10 Pulmonary Atresia and Intact Ventricular Septum
I. CASE
A. Fetal echocardiography findings
1. The fetal echo reveals situs solitus of the atria, levocardia, left aortic arch, and heart rate of 142 bpm.
2. The four-chamber view is abnormal, with a small right ventricle (RV), which is hypertrophied and has reduced systolic function, and a dilated right atrium (RA) (Fig. 10-1).
3. Cardiac axis and position are normal.
4. Cardiac size is increased (cardiothoracic ratio = 0.40).
5. The outflow assessment reveals normally related great arteries with significant asymmetry (aorta–to–pulmonary artery diameter ratio = 1:0.5).
6. The pulmonary valve is atretic, with no antegrade flow in systole and no pulmonary insufficiency.
7. There are good-sized confluent branch pulmonary arteries.
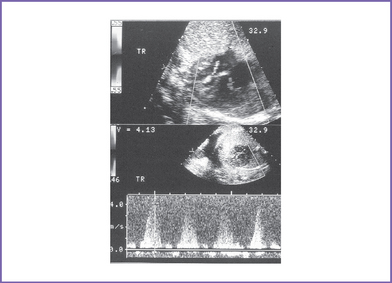
8. The tricuspid valve diameter is small (0.24 cm) (z score = −3), with normal inflow waves (E/A waves).
9. A narrow jet of tricuspid regurgitation (4 m/s) and a dP/dt (change in pressure per change in time) of 600 mm Hg per second.
10. There are possible RV coronary sinusoids.
11. The aortic arch is leftward, with normal antegrade flow.
12. The ductal arch shows flow reversal in systole.
13. There is unrestricted flow through the foramen ovale, with a right- to-left shunt.
14. The pulmonary venous flow pattern is normal. There is also normal flow in the inferior vena cava (IVC), ductus venosus, and umbilical vein.
15. The left ventricular (LV) Tei index (myocardial performance index) is normal to mildly increased (0.48). The RV Tei index could not be calculated due to the pulmonary atresia.
D. Fetal management and counseling
1. Amniocentesis should be offered, although chromosomal anomalies are unlikely with pulmonary atresia with intact ventricular septum.
2. Follow-up includes serial antenatal studies at 4-week intervals.
a. Assess RV size and function and endocardial fibroelastosis.
b. Look for congestive heart failure (CHF), which can develop if there is:
d. Assess the growth of pulmonary arteries and branches. Ductal flow has already been shown to be reverse of normal due to the atretic pulmonary valve.
a. Conditions for vaginal delivery.
b. Conditions for cesarean delivery.
c. Cesarean section practice varies with institution preference.
E. Neonatal management
a. Administer prostaglandin E1 (PGE1) infusion to keep the ductus open to increase pulmonary blood flow and raise arterial oxygen saturation.
b. Oxygen administration can increase oxygen saturation by decreasing pulmonary venous regurgitation and increasing blood flow.
c. Therapeutic balloon atrial septostomy should be performed in the rare situation of restrictive patent foramen ovale.
d. Cardiac catheterization and angiography are recommended to demonstrate coronary sinusoids (by RV angiogram) and rule out coronary artery anomalies such as stenosis. In many pediatric cardiac centers, radiofrequency perforation of the RV outflow tract (RVOT) is possible at cardiac catheterization, eliminating the need for surgery when the RV can otherwise sustain sufficient pulmonary circulation.
F. Follow-up
None of the surgical procedures is curative.
1. Patients who had a Fontan operation.
a. Arrhythmia, reduced heart function, and neurodevelopmental problems are possible sequelae.
b. Women who have undergone the Fontan procedure can become pregnant.
c. Transplantation is a possibility in young adult life if LV dysfunction develops.
2. For patients who had RVOT reconstruction and two-ventricle repair, surgery might be necessary for recurrent RV outflow obstruction or conduit replacement.
G. Risk of recurrence
1. When there is a previous baby with the same condition, this rare congenital heart defect may be an autosomal recessive trait.
2. In the absence of positive family history and normal karyotype, including FISH for 22q11.2, we assume a multifactorial inheritance, and hence the estimated recurrence risk is around 2%.
H. Outcome of this case
1. The baby was born at term with good Apgar scores and good weight.
2. Moderate cyanosis was noted at birth (pulse oximeter reading was 76%-82%).
3. Prostaglandin E1 (PGE1) infusion was started.
4. Postnatal echocardiography confirmed the diagnosis of pulmonary atresia, moderate tricuspid regurgitation, and intact ventricular septum.
5. The pulmonary arteries were of good size and had confluent pulmonary artery branches.
6. The foramen ovale and ductus arteriosus were patent and good sized, with left-to-right shunting.
7. There was retrograde flow in the left anterior descending artery and the left main coronary artery, and there was evidence of a proximal left main coronary artery stenosis.
8. Cardiac catheterization confirmed the presence of RV-dependant coronary circulation. The presence of RV-dependant coronary circulation made a univentricular repair the only surgical option.
9. The infant had a palliative A-P shunt at day 5 of postnatal life.
10. At 4 months of age, the baby had a successful bidirectional Glenn operation.
11. Two years later, the baby had a successful external Fontan procedure.
II. YOUR HANDY REFERENCE
A. Pulmonary atresia with intact ventricular septum
a. Pulmonary atresia with intact ventricular septum (PAIVS) accounts for 2.5% to 4% of congenital heart disease and occurs in 1 in 2200 live births.
b. PAIVS accounted for 5% of Sharland and coworkers’ (1996) fetal series.
c. In Sharland and coworkers’ 1996 fetal series, two forms of PAIVS were noted.
a. Pulmonary atresia with intact ventricular septum lies at the severe end of the spectrum of congenital heart disease. Factors that affect the outcome include:
b. In 2005, Daubeney and coworkers reported predictors of early and medium-term outcome in a population-based study in the United Kingdom and Ireland.
3. Clues to fetal sonographic diagnosis.
a. Normal cardiothoracic ratio.
b. Hypoplastic right ventricle, poorly contracting thick-walled ventricle.
c. Small tricuspid valve annulus (compare to mitral valve annulus and get a z score for the annulus).
d. Tricuspid regurgitation (usually at high Doppler velocity).
e. Variable size of the main pulmonary artery and branches.
f. Absence of forward flow across the pulmonary valve or main pulmonary artery.
g. No antegrade flow across the pulmonary valve (in systole).
h. Reverse flow in the arterial duct (aorta to pulmonary).
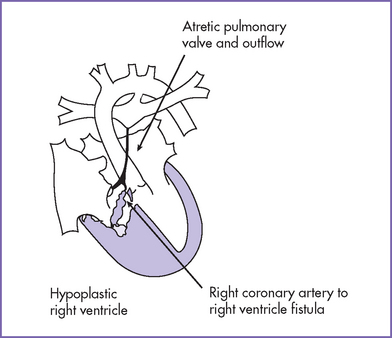
i. Fistulas from the right ventricle to the coronary arteries, which can result in flow reversal in the coronary arteries, particularly in systole, when the RV pressure is highest. Rarely, where there is no proximal stenosis of the coronary arteries, flow from the coronaries into the aortic root may be seen.
j. Ventriculocoronary connections.
4. Associated syndromes and extracardiac anomalies.
a. PAIVS is not commonly associated with extracardiac malformations, chromosomal anomalies, or dysmorphic features.
b. A case associated with trisomy 18 has been reported.
5. Cardiovascular profile score helps in assessing the degree of fetal heart failure and hence in anticipating the timing of delivery.
6. Immediate postnatal management for patients without prenatal diagnosis of PAIVS.
b. Ask for an echocardiogram and immediately refer the patient to a tertiary center with cardiac facilities.
7. Nonsurgical options consist of transcatheter radiofrequency/laser wire or guidewire perforation of the valve combined with balloon dilation in suitable patients.
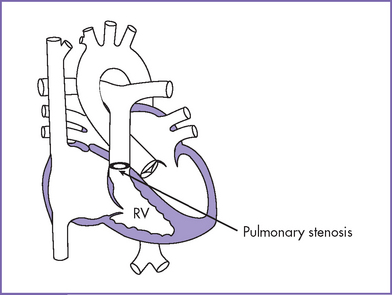
8. Pathophysiology (Fig. 10-2).
a. The pulmonary valve is atretic, and the interventricular septum is intact. An interatrial communication (either ASD or patent foramen ovale) and patent ductus arteriosus are necessary for survival.
b. The RV size is variable and is related to survival.
c. The high pressure in the RV is often decompressed through dilated coronary sinusoids into the left or right coronary artery (Figs. 10-3 and 10-4). Tricuspid regurgitation is commonly present.
d. RA dilation and hypertrophy may be one of the earliest clues to RVOT obstruction. With redistribution of flow to the left atrium (LA) and LV, the left heart chambers dilate to accommodate the preload that is critical to sustain the equivalent of a combined ventricular output.
e. Pulmonary blood flow in utero depends on retrograde flow through the ductus arteriosus. This confirms the diagnosis of critical pulmonary outflow obstruction. Critical pulmonary valve stenosis and pulmonary atresia may be difficult to distinguish both before and after birth. Rarely, there is obvious pulmonary insufficiency with pulmonary valve stenosis that suggests patency of the valve.
f. The RV has a tendency to grow at a reduced rate with advancing gestation. In the second trimester, significant endocardial fibroelastosis is unlikely, although this is a feature commonly observed in fetal hearts with severe pulmonary valve stenosis and can be found in infants with PAIVS. This is in contrast to the analogous situation in the left heart, where atresia of the aortic valve associated with an intact ventricular septum and a patent mitral valve is often associated with endocardial fibroelastosis when seen early in the second trimester.
g. The tricuspid valve orifice is usually hypoplastic compared to normal for the gestational age, and the tricuspid valve opening appears restricted. The distinction from tricuspid atresia can be difficult; however, a narrow jet of tricuspid regurgitation can differentiate between the two.
h. The pulmonary trunk size can vary and does not always correlate with the size of the RV. Thus, a hypoplastic RV can be associated with a normal-sized pulmonary trunk and vice versa.
i. Connections between the coronary arteries and the RV or sinusoids are often encountered in patients with the smallest RVs.
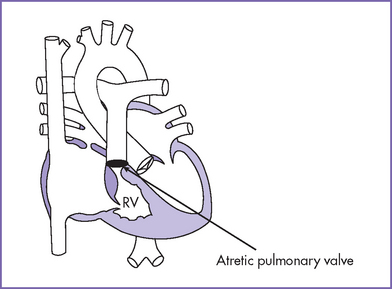
Fig. 10-2 Pulmonary atresia with intact interventricular septum. RV, right ventricle.
(Modified from Mullins CE, Mayer DC: Congenital Heart Disease: A Diagrammatic Atlas. New York, Liss, 1988.)
 B.Pulmonary atresia with dilated right ventricle
B.Pulmonary atresia with dilated right ventricle
1. There is usually an abnormality of the tricuspid valve, which in the majority is either Ebstein’s malformation or valve dysplasia. Rarely, there is unguarded tricuspid valvar orifice or Uhl’s anomaly of the RV.
a. In some of these, the pulmonary outlet obstruction evolves to lack of sufficient flow through the outflow tract.
b. If the tricuspid insufficiency is so severe that the RV cannot generate a pressure in systole to open the pulmonary valve, the pulmonary valve might not grow and might even fuse closed.
2. Pulmonary atresia with a dilated RV is readily detectable prenatally because the cardiothoracic ratio is usually increased due to the associated tricuspid regurgitation.
3. Massive cardiomegaly can inhibit the normal development of the lungs.
4. The pulmonary trunk can vary in size.
5. Functional pulmonary atresia can occur and may be difficult to differentiate from anatomic pulmonary atresia. Reversal of ductal flow is seen in both situations.
6. Coronary artery anomalies are not usually encountered because the RV is decompressed by the tricuspid regurgitation.
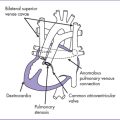
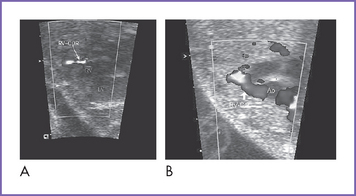
7. Reversal of flow in the ductus venosus during atrial systole or an increased pulsatility index can occur in foramen ovale–dependent circulations such as these (Fig. 10-5).
1. Isolated pulmonary regurgitation in a structurally normal heart is an unusual finding during fetal life.
a. It is reported to be 0.5% in one series in a low-risk group of normal pregnancies.
b. In all cases reported in this study, pulmonary regurgitation resolved with time and there was no postnatal anomaly.
2. Pulmonary regurgitation can occur secondary to increased load on the RV and increased diastolic pulmonary artery pressure from increased afterload such as is seen with:
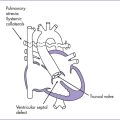
Pulmonary valve stenosis is shown in Figure 10-6.
a. Isolated pulmonary valve stenosis represented 0.8% of cases of structural cardiac malformation diagnosed in fetal life in Sharland’s series.
b. The majority of cases detected in the fetus fall at the severe end of the spectrum, and there is significant overlap with PAIVS.
2. Clues to fetal sonographic diagnosis.
b. Degree of pulmonary valve obstruction.
3. Associated syndromes and extracardiac anomalies.
a. Pulmonary valve stenosis can occur as part of Noonan’s, Alagille’s, or Williams’ syndrome.
b. Pulmonary valve stenosis can occur in the recipient twin as a consequence of the twin-to-twin transfusion syndrome.
c. Pulmonary valve stenosis is a common component of many forms of complex heart disease.
d. It is very rare for isolated pulmonary valve stenosis to be associated with chromosomal anomalies. Amniocentesis is therefore not usually offered in such a situation.
4. Fetal management and counseling.
a. Management and counseling depend on the severity of the lesion and the gestational age at which the diagnosis is made.
b. The RV and the pulmonary trunk can fail to grow as the pregnancy advances. This is important in counseling, especially with regard to the possibility of biventricular surgical repair at birth.
c. Fetal intervention to dilate the stenotic pulmonary valve early in gestation has been done.
5. Risk of recurrence is around 2% if one sibling or the father is affected and around 6.5% if the mother is affected.
III. TAKE-HOME MESSAGE
A. Diagnosis
1. Pulmonary regurgitation in a structurally normal valve could be a sign of RV systolic dysfunction or may be present when there is severe AV valve regurgitation.
2. The pulmonary valve stenosis gradient in utero does not reflect the severity of the stenosis. The flow through the ductus arteriosus is most useful in predicting the severity of RVOT obstruction in pulmonary valve stenosis.
B. Prognosis
1. The intrauterine prognosis of critical pulmonary valve stenosis or PAIVS depends on both the size of the foramen ovale (to allow right-to-left shunt) and LV function to support the combined cardiac output.
2. The outcome of PAIVS is determined by the size of the RV and the tricuspid valve, the morphology of the RV outflow obstruction (e.g., infundibular atresia versus valvar atresia), and the presence of RV-dependent coronary circulation
3. A significantly hypoplastic RV will not likely grow and thus is not likely to allow biventricular repair or palliation in the future.
C. Treatment
1. RV-dependent coronary circulation is a contraindication to any attempt to open the RV outlet as part of surgical repair.
2. Significant coronary artery stenosis is not detectable before birth with current technology. However, it can significantly affect the management options and long-term outcome of an affected infant.
Ali Khan MA, al-Yousef S, Huhta JC, et al. Critical pulmonary valve stenosis in patients less than 1 year of age: Treatment with percutaneous gradational balloon pulmonary valvuloplasty. Am Heart J. 1989;117(5):1008-1014.
Allan LD, Crawford DC, Tynan MJ. Pulmonary atresia in prenatal life. J Am Coll Cardiol. 1986;8(5):1131-1136.
Ansari A, Goltz D, McCarthy KP, et al. The conduction system in hearts with pulmonary atresia and intact ventricular septum. Ann Thorac Surg. 2003;75(5):1502-1505.
Arzt W, Tulzer G, Aigner M, et al. Invasive intrauterine treatment of pulmonary atresia/intact ventricular septum with heart failure. Ultrasound Obstet Gynecol. 2003;21(2):186-188.
Chitayat D, McIntosh N, Fouron JC. Pulmonary atresia with intact ventricular septum and hypoplastic right heart in sibs: A single gene disorder? Am J Med Genet. 1992;42(3):304-306.
Daubeney PE, Wang D, Delany DJ, et alUK and Ireland Collaborative Study of Pulmonary Atresia with Intact Ventricular Septum. Pulmonary atresia with intact ventricular septum: Predictors of early and medium-term outcome in a population-based study. J Thorac Cardiovasc Surg. 2005;130(4):1071.
de Leval M, Bull C, Stark J, et al. Pulmonary atresia and intact ventricular septum: Surgical management based on a revised classification. Circulation. 1982;66(2):272-280.
Freedom RM, Harrington DP. Contributions of intramyocardial sinusoids in pulmonary atresia and intact ventricular septum to a right-sided circular shunt. Br Heart J. 1974;6:1061-1065.
Gutgesell HP, Huhta JC, Latson LA, et al. Accuracy of two-dimensional echocardiography in the diagnosis of congenital heart disease. Am J Cardiol. 1985;55(5):514-518.
Hornberger LK, Benacerraf BR, Bromley BS, et al. Prenatal detection of severe right ventricular outflow tract obstruction: pulmonary stenosis and pulmonary atresia. J Ultrasound Med. 1994;13(10):743-750.
Huhta JC, Edwards WD, Tajik AJ, et al. Pulmonary atresia with intact ventricular septum, Ebstein’s anomaly of hypoplastic tricuspid valve and double-chamber right ventricle: Two-dimensional echocardiographic–anatomic correlation. Mayo Clin Proc. 1982;57(8):515-519.
Huhta JC, Piehler JM, Tajik AJ, et al. Two-dimensional echocardiographic detection and measurement of the right pulmonary artery in pulmonary atresia–ventricular septal defect: Angiographic and surgical correlation. Am J Cardiol. 1982;49(5):1235-1240.
Khoshhal S, Sandor GG, Duncan WJ. Pulmonary atresia with intact ventricular septum and antenatal left ventricular failure. Cardiol Young. 2004;14(3):335-337.
Ladusans EJ, Qureshi SA, Parsons JM, et al. Balloon dilatation of critical stenosis of the pulmonary valve in neonates. Br Heart J. 1990;63(6):362-367.
Maeno YV, Boutin C, Hornberger LK, et al. Prenatal diagnosis of right ventricular outflow tract obstruction with intact ventricular septum, and detection of ventriculocoronary connections. Heart. 1999;81(6):661-668.
Qureshi SA. Catheter treatment of congenital heart disease. Br J Hosp Med. 1993;50(9):523-528.
Qureshi SA. Collaborative approach in the management of pulmonary atresia with intact ventricular septum. J Interv Cardiol. 2001;14(3):377-384.
Schneider C, McCrindle BW, Carvalho JS, et al. Development of z-scores for fetal cardiac dimensions from echocardiography. Ultrasound Obstet Gynecol. 2005;26(6):599-605.
Sharland GK, Tynan M, Qureshi SA. Prenatal detection and progression of right coronary artery to right ventricle fistula. Heart. 1996;76(1):79-81.
Smrcek JM, Germer U, Gembruch U. Functional pulmonary valve regurgitation in the fetus. Ultrasound Obstet Gynecol. 1998;12(4):254-259.

 C.
C. D.
D.