the atretic pulmonary valve and the well-formed pulmonary trunk, and distinguishes a small right ventricle with a small tricuspid valve from a dilated right ventricle with tricuspid regurgitation.12,13,38 The malformation can be recognized in utero.53
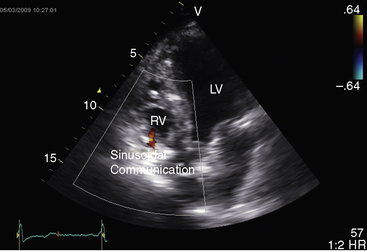
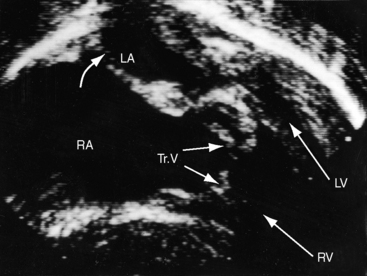
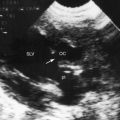
Hypoplasia of the right ventricle is judged as mild, moderate, or severe (Figure 24-14 and Video 24-1), and the inlet, trabecular, and outlet portions can be imaged.38 Deep endocardial recesses represent blind penetrations of the proximal segments of ventriculocoronary arterial communications (see Figure 24-14). Retrograde flow into the aorta is detected with pulsed Doppler or can be suspected because of dilation of a proximal coronary artery. Color flow imaging with Doppler interrogation confirms the absence of flow across the atretic pulmonary valve, which appears as an immobile dense line that moves but fails to open. Initial patency of the ductus is identified with color flow imaging, which also detects a right-to-left shunt across a restrictive interatrial communication.
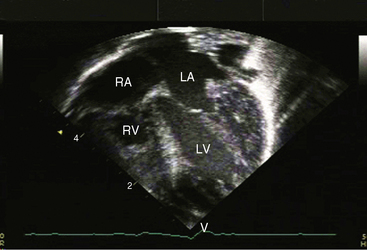
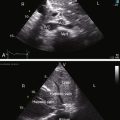
When the right ventricle is dilated, echocardiography identifies the Ebstein’s-like malformation of the tricuspid valve with displacement and immobility of the septal and posterior leaflets (Figures 24-15A and 24-16A). Color flow imaging establishes the degree of tricuspid regurgitation (Figures 24-15B, and 24-16B). The right atrium can be immense (see Figures 24-15A, and 24-16A). Ebstein’s anomaly with functional pulmonary atresia in which a patent but stenotic pulmonary valve fails to open can be distinguished from anatomic pulmonary atresia and a malformed Ebstein’s-like tricuspid valve.12
1 Peacock T.B. On malformations of the human heart: with original cases. London: John Churchill & Sons; 1858.
2 Hunter J.. Medical observations and inquiries: Society of Physicians in London. 1784.
3 Braunlin E.A., Formanek A.G., Moller J.H., Edwards J.E. Angio-pathological appearances of pulmonary valve in pulmonary atresia with intact ventricular septum. Interpretation of nature of right ventricle from pulmonary angiography. Br Heart J. 1982;47:281-289.
4 Daubeney P.E.F., Delany D.J., Anderson R.H., et alUnited K, Ireland Collaborative Study of Pulmonary Atresia with Intact Ventricular S. Pulmonary atresia with intact ventricular septum: range of morphology in a population-based study. J Am Coll Cardiol. 2002;39:1670-1679.
5 Shinebourne E.A., Rigby M.L., Carvalho J.S. Pulmonary atresia with intact ventricular septum: from fetus to adult: congenital heart disease. Heart. 2008;94:1350-1357.
6 Daubeney P.E., Sharland G.K., Cook A.C., Keeton B.R., Anderson R.H., Webber S.A. Pulmonary atresia with intact ventricular septum: impact of fetal echocardiography on incidence at birth and postnatal outcome. UK and Eire Collaborative Study of Pulmonary Atresia with Intact Ventricular Septum. Circulation. 1998;98:562-566.
7 Ekman J., Sunnegardh J., Hanseus K. Outcome of children born with pulmonary atresia and intact septum in Sweden from 1980 to 1990. Scand Cardiovasc J. 2001;35:192.
8 Shams A., Fowler R.S., Trusler G.A., Keith J.D., Mustard W.T. Pulmonary atresia with intact ventricular septum: report of 50 cases. Pediatrics. 1971;47:370-377.
9 Zuberbuhler J.R., Anderson R.H. Morphological variations in pulmonary atresia with intact ventricular septum. Br Heart J. 1979;41:281-288.
10 Bulkley B.H., D’Amico B., Taylor A.L. Extensive myocardial fiber disarray in aortic and pulmonary atresia. Relevance to hypertrophic cardiomyopathy. Circulation. 1983;67:191-198.
11 Oosthoek P.W., Moorman A.F., Sauer U., Gittenberger-De Groot A.C. Capillary distribution in the ventricles of hearts with pulmonary atresia and intact ventricular septum. Circulation. 1995;91:1790-1798.
12 Smallhorn J.F., Izukawa T., Benson L., Freedom R.M. Noninvasive recognition of functional pulmonary atresia by echocardiography. Am J Cardiol. 1984;54:925-926.
13 Gewillig M., Dumoulin M., Van Der Hauwaert L.G. Transient neonatal tricuspid regurgitation: a Doppler echocardiographic study of three cases. Br Heart J. 1988;60:446-451.
14 Freedom R.M., Jaeggi E., Perrin D., Yoo S.-J., Anderson R.H. The “wall-to-wall” heart in the patient with pulmonary atresia and intact ventricular septum. Cardiol Young. 2006;16:18-29.
15 Amin P., Levi D.S., Likes M., Laks H. Pulmonary atresia with intact ventricular septum causing severe left ventricular outflow tract obstruction. Pediatr Cardiol. 2009;30:851-854.
16 Peraira Moral J.R., Burguens Valero M., Garcia-Guereta Silva L. Pulmonary valve atresia with intact ventricular septum and severe aortic stenosis. Pediatr Cardiol. 2005;26:117-118.
17 L’Ecuyer T.J., Poulik J.M., Vincent J.A. Myocardial infarction due to coronary abnormalities in pulmonary atresia with intact ventricular septum. Pediatr Cardiol. 2001;22:68-70.
18 Kasznica J., Ursell P.C., Blanc W.A., Gersony W.M. Abnormalities of the coronary circulation in pulmonary atresia and intact ventricular septum. Am Heart J. 1987;114:1415-1420.
19 O’Connor W.N., Stahr B.J., Cottrill C.M., Todd E.P., Noonan J.A. Ventriculocoronary connections in hypoplastic right heart syndrome: autopsy serial section study of six cases. J Am Coll Cardiol. 1988;11:1061-1072.
20 Craver R.D., Caspi J. Pulmonary atresia, intact ventricular septum, right ventricle-dependent coronary artery circulation, and systemic collateral arteries supplying the left coronary artery. Pediatr Dev Pathol. 2006;9:152-156.
21 Dyamenahalli U., Mccrindle B.W., Mcdonald C., et al. Pulmonary atresia with intact ventricular septum: management of, and outcomes for, a cohort of 210 consecutive patients. Cardiol Young. 2004;14:299-308.
22 L’Ecuyer T.J., Poulik J.M., Vincent J.A. Myocardial infarction due to coronary abnormalities in pulmonary atresia with intact ventricular septum. Pediatr Cardiol. 2001;22:68-70.
23 Sandor G.G.S., Cook A.C., Sharland G.K., Ho S.Y., Potts J.E., Anderson R.H. Coronary arterial abnormalities in pulmonary atresia with intact ventricular septum diagnosed during fetal life. Cardiol Young. 2002;12:436-444.
24 Sarkola T., Boldt T., Happonen J.-M., Karikoski R., Eronen M. Atresia of proximal coronary arteries in pulmonary atresia with intact ventricular septum: fetal and neonatal findings. Fetal Diagn Ther. 2008;24:413-415.
25 Grant R. An unusual anomaly of the coronary vessels in the malformed heart of a child. Heart. 1926;13:273-283.
26 Hutchins G.M., Kessler-Hanna A., Moore G.W. Development of the coronary arteries in the embryonic human heart. Circulation. 1988;77:1250-1257.
27 Wald R.M., Juraszek A.L., Pigula F.A., Geva T. Echocardiographic diagnosis and management of bilateral coronary ostial atresia in a patient with pulmonary atresia and intact ventricular septum. J Am Soc Echocardiogr. 2006;19:939.e931-939.e933.
28 Engberding R., Bender F. Identification of a rare congenital anomaly of the myocardium by two-dimensional echocardiography: persistence of isolated myocardial sinusoids. Am J Cardiol. 1984;53:1733-1734.
29 Shimizu T., Ando M., Takao A. Pulmonary atresia and intact ventricular septum and corrected transposition of the great arteries. Br Heart J. 1981;45:471-474.
30 Steeg C.N., Ellis K., Bransilver B., Gersony W.M. Pulmonary atresia and intact ventricular septum complicating corrected transposition of the great vessels. Am Heart J. 1971;82:382-386.
31 Baffa J.M., Chen S.L., Guttenberg M.E., Norwood W.I., Weinberg P.M. Coronary artery abnormalities and right ventricular histology in hypoplastic left heart syndrome. J Am Coll Cardiol. 1992;20:350-358.
32 Sauer U., Gittenberger-De Groot A.C., Geishauser M., Babic R., Buhlmeyer K. Coronary arteries in the hypoplastic left heart syndrome. Histopathologic and histometrical studies and implications for surgery. Circulation. 1989;80:I168-I176.
33 Powell A.J., Mayer J.E., Lang P., Lock J.E. Outcome in infants with pulmonary atresia, intact ventricular septum, and right ventricle-dependent coronary circulation. Am J Cardiol. 2000;86:1272-1274. A1279
34 Hubbard J.F., Girod D.A., Caldwell R.L., Hurwitz R.A., Mahony L.A., Waller B.F. Right ventricular infarction with cardiac rupture in an infant with pulmonary valve atresia with intact ventricular septum. J Am Coll Cardiol. 1983;2:363-368.
35 Scognamiglio R., Daliento L., Razzolini R., et al. Pulmonary atresia with intact ventricular septum: a quantitative cineventriculographic study of the right and left ventricular function. Pediatr Cardiol. 1986;7:183-187.
36 Ansari A., Goltz D., Mccarthy K.P., Cook A., Ho S.Y. The conduction system in hearts with pulmonary atresia and intact ventricular septum. Ann Thorac Surg. 2003;75:1502-1505.
37 Venables A.W. The patterns of pulmonary circulation in pulmonary atresia. Br Heart J. 1964;26:760-769.
38 Leung M.P., Mok C.K., Hui P.W. Echocardiographic assessment of neonates with pulmonary atresia and intact ventricular septum. J Am Coll Cardiol. 1988;12:719-725.
39 Chitayat D., Mcintosh N., Fouron J.C. Pulmonary atresia with intact ventricular septum and hypoplastic right heart in sibs: a single gene disorder? Am J Med Genet. 1992;42:304-306.
40 Eriksen N.L., Buttino L.Jr., Juberg R.C. Congenital pulmonary atresia with intact ventricular septum, tricuspid insufficiency, and patent ductus arteriosus in two sibs. Am J Med Genet. 1989;32:187-188.
41 Grossfeld P.D., Lucas V.W., Sklansky M.S., Kashani I.A., Rothman A. Familial occurrence of pulmonary atresia with intact ventricular septum. Am J Med Genet. 1997;72:294-296.
42 De Stefano D., Li P., Xiang B., Hui P., Zambrano E. Pulmonary atresia with intact ventricular septum (PA-IVS) in monozygotic twins. Am J Med Genet. 2008;146A:525-528. Part A
43 Kieffer S.A., Carey L.S. Radiological aspects of pulmonary atresia with intact ventricular septum. Br Heart J. 1963;25:655-662.
44 Allanby K.D., Brinton W.D., Campbell M., Garnder F. Pulmonary atresia and the collateral circulation to the lungs. Guys Hosp Rep. 1950;99:110-152.
45 Abbott M. Atlas of Congenital Cardiac Diseases. New York: American Heart Association; 1936.
46 Bostoen H., Robicsek F., Sanger P.W. Atresia of the pulmonary valve with normal pulmonary artery and intact ventricular septum in a 21 year old woman. Coll Works Cardiopulm Dis. 1966;11:753-758.
47 Costa A. Atresia congenita dell’ostio della polmonare, con setto interventricolare chiuso e dotto di Botallo persistente in uomodi 20 anni. Clin Med Ital. 1930;61:567.
48 Robicsek F., Bostoen H., Sanger P.W. Atresia of the pulmonary valve with normal pulmonary artery and intact ventricular septum in a 21-year-old woman. Angiology. 1966;17:896-901.
49 Mcarthur J.D., Munsi S.C., Sukumar I.P., Cherian G. Pulmonary valve atresia with intact ventricular septum. Report of a case with long survival and pulmonary blood supply from an anomalous coronary artery. Circulation. 1971;44:740-745.
50 Ogden J.A. Secondary coronary arterial fistulas. J Pediatr. 1971;78:78-85.
51 Gamboa R., Gersony W.M., Nadas A.S. The electrocardiogram in tricuspid atresia and pulmonary atresia with intact ventricular septum. Circulation. 1966;34:24-37.
52 Celermajer J.M., Bowdler J.D., Gengos D.C., Cohen D.H., Stuckey D.S. Pulmonary valve fusion with intact ventricular septum. Am Heart J. 1968;76:452-465.
53 Patel C.R., Shah D.M., Dahms B.B. Prenatal diagnosis of a coronary fistula in a fetus with pulmonary atresia with intact ventricular septum and trisomy 18. J Ultrasound Med. 1999;18:429-431.