Chapter 14 Psychosis of Epilepsy
Introduction
Psychotic disorders are an uncommon psychiatric comorbidity in patients with epilepsy, but their prevalence is higher than in the general population. An association between psychotic phenomena and epilepsy was described in ancient Greek and Roman literature. For example, Hippocrates and Aristotle considered Hercules to have suffered from epilepsy and to have killed his children in the midst of a “fit of madness.”1 Until the 19th century, epilepsy continued to be associated with “madness” in one form or another to the point where various authors considered psychotic episodes to be an “epileptic equivalent.”2 Indeed, Falret in 1854 and Morel in 1873 referred to psychotic disorders in patients with epilepsy as larval epilepsy, epileptic mania, and grands maux intellectuels.3,4 Kraepelin described the temporal relation between seizure occurrence and the development of psychotic episodes, such as postictal psychosis, and also recognized their occurrence independent of seizures (interictal psychotic episodes).5
In the 1950s, several investigators recognized that patients with epilepsy, especially those with temporal lobe epilepsy (TLE), could have a psychotic disorder that differed in many ways from the schizophrenias. Hill in 19536 and Pond in 19577 observed that these patients did not display the lack of affect and the “asocial or withdrawn attitude” that was typical of patients with schizophrenia. In their classic paper, Slater et al.8 also emphasized the differences described by Hill and Pond. Because the psychotic episodes in these patients included paranoid delusions with visual and auditory hallucinations Slater et al. introduced the term “schizophrenia-like psychosis.” Further systematic analyses of psychiatric phenomena by others continued to depict a unique psychotic disorder that, while sharing pivotal symptoms, differed in important ways from the psychoses that affected nonepileptic patients.
Today, psychotic disorders identified in patients with epilepsy are commonly referred in the medical literature as psychosis of epilepsy (POE). This is a term applied to a group of psychotic disorders with distinct phenomenology and etiopathogenic mechanisms that are likely to be closely related to the seizure disorder.9 For example, in a review of the literature, Ferguson and Rayport described how episodic psychosis of epilepsy is related to seizure recurrence and remits when seizures are controlled.9,10 The purpose of this chapter is to review the clinical manifestations of the various forms of POE, their potential pathogenic mechanisms, and current management.
EPIDEMIOLOGIC ASPECTS
The prevalence rates of primary schizophreniform disorders in the general population range between 0.4 and 1%. In contrast, the prevalence of POE has been estimated to be between 7 and 10%.2 Unfortunately, data about POE derived from population-based studies are limited. In a retrospective study from a tertiary center, Mendez et al found psychotic disorders to be prevalent in 9% of patients with epilepsy and in 1% in migraneurs.11 In a study by Matsuura et al. carried out in Japan, psychotic disorders were found in 6% of patients with epilepsy.12 Overall, the incidence of schizophrenia-like psychosis is believed to be 6 to 12 times higher in patients with epilepsy than in the general population.
The relation between psychotic disorders and epilepsy has long attracted considerable interest. At the beginning of the last century, von Meduna described an inverse relationship between the occurrence of psychotic episodes and seizures.2 Such observations ultimately led to the use of iatrogenic convulsions, initially with camphor and later with electroconvulsive therapy (ECT), for the treatment of serious psychotic disorders. In a case-control study of new-onset epilepsy in older adults, Hesdorffer et al. concluded that a prior diagnosis of schizophrenia was protective against developing epilepsy.13,14 However, this does not appear to be the case in pediatric populations. For example, Jablinsky et al. found that epilepsy in children increased the risk of schizophrenia by a factor of 2 in a case-control study of psychiatric illness in developing countries.15 The relation between febrile seizures and the risk of schizophrenia is disputed: A Danish study found a positive correlation,16 whereas no association was found in a ten-country study by the WHO.
Clinical Features
The traditional classification of POE has been based on the temporal relationship between the psychotic episode and occurrence of seizures. Psychotic episodes are divided into ictal (e.g., the psychotic symptoms are the clinical expression of the seizure), postictal (e.g., episodes occurring up to 120 hours after the seizure); and interictal (e.g., episodes that are independent of seizures).17 A fourth manifestation of POE is the alternative psychotic episode (APE), also known as forced normalization, in which psychotic episodes occur in patients who become seizure free after having had persistent seizures for many years.18,19 Rayport and Ferguson proposed that POE be grouped into two categories9,10: episodic psychosis of epilepsy and chronic (or nonepisodic) psychosis of epilepsy. The former has been identified primarily in patients with TLE and, according to these authors, are closely linked to the effectiveness of seizure control. Episodic psychotic episodes last from a few days to several weeks. They can have a prolonged course and carry a guarded prognosis. Rayport and Ferguson also pointed out that episodic exacerbations can occur in patients with nonepisodic disorders if the seizures worsen. Thus, Rayport and Ferguson’s episodic psychosis of epilepsy corresponds to postictal psychotic episodes (PIPE) and APE, whereas the nonepisodic psychoses are equivalent to the interictal psychosis of epilepsy (IPE). Finally, in addition to IPE, PIPE, and APE, any classification should also include psychotic processes resulting from iatrogenic effects of antiepileptic drugs (AEDs) or surgical treatment.
Interictal Psychosis of Epilepsy
Persons with epilepsy can have interictal psychotic disorders that are clinically indistinguishable from primary schizophreniform disorders.20 However, these patients are older at onset of psychotic symptoms than people without epilepsy. According to the Diagnostic and Statistical Manual of Mental Disorders,21 a diagnosis of schizophrenia requires symptoms from at least two of the following five categories to have been present for a minimum period of 1 month: delusions, hallucinations, disorganized speech, grossly disorganized or catatonic behavior, and negative symptoms (i.e., affective flattening, alogia, avolition). In addition, patients have to display dysfunction in social and occupational domains or in self-care for a minimum of 6 months (including at least 1 month of negative symptoms or at least two of the symptom categories listed earlier), but of lesser severity.
However, as already mentioned, POE is remarkable for the absence of negative symptoms as well as a better premorbid condition. It is also only rarely accompanied by deterioration of a patient’s personality.8 This point is illustrated in Slater’s observation that “the delusions and hallucinations of patients with POE were empathizable (the patient remains in our world).”8 There is general agreement about the lesser severity and also that better response to therapy can be anticipated in POE.22
Postictal Psychotic Episodes
In the past, IPE was thought to be the most frequent POE. Since the advent of video-electroencephalogram (V-EEG), however, PIPE have been recognized with increasing frequency, and some investigators are now suggesting that their prevalence is greater than IPE. Furthermore, several studies have now demonstrated that PIPE can progress to chronic IPE 23,24 (see discussion later in chapter).
Although PIPE are of limited duration and remit following treatment with low doses of antipsychotic drugs or, sometimes, even benzodiazepines, they must nonetheless be taken seriously as they are associated with increased mortality, which results from an elevated incidence of suicide. In addition, PIPE are associated with more severe forms of epilepsy that are often not amenable to surgical treatment (see discussion later in chapter). Postictal psychotic phenomena can present as isolated symptoms or as fully developed psychotic episodes.
POSTICTAL PSYCHOTIC SYMPTOMS
Kanner et al. investigated the prevalence of postictal psychiatric symptoms in 100 consecutive patients with pharmaco-resistant focal epilepsy during a 3-month period.25 The postictal period was defined as the 72 hours that followed recovery from the last seizure. Questions were asked about the frequency of occurrence of each symptom. Only symptoms that were identified after more than 50% of seizures were included in this study to reflect a “habitual” occurrence. To ensure that patients were reporting postictal psychiatric symptoms, each question in the survey also asked about occurrence of the same symptoms during the interictal period. When similar symptoms were identified during both interictal and postictal periods, the only symptoms considered to be postictal were those that were significantly more severe during the postictal period. These were then classified as a postictal exacerbation of interictal psychiatric symptoms. Among the 100 patients, 79 patients had seizures of temporal origin, and 21 had seizures of extratemporal origin. Half of the patients had only complex partial seizures (CPS); the other half had both CPS and generalized tonic-clonic (GTC) seizures. Fifty-two patients had a history of psychiatric conditions: depression, anxiety disorders, and attention deficit disorders. Seven patients experienced postictal psychotic symptoms after more than 50% of their seizures; the duration of the postictal psychotic symptoms, ranged between 1 and 108 hours (median: 15 hours). Such symptoms always occurred together with postictal symptoms of depression and anxiety.
POSTICTAL PSYCHOTIC EPISODES
As mentioned previously, postictal psychiatric symptoms may cluster into PIPE, which correspond to approximately 25% of POE.26 The prevalence of PIPE has been estimated to range between 6 and 10% among patients with pharmaco-resistant epilepsy.27,28 Since the advent of V-EEG monitoring more than four decades ago, recognition of PIPE has increased substantially. This is not surprising because the circumstances of V-EEG are such that the likelihood of psychiatric symptoms occurring is increased (due, for example, to the facilitation of frequent seizures over a short time period following discontinuation or dose reduction of AEDs). In a 1996 study, the yearly incidence of postictal psychiatric disorders among patients with focal epilepsy undergoing V-EEG was 7.9%.27 The majority (6.4%) presented as PIPE. Among the 10 patients with PIPE, four patients had a delusional psychosis, one patient had a mixed manic depressive–like psychosis, two had psychotic depression, one had a hypomanic-like psychosis, and one had a manic-like psychosis. The tenth patient presented with bizarre behavior associated with a thought disorder. In every case, the onset of symptoms lagged the last seizure by a mean period of 24 hours (range 12 to 72 hours). The mean duration of the PIPE was 69.6 hours (range 24 to 144). In five patients, psychotic episodes remitted with low doses of a neuroleptic drug (2 to 5 mg/day of haloperidol), although one patient required high doses of haloperidol (40 mg/day), and remission occurred in four without pharmacotherapy. Six of the 10 patients had an average of 2.4 PIPE prior to V-EEG, whereas in the remaining four patients, it was the first one. Other authors have reported similar findings with respect to clinical characteristics, course, and response to pharmacotherapy.29–34 Common findings among the different case series include (1) a symptom-free interval between the last seizure and onset of psychiatric symptoms; (2) a relatively short duration; (even shorter durations were noted among our patients, as only two had episodes lasting more than five days, while this was true in 5 of 9 patients in Savard’s30 and in 7 of 14 patients in Logsdail’s series29; (3) affect-laden symptoms; (4) clustering of symptoms into delusional and affective-like psychosis; (5) increased numbers of secondarily generalized tonic-clonic seizures before the onset of PIPE; (6) onset of PIPE after having had seizures for more than 10 years; and (7) a prompt response to low-dose neuroleptic medication or benzodiazepines.
Kanemoto et al. studied the clinical differences between PIPE and acute and chronic IPE.33 They noted that patients with PIPE were more likely to experience grandiose and religious delusions in the presence of elevated moods as well as a sense of mystic fusion of the body with the universe. On the other hand, perceptual delusions or commenting voices were less frequent in PIPE, whereas feelings of impending death were common among patients with PIPE.
Various investigators have tried to identify the pathogenic mechanisms of PIPE. For example, Kanner and Ostrovskaya compared 18 consecutive adults with focal seizure disorders and PIPE, and 36 patients with focal epilepsy but without PIPE. Data analysis included the number and location of ictal foci recorded by V-EEG, seizure type, etiology, age at seizure onset, duration of seizure disorder, MRI abnormalities, and psychiatric history before the index V-EEG (other than PIPE).35 Significant differences included the presence of bilateral independent ictal foci on V-EEG (these were identified as an independent predictor of the development of PIPE in univariate and multivariate analyses). The logistic regression model correctly classified 89% of patients. A second independent predictor of PIPE was the occurrence of only secondarily generalized tonic-clonic seizures. Conversely, the occurrence of PIPE and cryptogenic focal epilepsy were predictive of bilateral independent ictal foci in univariate analyses. In a recent study that included 59 consecutive patients with focal epilepsy and a history of PIPE, and 94 controls with focal epilepsy and no history of PIPE, Alper et al. found that predictors of PIPE included ambiguous or extratemporal localization, a family history of psychiatric disorders, abnormal interictal EEGs, and encephalitis.36 Other investigators have also identified bilateral independent interictal32 and ictal34 foci, as well as the presence of secondarily GTC.29,30,34
Patients with a family history of psychiatric symptoms, who have a cluster of secondarily GTC seizures in the course of V-EEG monitoring, and who are found to have bilateral independent ictal foci should be watched carefully for development of PIPE. Symptoms typically become evident between 12 and 72 hours after the last seizure. Occurrence of PIPE has implications with respect to the localization of ictal foci. As suggested by the data cited earlier, development of PIPE should raise the possibility of bilateral independent ictal foci. This is especially important in patients who are being considered for epilepsy surgery. The consequence is that such patients may require longer V-EEG monitoring and, possibly, use of intracranial electrodes. If recordings with depth or subdural electrodes are undertaken, prophylactic treatment with low-dose atypical antipsychotic drugs can avert the occurrence of PIPE during the period of invasive V-EEG monitoring.27
RELATION BETWEEN PIPE AND IPE
There appears to be a bidirectional relationship between PIPE and IPE. For example, in a retrospective study of 18 consecutive adults with focal seizure disorders and PIPE and 36 patients with focal seizure disorders not accompanied by PIPE, Kanner and Ostrovskaya found that seven patients with PIPE and one control patient went on to develop IPE.24 Predictors of developing IPE in univariate logistic regression analyses included a history of PIPE, male gender and having bilateral ictal foci.
Whether preventing further PIPE protects patients from developing IPE has not been established. Such risk, however, supports the recommendation to consider epilepsy surgery whenever possible, even if it is only palliative.24 Remission of IPE and PIPE has been reported after epilepsy surgery in a woman who had had temporal lobe epilepsy since the age of 15 years.37 She developed recurrent PIPE at the age of 35, and this evolved to chronic refractory IPE. Presurgical evaluation demonstrated right hippocampal atrophy and a right mesial temporal epileptogenic focus. Following a right temporal lobe resection, she has been seizure free, and the IPE has remitted.
Although an association between a history of PIPE and development of IPE has been identified in nonsurgical case series,23,37 this has not been reported in studies of patients who have undergone epilepsy surgery. However, Kanemoto et al. found a significant risk of postsurgical mood disorders in patients with a history of presurgical PIPE.38 Indeed, preoperative occurrence of PIPE was five times more frequent among patients with postoperative mood disorders (38%) than among those without (7%). The relation between mood disorders and PIPE was further established in a study by Alper et al. that found a higher prevalence of mood disorders among first- and second-degree relatives as the only psychiatric variable that predicted the development of PIPE on logistic regression analyses (odds ratio = 3.49, P = 0.001).39
A bidirectional relationship between PIPE into IPE was also reported by Tarulli et al., who conducted a retrospective study of 43 patients with PIPE.23 Five (13.9%) patients developed IPE after multiple documented PIPE, whereas in one patient IPE preceded PIPE. The length of time between PIPE and IPE ranged from 7 to 96 months. Adachi et al. also reported a bidirectional relationship between IPE and PIPE (which they called “bimodal psychosis”) in a study of 14 patients.40 Ten patients who had PIPE went on to develop IPE, whereas four patients who had IPE that remitted later developed PIPE. Mean age at onset of epilepsy was 10.8 ± 4.3 years, at the time of the first IPE 24.4 ± 6.1 years, and at the time of the initial PIPE 33.8 ± 4.5 years. These patients did not differ with respect to the epilepsy-related characteristics found in patients with only PIPE: They had bilateral EEG abnormalities and borderline (or decreased) intellectual function.
In the study by Tarulli et al., the symptomatology of PIPE and IPE was similar or identical in five of six cases, and this was also true in our study. However, differences were found in other studies. For example, Kanemoto et al. compared the clinical semiology among 30 patients with PIPE, 33 patients with acute IPE, and 25 patients with chronic IPE.41 The most striking feature that distinguished PIPE from both acute interictal and chronic psychoses was “the relatively frequent occurrence of grandiose delusions as well as religious delusions in the setting of markedly elevated moods and feeling of mystic fusion of the body with the universe” in pipe. In addition, patients with PIPE exhibited few schizophreniform psychotic traits such as perceptual delusions or auditory hallucinations. Further studies are needed to clarify this point.
ICTAL PSYCHOTIC EPISODES
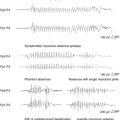
This form of psychosis should always be considered in the differential diagnosis of PIPE and IPE. Ictal psychotic episodes are typically an expression of nonconvulsive status epilepticus (NCSE) presenting as simple partial (SPSE), complex partial (CPSE), or absence status (ASE).42 In the case of SPSE, diagnosis can be difficult, as scalp recordings may not detect ictal patterns.43 SPSE can manifest as hallucinations and illusions. Ictal hallucinations include visual and, less frequently, auditory hallucinations. Although ictal visual hallucinations are usually brief and stereotyped, there are reports of long-lasting hallucinations. In contrast to “psychotic hallucinations,” patients are able to realize that their hallucinations reflect unreal phenomena. This is illustrated in the case of a 54-year-old man who began to have recurrent complex visual hallucinations and illusions described as “puffs of smoke,” “a wall filled with water and fish,” and “the American flag on a pole, growing larger.” He said that he knew they were not real, and that he had no fear of them. These hallucinations recurred over a period of 7 weeks and remitted after administration of antiepileptic drugs (AEDs). The EEG demonstrated a focal ictal discharge over the right temporal area that was concurrent with the hallucinations.44
Elementary visual hallucinations have also been reported as illustrated in the case of a 26-year-old man with SPSE who presented with images of “snowing on TV,” “flickering lights,” and “rotating colored balls” in the right upper visual field that persisted for several days. Magnetoencephalography showed continuous periodic epileptiform discharges over the left posterior superior temporal region, whereas simultaneous EEG showed rhythmic theta waves and sporadic spikes over the left temporal region. Symptoms disappeared after the patient was treated with AEDs.45 Transient cortical blindness has also been reported as a manifestation of occipital lobe SPSE.46,47
Absence status epilepticus has been associated with bizarre behaviors that patients may be unaware of and are thus usually reported by relatives or friends. For example, Olnes at al. described a man who was unable to dress himself after a shower, roamed about his house until his wife could assist him, and went to bed wearing his coat and boots.48 After driving to work, he could not open his locker and when assisted he stated, “I can’t get my truck started.” He put two cups in an empty dishwasher and ran it without detergent. He lit a cigarette as if to smoke but stared at it for several minutes. These behaviors persisted for several days. A neurological evaluation revealed that he was oriented to person, place, year, and hospital. He had difficulty concentrating and had limited short-term recall. He was unable to draw a clock face after several attempts. An EEG demonstrated generalized spikes, polyspikes, and slow waves consistent with atypical ASE. The patient was treated with intravenous lorazepam, and his mental status improved within minutes. When he was again asked to draw a clock face, he was able to do so.
Some patients become depressed, agitated, and occasionally hostile in the midst of an ASE. Other common phenomena have been described in the following way: “sensation of viewing the world through a different medium,” “a feeling of not being in the same world as everyone else,” “uncontrollable rush of thoughts,” “a feeling of fear of losing control of my mind,” “a feeling of closeness,” “a funny feeling that I cannot elaborate,” “a strange feeling of not being myself,” “edgy, worried, and uncomfortable,” “my character changes completely, I become extremely snappy … have a severe headache,” or “weird.”49,50
In addition, isolated reports of ictal catatonia as an expression of NCSE have been published.51,52 For example, Kanemoto et al. described the case of a 78-year-old man who presented with a 1-week history of episodes of mutism alternating with psychomotor agitation.52 On examination, the patient was awake but had a fixed stare. Occasionally, when questioned repeatedly, the patient answered in a whisper with appropriate but fragmented words. His arms and legs were maintained in indefinite and passively placed bizarre postures. There were no focal neurological signs, and he had no history of either epilepsy or psychiatric illness. The EEG showed continuous generalized spike and wave discharges (1.5 to 2 Hz) consistent with atypical ASE. An interview with his wife revealed that he had discontinued benzodiazepines acutely. Administration of diazepam resulted in remission of the abnormal behavior, including catatonic symptoms. Without EEG recording, the ictal nature of the catatonia would likely have remained unrecognized. EEG examination should be considered in patients with catatonia, especially those that occur de novo in elderly persons.
ALTERNATIVE PSYCHOSIS OR FORCED NORMALIZATION
In 1953, Landoldt reported an inverse relation between seizure control and the development of psychotic symptoms in patients who had suffered from persistent seizures for a long time.18 Landoldt described a “normalization” of EEG recordings with the appearance of psychiatric symptoms and coined the term “forced normalization”; Tellenbach proposed the term “alternative psychosis.”19 This antagonism between psychosis and epilepsy has been considered by some as the explanation for the therapeutic effect of electroconvulsive therapy (ECT) of psychotic disorders. This phenomenon is relatively rare. Landoldt reported 47 cases between 1951 and 1958. Single case studies were reported by others, and in 1988 Schmitz estimated the prevalence of alternative psychosis to be 1% among 697 patients followed at a university epilepsy center.53 Forced normalization has been reported in patients with TLE and generalized epilepsies. As with other forms of POE, Wolf identified the psychotic manifestations of an APE after a 15.2-year history of epilepsy in 23 patients.54 Both Landoldt and Wolf reported a pleomorphic clinical presentation with a paranoid psychosis without clouding of consciousness being the most frequent manifestation. As with other POE, a richness of affective symptoms has been identified.
The phenomenon of forced normalization has been observed following the use of various AEDs, including phenytoin and primidone,55,56 carbamazepine and valproic acid,57 and vigabatrin.58 In these cases, the psychotic disorder was thought to result from the suppression of seizures and not from an adverse event of the AED.59
The pathogenic mechanisms mediating APE are yet to be established. Some hypotheses have been proposed. Trimble postulated that an excess of dopaminergic effect is responsible for the seizure cessation and the psychotic symptomatology.2 Rayport and Ferguson suggested that forced normalization is not the expression of seizure cessation, but, rather, of a “voltage depression or suppression” in neocortical derivations concurrent with ictal activity in amygdala or hippocampal structures.9,10 Depth electrode studies by Wieser appear to lend support to this hypothesis.60
The treatment of APE remains the source of debate. Ried and Mothersill, for example concluded that the treatment of alternative psychotic episodes should include the reduction and/or discontinuation of AED, until overt seizure recurrence causes remission of psychotic symptoms.59 The rapidity with which AED should be tapered is not clear. Tellenbach suggested a rapid tapering under EEG monitoring. Landoldt advocated the use of ECT or metrazol if necessary.18,19 Following the recurrence of seizures and the remission of psychotic symptoms, AEDs should be reintroduced slowly.59
IATROGENIC PSYCHOTIC EPISODES
AED-Related Psychotic Episodes
Psychotic symptoms and episodes as an expression of a toxic phenomenon has been reported with most AEDs, including the first-generation AEDs ethosuximide, phenytoin, phenobarbital, and primidone.61,62 as well as the newer AEDs, like vigabatrin, topiramate, levetiracetam, and zonisamide.63–65 The clinical differentiation between APE and a toxic reaction can be difficult if a seizure-free state followed the introduction of the AED.
Psychotic disorders can occasionally follow the discontinuation of AEDs, particularly those with mood stabilizing properties. Ketter et al. reported the development of anxiety and depression, primarily, but also of some cases who experienced psychosis among 32 inpatients who were withdrawn from carbamazepine, phenytoin, and valproic acid.66 Acute withdrawal from benzodiazepines is well known to result in an acute psychotic episode.67
Postsurgical Psychotic Episodes
Postsurgical psychotic disorders are a relatively rare complication of epilepsy surgery with prevalence rates estimated to range between 3 and 10% among patients undergoing an anterior-temporal lobe resections. Several case series have included a mixture of patients with presurgical and de novo postsurgical psychotic disorders. The first important series was that by Falconer, who reported on the prevalence of de novo postsurgical psychotic episodes among 100 patients who had undergone an anterior temporal lobe resection. Seven patients developed de novo postoperative psychosis.68 Jensen and Vaernet reported de novo psychotic disorders in 9 of 74 patients.69 Trimble calculated that the prevalence rate of postoperative de novo psychoses ranged between 3.8 and 35.7% (mean, 7.6%). He suggested that at least some of these cases may be an expression of forced normalization.70 Many epilepsy centers currently do not consider patients with a preoperative history of psychosis as potential candidates for epilepsy surgery. Thus, the lower prevalence rates of more recent reports of postsurgical psychotic episodes are primarily de novo psychoses.
De novo postsurgical psychotic episodes can mimic schizophreniform disorders, manic episodes, or present as PIPE. In a study carried out at the Rush Epilepsy Center, Kanner et al. identified de novo psychotic episodes in four patients within the first 6 months after an anterior temporal lobe resection. Manifestations consisted of manic episodes in two and paranoid episodes in two [unpublished data]. Symptoms remitted in three patients using pharmacotherapy without need for hospitalization. One patient had to be hospitalized in a psychiatric unit. Two of the patients had lesional epilepsy; in one it was caused by dysembryoplastic neuroepithelioma (DNET) and in the other by a ganglioglioma. Shaw et al. reported 11 patients with postoperative new-onset schizophreniform psychosis among 320 consecutive patients (3.2%) who underwent anterior temporal lobe resection.71 Psychotic symptoms became apparent within the first postoperative year in all patients. These 11 patients were compared to a control group of 33 postsurgical patients who did not develop psychotic symptoms. Symptomatic patients were significantly more likely to have bilateral epileptiform activity, a smaller amygdala in the nonoperated side, and pathologies other than mesial temporal sclerosis.
In a study of 57 consecutive patients who underwent anterior temporal lobe resection, Leinonen et al. identified five (8.8%) who developed postoperative psychotic episodes.72 Two (3.5%) patients had experienced PIPE before surgery and continued to have similar episodes postoperatively. Among the other three patients, two (3.5%) experienced a definite and one (1.8%) a probable de novo schizophreniform psychotic disorder.
Stevens identified a de novo psychotic disorder within the first 12 months after surgery in 14 patients who had undergone an anterior temporal lobe resection and who were followed for a period of 20 to 30 years.73 Some investigators have associated the risk of postsurgical psychotic episodes with a right temporal seizure focus. For example, Mace et al. reported seven consecutive patients who developed a de novo psychotic disorder following a right anterior temporal lobe resection: one developed a delusional depression and four a schizophrenic-like psychosis, and one patient was diagnosed with Capgras’ syndrome.74 Nonetheless, the relation between side-of-seizure focus and the risk of developing postsurgical psychosis cannot be decided on the bases of these small case series.
Other authors have associated the presence of gangliogliomas or DNET with the development of de novo postsurgical psychotic disorders. Andermann et al. reported six patients from four centers who experienced a de novo postoperative psychotic disorder presenting as schizophreniform-like episodes with paranoid and depressive symptomatology.75 This association remains to be established in larger studies, however.
Postsurgical psychiatric complications of anterior temporal lobe resection can present as manic episodes. For example, Carran et al. identified 16 patients who developed a de novo manic episode from a case series of 415 consecutive patients (corresponding to a prevalence of 3.8%) who had undergone anterior temporal lobe resection at the Comprehensive Epilepsy Center of Jefferson University Medical Center.76 These patients were compared to a control group of asymptomatic patients matched for age and gender and a second group of 30 patients who experienced a postsurgical depression. Manic episodes occurred within the first postoperative year and were short-lived in all but one patient. Compared to the two control groups, patients with postsurgical mania were more likely to display bilateral EEG abnormalities and to have a right temporal seizure focus, although this difference did not reach significance when compared to the depressed group. Both symptomatic postsurgical patients were more likely to have experienced GTC seizures before surgery and to have had continuing seizures postoperatively.
Postictal psychotic episodes can also occur de novo after anterior temporal lobe resections. For example, Christodoulou et al. reported three cases (1%) among 282 consecutive patients who had undergone an anterior temporal lobe resection.77 All three patients had seizures predominantly from the contralateral (nonsurgical) site or had bilateral independent seizures, whereas none of the patients who failed surgery but continued to have seizures from the site of the surgery developed de novo postictal psychosis. This supports the conclusion that patients with PIPE (chronic or de novo) have bilateral independent temporal lobe dysfunction. Manchanda et al. identified four patients (1.3%) who developed a de novo PIPE among a group of 298 consecutive patients who had undergone an anterior temporal lobe resection.78 All four patients had a right-sided resection and had no preoperative psychiatric history.
A history of psychosis should not be considered a contraindication to epilepsy surgery, provided the patient can cooperate during the presurgical evaluation and has a clear understanding of the nature of the surgical procedure, the potential risks and probability of seizure freedom. This view is supported by the paper of Reutens et al. on five patients with medically intractable epilepsy and chronic psychosis who had temporal lobe resection for epilepsy.79 Postoperatively, all patients were seizure free. Surgery had no apparent effect on the course or severity of the patients’ psychoses. However, seizure freedom allowed two of the five patients to return to work or a supervised workshop.
Although it is often difficult to predict which patients will develop psychiatric complications after epilepsy surgery, several studies have suggested potential risk factors for the development of psychosis. Among these are surgery over the age of 30 years, family history of psychosis,80 and as stated earlier, the presence of a ganglioglioma or DNET.75 In addition, several studies have examined the issue of postoperative psychosis and the laterality of surgery. Most studies report psychosis to be more common in patients following right temporal lobectomy.72,75,78 The explanation for this lateralizing correlation is yet to be established.
PHARMACOLOGIC TREATMENT OF POE
APDs are grouped into the first generation or “conventional” APD (CAPD) which include 18 drugs developed between the 1950s and the 1970s and second-generation or “atypical” (AAPD), which include six drugs. The mechanism of action of CAPD consists of the dopamine (DA-2) receptors blockade, both at the level of mesocortical, nigrostriatal, and tuberoinfundibular DA pathways.81 These mechanisms are responsible for their antipsychotic effect, but can also cause “emotional blunting” and cognitive symptoms that often lead to confusion with the “negative” symptoms of schizophrenia. Blockade at the nigrostriatal pathways can be associated with acute and chronic movement disorders, presenting as parkinsonian symptoms, as well as dystonic and dyskinetic movements, whereas blockade at the tuberoinfundibular pathways results in increased secretion of prolactin. In addition to their DA blockade properties, most of these CAPDs have muscarinic cholinergic, alpha-1 and histaminic blocking properties, responsible for anticholinergic adverse effects, weight gain, sedation, dizziness, and orthostatic hypotension.
Atypical APDs are dopamine-serotonin antagonists that target DA-2 and 5HT-2A receptors.81 Their main difference with CAPDs is the absence or mild occurrence of extrapyramidal adverse events and of hyperprolactinemia. In addition, this class of drugs has less blunting of effect, and several of these AAPD have mood-stabilizing properties. Hence, AAPDs have in large part replaced the CAPDs. The six AAPDs available include clozapine, risperidone, olanzapine, ziprasidone, quietapine, and aripiprazole.
The proconvulsant properties of APDs have been recognized for a long time and often led to some clinicians’ reluctance to use these drugs in patients with epilepsy for fear of worsening seizure frequency and severity. Among the CAPDs, the incidence rates of seizures in nonepileptic patients have ranged between 0.5 and 1.2%.82,83 The risk is higher with certain drugs and in the presence of the following factors: (1) a history of epilepsy, (2) abnormal EEG recordings, (3) history of CNS disorder, (4) rapid titration of the CAPD dose, (5) high doses of AP, and (6) the presence of other drugs that lower the seizure threshold. For example, when chlorpromazine is used at doses above 1000 mg/day, the incidence of seizures was reported to increase to 9%, in contrast to a 0.5% incidence when lower doses are taken.82 Fluphenazine, perphenazine, and trifluoperazine are the other CAPDs that can cause seizures in a dose-related manner. Haloperidol and molindone are among the CAPDs with a lower seizure risk.83
In a recent study, Alper et al. compared the incidence of seizures between (nonepileptic) patients randomized to an AAPD or placebo in the course of regulatory studies submitted to the FDA between 1985 and 2004.84 The incidence of seizures was higher among those randomized to clozapine and olanzapine, but there was no difference in seizure occurrence between patients randomized to placebo and the other four AAPDs. In nonepilepsy patients, clozapine has been reported to cause seizures in 4.4% when used at doses above 600 mg/day, whereas at a dose lower than 300 mg, the incidence of seizures has been found to be less than 1%.81 On the other hand, in patients with epilepsy, clozapine can increase the seizure frequency at any dose.85
Most APDs can cause EEG changes consisting of slowing the background activity above all when used at high doses. In addition some of these drugs, and particularly clozapine, can cause interictal sharp waves and spikes; this epileptiform activity, however, is not predictive of seizure occurrence.85 There are data suggesting that a severe disorganization of the EEG recordings is a more likely predictor of seizure occurrence. As a rule, any APD should be started at low doses and should undergo slow-dose increments to minimize the risk of seizures in patients with epilepsy.
Pharmacokinetic Interaction Between AEDs and APD
Pharmacodynamic Interactions
Worsening of CNS-related adverse events, including sedation, ataxic gait, and cognitive disturbances, are the most frequent consequences of the pharmacodynamic interactions between APDs and AEDs with sedative properties.85
Clozapine and carbamazepine can cause leucopenia on their own, and their combination can worsen the severity of the leucopenia.85 In addition, the combination of these two drugs has been also reported to cause an increased risk of neuroleptic malignant syndrome. Thus, this combination should be avoided. In addition all six AAPDs can cause weight gain and type II diabetes mellitus, with olanzapine having the greatest impact on weight gain and aripiprazole the least. Thus, glycemias and lipid profiles need to be monitored in all patients on a regular basis, but particularly in patients taking AEDs that are associated with an increased risk of weight gain, such as valproic acid, gabapentin, pregabalin, and carbamazepine.
Electroshock therapy (ECT) is not contraindicated in patients with severe POE and should be considered in patients with epilepsy with psychotic depressive or manic episodes that fail to respond to APD.86–92 Blackwood et al. found that the incidence of seizures in patients following treatment with ECT was no higher than in the general population.87 In fact, several studies have shown that ECT increases the seizure threshold by 50 to 100%,86,89,92 Krystal and Coffey have suggested the use of unilateral ECT in the nondominant frontotemporal region.91 AEDs should be withheld in the mornings before a treatment, but otherwise should be kept at baseline doses.
CONCLUSIONS
Psychotic disorders are the least frequent psychiatric comorbidities in patients with epilepsy. They are seen most often in the setting of drug-resistant epilepsy. In contrast to primary psychotic disorders, epilepsy-associated psychoses tend to be more responsive to pharmacotherapy using antipsychotic drugs. Although some antipsychotic drugs can lower the seizure threshold, several antipsychotic drugs, both conventional and atypical, appear to be safe. Their appropriate use should never be withheld for fear of exacerbating seizures in patients with epilepsy. Started at low dosage and using gradual titration schedules, the risk of seizures can be minimized in both nonepileptic patients and in patients with epilepsy.
1. Temkin O. The Falling Sickness. A History of Epilepsy from the Greeks to the Beginnings of Modern Neurology, 2nd. Baltimore: The John Hopkins University Press, 1971.
2. Trimble MR. Psychosis of Epilepsy. New York: Raven Press, 1991.
3. Falret JP. Mémoire sur la folie ciculaire. Bull. Acad. Med. (Paris). 1854;19:382-400.
4. Morel BA. Discussion sur l’Épilepsie larvée. Ann. Med. Psychol. 1873;9(series 5):155-163.
5. Kraepelin E. Psychiatrie, 8th. Barth: Leipzig, 1923.
6. Hill D. Psychiatric disorders of epilepsy. Med Press. 1953;229:473-475.
7. Pond DA. Psychiatric aspects of epilepsy. J Indian Med Prof. 1957;3:1442-1451.
8. Slater E, Beard AW, Glithero E. The schizophrenia-like psychosis of epilepsy. v. Discussion and conclusions. Br J Psychiatry. 1963;109:95-150.
9. Ferguson SM, Rayport M. Psychosis in epilepsy. In: Blumer D, editor. Psychiatric Aspects of Epilepsy. Washington, DC: American Psychiatric Press; 1984:229-270.
10. Rayport M, Ferguson SM. Psychosis of epilepsy. In: Ettinger AB, Kanner AM, eds. Psychiatric Aspects of Epilepsy: A Practical Guide to Diagnosis and Treatment. Baltimore: Lippincott, Williams and Wilkins. In press.
11. Mendez MF, Grau R, Doss RC, Taylor JL. Schizophrenia in epilepsy: seizure and psychosis variables. Neurology. 1993;43:1073-1077.
12. Matsuura M, Adachi N, Muramatsu R, et al. Intellectual disability and psychotic disorders of adult epilepsy. Epilepsia. 2005;46(Suppl 1):11-14.
13. Hesdorffer DC, Hauser WA, Annegers JF, et al. Psychiatric diagnoses preceding unprovoked seizures in adults: a population-based case-control study. Epilepsia. 1992;33(Suppl 3):16.
14. Hesdorffer DC, Hauser WA. Epidemiological considerations. In: Ettinger AB, Kanner AM, editors. Psychiatric Issues in Epilepsy: A Practical Guide to Diagnosis and Treatment. 2nd. Baltimore: Lippincott Williams and Wilkins; 2007:1-16.
15. Jablinsky A, Sartorius N, Ernberg G, et al. Schizophrenia: manifestations, incidence and course in different cultures. A World Health Organization ten-country study. Psychol Med Monogr Suppl. 1992;20:1-97.
16. Vestergaard M, Pedersen CB, Christensen J, et al. Febrile seizures and the risk of schizophrenia. Schizophr Res. 2005;73:343-349.
17. Kanner AM. Psychosis of epilepsy: a neurologist’s perspective. Epilepsy & Behav. 2000;1:219-227.
18. Landoldt H. Some clinical electroencephalographical correlations in epileptic psychosis (twilight states). Electroencephalogr Clin Neurophysiol. 1953;5:121. (abstract)
19. Tellenbach H. Epilepsie als Anfallseiden und als Psychose. Uber alternative psychosen paranoider Pragung bei “forcierter Normalisierung” (Landoldt) des Elektroencephalogramms Epileptischer. Nervarzt. 1965;36:190.
20. Perez MM, Trimble MR, Murray NMF, Reider I. Epileptic psychosis: an evaluation of PSE profiles. Br J Psychiat. 1985;146:155-163.
21. Diagnostic and Statistical Manual of Mental Disorders. Washington, DC: American Psychiatric Association. 1994;4th.
22. Toone BK, Garralda ME, Ron MA. The psychosis of epilepsy and the functional psychosis: a clinical and phenomenological comparison. Br. J Psychiat. 1982;141:256-261.
23. Tarulli A, Devinsky O, Alper K. Progression of postictal to interictal psychosis. Epilepsia. 2001;42(11):1468-1471.
24. Kanner AM, Ostrovskaya A. Long-term significance of postictal psychotic episodes II. Are they predictive of interictal psychotic episodes? Epilepsy & Behav. 2008;12(1):154-156.
25. Kanner AM, Soto A, Gross-Kanner H. Prevalence and clinical characteristics of postictal psychiatric symptoms in partial epilepsy. Neurology. 2004;62:708-713.
26. Dongier S. Statistical study of clinical and electroencephalographic manifestations of 536 psychotic episodes occurring in 516 epileptics between clinical seizures. Epilepsia. 1959/1960;1:117-142.
27. Kanner AM, Stagno S, Kotagal P, Morris HH. Postictal psychiatric events during prolonged video-electroencephalographic monitoring studies. Arch Neurol. 1996;53:258-263.
28. Lancman ME, Craven WJ, Asconape JJ, Penry JK. Clinical management of recurrent postictal psychosis. J Epilepsy. 1994;7:47-51.
29. Logsdail SJ, Toone BK. Postictal psychosis: a clinical and phenomenological description. Br J Psychiat. 1988;152:246-252.
30. Savard G, Andermann F, Olivier A, Remilliard GM. Postictal psychosis after complex partial seizures: a multiple case study. Epilepsia. 1991;32:225-231.
31. So NK, Savard G, Andermann F, Olivier A, Remillard GM. Acute postictal psychosis: a stereo-EEG study. Epilepsia. 1990;31:188-193.
32. Devinsky O, Abrahmson H, Alper K, et al. Postictal psychosis: a case control study of 20 patients and 150 controls. Epilepsy Res. 1995;20:247-253.
33. Kanemoto K, Kawasaki J, Kawai J. Postictal psychosis: a comparison with acute interictal and chronic psychoses. Epilespia. 1996;37:551-556.
34. Umbricht D, Degreef G, Barr WB, Lieberman JA, Pollack S, Schaul N. Postictal and chronic psychosis in patients with temporal lobe epilepsy. Am J Psychiatry. 1995;152:224-231.
35. Kanner AM, Ostrovskaya A. Long-term significance of postictal psychotic episodes I. Are they predictive of bilateral ictal foci? Epilepsy & Behav. 2008;12(1):150-153. Jan
36. Alper A, Kuznieky R, Carlson C, et al. Postictal psychosis in partial epilepsy: a case-control study. Ann Neurol. 2008;63(5):602-610.
37. Marchetti RL, Tavares AG, Gronich G, Fiore LA, Ferraz RB. Complete remission of epileptic psychosis after temporal lobectomy: case report. Arq Neuropsiquiatr. 2001;59(3-B):802-805.
38. Kanemoto K, Kim Y, Miyamoto T, Kawasaki J. Presurgical postictal and acute interictal psychoses are differentially associated with postoperative mood and psychotic disorders. J Neuropsychiatry Clin Neurosci. 2001;13(2):243-247.
39. Alper K, Devinsky O, Westbrook L, et al. Premorbid psychiatric risk factors for postictal psychosis. J Neuropsychiatry Clin Neurosci. 2001;13(4):492-499.
40. Adachi N, Kato M, Sekimoto M, et al. Recurrent postictal psychosis after remission of interictal psychosis: further evidence of bimodal psychosis. Epilepsia. 2003;44(9):1218-1222. Sep
41. Kanemoto K, Kawasaki J, Kawai J. Postictal psychosis: a comparison with acute interictal and chronic psychoses. Epilepsia. 1996;37:551-556.
42. Kaplan PW. Behavioral manifestations of nonconvulsive status epilepticus. Epilepsy Behav. 2002;3(2):122-139.
43. Devinsky O, Kelly K, Porter WH. Clinical and electroencephalographic features of simple partial seizures. Neurology. 1988;43:1347-1352.
44. Sowa MV, Pituck S. Prolonged spontaneous complex visual hallucinations and illusions as ictal phenomena. Epilepsia. 1989;30:524-526.
45. Oishi M, Otsubo H, Kameyama S, et al. Ictal magnetoencephalographic discharges from elementary visual hallucinations of status epilepticus. J Neurol Neurosurg Psychiatry. 2003;74(4):525-527.
46. Sawchuk KS, Churchill S, Feldman E, Drury I. Status epilepticus amauroticus. Neurology. 1997;49:1467-1469.
47. Barry E, Sussman NM, Bosley TM, Harner RN. Ictal blindness and status epilepticus amauroticus. Epilepsia. 1985;26:577-584.
48. Olnes MJ, Golding A, Kaplan PW. Nonconvulsive status epilepticus resulting from benzodiazepine withdrawal. Ann Intern Med. 2003;139:956-958.
49. Agathonikou A, Panayiotopoulos CP, Giannakodimos S, Koutroumanidis M. Typical absence status in adults: diagnostic and syndromic considerations. Epilepsia. 1988;39:1265-1276.
50. Panayiotopoulos CP. Syndromes of idiopathic generalized epilepsies not recognized by the International League Against Epilepsy. Epilepsia. 2005;46(Suppl 9):57-66.
51. Lim J, Yagnik P, Schraeder P, Wheeler S. Ictal catatonia as a manifestation of nonconvulsive status epilepticus. J Neurol Neurosurg Psychiatry. 1986;49:833-836.
52. Kanemoto K, Miyamoto T, Abe R. Ictal catatonia as a manifestation of de novo absence status epilepticus following benzodiazepine withdrawal. Seizure. 1999;8:364-366.
53. Schmitts B, Wolf P. Psychosis in epilepsy. In: Devinsky O, Theodore WH, editors. Epilepsy and Behavior. New York: Wiley-Liss; 1991:97-128.
54. Wolf P, Trimble MR. Biological antagonism and epileptic psychosis. Br J Psychiat. 1985;146:272-276.
55. Gibbs FA. Ictal and non-ictal psychiatric disorders in temporal lobe epilepsy. J Nerv Ment Dis. 1951;113:522-528.
56. Wolf P. Acute behavioral symptomatology at disappearance of epileptiform EEG abnormality; paradoxical or “forced normalization. In: Smith D, Treiman D, Trimble M, editors. Neurobehavioral Problems in Epilepsy. Advances in Neurology,, 55. New York: Raven Press; 1991:127-142.
57. Pakalnis A, Drake JK, Kellum JB. Forced normalization: acute psychosis after seizure control in seven patients. Arch Neurol. 1987;44:289-292.
58. Sander JWAS, Hart YM, Trimble MR, Shorvon SD. Vigabatrin and psychosis. J Neurol Neurosurg Psychiatry. 1991;54:435-439.
59. Ried S, Mothersill IW. Forced normalization: the clinical neurologist’s view. In: Trimble MR, Schmitz B, editors. Forced Normalization and Alternative Psychoses of Epilepsy. Bristol, PA: Wrightson Biomedical Publishing Ltd; 1998:77-94.
60. Wieser HG. Depth recorded limbic seizures and psychopathology. Neuroscience Biobehav Rev. 1983;7:427-443.
61. Perlo VP, Schwab RS. Unrecognized dilantin intoxication. In: Locke S, editor. Modern Neurology. Boston: Little Brown; 1969:589-597.
62. Rivinus TM. Psychiatric effects of the anticonvulsant regimens. J Clin Psychopharmacol. 1982;2:165-192.
63. Kanner AM, Wuu J, Faught E, Tatum WO, Fix A, French JA. A past psychiatric history may be a risk factor for topiramate-related psychiatric and cognitive adverse events. Epilepsy & Behav. 2003;4:548-552.
64. Mula M, Trimble MR, Yuen A, Liu RS, Sander JW. Psychiatric adverse events during levetiracetam therapy. Neurology. 2003;61(5):704-706. Sep 9
65. Miyamoto T, Kohsaka M, Koyama T. Psychotic episodes during zonisamide treatment. Seizure. 2000;9(1):65-70. Jan
66. Ketter TA, Marlow BA, Flamini R, et al. Anticonvulsant withdrawal: emergent psychopathology. Neurology. 1994;44:55-61.
67. Sironi VA, Franzini A, Ravaghati L, Marossero F. Interictal psychoses in temporal lobe epilepsy during withdrawal of anticonvulsant therapy. J Neurol Neurosurg Psychiatry. 1979;42:724-730.
68. Taylor DC. Mental state and temporal lobe epilepsy. Epilepsia. 1972;13:727-765.
69. Jensen I, Vaernet K. Temporal lobe epilepsy: Follow-up investigation of 74 temporal lobe resected patients. Acta Neurochirurgica. 1977;37:173-200.
70. Trimble MR. Behaviour changes following temporal lobectomy, with special reference to psychosis (editorial). J Neurol Neurosurg Psychiatry. 1992;55(2):89-91.
71. Shaw P, Mellers J, Henderson M, Polkey C, David AS, Toone BK. Schizophrenia-like psychosis arising de novo following a temporal lobectomy: timing and risk factors. J Neurol Neurosurg Psychiatry. 2004;75:1003-1008.
72. Leinonen E, Tuunainen A, Lepola U. Postoperative psychoses in epileptic patients after temporal lobectomy. Acta Neurol Scand. 1994;90(6):394-399.
73. Stevens JR. Psychiatric consequences of temporal lobectomy for intractable seizures: a 20 to 30-year follow-up of 14 cases. Psychol Med. 1990;20(3):529-545.
74. Mace CJ, Trimble MR. Psychosis following temporal lobe surgery: a report of six cases. J Neurol Neurosurg Psychiatry. 1991;54(7):639-644.
75. Andermann LF, Savard G, Meencke HJ, McLachlan R, Moshe S, Andermann F. Psychosis after resection of ganglioglioma or DNET: evidence for an association. Epilepsia. 1999;40(1):83-87.
76. Carran MA, Kohler CG, O’Connor MJ, Bilker WB, Sperling MR. Mania following temporal lobectomy. Neurology. 2003;61:770-774.
77. Christodoulou C, Koutroumanidid M, Hennessy MJ, et al. Postictal psychosis after temporal lobectomy. Neurology. 2002;59(9):1432-1435. Nov 12
78. Manchanda R, Miller H, McLachlan RS. Post-ictal psychosis after right temporal lobectomy. J Neurol Neurosurg Psychiatry. 1993;56(3):277-279.
79. Reutens DC, Savard G, Andermann F, et al. Results of surgical treatment in temporal lobe epilepsy with chronic psychosis. Brain. 1977;120:1929-1936.
80. Glosser G, Zwil AS, Glosser DS, et al. Psychiatric aspects of temporal lobe epilepsy before and after anterior temporal lobectomy. J Neurol Neurosurg Psychiatry. 2000;68:53-58.
81. Stahl SM. Antipsychotic agents. In: Stahl SM, editor. Essential Pharmacology: Neuroscientific Basis and Practical Applications. 2nd. New York: Cambridge University Press; 2000:401-458.
82. Logothetis J. Spontaneous epileptic seizures and EEG changes in the course of phenothiazine therapy. Neurology. 1967;17:869-877.
83. Whitworth AB, Fleischhacker WW. Adverse effects of antipsychotic drugs. Int Clin Psychopharmacol. 1995;9(Suppl 5):21-27.
84. Alper K, Schwartz KA, Kolts RL, Khan A. Seizure incidence in psychopharmacological clinical trials: an analysis of Food and Drug Administration (FDA) summary basis of approval reports. Biol Psychiatry. 2007;62(4):345-354. 15
85. Zaccara G, Messori A, Cincotta M. Clinical studies of pharmacodynamic interactions between antiepileptic drugs and other drugs. In: Majkowski J, Bourgeois B, Patsalos P, Mattson R, editors. Antiepileptic Drugs: Combination Therapy and Interactions. Cambridge, UK: Cambridge University Press; 2005:241-254.
86. Post R, Putnam F, Uhde T. Electroconvulsive therapy as an anticonvulsant: implications for its mechanisms of action in affective illness. In: Malitz S, Sackeim H, editors. Electroconvulsive Therapy: Clinical and Basic Research Issues. New York: New York Academy of Sciences, 1986.
87. Blackwood DHR, Cull RE, Freeman CP, et al. A study of the incidence of epilepsy following ECT. J Neurol Neurosurg Psychiatry. 1980;43:1098-1102.
88. Abrams R. Electroconvulsive therapy in the high-risk patient. In: Abrams R, editor. Electroconvulsive Therapy. New York: Oxford University Press; 1997:81-113.
89. Sackeim HA. The anticonvulsant hypothesis of the mechanisms of action of ECT: current status. The Journal of ECT. 1999;15:5-26.
90. Viparelli U, Viparelli G. ECT and grand mal epilepsy. Convulsive Ther. 1992;8:39-42.
91. Coffey CE, Lucke J, Weiner RD, et al. Seizure threshold in electroconvulsive therapy (ECT). II. The anticonvulsant effect of ECT. Biol Psychiatry. 1995;37:777-788.
92. Fink M, Kellner C, Sackheim HA. Intractable seizures, status epilepticus and ECT. JECT Lett. 1999;15:282-284.