Pulmonary Air Leak Syndromes
By the end of this chapter, the reader will be able to:
• List the anatomic alterations of the lungs associated with pulmonary air leak syndrome.
• Describe the causes of pulmonary air leak syndrome.
• List the cardiopulmonary clinical manifestations associated with pulmonary air leak syndrome.
• Describe the general management of pulmonary air leak syndrome.
• Describe the clinical strategies and rationales of the SOAP presented in the case study.
• Define key terms and complete self-assessment questions at the end of the chapter and on Evolve.
Pulmonary air leak syndromes (also called air block syndromes) in the infant comprise a large spectrum of clinical entities, including pulmonary interstitial emphysema (PIE), followed by, in severe cases, pneumomediastinum, pneumothorax, pneumopericardium, pneumoperitoneum, and, in rare cases, intravascular air embolism. Pulmonary air leak syndromes are common complications of mechanical ventilation in premature infants, especially when very high pressures are used. They are often seen in infants being treated for respiratory distress syndrome (see Chapter 34).
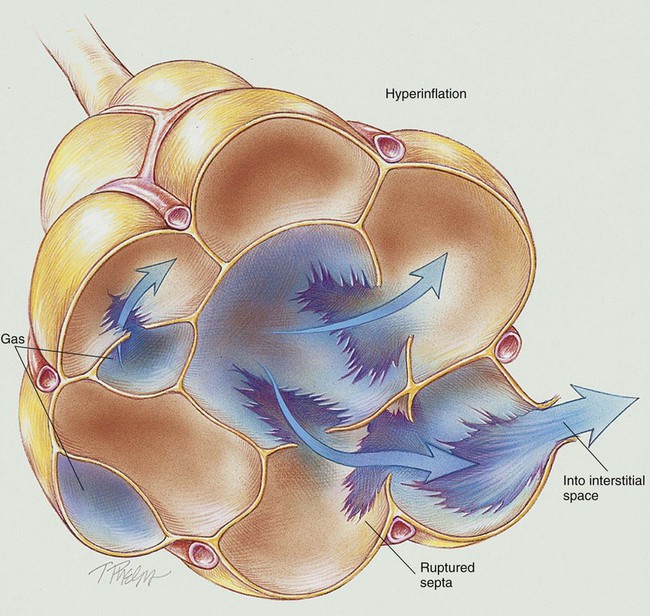
Anatomic Alterations of the Lungs
Pulmonary Interstitial Emphysema
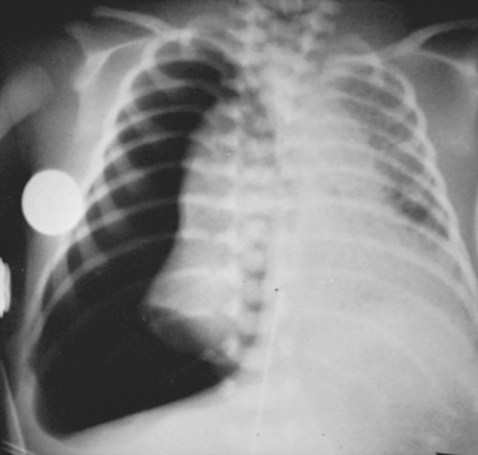
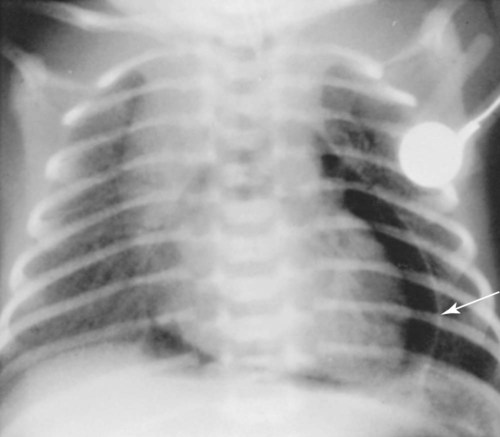
Virtually all pulmonary air leak syndromes begin with some degree of PIE. When high airway pressures are applied to an infant’s lungs (e.g., during mechanical ventilation), the distal airways and alveoli often become overdistended—that is, they develop bleb or emphysema-like areas—and rupture (see Figure 35-1). In addition, gas trapping from an insufficient expiratory time can also cause alveolar overdistention and rupture. Once the gas escapes, it is forced into (1) the loose connective tissue sheaths that surround the airways and pulmonary capillaries, and (2) the interlobular septa containing pulmonary veins. In severe cases, the gas continues to spread peripherally by dissecting along the peribronchial and perivascular spaces to the hilum of the lung, producing the classic radiographic appearance of PIE that shows bubbles of air in the interstitial cuffs (Figure 35-2 and Figure 35-3).
A pneumothorax may occur because of the alveolar overdistention and subsequent rupture commonly associated with a PIE (Figure 35-4). A pneumopericardium can develop from direct tracking of interstitial air along the great vessels into the pericardial sac (Figure 35-5). Gas pressure in the pericardium restricts atrial and ventricular filling, resulting in a decreased stroke volume and, ultimately, a reduced cardiac output and systemic hypotension. During the late stages, inflammatory changes of the airways lead to increased capillary leakage and excessive bronchial secretions.
The major pathologic changes associated with pulmonary air leak syndromes are as follows:
General Management of Pulmonary Air Leak Syndromes
Prevention is the best treatment for pulmonary air leak syndromes. These syndromes may be prevented by the use of low mechanical ventilator pressures and the maintenance of good ventilation and oxygenation. Selective intubation of the unaffected or less affected lung may allow the injured lung time to heal. High-frequency ventilation has been successful in treating infants with pulmonary air leak syndromes. Survivors of pulmonary air leak syndromes often develop bronchopulmonary dysplasia (see Chapter 37) as a result of overly vigorous mechanical ventilation. Finally, the respiratory care practitioner must always be on alert for signs and symptoms of subcutaneous emphysema, pneumothorax, pneumopericardium, pneumoperitoneum, and intravascular air embolism. Mechanical removal of free air from the intrathoracic cavity, pericardium, or mediastinum may sometime be necessary, especially if vascular collapse is present.
Respiratory Care Treatment Protocols
Oxygen Therapy Protocol
Oxygen therapy is used to treat hypoxemia, decrease the work of breathing, and decrease myocardial work. Because of the hypoxemia that often develops in pulmonary air leak syndromes, supplemental oxygen may be required (see Oxygen Therapy Protocol, Protocol 9-1).
Bronchopulmonary Hygiene Therapy Protocol
Because of the excessive airway secretions and mucous accumulation associated with pulmonary air leak syndromes, a number of bronchial hygiene treatment modalities may be used to enhance the mobilization of bronchial secretions (see Bronchopulmonary Hygiene Therapy Protocol, Protocol 9-2).
Mechanical Ventilation Protocol
Mechanical ventilation may be necessary to provide and support alveolar gas exchange and eventually return the patient to spontaneous breathing. As previously mentioned, prevention is the best treatment for pulmonary air leak syndromes. Selective intubation of the unaffected or less affected lung may allow the injured lung time to heal. High-frequency ventilation has been successful in treating infants with pulmonary air leak syndromes (see Mechanical Ventilation Protocols, Protocol 9-5, Protocol 9-6, and Protocol 9-7).
CASE STUDY
Pulmonary Air Leak Syndromes
Admitting History and Physical Examination
Respiratory Assessment and Plan
O Skin: Pink. HR 155 bpm, BP 68/35. Crackles and rhonchi throughout. ABGs: pH 7.34, Paco2 42, 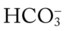 22, Pao2 53, and Sao2 89%. CXR: Reexpanded right lung with segmental atelectasis.
22, Pao2 53, and Sao2 89%. CXR: Reexpanded right lung with segmental atelectasis.
• Preterm infant in severe respiratory distress
• Right tension pneumothorax, treated
• Atelectasis—right lung (CXR)
• Adequate ventilation and oxygenation on present ventilator settings (ABGs)
P Continue Mechanical Ventilation Protocol. Attempt to reduce Fio2 per Oxygen Therapy Protocol. Continue Lung Expansion Therapy Protocol (PEEP +5 cm H2O). Start Bronchopulmonary Hygiene Therapy Protocol (chest physical therapy [CPT] qid). Monitor closely (vital signs, color, ABGs, transillumination).
Discussion
In this case the clinical manifestations associated with Atelectasis (see Figure 9-8) were quickly identified when the respiratory therapist noted the possibility of a pneumothorax by pointing out that the baby’s breath sounds were absent over the right lung and severely decreased over the left upper and middle lobes. Transillumination further supported the presence of a pneumothorax. The chest radiograph confirmed a right lung pneumothorax. To help in the monitoring and the identification of a possible pneumothorax, the respiratory care practitioners often make a simple ballpoint pen mark at the PMI on the chests of infants at risk for pneumothorax. The PMI is the point at which the impulse of the left ventricle is felt most strongly. If the PMI moves away from the mark, the practitioner has a good and timely clinical indication that the baby has developed a pneumothorax—even before a chest x-ray examination can be performed.


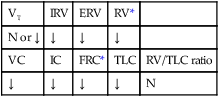
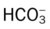

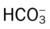
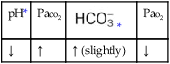
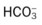 values will be lower than expected for a particular Pa
values will be lower than expected for a particular Pa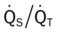
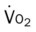
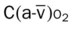
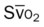

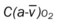 , Arterial-venous oxygen difference; DO2, total oxygen delivery; O2ER, oxygen extraction ratio;
, Arterial-venous oxygen difference; DO2, total oxygen delivery; O2ER, oxygen extraction ratio; 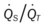 , pulmonary shunt fraction;
, pulmonary shunt fraction; 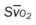 , mixed venous oxygen saturation;
, mixed venous oxygen saturation; 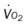 , oxygen consumption.
, oxygen consumption.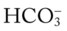 19, Pa
19, Pa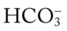 21, Pa
21, Pa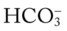 22, and Pa
22, and Pa