Frequently associated with loculated ascites of similar attenuation to individual implants
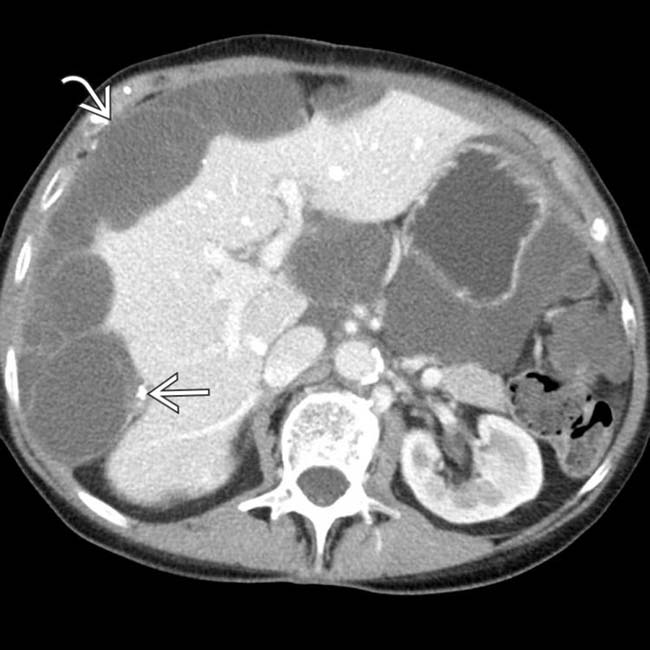
 Dominant cystic or solid mass often present in right lower quadrant (in expected location of appendix)
Dominant cystic or solid mass often present in right lower quadrant (in expected location of appendix) Metastases to ovary are common, so cystic masses in ovaries may not represent primary ovarian neoplasm
Metastases to ovary are common, so cystic masses in ovaries may not represent primary ovarian neoplasm• MR: Implants usually low signal on T1WI and high signal on T2WI with possible internal enhancement on T1WI C+
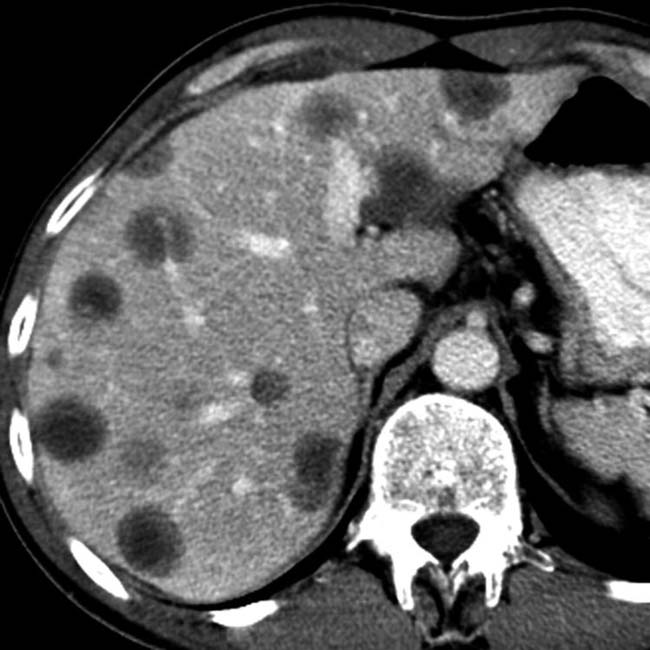
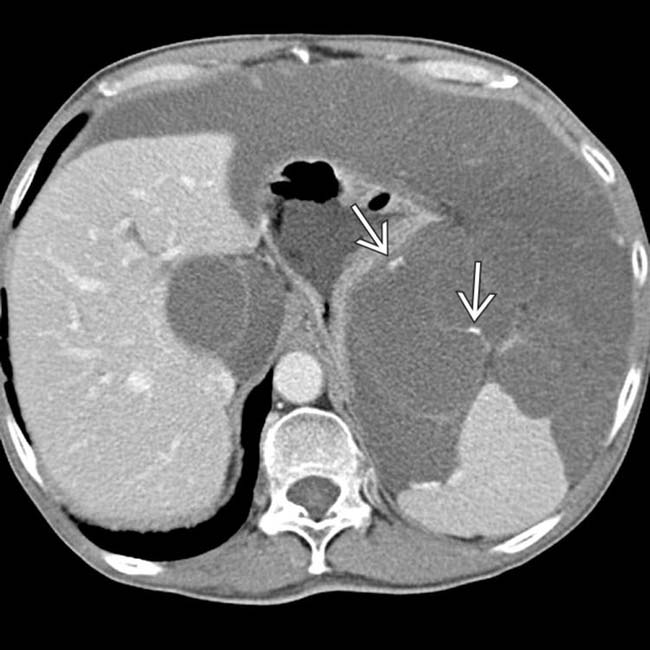
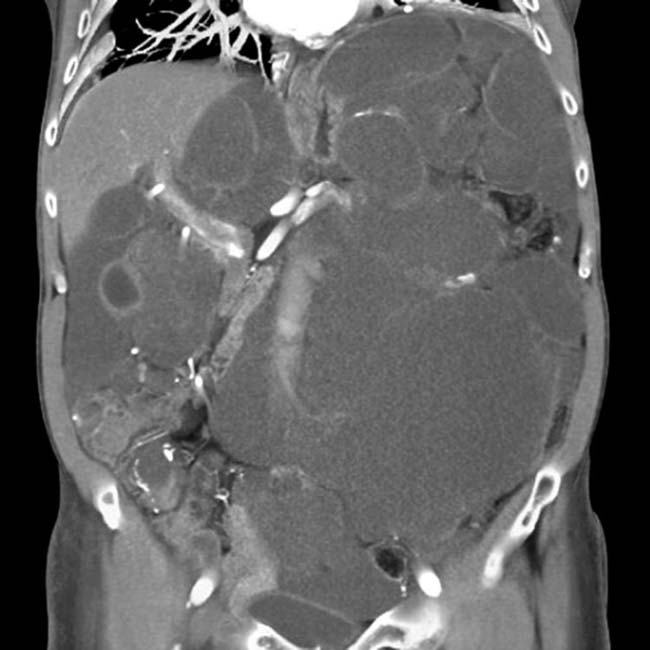
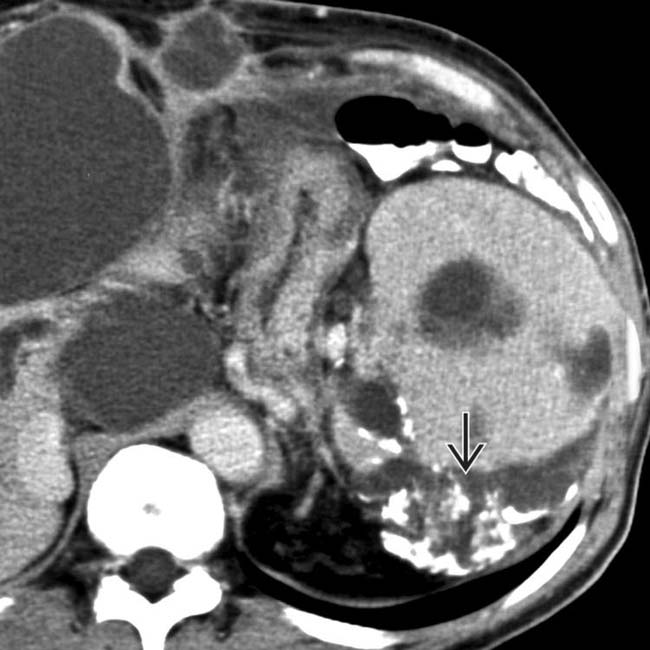
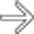
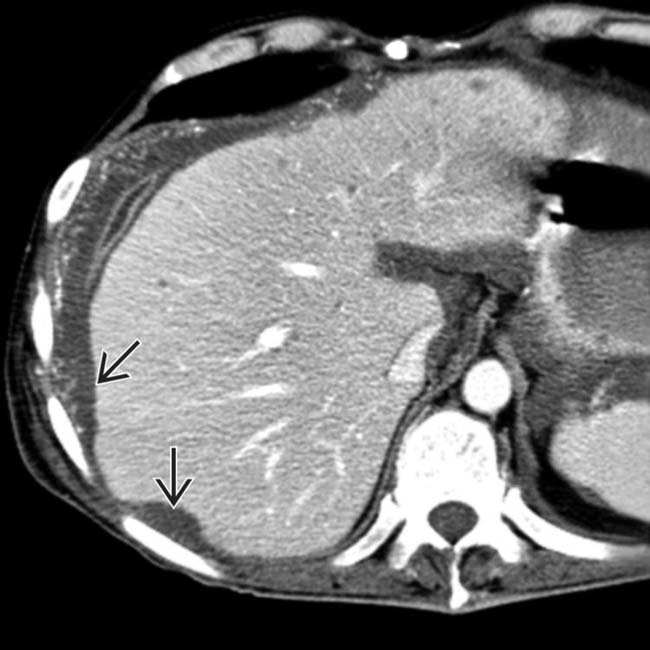
 “scalloping” the border of the liver, a characteristic appearance of pseudomyxoma peritonei. At least 1 implant demonstrates peripheral calcification
“scalloping” the border of the liver, a characteristic appearance of pseudomyxoma peritonei. At least 1 implant demonstrates peripheral calcification  .
.
 .
.
IMAGING
General Features
CT Findings
• Low-attenuation masses (usually < 20 HU) scattered throughout peritoneum with central displacement of bowel loops
• Dominant cystic or solid mass often present in right lower quadrant (in expected location of appendix)
DIFFERENTIAL DIAGNOSIS
Peritoneal Carcinomatosis Without Mucinous Ascites
Peritoneal Carcinomatosis With Mucinous Ascites
• Some authors use the term PMP for disseminated spread of any mucin-producing neoplasm (not simply cases with appendiceal primary)
• Implants may appear low density (with “scalloping” of liver and spleen) and be indistinguishable from classic PMP due to appendiceal primary neoplasm
PATHOLOGY
General Features
Staging, Grading, & Classification
• Ronnet classification (1995)
 Type I: Adenomucinosis (also known as disseminated peritoneal adenomucinosis or DPAM)
Type I: Adenomucinosis (also known as disseminated peritoneal adenomucinosis or DPAM)
 Type I: Adenomucinosis (also known as disseminated peritoneal adenomucinosis or DPAM)
Type I: Adenomucinosis (also known as disseminated peritoneal adenomucinosis or DPAM)
CLINICAL ISSUES
Presentation
Natural History & Prognosis
• Slowly progressive process with progressive accumulation of implants and loculated ascites and development of multiple bowel obstructions
Treatment
• Primary treatment is cytoreductive surgery (Sugarbaker procedure) with debulking of intraperitoneal implants and infusion of heated intraperitoneal therapy
• Some groups have reported using systemic chemotherapy, radiotherapy, or intraperitoneal isotope therapy, although little supportive evidence
 in the right lower quadrant found to be the result of a ruptured appendiceal mucocele. No other implants were present elsewhere.
in the right lower quadrant found to be the result of a ruptured appendiceal mucocele. No other implants were present elsewhere.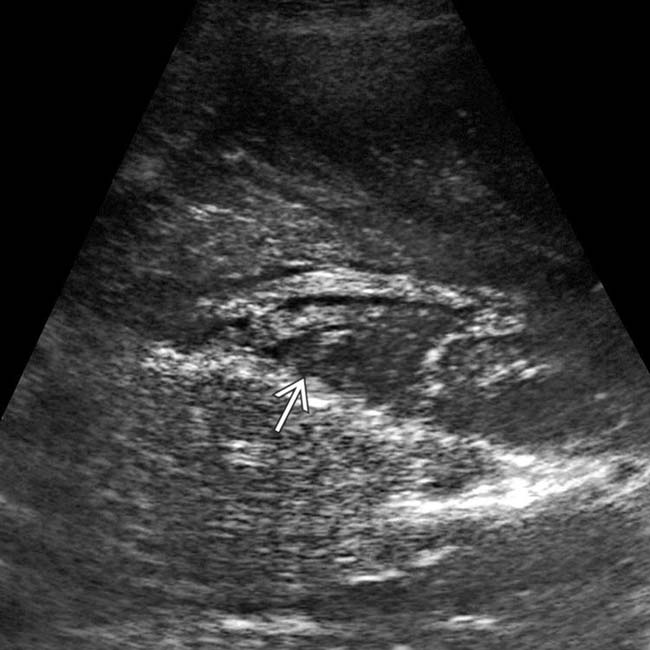
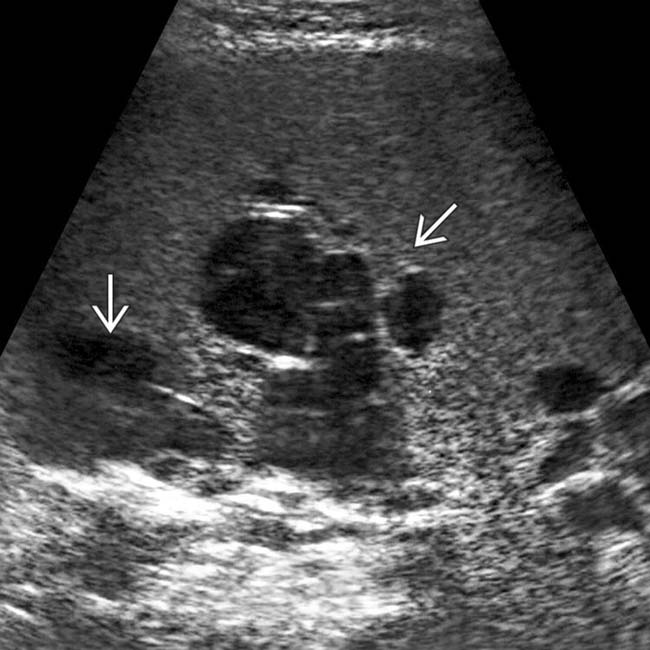
 . Despite appearing cystic on CECT, implants on US can appear solid or echogenic.
. Despite appearing cystic on CECT, implants on US can appear solid or echogenic.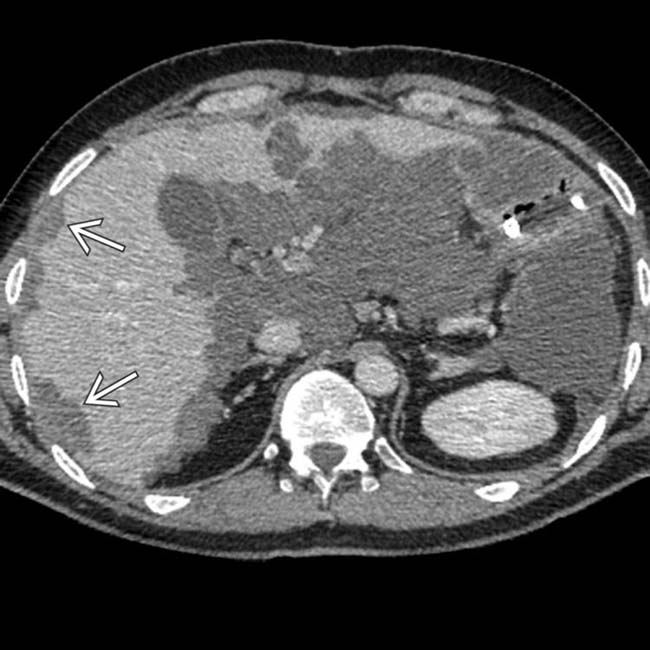
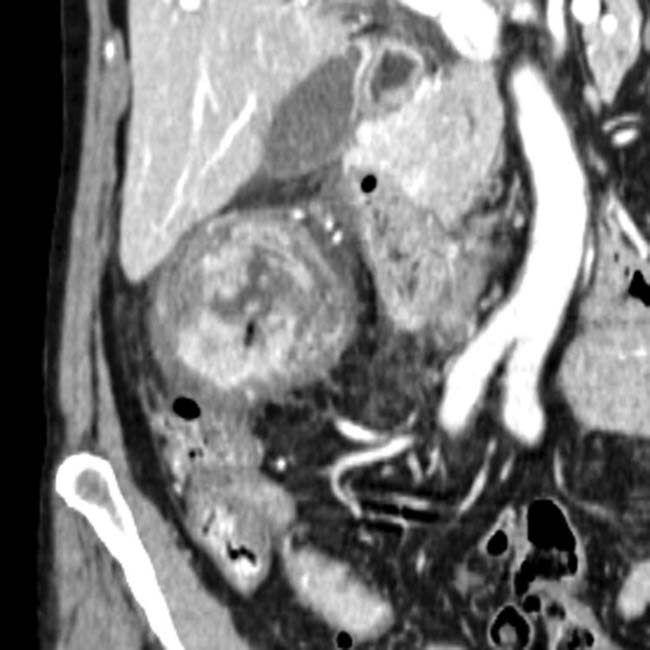
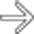
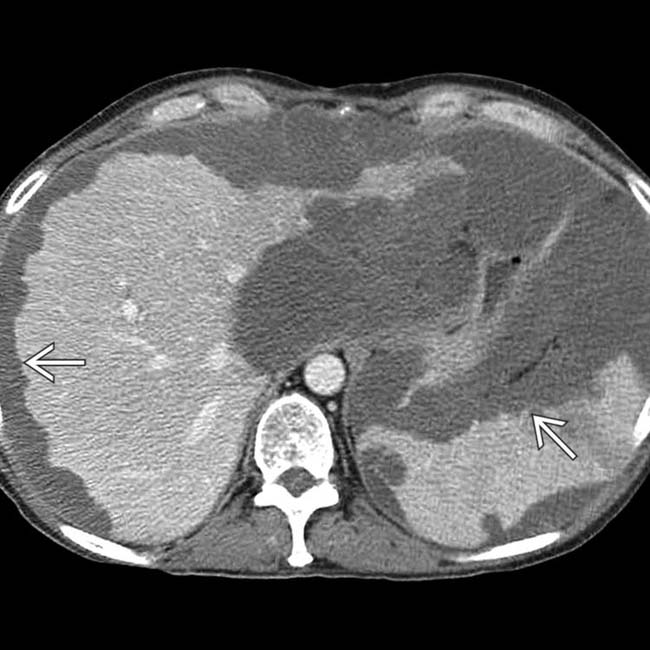
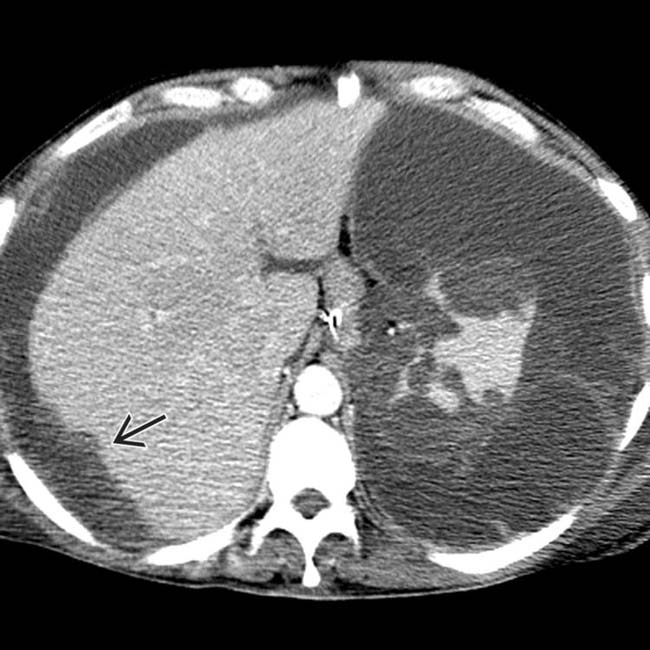
 . The implants are low density, but slightly higher in attenuation (∼ 20 HU) than simple fluid.
. The implants are low density, but slightly higher in attenuation (∼ 20 HU) than simple fluid.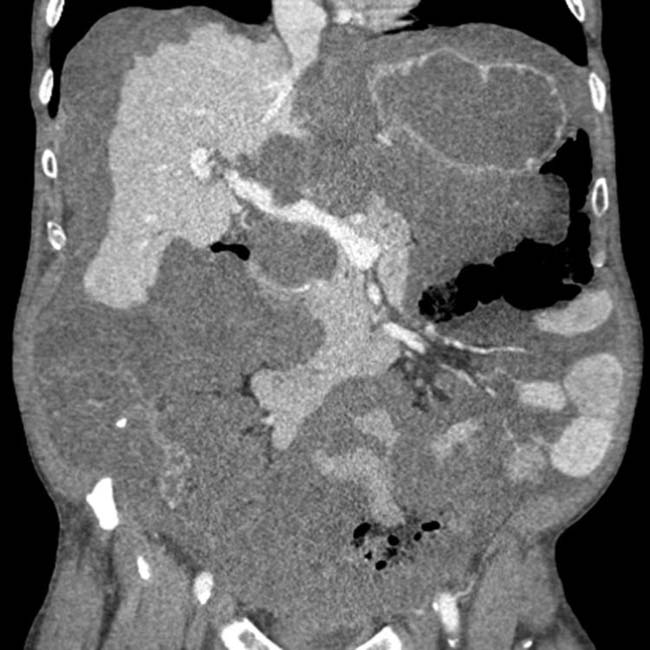
 .
.
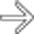
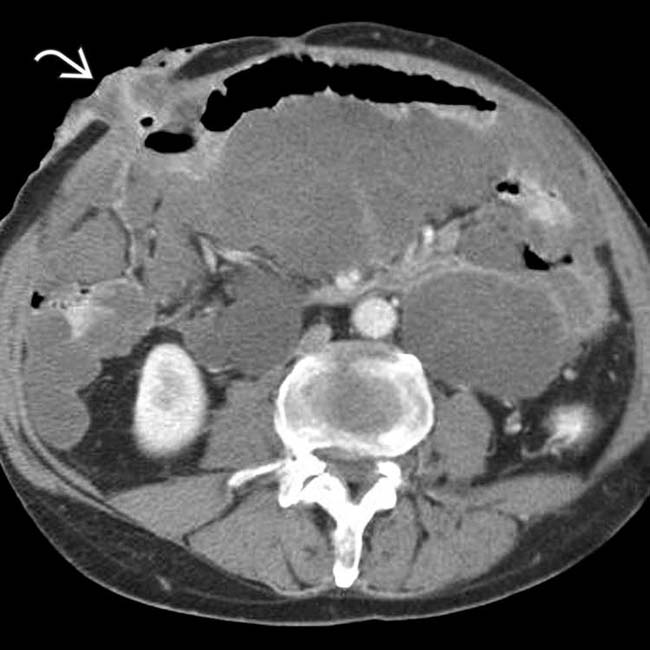
 . Calcifications, often curvilinear and peripheral, are not uncommonly seen with implants in pseudomyxoma.
. Calcifications, often curvilinear and peripheral, are not uncommonly seen with implants in pseudomyxoma.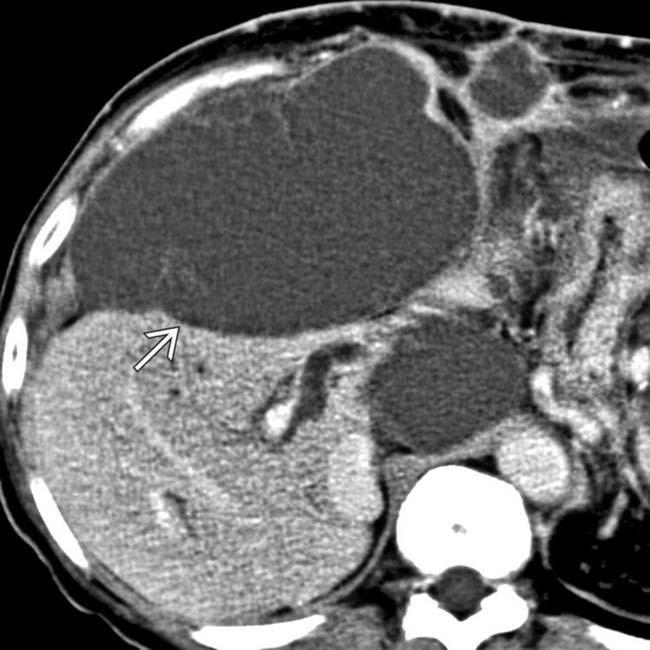
 .
.
 .
.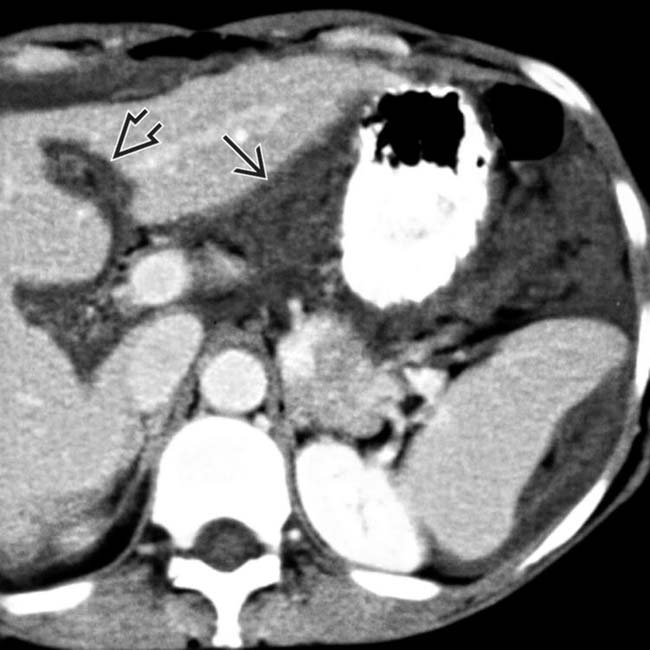
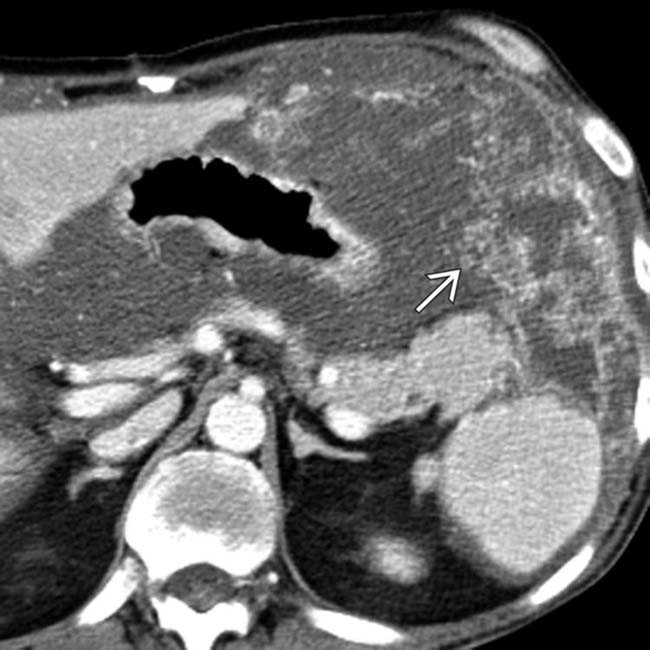
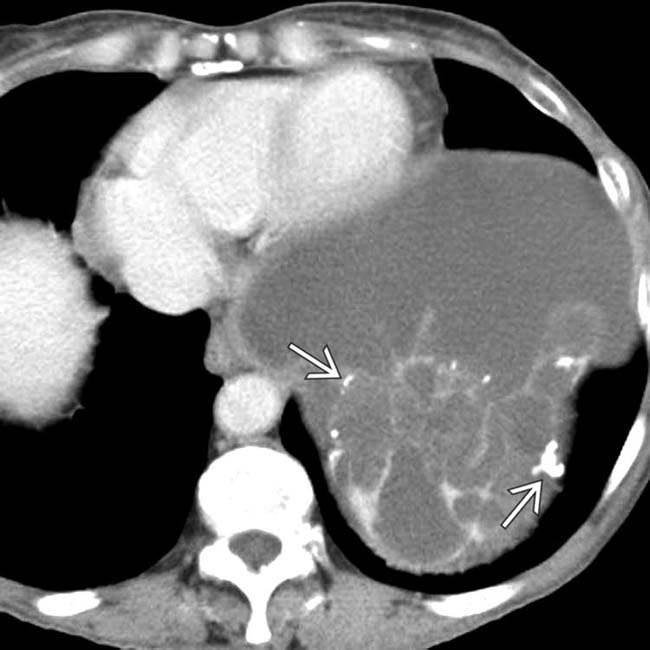
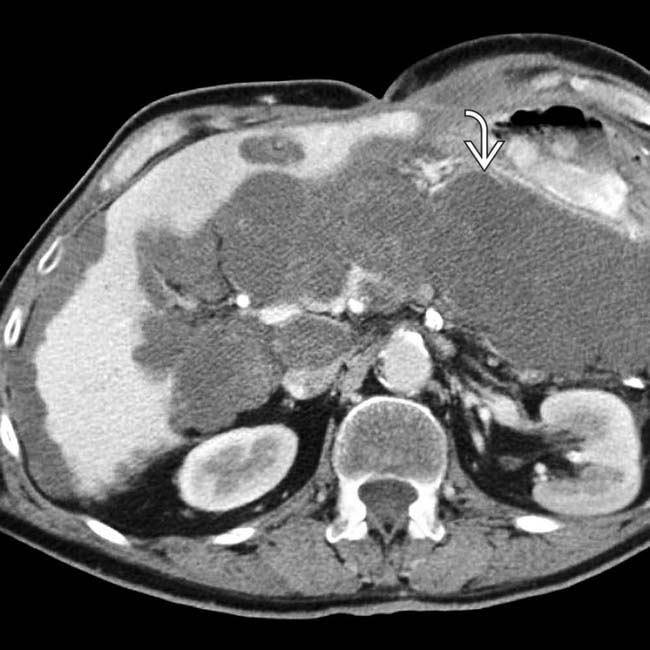
 and falciform
and falciform  ligaments.
ligaments.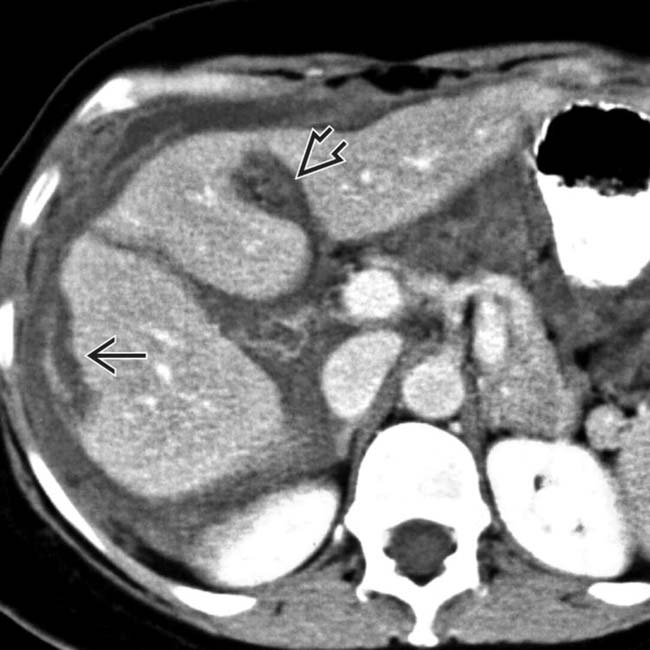
 and falciform ligament implant
and falciform ligament implant  in a patient with pseudomyxoma peritonei.
in a patient with pseudomyxoma peritonei.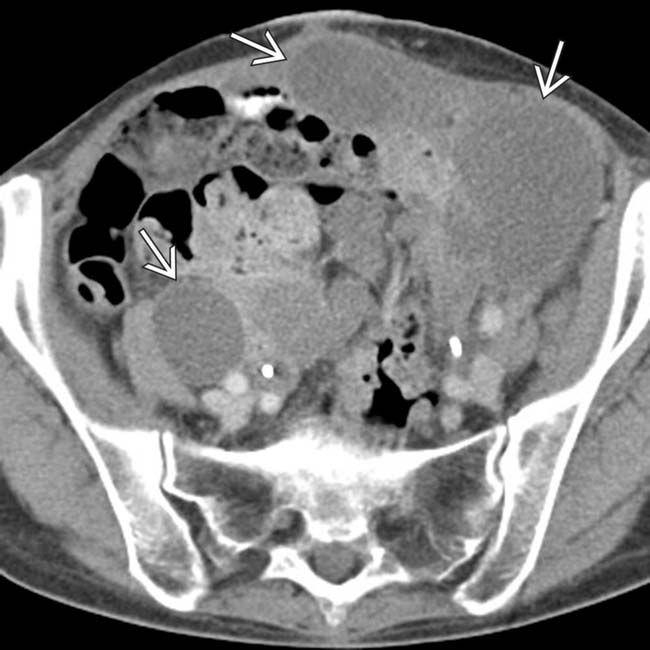
 along the periphery of the pelvic cavity.
along the periphery of the pelvic cavity.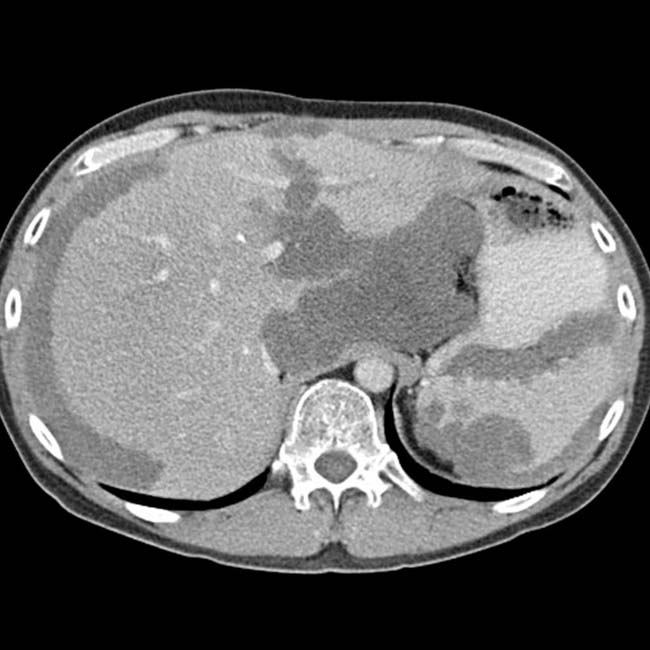
 .
.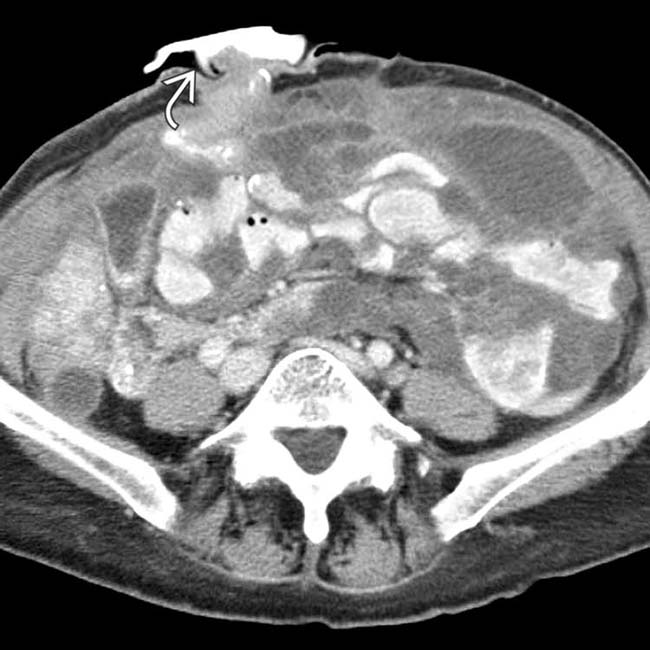
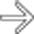
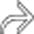 , which was performed to alleviate the bowel obstruction.
, which was performed to alleviate the bowel obstruction.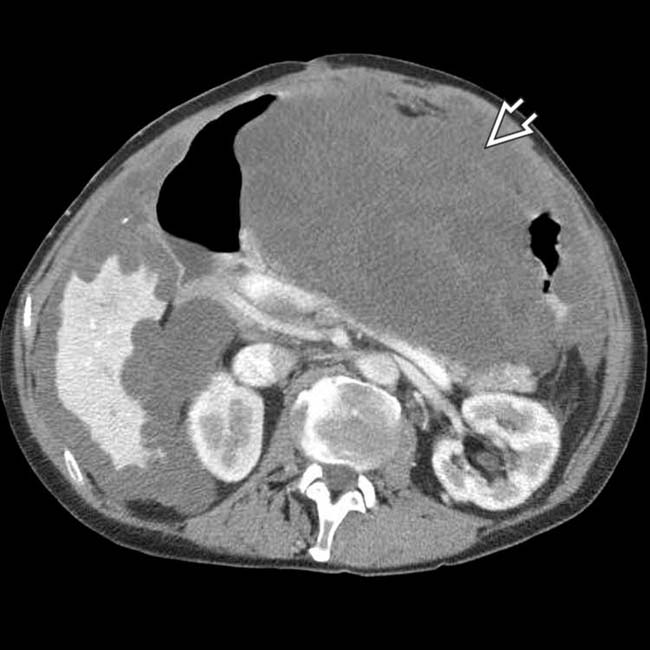
 , often indicative of malignant or infected ascites.
, often indicative of malignant or infected ascites.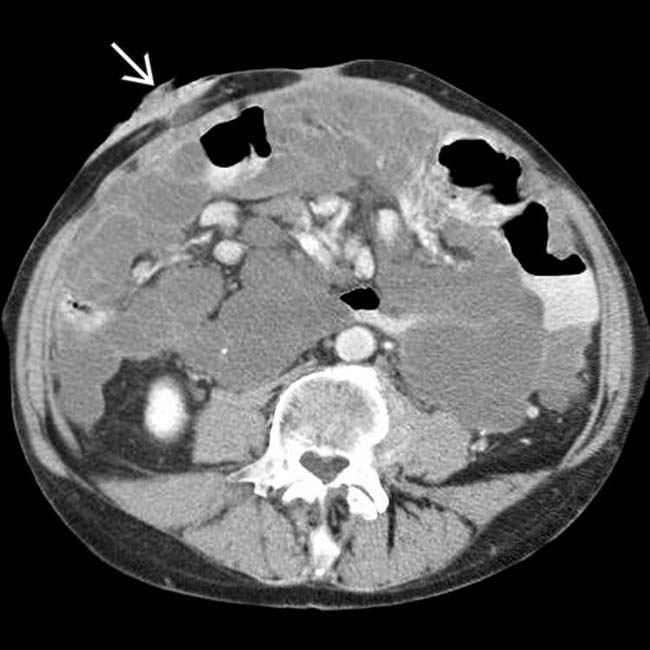
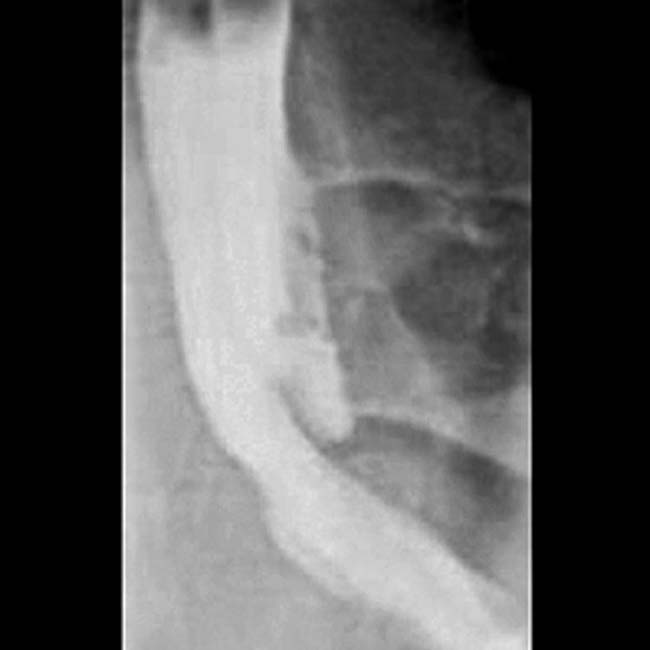
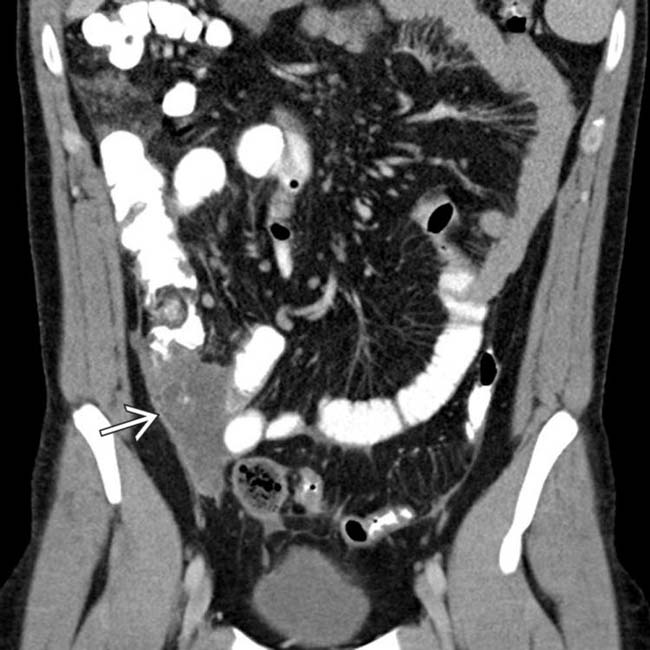
 , reflecting the obstruction due to bowel and mesentery compression by the peritoneal tumor.
, reflecting the obstruction due to bowel and mesentery compression by the peritoneal tumor.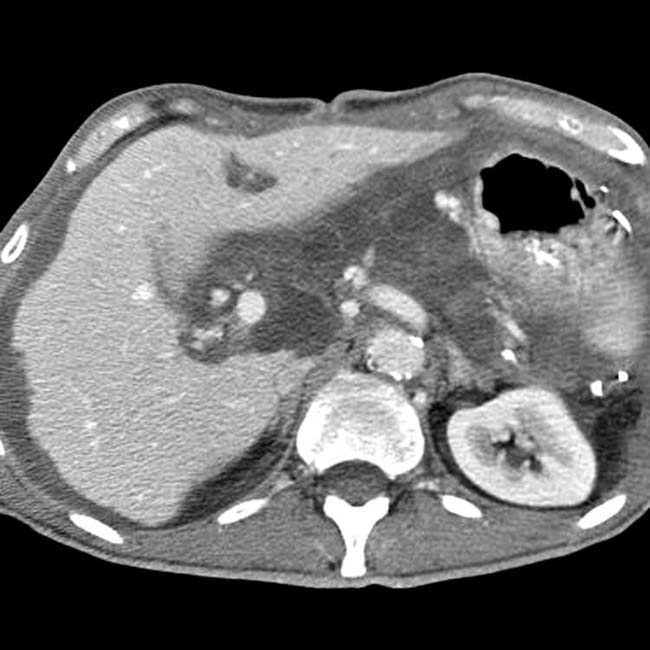
 .
.
 .
.
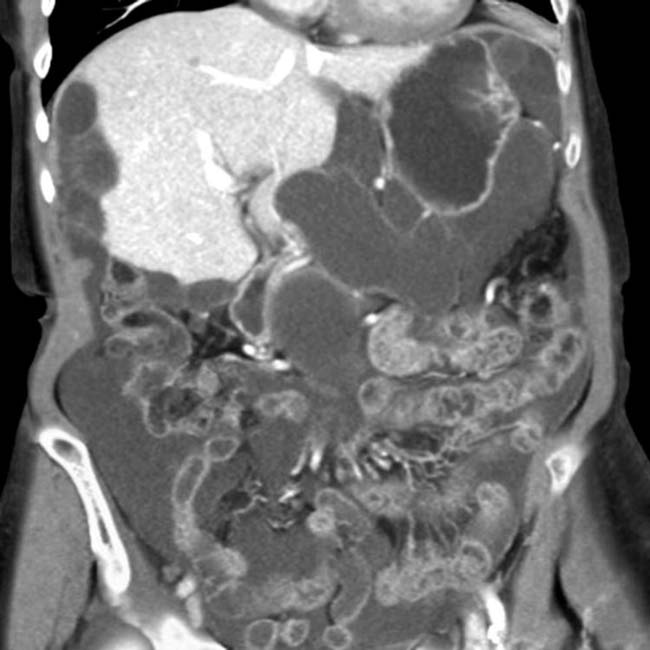
 , which was required because the loculated mucinous peritoneal metastases resulted in compression, distortion, and partial obstruction of the bowel.
, which was required because the loculated mucinous peritoneal metastases resulted in compression, distortion, and partial obstruction of the bowel.






































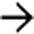 .
.
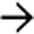 due to peritoneal tumor implants.
due to peritoneal tumor implants.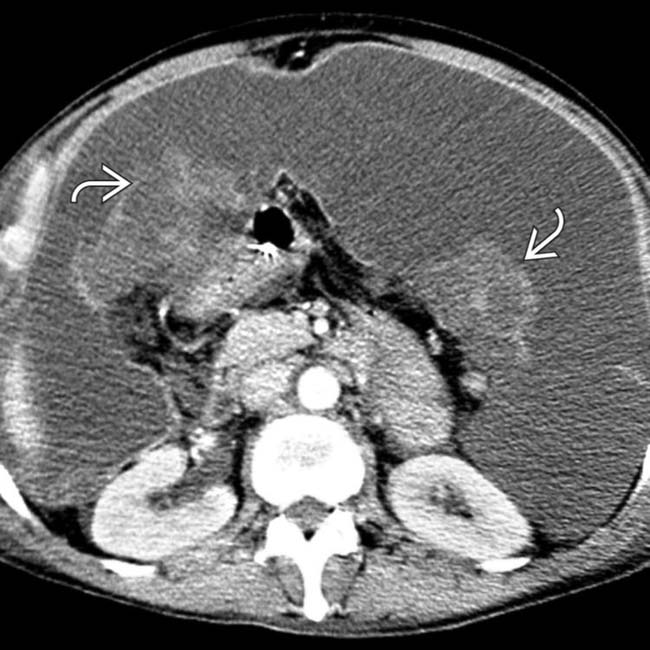
 .
.