CHAPTER 48 Ptosis surgery
Introduction
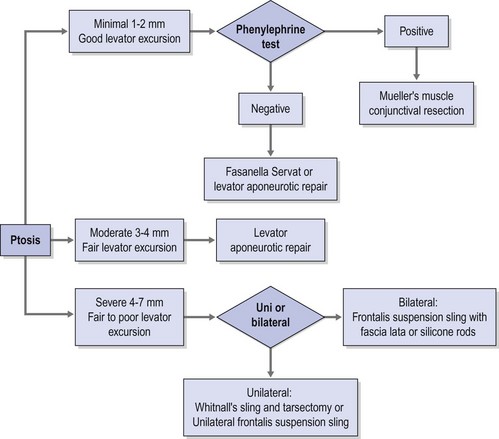
A practical clinical algorithm that allows the surgeon to select a reliable procedure for the management and treatment of the ptosis patient is provided. The algorithm’s goal is to help the surgeon with the selection of the best surgical procedure based on the amount of ptosis and levator function present the time of diagnosis, to achieve successful correction of the ptosis present with good postoperative results and patient satisfaction (Fig. 48.1).
Epidemiologic consideration and terminology
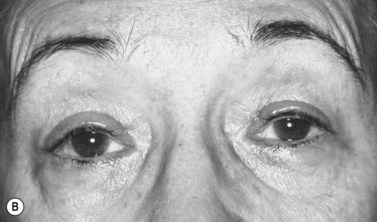
Congenital ptosis can affect one or both eyes; however, in approximately 70% of known cases, congenital ptosis has unilateral affectation. Congenital ptosis may be present at birth, or it may develop later in life. A droopy eyelid(s) that is present at birth, or that develops within the first year of life, is considered congenital in nature. In most cases of congenital ptosis, the problem is isolated and does not affect the vision if the eyelid doesn’t obstruct the visual axis. This holds particularly true in the critical period of visual development, corresponding to the first 3 months of life. The pupil obstruction caused by the droopy eyelid obscures the pediatric patient’s visual field, and permanent loss of vision may occur as a result of amblyopia. Congenital ptosis has no mortality and its morbidity arises from occlusion (deprivation) amblyopia, astigmatism induced from the compression of the droopy eyelid on the corneal tissue, and ocular torticollis. The astigmatism induced by congenital ptosis is generally anisometropic and can be uni or bilateral depending on whether one or both eyelids are affected by the condition. In instances where the degree of anisometropia is significant, anisometropic amblyopia can also develop. In the presence of amblyopia and/or functional limitation caused by congenital ptosis, surgery must be performed to correct the problem early in life, since recent evidence supports that the incidence of deprivation or anisometropic amblyopia in congenital ptosis can be reduced significantly1.
Congenital ptosis can also be seen as part of many congenital syndromes including blepharophimosis syndrome, and certain forms of congenital fibrosis of the extraocular muscles syndromes (CFEOM)2, Marcus Gunn jaw winking syndrome, and congenital Horner syndrome
Clinical features, diagnosis, and differential diagnosis
Although there are numerous classifications for ptosis; such as congenital versus acquired, neurogenic, myogenic, traumatic, and mechanical3, none of those classifications provides a complete approach to the ptosis patient, nor does any of them guide the clinician to the development of a system for adequate repair. Classifying ptosis as minimal = 0.5–1.5 mm, moderate = 2.0–3 mm, or severe >3 mm based on the amount of ptosis provides a practical framework for a logical system of repair when this system is used in conjunction with the amount of levator excursion. This last measurement is an indirect measurement of the viability of the levator palpebrae superioris and an excellent predictor of the surgical outcome. Using this matrix the appropriate choice of surgical strategy can then be applied to each of these three scenarios.
Preoperative assessment
Assessment of ptosis and documentation
Ptosis is documented by the margin to reflex distance 1 (MRD1)4, which is the distance from the central pupillary light reflex to the upper eyelid margin, measured in millimeters. It is important to document the amount of ptosis to the nearest 0.5 mm, if possible. The margin to reflex distance 2 (MRD2) is the distance from the central pupillary light reflex to the lower eyelid margin. The MRD1 plus the MRD2 should equal the palpebral fissure.
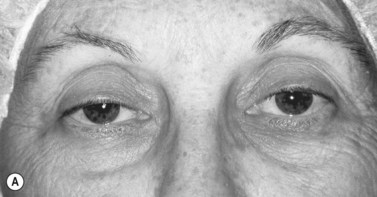
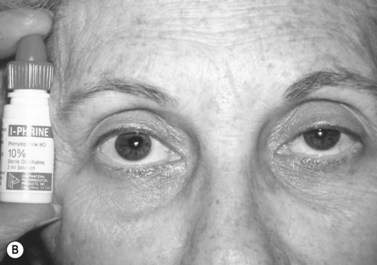
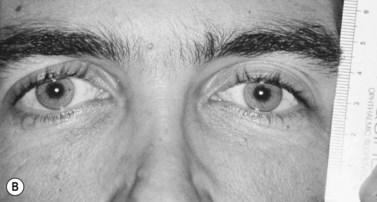
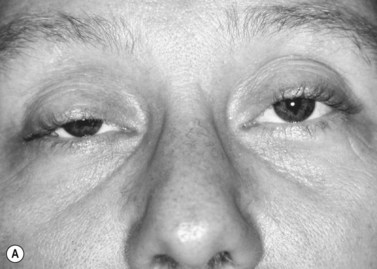
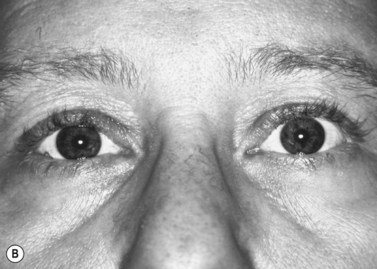
Patients with minimal ptosis (2 mm or less) should have a phenylephrine test performed in the involved eye or eyes after appropriate ptosis measurements have been documented. Either 2.5% or 10% phenylephrine is instilled in the affected eye or eyes. We prefer to use 2.5% phenylephrine. Usually two drops are instilled and the patient is re-examined 5 minutes later. The MRD1 is rechecked in the affected and unaffected eyes (Fig. 48.2). A rise in the MRD1 of 1.5 mm or greater is considered a positive phenylephrine test. This indicates that Müller’s muscle is viable, and the Müller’s muscle conjunctival resection procedure can be performed, also giving the patient a reasonable prediction of the desired result.
The contralateral eye must also be rechecked in patients with unilateral ptosis. When the ptotic eye is occluded, if the MRD1 decreases appreciably in the opposite eye, this usually indicates that bilateral ptosis is present; this finding is consistent with Hering’s law5. The patient may require bilateral surgery. A negative phenylephrine test precludes the use of the Müller’s muscle conjunctival resection procedure, because the outcome of the procedure is unpredictable in this setting. Callahan and Beard3 have stated that minimal or mild ptosis is 2 mm or less, moderate ptosis is 3–4 mm, and severe ptosis is 4 mm or greater. Usually patients with minimal ptosis will have good levator excursion. The moderate ptosis patients usually have good to fair excursion, and typically patients with severe ptosis have poor levator excursion.
Operation techniques
Surgical options based on levator function
For patients with minimal ptosis (2 mm or less) there are three surgical options: (i) Müller’s muscle conjunctival resection, (ii) the Fasanella–Servat procedure, or (iii) levator aponeurotic surgery. If the phenylephrine test is positive, the Müller’s muscle conjunctival resection procedure is the most precise and predictable surgical option4. For many ptosis surgeons, this is the preferred approach for minimal ptosis because of its ease, predictability, and the ability to grade or titrate the correction according to the pre-established nomograms. The surgeon can also calculate, with time and personal experience, his or her own nomogram6. If, however, the phenylephrine test is negative, one must consider other procedures because of the unpredictability of Müller’s muscle conjunctival resection in this subset of patients. The Fasanella–Servat procedure is the next option for minimal ptosis and a negative phenylephrine test. The Fasanella–Servat procedure is nearly as predictable as Müller’s muscle conjunctival resection and equally easy to perform7. Because it is in a sense a tarsectomy with little Müller’s muscle resected, it is viable in the absence of a positive phenylephrine test.
Levator aponeurosis repair is the third option for minimal ptosis. Many surgeons prefer this technique because it allows them to set the eyelid height while the patient is on the operating table. It is particularly useful for patients who have contour abnormalities and who have ptosis that requires concomitant blepharoplasty5. There are, however, many variables that may affect the results, including the need for patient cooperation, the effects of sedation or local anesthetic infiltration, and the need to over-correct the affected side or sides. It is also difficult to grade the degree of correction under general anesthesia. Indeed, many reports have suggested that predictability and success with this procedure may vary by as much as 2 mm between the two affected eyelids8,9. In the setting of minimal ptosis, however, success ought to be judged to within 0.5 mm. Nonetheless, levator aponeurotic repair is useful for many minimal ptosis patients.
Bilateral severe ptosis patients, or patients with very poor levator excursion, need some type of frontalis suspension. Congenital severe ptosis with little levator excursion is best served with autogenous fascia lata sling grafts. Non-autogenous materials are available and can be used, if necessary; however, the long-term results are poorer than with autogenous materials10. Acquired severe ptosis, as seen with third nerve palsy, progressive external ophthalmoplegia, or oculopharyngeal dystrophy, is best treated by frontalis suspension sling with a silicone rods11 (Silastic) or more recently expanded polytetrafluoroethylene (ePTFE) because of its adjustability and the possibility for subsequent removal if the cornea becomes compromised.
Müller’s muscle conjunctival resection
Müller’s muscle conjunctival resection is reserved for patients with minimal ptosis (2 mm or less) with normal levator excursion and a positive phenylephrine test. Putterman and Urist12 originally described this technique in 1975. Various modifications have since been described4,13.
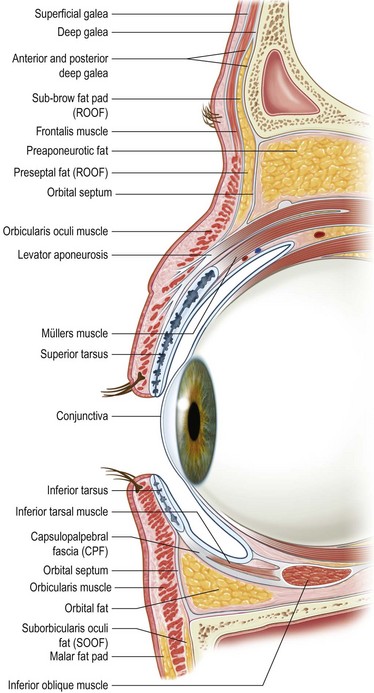
Müller’s muscle is a smooth muscle that originates from the undersurface of the levator and inserts with a 0.5–1 mm tendon into the superior tarsal plate14. When denervated in Horner syndrome, this muscle relaxes causing 2–3 mm of clinical blepharoptosis. The levator aponeurosis has been shown to insert on the anterior surface of the tarsus within 7–8 mm of the upper tarsal border. Additional interdigitations with the intermuscular septum of the orbicularis oculi form the eyelid crease15. Whitnall, however, recognized Müller’s muscle as another important primary attachment or insertion of the levator. When Müller’s muscle is advanced it strengthens the posterior lamella and appears to plicate the levator aponeurosis, with healing and subsequent scarring in the posterior lamella. This plication is successful in maintaining constant elevation of the upper eyelid in the open eyelid positioning primary gaze4.
Surgical technique
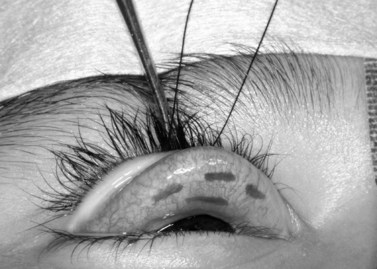
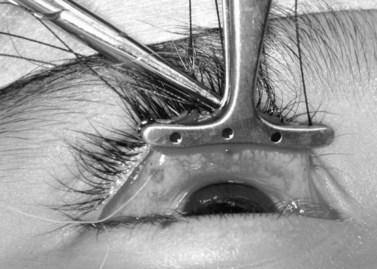
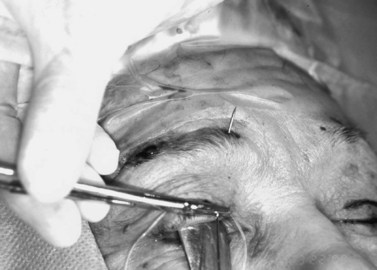
A frontal nerve block is unnecessary for the procedure; 1% or 2% xylocaine is used as a regional block for the upper eyelid. Epinephrine is omitted to avoid stimulation of Müller’s muscle. Approximately 5 cc of the solution, mixed with hyaluronidase (10 cc of anesthetic is mixed with 150 units of hyaluronidase), is injected just below the superior orbital rim. Tetracaine topical anesthetic eye drops are then placed on the conjunctival surface. A 4-0 silk suture is placed through the tarsus at the eyelid margin in the pupillary axis. The eyelid is reflected over a Desmarres retractor. Marks are made at one-half the distance of the total resection amount medially, centrally, and laterally, measured with a caliper, and beginning 0.5 mm above the tarsal plate. Another mark is made centrally to measure the total extent of resection desired7 (Fig. 48.3).
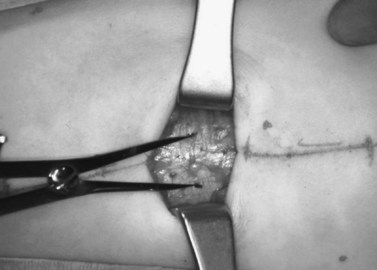
Three 4-0 silk traction sutures are placed through the conjunctiva and Müller’s muscle centrally, medially, and laterally at the halfway marks. Each bite is approximately 3 mm long and deep to the underlying Müller’s muscle but should not penetrate to the levator aponeurosis or orbicularis muscle. The sutures are separated into two bundles and tied on themselves, to be used as traction sutures to elevate the required amount of conjunctiva and Müller’s muscle to be resected (Fig. 48.4)7.
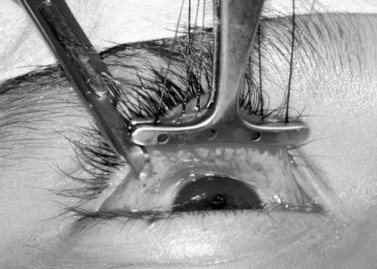
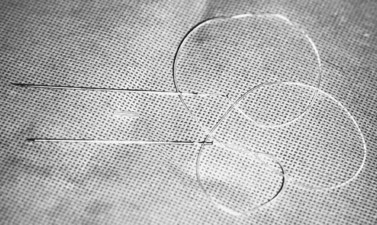
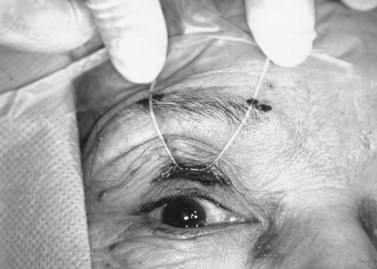
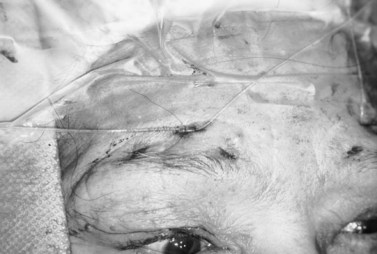
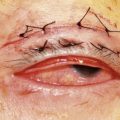
A 6-0 plain horizontal mattress suture is placed under the clamp in a running fashion, approximately 0.5–1 mm below the clamp (Fig. 48.5). The conjunctiva is closed with a running baseball stitch in the reverse direction of the original pass. The suture is tied on itself (Fig. 48.6)7. Exteriorizing the suture is not required. The clamped tissues are excised with a no. 15 blade metal on metal (Fig. 48.7). The eyelid is returned to its anatomic position, and the eyelid margin suture is removed.
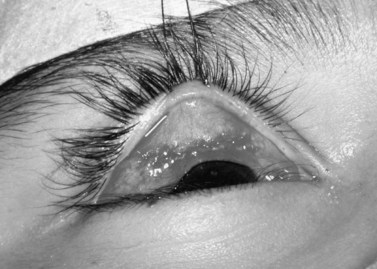
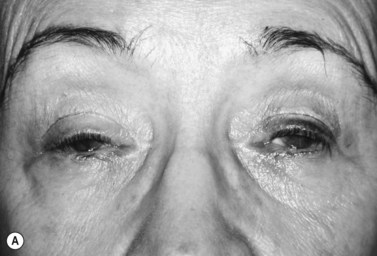
Alternatively a 6-0 Prolene suture can be used; for this modification, the suture should enter from the anterior lamella full thickness and placed under the clamp in the same way described above. This suture needs to be exteriorized and, after excising Müller’s muscles and conjunctiva, conjunctival closure is not necessary. Both ends of the exteriorized Prolene suture are tied to each other once the eyelid is back to its anatomic position. The suture is removed after 5–7 days. Antibiotic ointment is placed in the eye and no patch is necessary. Excellent results can be seen, with minimal ptosis ranging between 1 and 2 mm (Fig. 48.8). The advantages of this technique are that it is quick, predictable, and quantifiable. Late failures are rare. Complications include a rare superior corneal abrasion, under-correction, or over-correction. Usually an abrasion heals spontaneously if it is small. A bandage contact lens can also be placed if desired. Over-correction is rare with this technique. If it occurs, the plain suture can be cut under topical anesthetic in the office, and the wound can be separated gently with a cotton swab. Under-correction requires another procedure at a later date (Table 48.1).
Table 48.1 Resection nomogram for Müller’s muscle conjunctival resection
| Amount of ptosis in mm | Amount of MMCR resection |
|---|---|
| 0.5–1 mm | 4 mm |
| 2 mm | 8 mm |
| 3 mm | 10 mm |
| >3 mm | Not recommended |
Courtesy of S. Dresner.
Levator aponeurosis repair
The levator palpebrae superioris extends from the annulus of Zinn traveling anteriorly through the superior orbit to Whitnall’s ligament, which serves as a suspensory ligament for the upper eyelid (Fig. 48.9). At this point the muscle appears aponeurotic and has a whitish color. The levator aponeurosis courses downward to insert on the inferior two-thirds of the anterior surface of the tarsal plate, the fibrous septi of the orbicularis, and the subcutaneous tissues16. Anterior to the levator aponeurosis is the preaponeurotic fat pad and the orbital septum.
Surgical technique
After the local anesthetic is injected into the eyelid, topical tetracaine eye drops are placed on the conjunctival surface. The patient is prepped, and protective corneal shields can be placed over the globes. The incision can be made with a no. 15 Bard–Parker blade, a Colorado needle tip (Colorado Biomedical, 6851 Highway 73 Evergreen, CO 80439) in cut mode, or a CO2 laser set to incisional mode. A skin muscle flap is developed to expose the orbital septum. The septum is incised over the upper one-third to avoid incising or damaging the underlying levator (Fig. 48.10)17. The pre-aponeurotic fat pad is reflected upward to reveal the whitish aponeurosis beneath. A high temperature hand-held cautery is used to disinsert the aponeurosis from the tarsal plate, which separates the aponeurosis from the underlying Müller’s muscle (Fig. 48.11). Dissection is carried upward, as high as Whitnall’s ligament if necessary. A double 5-0 Vicryl suture is placed partial thickness through the central portion of the upper tarsus in two 3 mm bites. This suture is then taken up through the aponeurosis at the desired height (Fig. 48.12). This is temporarily tied and the level is examined. Usually sitting the patient up on the table gives a more accurate assessment. A 1–1.5 mm over-correction is desirable because the protractors (orbicularis) are paralyzed by local anesthetic and there can be some stimulation of Müller’s muscle by the epinephrine. Additional sutures can be placed medially and laterally for contour adjustment; often, however, they are unnecessary. The excess levator aponeurosis is trimmed. A strip of skin-orbicularis flap superiorly can be excised if necessary, or bilateral blepharoplasties can be performed. The wound is then closed with a 6-0 suture of choice with supratarsal fixation on every other bite of the suture. Excellent results can be obtained with this approach (Fig. 48.13).
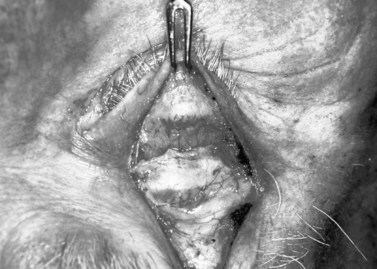
Fig. 48.11 Levator aponeurosis repair. The levator is dissected off of the tarsus with a hand-held cautery.
Whitnall sling procedure
Surgical technique
Because this technique is often performed under general anesthesia, an empiric formula needs to be used to set the height of the lid margin. The gaping technique described by McCord18 suggests that in congenital ptosis, 3 mm should be added to the amount of ptosis present; this numeric value equals the amount of gaping or lagophthalmos that will be established on the operating room table. For instance, if there is 3 mm of ptosis present, the eyes should be left open or ‘gaped’ 6 mm on the operating table. Another formula that can be used is to subtract the levator excursion in the affected eyelid from the levator excursion on the normal side; this number is then multiplied by 1.2 to calculate the amount of levator aponeurotic advancement. For example, if one ptotic side has a levator excursion of 6 mm and the unaffected side has a levator excursion of 14 mm; the difference in levator function is 8 mm. This value of 8 mm is then multiplied by 1.2, for a total of 9.6 mm of levator advancement.
If additional elevation is required, a tarsectomy can be performed at the same time that the Whitnall’s sling is being performed; up to 2–4 mm of tarsus can be excised at the time of surgery19. With this method, each millimeter of tarsus is equivalent to 2 mm of aponeurosis advancement.
Complications with aponeurosis surgery include contour abnormalities, over-correction, under-correction, and conjunctival prolapse, which is rare. Complications are usually best addressed 1 week into the postsurgical period9. With over-correction, the wound can be opened and the aponeurosis sutures cut. The aponeurosis is then recessed slightly with cotton swabs and the eyelid level is reassessed. Under-corrections are opened and the aponeurosis advanced appropriately to the desired position, and contour abnormalities are handled in a similar fashion.
Frontalis suspension using fascia lata
Patients with severe ptosis and poor levator function are candidates for frontalis suspension with autogenous fascia lata. Patients with synkinetic ptosis (Marcus–Gunn jaw-winking ptosis) may also be candidates for this procedure with or without levator extirpation. Generally, autogenous fascia lata gives more predictable and long-lasting results10. Eye bank preserved tissues can be utilized when the patient is younger than 3 years of age or at the family’s request.
Autogenous fascia lata is easily harvested ‘freehand’, obviating the need for any kind of fascial strippers. An incision of approximately 3–4 cm is marked in the mid-thigh longitudinally, halfway between the head of the fibula and the anterior superior iliac spine (Fig. 48.14). Although this procedure is usually performed under general anesthesia, 0.5% marcaine with 1 : 100 000 epinephrine is injected subcutaneously for hemostasis and postoperative analgesia. The foot is pronated slightly by a non-scrubbed assistant, or it can be taped to immobilize the leg and place the fascia lata on stretch. The incision is begun with a no. 15 Bard–Parker blade, and dissection is carried down through the subcutaneous fat to the fascia. For bilateral surgery, the harvested strip of fascia needs to be at least 6 mm in width and 8 to 10 cm in length. Two incisions are made 8–10 cm apart into the fascia with a no. 15 Bard–Parker blade. The fascia is exposed superiorly and inferiorly by dissecting bluntly with small ‘peanuts’ (small gauze-wrapped cotton balls). A surgical assistant moves along the incisions with army–navy retractors to expose the field. Using long Metzenbaum scissors, the fascial strips are incised lengthwise. Each strip is transected at both ends with curved scissors or Jorgenson’s scissors, and pulled out of the wound. The fascia lata is not repaired primarily. The subcutaneous tissues are closed with 4-0 or 5-0 Vicryl sutures, and the skin can be closed with a 5-0 plain gut suture.
Frontalis suspension using a silicone (silastic) rod
Silicone rod frontalis suspension is recommended in patients with progressive neuromuscular disorders and third nerve palsy because of the possibility of recovery from illness, possible favorable response to therapeutics, and allowance for postoperative adjustment. The 1 mm solid silicone (Silastic) rods are available commercially (Fig. 48.15). Some of the available silicone rod packages like the Seiff frontalis suspension set from BD Visitec™ Catalog# 585192 come with passing needles and a silicone sleeve, which eliminate the need for the Wright fascia lata needle (BD 1 Becton Drive, Franklin Lakes, NJ USA 07417 Ph: 201.847.6800). Some surgeons that find the metal trochard on the Seiff set to be not sturdy enough to withstand torque without bending, and they still prefer to use the Wright fascia needle. Using this instrument has two minor disadvantages that can be easily overcome: the Wright needle to pass the silicone needs to be done in a retrograde fashion and silicone has to be threaded into the needle. The silicone thread used by the authors (NU) when using the fascia needle is the Dyna Rod Prosthesis (Roger Klein Instruments, PO Box 501. Palmer, PR 00721 Ph: 787 8873405 Fax:787 888 8071). Other materials like ePTFE (Gore-Tex®) are available for this purpose, but the authors do not use this material routinely.
Surgical technique
One percent xylocaine with epinephrine and hyaluronidase is injected under the lid crease incision and just above the eyebrow centrally, medially, and laterally. The lid crease incision is made and the tarsal plate is exposed by dissection through the orbicularis. The silicone rod is sewn onto the tarsal plate with three to five interrupted 6-0 braided nylon or polypropylene sutures (Fig. 48.16). The rod is then pulled up to the eyebrow to mark the medial and lateral brow incisions. This will help to optimize the contour of the upper eyelid margin. Incisions are made down to the periosteum. With the globe protected, in a similar fashion as described above, the rods are passed through the preaponeurotic space to the medial and lateral brow incisions with the passing needles (Fig. 48.17) or the Wright fascia needle. The medial and lateral ends of the silicone rod are then pulled up and crossed centrally to mark the central incision above the pupillary optic axis, which is usually above the pupil or just slightly medial to it. The two ends of the rod are passed through a small silicone sleeve to secure them at optimum length and tension. This maneuver can be performed using either a Watzke or a Lambert silicone sleeve spreader. The lid level is set between 1 and 3 mm above the pupil, depending on the condition being treated. (When dealing with adult myogenic or neurogenic ptosis, the surgeon should not elevate the margin of the superior eyelid to the limbus, as for correction of congenital ptosis.) The rods are trimmed and left with 5–8 mm at either end for possible future adjustment. The ends of these rods are then tucked into the wound. A 6-0 braided nylon or polypropylene suture is sewn around the sleeve, cutting into the silicone sleeve, which is then sewn superiorly to the deeper frontalis muscle (Fig. 48.18). The brow incisions are closed and the lid crease incision is closed, usually with supratarsal fixation every other bite to create a defined lid crease. One can adjust the lid level and contour postoperatively in the office by exposing the silicone sleeve under the central suprabrow incision. This is best done within the first few weeks after surgery, because once a pseudocapsule forms around the rods adjustment may prove to be more difficult. If indicated, the rod can be entirely removed at any time postoperatively. The silicone rod offers the extra advantage and flexibility of adjustability, and results comparable to those obtained with fascia lata techniques can be achieved (Fig. 48.19).
1 Lin LK, Uzcategui N, Chang EL. Effect of surgical correction of congenital ptosis on amblyopia. Ophthal Plast Reconstr Surg. 2008;24(6):434-436.
2 Yamada K, Chan WM, Andrews C, et al. Identification of KIF21A mutations as a rare cause of congenital fibrosis of the extraocular muscles type 3 (CFEOM3). Invest Ophthalmol Vis Sci. 2004;45(7):2218-2223.
3 Callahan M, Beard C. Ptosis, 4th edn. Birmingham: Aesculapius; 1990.
4 Dresner SC. Further modifications of the Müller’s muscle conjunctival resection procedure. Ophthalmic Plast Reconstr Surg. 1991;7:114-122.
5 Meyer DR, Wobig JL. Detection of contralateral eyelid retraction associated with blepharoptosis. Ophthalmology. 1992;99:366-369.
6 Mercandetti M, Putterman AM, Cohen ME, et al. Internal levator advancement by Müller’s muscle-conjunctival resection: technique and review. Arch Facial Plast Surg. 2001;3:104-110.
7 Dresner SC. Minimal ptosis management. In: Kikkawa DO, editor. Aesthetic Ophthalmic Plastic Surgery. Philadelphia: Lippincott-Raven; 1997:151-162.
8 Older JJ. Ptosis repair and blepharoplasty in the adult. Ophthalmic Surg. 1995;4:304-308.
9 Berlin AJ, Vestal KP. Levator aponeurosis surgery. Ophthalmology. 1989;96:1033-1037.
10 Crawford JS. Repair of ptosis using frontalis muscle and fascia lata: a 20 year review. Ophthalmic Surg. 1977;8:31-40.
11 Older JJ, Dunne PB. Silicone slings for the correction of ptosis associated with progressive external ophthalmoplegia. Ophthalmic Surg. 1984;15:379-381.
12 Putterman AM, Urist MJ. Muller muscle-conjunctival resection. Arch Ophthalmol. 1975;93:619-623.
13 Weinstein GW, Buerger GF. Modifications of the Müller’s muscle-conjunctival resection operation for blepharoptosis. Am J Ophthalmol. 1982;93:647.
14 Beard C. Müller’s superior tarsal muscle: anatomy, physiology and clinical significance. Ann Plast Surg. 1985;14:324-333.
15 Putterman AM. A clamp for strengthening Müller’s muscle and the treatment of ptosis: modification, theory and a clamp for Fasanella–Servat operation. Arch Ophthalmol. 1972;87:665-667.
16 McCord CD, Tannenbaum M. Oculoplastic Surgery. New York: Raven Press; 1987.
17 Linberg JV, Vasquez RJ, Chao GM. Aponeurotic ptosis repair under local anesthesia. Prediction of results from operative lid height. Ophthalmology. 1988;95:1046-1052.
18 McCord CD. An external tarso-aponeurectomy. Trans Am Acad Ophthalmol Otol. 1975;79:683.
19 Anderson RL, Dixon RS. Aponeurotic ptosis surgery. Arch Ophthalmol. 1979;79:1123-1128.

 , which means ‘to fall’. In ophthalmology and oculoplastics this term invariably and unequivocally refers to the pathologic condition in which the upper eyelid margin is inferiorly displaced. In the setting of a patient with ptosis, the abnormal eyelid position may cover a significant portion of the cornea and hence the pupillary aperture, sometimes to a degree that is enough to cause visual impairment from the obstruction created. A more suitable term to describe the condition would be that of blepharoptosis, but for the purposes of this chapter the terms ptosis and blepharoptosis will be used interchangeably.
, which means ‘to fall’. In ophthalmology and oculoplastics this term invariably and unequivocally refers to the pathologic condition in which the upper eyelid margin is inferiorly displaced. In the setting of a patient with ptosis, the abnormal eyelid position may cover a significant portion of the cornea and hence the pupillary aperture, sometimes to a degree that is enough to cause visual impairment from the obstruction created. A more suitable term to describe the condition would be that of blepharoptosis, but for the purposes of this chapter the terms ptosis and blepharoptosis will be used interchangeably.