Chapter 70 Provocative Testing for Arrhythmias
Bradyarrhythmia
Atrioventricular Block
The AV node and the His-Purkinje system are innervated differently by the autonomic nervous system, with the AV node being highly innervated by sympathetic and parasympathetic nerves and the His-Purkinje system being poorly innervated.1 These differences are the basis of provocative maneuvers to determine further the exact location of an AV block.
Carotid sinus massage increases vagal tone and worsens AV nodal block.2 Isoproterenol, exercise, and atropine are known to increase sympathetic tone, improving AV nodal conduction.2 Because of these changes on rate impulse conduction through the AV node, these maneuvers exert different effects on infranodal block; for example, after carotid sinus massage, the sinus rate drops, and therefore the presence of a functional infranodal block can disappear, depending on whether a lower impulse rate approaches the infranodal conduction system, re-establishing 1 : 1 conduction. However, increasing the impulse rate (e.g., by atropine) could worsen the functional infranodal block. So in regard to infranodal block, carotid sinus massage improves infranodal block, and exercise and atropine worsen infranodal block.
Some authors have tried to evaluate the role of adenosine or its precursor adenosine 5′-triphosphate (ATP) to diagnose high-degree AV block as the underlying mechanism for unexplained syncope.3 Adenosine binds to the A1-receptor, thus exerting its effects of depressing sinus node automaticity, slowing or blocking AV node conduction, and shortening and hyperpolarizing the atrial action potential as well as decreasing automaticity in Purkinje fibers.4,5
Brignole et al suggested that an ATP test could be valuable for diagnosis of paroxysmal AV block based on a potentially higher ATP susceptibility of patients with syncope.6 However, the same group published data showing that ATP could predict AV block only in a few patients.7 Another study by Fragakis et al investigating adenosine in the context of sick sinus syndrome and unexplained syncope failed to demonstrate a valuable role for adenosine in confirming AV block as a cause of syncope as well.8 As a result, the usefulness of adenosine in detecting AV block has not been established.
During invasive EPS, the class I antiarrhythmic drug ajmaline can be used to unmask infra-Hisian conduction abnormalities.2 A dose of 1 mg/kg is used in patients with normal systolic function. If the infra-Hisian conduction is pathologic, a second- or third-degree infra-Hisian block or an H-V interval greater than 120 ms occurs a few minutes after ajmaline infusion. Theoretically, ajmaline could be replaced by other class I antiarrhythmic drugs such as flecainide or procainamide, but the elimination half-life of ajmaline is shorter, which is an advantage for its use in a screening test.
Syncope
Carotid sinus hypersensitivity can be the underlying disease leading to syncope caused by a sinus pause. It can easily be tested for by performing carotid sinus massage over the region of carotid bifurcation (i.e., the maximal point of carotid pulsation between the angle of the mandible and the superior border of the thyroid cartilage) under continuous ECG and blood pressure monitoring for 5 seconds on both sides, one after another, in the supine, 70-degree, head-up tilt position.9 The test result is assumed to be positive if a sinus pause greater than 3 seconds (cardio-inhibitory subtype), a decrease of systolic blood pressure greater than 50 mm Hg (vasodepressor subtype), or both are observed. However, such sinus pauses can be observed in healthy individuals as well, so carotid sinus massage cannot definitively distinguish healthy individuals from sick individuals.10,11
Tilt-table testing (TTT) is a widely used provocative test method for the assessment of syncope. Several protocols have been developed, with the Bruce protocol being the most widely used (see Chapters 48 and 70). In addition, isoproterenol or nitrates are used as provocative agents to increase the sensitivity and decrease the specificity of the TTT.2
ATP can be used for the evaluation of cardio-inhibitory syncope.13 According to a protocol developed by Flammang and colleagues, a rapid bolus of 20 mg ATP is administered. The ATP test result is considered positive if complete AV block greater than 10 seconds occurs or if the longest R-R interval after ATP administration is greater than 6 seconds.14,15 Head-up TTT and the ATP test do not correlate well, which has led to the hypothesis that the ATP test reveals a form of syncope different from that detected by the head-up TTT or carotid sinus massage.7,16 The ATP test is not routinely used; additional clinical trials are needed. According to the guidelines published by the European Society of Cardiology, the adenosine test is a class IIb recommendation because of its low predictive value.17
Sick Sinus Syndrome or Sinus Node Dysfunction
Sick sinus syndrome is a clinically heterogeneous disease. Underlying pathophysiological mechanisms include changes in intrinsic sinus node properties, sinoatrial (SA) conduction properties, and extrinsic factors such as autonomic regulation.18 To distinguish endogenous sinus node dysfunction from autonomic dysregulation responsible for sick sinus syndrome, intrinsic heart rate (IHR) can be determined with propranolol and atropine.19 With the patient under continuous ECG monitoring, 0.2 mg/kg propranolol is administered at 1 mg/min, followed by a single bolus of 0.04 mg/kg atropine over 2 minutes. IHR is determined as the maximum sinus rate after injection of atropine. Based on the formula IHR = 118.1 – (0.57 × Age), normal IHR is assumed to be within ±14% of the 95% confidence interval (CI) for age 45 years or younger or within ±18% for age 45 years or older. An abnormal IHR therefore represents dysfunction of intrinsic sinus node properties. In contrast, autonomically mediated sick sinus syndrome shows a normal IHR after autonomic blockade. By eliminating (para)sympathetic activity, propranolol or atropine testing facilitates discrimination of intrinsic sinus node dysfunction and autonomic dysregulation. Further evaluation of intrinsic sinus node dysfunction can be achieved by determining sinus node recovery time (SNRT) and SA conduction time (SACT) during EPS (see Chapter 39).
Fragakis et al evaluated the diagnostic value of adenosine in detecting sick sinus syndrome in a study with 19 patients, 12 of whom were control patients and 7 with syncope of unknown origin.8 The investigators measured the maximum corrected SNRT after atrial overdrive pacing at different cycle lengths and compared it with the longest sinus pause after adenosine administration corrected to basic cycle length. With a bolus of 0.15 mg/kg adenosine, they showed that this noninvasive test is at least comparable with the measurement of corrected SNRT in diagnosing sick sinus syndrome. However, they could not show any value for the use of adenosine in the diagnosis of AV block.
Another study by Burnett et al investigated adenosine as a provocative agent in 10 patients with sick sinus syndrome. By validating their results with EPS, they demonstrated that the lengthening of the sinus cycle length corrected to the basic cycle length after adenosine infusion offers a sensitivity of 80% and a specificity of 97% for the diagnosis of sick sinus syndrome.20
Furthermore, administration of ajmaline has been shown to increase the sensitivity of EPS in the detection of sick sinus syndrome.2 The test result is assumed to be positive if the corrected sinus node time is greater than 550 ms after ajmaline infusion.
Chronotropic Incompetence
Athletes often show resting bradycardia, but they can increase their heart rate adequately with exercise. In contrast, patients with chronotropic incompetence or sinus node dysfunction and resting bradycardia cannot appropriately accelerate their heart rate. A physical exercise stress test can be useful in differentiating nonpathologic bradycardia at rest from chronotropic incompetence. In addition, drug testing can be performed by increasing the heart rate after infusion of isoproterenol and atropine based on the sympathetic stimulation and parasympathetic blockade, respectively. Vavetsi et al studied 100 patients with sinus bradycardia but no obvious cardiac disease.21 According to their protocol, 2 mg atropine was injected, followed by incremental isoproterenol infusion starting at a dose of 2.4 mg/min up to a maximum of 7.2 mg/min (2 to 3 minutes and 1.2 mg/min isoproterenol per step). By measuring the maximum heart rate, they defined the chronotropic reserve. As a result, they clearly identified patients with deficient chronotropic response who required pacemaker implantation.
Supraventricular Tachyarrhythmias
In the surface ECG, SVTs are easily recognized by narrow-complex tachycardia. However, distinguishing different forms of SVT, as well as identifying SVT with pre-existing bundle branch block presenting as wide-complex tachycardia, remains a clinical challenge. In this regard, adenosine or ATP is widely used for the diagnosis and therapy of tachyarrhythmias (Figure 70-1).
Atrioventricular Nodal Re-entrant Tachycardia and Atrioventricular Re-entrant Tachycardia
Dual conduction properties (fast and slow) exist in the AV node as the basis of AV nodal re-entrant tachycardia (AVNRT).22 Adenosine is routinely used to terminate AVNRT.4 However, AVNRT is a paroxysmal SVT that often self-terminates before ECG documentation can be performed. Thus patients with palpitations and tachycardia often remain undiagnosed and do not receive adequate therapy (e.g., ablation). In these cases, adenosine can be used for diagnostic purposes because the two pathways may respond differently to adenosine. A higher dose is necessary to block conduction in the slow pathway compared with the fast pathway, resulting in an abrupt increase in A-H and P-Q intervals detectable in intracardiac or surface ECG recordings, respectively.23,24
With the patient under continuous ECG monitoring, adenosine is administered during sinus rhythm as a bolus of 6 mg (followed by a bolus of 0.9% saline) with additional 6-mg doses to a maximum of 18 mg or until second- or third-degree AV block occurs.4,25 Some authors suggest a starting dose of 3 mg adenosine followed by further 3-mg steps or a dose regimen based on body weight, starting at 0.05 mg/kg with further application of 0.05 mg/kg.4,24 In general, dose reduction should be considered in patients with concomitant dipyridamole therapy or those with heart transplants because of the increased effect of adenosine in these patients.4 Dual AV node physiology is considered if a sudden increase greater than 50 ms of the P-Q interval is seen, AV nodal echo beats occur, or AVNRT develops.
Tebbenjohanns et al used adenosine in 37 patients with symptoms of paroxysmal tachycardia but without ECG documentation who underwent EPS.24 They used an average adenosine dose of 10.3 ± 4.2 mg and identified a sudden increase in the P-Q interval on the surface ECG in 76% of the patients with inducible AVNRT but in only 5% of the patients without AVNRT. Thus the adenosine test had a sensitivity of 76%, a specificity of 95%, a positive predictive value (PPV) of 93%, and a negative predictive value (NPV) of 83%, thus proving to be a suitable noninvasive bedside test for the diagnosis of AVNRT in patients at risk.
Viskin and coworkers developed a test protocol that used ATP to distinguish tachycardias caused by accessory pathways (e.g., AVNRT or AV re-entrant tachycardia [AVRT]) from other forms of tachycardia (e.g., AF).26 They included 146 patients with palpitations of unknown etiology. The ATP test was performed as a bedside test with the patient under continuous ECG monitoring. The investigators started with a bolus of 10 mg ATP followed by additional injections of 10 mg ATP (to a maximum of 60 mg). The test result was considered positive when a dual AV node physiology or a concealed accessory pathway became obvious by a more than 50-ms P-R interval increment or decrement or by the occurrence of AVNRT or AVRT. The test result was considered negative when second- or third-degree heart block occurred without any of the criteria mentioned above. To confirm the test results, EPS was performed on all patients; it showed a sensitivity of 71%, a specificity of 76%, a PPV of 93%, and an NPV of 37% of the ATP test in regard to predicting AVNRT or AVRT.26
Belhassen et al evaluated the diagnostic role of ATP in 96 patients during EPS.27 They showed that ATP injected during sinus rhythm in an incremental dosage, starting at 10 mg to a maximum of 60 mg, identified dual AV nodal physiology in 75% of the patients with inducible AVNRT. In addition, they demonstrated that ATP can be used to confirm the results of a successful radiofrequency ablation of the slow pathway.
Distinguishing Atrial Flutter from Atrioventricular Nodal Re-entrant Tachycardia or Atrioventricular Re-entrant Tachycardia
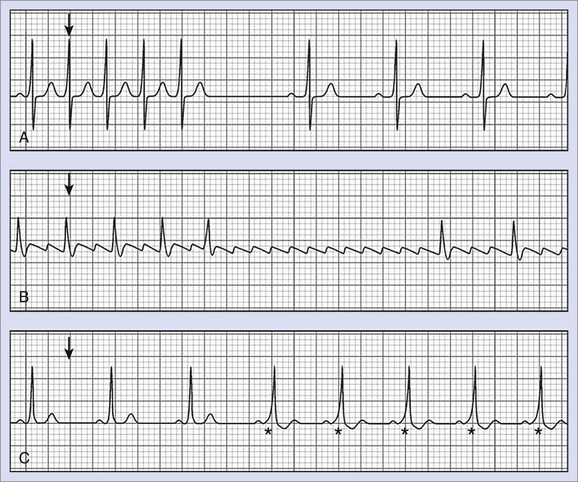
Atrial flutter with 2 : 1 block normally presents with heart rates of 140 to 150 beats/min. Similarly, AVNRT or AVRT displays a comparable heart rate spectrum. Adenosine can be used to discriminate between AVNRT or AVRT and atrial flutter with 2 : 1 block.4 Because of the transient AV block caused by adenosine, electrical atrial activity will be unmasked, showing characteristic P waves and leading to the correct diagnosis of atrial flutter (Figure 70-2).
Identification of Accessory Bundles by Adenosine and Adenosine 5′-Triphosphate
Furthermore, adenosine, given during sinus rhythm, has been shown to unmask accessory AV conduction bundles with latent pre-excitation, which have only been apparent during atrial arrhythmia or atrial pacing.28,29 Despite the absence of pre-excitation in sinus rhythm, these patients are at risk of rapid ventricular heart rate when AF occurs. By slowing or transient block of conduction to the ventricles through an AV node, a concealed accessory pathway can be exposed (see Figure 70-2). Garratt et al investigated 22 patients with a history of documented SVT and a normal ECG at sinus rhythm. They demonstrated that adenosine is capable of unmasking latent pre-excitation with high sensitivity and specificity.29
In summary, adenosine is a useful drug for the management and diagnosis of tachycardias. By inducing a transient AV block, re-entry tachycardias involving the AV node are terminated and the ventricular rate is slowed, thus unmasking atrial activity when atrial tachyarrhythmias are present. Adenosine has no influence on VTs.4,30 Of note, the same effect of transient AV nodal conduction slowing can be achieved by physical methods to stimulate the vagal nerve, including carotid sinus massage, the diving reflex, the Valsalva maneuver, or deep inspiration.31
However, adenosine can, as with most drugs, have certain adverse effects. It can induce AF in patients at risk because of its ability to shorten the atrial action potential duration in a dose-dependent and rate-dependent manner.32 Because of its sympathetic stimulation, adenosine also can cause a 1 : 1 conduction in atrial flutter.33 In addition, in patients with AF and accessory pathways, adenosine can enhance antegrade AV conduction of the high atrial rate via the accessory bundle, resulting in a fast ventricular rate with wide QRS complexes (FastBroadIrregular tachycardia), which also can lead to ventricular fibrillation (VF). Therefore adenosine should always be administered with caution and after taking the required measures to establish the safety of the patient.
Isoproterenol During Electrophysiological Study
Many SVTs are currently treated with radiofrequency ablation procedures. However, inducing the specific arrhythmia during EPS is important before performing an ablation. For this purpose, isoproterenol infusion is widely used in combination with programmed stimulation, especially when programmed atrial stimulation alone fails to induce the clinical arrhythmia.2 Furthermore, failure of arrhythmia re-induction after ablation (with or without isoproterenol) confirms the successful outcome of the procedure.
Atrial Fibrillation
Oral et al investigated the role of isoproterenol to assess the inducibility of paroxysmal AF during catheter ablation.34 They enrolled 80 patients with a history of paroxysmal AF and 20 healthy controls receiving an incremental dosage of isoproterenol starting at 5 µg/min followed by 10, 15, and 20 µg/min every 2 minutes or until AF was induced. They induced AF in 84% of patients with AF but in only one of the control subjects (5%). They revealed inducibility of vagotonic AF in 88%, adrenergic AF in 100%, and nonspecific AF in 79%. Overall sensitivity was 88%, and specificity was 95% for induction of AF in patients with paroxysmal AF regardless of the clinical subtype.
In the case of vagally induced AF, carotid sinus massage or ATP at a dosage of 10 to 20 mg can be useful to induce AF.2,35
Oral et al were able to demonstrate the value of isoproterenol infusion after catheter ablation of paroxysmal AF in predicting clinical outcome more accurately compared with the widely used rapid atrial pacing.36
ATP also can be used for the same purpose.37 Ninomiya et al used 30 mg of ATP after isolation of all four pulmonary veins during isoproterenol infusion in 21 patients with AF. In all patients, electrical isolation was achieved initially. However, spontaneous reconduction was observed in 12 pulmonary veins, with 8 more pulmonary veins showing re-conduction only after ATP administration. Thus their results indicated the usefulness of ATP to detect early reconduction after pulmonary vein isolation.
Arentz et al performed a study on 29 patients with paroxysmal or persistent AF who underwent pulmonary vein isolation.38 They used adenosine at a dose of 12 to 18 mg injected after the successful isolation of pulmonary veins to unmask the so-called dormant conduction, that is, restored conduction through a previously isolated pulmonary vein. Adenosine induced transient conduction in 25% of successfully isolated pulmonary veins. However, a second EPS was performed in 14 patients that revealed a non-significant higher rate of restored conduction in previously “adenosine-positive” pulmonary veins. To identify the potential underlying mechanism, Datino and coworkers studied the effects of adenosine on canine pulmonary veins.39 They illustrated that adenosine selectively hyperpolarized canine pulmonary veins by increasing the inward rectifier potassium current IKAdo, restoring the excitability of isolated pulmonary veins with dormant conduction.
Ventricular Tachyarrhythmias
General Drug Effects on Inducibility of Ventricular Tachycardia and Differentiation of Ventricular Tachycardia Mechanisms
The most common method to induce VT during EPS is programmed stimulation by specific stimulation protocols. However, in some special variants of VT (e.g., CPVT) or if programmed stimulation fails to induce VT, drugs can be used to induce tachycardia. The drug of first choice in this context is isoproterenol because of its adrenergic stimulation effects. However, data on the use of isoproterenol regarding tachycardia induction rates are highly controversial in the literature, varying from less than 5% to 20%.40–42 With CPVT or repetitive monomorphic tachycardia, the use of isoproterenol seems to be established mainly on the basis of initiation mechanisms via triggered activity caused by delayed afterdepolarizations (DADs). In addition, epinephrine, atropine, and aminophylline are alternative agents to facilitate induction of these arrhythmias if isoproterenol fails to induce the arrhythmia during programmed stimulation. The use of catecholaminergic drugs also may be a useful alternative to induce tachycardias caused by abnormal automaticity; programmed stimulation is, however, highly unreliable for initiating this type of arrhythmias. In general, tachycardia induction requiring the use of catecholamines or other pharmacologic agents that increase intracellular cyclic adenosine monophosphate (cAMP) suggests an arrhythmia initiation mechanism caused by triggered activity via DADs; these agents should have less or only indirect effects on early afterdepolarization (EAD)–related arrhythmias. Another drug-specific effect based on the DAD-related initiation is hypothesized by the administration of verapamil or adenosine terminating this type of tachycardia and is therefore called adenosine-sensitive or verapamil-sensitive tachycardias. The following sections focus on specific arrhythmias and arrhythmia syndromes to discuss potential provocation tests in more detail.
Long QT Syndrome
LQTS is characterized by prolonged ventricular repolarization (detectable as QTc prolongation) and polymorphic VT, named torsades de pointes tachycardia, which may lead to SCD.43 By highlighting the predominant underlying pathophysiology in general, two forms of LQTS are described: LQTS caused by mutations in ion channel genes (congenital LQTS) or QTS secondary to therapy with QTc-prolonging drugs (acquired LQTS).43,44 Diagnosis of LQTS remains difficult because episodes of tachycardia rarely occur and QTc prolongation alone is not sufficient for diagnosis because even healthy individuals can show a prolonged Q-T interval.45–47 In contrast, approximately 25% of genetically affected people present with normal or borderline QTc interval.48 In congenital LQTS, genetic testing is currently the most important step toward establishing diagnosis. However, genotyping results take weeks to months and, according to the technique used, a disease-related mutation may not be detected or a mutation previously not described may be detected. In the latter case, whether this mutation could lead to arrhythmia often is unclear.
Priori et al were able to show that the percentage of concealed LQTS depends on the genotype.49 Studying 647 patients with genetically diagnosed LQTS, they demonstrated normal ECG recordings in 36%, 19%, and 10% of the patients with LQT1, LQT2, and LQT3, respectively. Thus provocative test methods are still of clinical relevance because these tests are fast and easy to perform and can potentially guide subsequent genetic testing to a certain subtype of LQTS, thus enabling a faster and more economic genetic testing.
In 1984, Schechter et al suggested that LQTS is associated with abnormally large β-adrenergic–mediated after-depolarizations as an underlying mechanism leading to ventricular arrhythmias.50 This finding resulted in the development of an epinephrine stress test to unmask concealed LQTS. The two established major protocols used in clinical routine were developed by the groups of Ackerman and Shimizu.51–53
Epinephrine Testing in Long QT Syndrome
Ackerman Protocol
The protocol developed by Ackerman and coworkers consists of a sequentially increasing infusion of epinephrine, starting at a low dosage of 0.025 µg/kg/min for 10 minutes followed by 0.05, 0.1, and 0.2 µg/kg/min every 5 minutes.52 The protocol originally started with an epinephrine dosage of 0.05 µg/kg/min increased to a maximum dosage of 0.3 µg/kg/min, but after initial observations of gene-specific responses at low dosages and increased adverse effects at high dosages, the protocol was changed.51,54 Patients receive epinephrine infusion for 25 minutes in total. The entire procedure is monitored with permanent ECG recordings with a focus on Q-T, R-R, and QTc intervals. Changes in QT and QTc (ΔQT and ΔQTc) were determined by calculating the differences between maximal and minimal QT or QTc between epinephrine infusion and baseline, respectively. The criteria to stop epinephrine infusion are increased systolic blood pressure (>200 mm Hg), nonsustained VT, polymorphic VT, increased premature ventricular contractions (>10/min), T-wave alternans, or clinical symptoms such as headache or nausea.
Epinephrine induces hyperphosphorylation of the slowly activating delayed rectifier potassium channel IKs, which is an important ion channel for repolarization. Hyperphosphorylation results in increased activity of IKs, leading to shortening of the Q-T interval.55,56 Because of mutations in KCNQ1, patients with LQT1 display loss of IKs function with slowed repolarization and reduced response to epinephrine. Thus during epinephrine infusion, an imbalance of ion currents resulting in prolongation of the Q-T interval can be observed in patients with LQT1.57 Because of different gene mutations, other ion channels are dysfunctional in LQT2 and LQT3, resulting in an epinephrine-induced Q-T interval reduction caused by intact IKs channels observed in the study mentioned above.
The epinephrine stress test, according to Ackerman, has several advantages compared with other provocative tests in diagnosing LQTS. First, it is easy to standardize and interpret because it does not require the patient’s effort. Second, the gradually increasing dosage of epinephrine is better tolerated by patients, and false-positive responses are less frequently observed compared with a bolus-based method. However, disadvantages include induction of hypokalemia by epinephrine and possible adverse effects such as life-threatening arrhythmias. Patients taking β-blockers, who cannot be tested accurately, are excluded from this test type.52
Taken together, these study results indicate that epinephrine stress testing may be a useful tool in patients with LQT1 recently diagnosed through genetic testing, especially when the resting ECG is concealed and if the KCNQ1 mutation is novel.52
Shimizu Protocol
The second protocol for epinephrine stress testing is that developed by Shimizu and coworkers.53 This protocol consists of bolus administration of epinephrine 0.1 µg/kg followed by continuous infusion of 0.1 µg/kg/min for 5 minutes with the patient under ECG monitoring. Of special interest are QTc intervals 1 to 2 minutes after bolus injection, which represent the peak epinephrine effect, and 2 to 3 minutes after starting continuous infusion, which represent steady-state conditions.
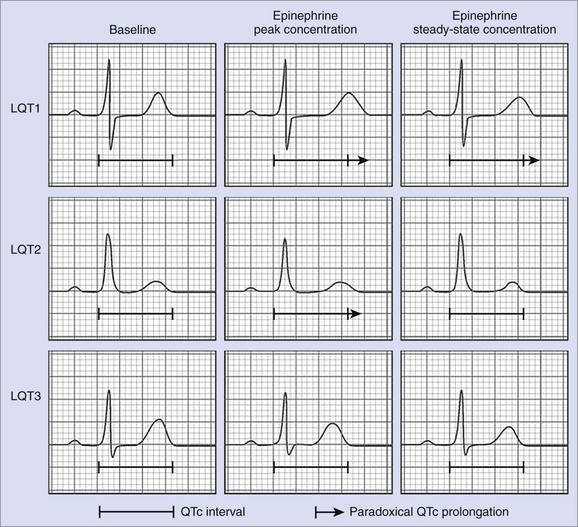
This protocol is based on experimental data showing that β-adrenergic stimulation differentially affects the action potential duration and the Q-T interval at peak and steady-state concentrations, depending on the underlying genotype.58 In LQT1, epinephrine cannot enhance dysfunctional IKs, leading to paradoxical QTc prolongation at peak epinephrine concentrations as well as under steady-state conditions. In LQT2, β-adrenergic stimulation results in an initial prolongation of the Q-T interval, followed by a Q-T interval shortening at steady-state concentrations. The underlying mechanism is assumed to be an initial enhancement of the sodium-calcium (Na+/Ca2+) exchange current with subsequent increase of IKs. In LQT3, however, epinephrine leads to persistent shortening of the Q-T interval at peak and steady-state concentrations (see Figure 70-2). Thus Shimizu’s protocol can be used to differentiate between the three most common subtypes of LQTS by monitoring the QTc interval at peak epinephrine concentrations after bolus administration and steady-state concentrations after continuous infusion.59
Adenosine Testing in Long QT syndrome
Sudden acceleration of the heart rate, typically seen in exercise and stress conditions, can trigger torsades de pointes tachycardias in LQTS. This is the mechanistic basis of tachycardia-mediated provocative stress tests as described above. Furthermore, sudden deceleration of heart rate, such as during a pause after a premature complex, can also initiate the onset of torsades de pointes.60 This knowledge led to the development of another provocative test protocol described by Viskin et al in which adenosine is used.61 As previously mentioned, adenosine causes transient AV block followed by marked sinus tachycardia when administered in sinus rhythm.5 In the study by Viskin et al, 38 patients in sinus rhythm (18 patients with diagnosed LQTS or high probability of LQTS and 20 control subjects) received intravenous adenosine starting with a 6-mg bolus.61 Adenosine administration was continued to a maximum of 24 mg by repetitive boluses until AV block (second or third degree) or sinus bradycardia or arrest of more than 3 seconds occurred. Measurements taken in this protocol are Q-T interval; QTpeak interval (from the beginning of QRS to the peak of the T wave); the difference between these two QT measurements, QTc; and the morphology of the T wave at the time of maximal bradycardia and tachycardia. Both groups of subjects developed a similar degree of adenosine-induced bradycardia, but the Q-T interval in patients with LQTS increased significantly. In addition, both groups showed a similar degree of subsequent tachycardia, but QTc prolongation was more pronounced in patients with LQTS.
Thus Viskin et al were able to demonstrate a proof of principle for using adenosine stress testing in the setting of LQTS.61 Because of the small number of patients enrolled in this study, these results are difficult to transfer to clinical practice. Patients in the LQTS cohort were either diagnosed with or were highly suspected to have LQTS. Hence, it is unclear whether the test could identify patients with lower probability of LQTS or whether the test protocol can discriminate different subtypes of LQTS as with epinephrine testing (according to Shimizu et al).53 Larger studies are needed to evaluate the potential role of adenosine stress testing in identifying LQTS.
Sotalol Test in Acquired Long QT Syndrome
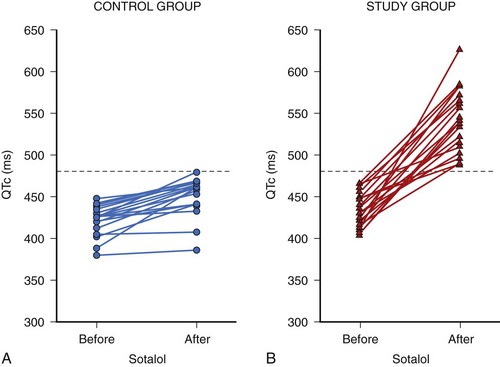
Acquired LQTS is caused by ion channel mutations. Current concepts postulate an intrinsic susceptibility to challenges of myocardial repolarization. This reduced repolarization reserve, the basis of which is still ill defined, is composed of rare as well as common genetic variants, together with acquired electrical remodeling, as seen during hypertrophy and heart failure. A large number of cardiac and noncardiac drugs induce disproportionate QTc prolongation and cause potentially lethal torsades de pointes arrhythmias in selected patients at risk.62,63 Therefore it is very important to identify patients at risk in a timely manner. To face this clinical challenge, Kääb and coworkers developed a drug-based provocative test protocol using the class II/III antiarrhythmic drug sotalol.64 In a pilot study, they conducted sotalol testing on 40 patients (20 control subjects and 20 patients with documented torsades de pointes). All patients fasted for 6 to 8 hours before testing, and blood pressure and heart rhythm were noninvasively monitored continuously from 1 hour before to 24 hours after testing. All patients had sinus rhythm at the beginning. After 1 hour of resting (baseline), intravenous d,l-sotalol was administered at a constant rate of 2 mg/kg (in 50 mL 0.9% saline) for 20 minutes. The longest Q-T and QTc intervals at baseline and 5 to 10 minutes after sotalol infusion were used for analysis. At baseline, no differences in Q-T and QTc intervals could be detected between the two groups. After sotalol infusion, QT increased from 404 ± 39 to 561 ± 68 ms and QTc increased from 434 ± 21 to 541 ± 37 ms, with QT duration in patients with torsades de pointes significantly longer than in control subjects. None of the control subjects had an increase of QTc greater than 480 ms, whereas all patients with torsades de pointes had an increase in QTc resulting in QTc greater than 480 ms after sotalol infusion, indicating 480 ms as a potential cutoff in diagnosing acquired LQTS (Figure 70-3).
Larger clinical trials are needed, however, to validate the results of this pilot study.
Effects of Ibutilide
From a study by Cheng et al, the class III antiarrhythmic ibutilide may be considered suitable for provocative testing in patients at risk for inducible LQTS.65 In this study, the investigators performed EPS on 21 patients without LQTS receiving 1 mg of the IKr blocker ibutilide. Ibutilide caused a reduction in baseline heart rates and prolongation of ventricular repolarization during sinus rhythm. They calculated the QT variability index (QTVI), which, when prolonged, was associated with an increased risk for arrhythmogenesis. QTVI remained unchanged during sinus rhythm after ibutilide infusion. Because variations in basic cycle lengths may increase the risk for ventricular arrhythmias, random interval atrial pacing was performed; it revealed a significant increase in QTVI after ibutilide infusion in patients with and without structural heart disease.60,65,66 On the basis of these results, it was suggested that an increased temporal lability of repolarization was caused by ibutilide during heart rate fluctuations. Therefore, despite some limitations of this study, such as the small number of patients enrolled, ibutilide administration in combination with programmed atrial pacing may be considered a useful tool in patients at risk for inducible LQTS in the future. However, further studies are required to develop an established and standardized test protocol in larger patient cohorts to confirm the validity of this test in patients with latent repolarization disease.
Exercise Stress Test
Another method to unmask concealed LQTS is the exercise test. A widely used protocol was developed by Bruce (see Chapter 53) and can be applied on a treadmill or a bicycle.12
Takenaka et al conducted exercise stress testing on 82 patients with genetically identified LQTS and 33 healthy control patients.67 They showed that the QTc interval and the interval between the peak and the end of the T wave (Tpe) were significantly prolonged in LQT1 associated with a morphologic change of the T wave to a broad-based pattern. In contrast, patients with LQT2 did not show any significant changes in QTc or Tpe interval but revealed a prominent notch on the T wave. LQT1 and LQT2 could be differentiated with an exercise stress test.
In another study by Wong et al, 159 patients with suspected LQTS performed an exercise stress test on a treadmill combined with postural ECG test recordings in the supine position and immediately thereafter in the vertical position.68 Each patient was genotyped to validate the results of the exercise stress test. These investigators demonstrated that patients with LQTS exhibited a greater prolongation of QTc with postural change compared with healthy subjects. During exercise, patients with LQT1 showed a marked increase in QTc prolongation that significantly differed from the slight prolongation of QTc in LQT2 or the unchanged QTc in healthy individuals. In conclusion, Wong et al validated exercise stress testing as a useful method in predicting LQTS, especially LQT1.
The different results of the various genotypes may support the finding that fatal cardiac events in LQT1 are more often associated with exercise.67
Unmasking Concealed Long QT Syndrome by Standing Maneuvers
Recently, investigators studied whether short, transient sinus tachycardia occurring while standing would be able to change the Q-T interval to establish an additional provocative test method to unmask concealed LQTS.69 Standing leads to sinus tachycardia causing Q-T interval shortening in healthy hearts. However, patients with LQTS often display a pathologic response to heart rate changes, as seen during epinephrine testing. To investigate this phenomenon, Viskin and colleagues conducted a standing test in 68 LQTS patients with different genotypes and 82 healthy control subjects and compared ECG parameters such as heart rate and Q-T and QTc intervals recorded with the patient beginning in the supine position and moving to the vertical position. Upon standing, both groups showed similar heart rate acceleration but significantly different Q-T interval changes. Healthy control subjects exhibited a shortening of the Q-T interval, whereas patients with LQTS showed a prolongation of the Q-T interval. Because of the heart rate acceleration, both groups showed a prolongation of the QTc interval, with both cohorts being significantly different. In a subgroup analysis, Q-T interval changes during sinus tachycardia induced by standing were particularly impaired in patients with LQT2, a finding that helped establish an additional useful test for the diagnosis of LQTS.
Brugada Syndrome
Another channelopathy predisposing to ventricular arrhythmias is Brugada syndrome, which was described for the first time by Brugada et al in 1992.70 Patients with Brugada syndrome commonly present with syncope, VT, or SCD depending on whether the arrhythmia is (self-)terminating. Interestingly, these patients do not show any structural heart disease but a specific ECG pattern of right bundle branch block (RBBB) with ST-segment elevation in precordial leads. The three types of ECG pattern are a coved ST-segment elevation (type 1), an ST-segment elevation with a saddleback-like T wave (type 2), and an only slightly elevated ST segment with either a coved-type configuration or a saddleback configuration (type 3).71 However, only the type 1 ECG pattern can be used to diagnose Brugada syndrome if other reasons for ST-segment elevation are excluded.71 In addition, the ECG in Brugada syndrome can be completely normal. As a consequence, provocative test methods have been developed to unmask concealed Brugada syndrome.72–74 This testing involves sodium channel blockers, with flecainide, procainamide, pilsicainide, propafenone, and ajmaline being the most frequently used (Figure 70-4). For a detailed description of provocative drug testing in Brugada syndrome, see Chapter 62.
Catecholaminergic Polymorphic Ventricular Tachycardia
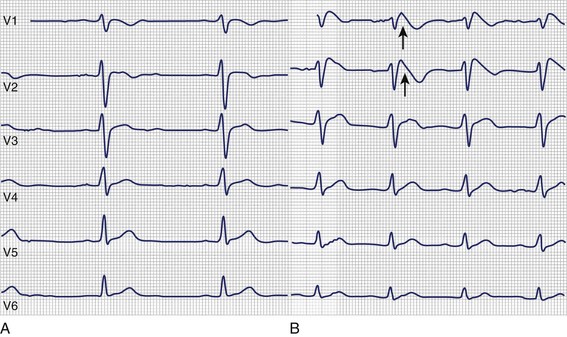
CPVT, which is an exercise-induced arrhythmia in patients with structurally normal hearts, causes syncope or SCD.75 Ventricular arrhythmia occurring during exercise is polymorphic with an alternating beat-to-beat QRS axis, the so-called bi-directional ventricular tachycardia (Figure 70-5). The resting ECG, however, is usually normal.76 Thus diagnosis of CPVT and differentiation from concealed LQTS is difficult. As a consequence, provocative stress testing with exercise or isoproterenol is often conducted.75
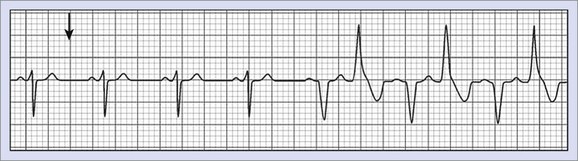
FIGURE 70-5 Bi-directional tachycardia seen in catecholaminergic polymorphic ventricular tachycardia.
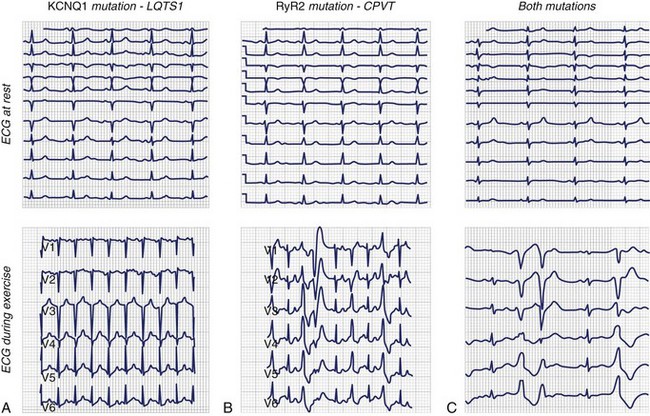
Beckmann et al reported a family with a history of recurrent SCD that affected nine family members during the past 30 years.77 Genetic testing revealed LQT1. However, one girl without KCNQ1 mutation died suddenly at the age of 13. Because other noncarrier family members showed symptoms such as syncope, an exercise stress test was performed by the family members; this led to the diagnosis of CPVT as a second inherited disease in the same family (Figure 70-6).
Krahn et al reported a study on 18 patients with unexplained cardiac arrest in which adrenaline and procainamide were used to unmask ventricular arrhythmias.78 Under continuous ECG monitoring, adrenaline was administered at a starting dose of 0.05 µg/kg/min followed by incremental doses up to 0.40 µg/kg/min to detect VT or QTc prolongation. After a 30-minute washout period, procainamide at a dose of 15 mg/kg was infused to unmask ST-segment elevations diagnostic for Brugada syndrome. Using this test protocol, 56% of the patients were diagnosed with CPVT, 11% with Brugada syndrome, and 33% with idiopathic VF. Interestingly, 50% of the patients with CPVT did not show any ECG abnormality during exercise stress test performed earlier. Thus the authors concluded that adrenaline was superior to exercise stress testing in unmasking CPVT. They suggested that a combined sympathetic stimulation with vagal blockade during exercise was less arrhythmogenic compared with sole sympathetic stimulation with adrenaline.
Despite the reported inducibility of CPVT with exercise in 100% and by isoproterenol in 75%, but not by programmed stimulation, Brunetti demonstrated one case of CPVT that was detected by continuous ECG recording but was not inducible by exercise.79,80
One main characteristic of CPVT is the reproducible provocation of ventricular tachyarrhythmia during exercise.81 Hence, repeated stress tests (exercise or drugs) can be used to monitor adequate therapy with β-blockers, the most frequently used drugs in CPVT. A tachyarrhythmia that is still inducible during exercise under β-blockade indicates ineffective therapy and should lead to increasing the dose of the β-blocker.80,82,83
Repetitive Monomorphic Ventricular Tachycardia (Type Gallavardin)
Another ventricular arrhythmia occurring in patients without an overt cardiac structural abnormality is repetitive monomorphic ventricular tachycardia (type Gallavardin, RMVT). It is defined by numerous monomorphic premature ventricular complexes, couplets, and runs of nonsustained VT. In most cases, a left bundle branch block (LBBB) pattern with normal or rightward axis during tachycardia can be observed.84 Because RMVT manifestation is exercise dependent, provocative test methods can be used to diagnose and differentiate it, especially from paroxysmal sustained idiopathic VT.
Hoffmann et al characterized 20 patients with RMVT, demonstrating the diagnostic value of exercise stress testing and isoproterenol infusion in these patients.84 Exercise testing on a treadmill induced runs of tachycardia or sustained VT in 85% of their patients. In addition, sensitivity for verapamil can be tested during exercise testing after induction of tachycardia. Because of its reproducibility, this exercise test can also be used to evaluate the effectiveness of drug therapy and establish the adequacy of therapy when tachycardia is no longer inducible during the same test protocol. Similar results have also been described for the isoproterenol test. By using 1 to 5 µg/min isoproterenol, tachycardia can be induced in 88% of these patients, but, interestingly, sole programmed right ventricular stimulation induced tachycardia in only 13% of the patients.
Key References
Ackerman MJ, Khositseth A, Tester DJ, et al. Epinephrine-induced QT interval prolongation: A gene-specific paradoxical response in congenital long QT syndrome. Mayo Clin Proc. 2002;77:413-421.
Arentz T, Macle L, Kalusche D, et al. “Dormant” pulmonary vein conduction revealed by adenosine after ostial radiofrequency catheter ablation. J Cardiovasc Electrophysiol. 2004;15:1041-1047.
Brembilla-Perrot B. Pharmacological testing in the diagnosis of arrhythmias. Minerva Cardioangiol. 2010;58:505-517.
Brugada R, Brugada J, Antzelevitch C, et al. Sodium channel blockers identify risk for sudden death in patients with ST-segment elevation and right bundle branch block but structurally normal hearts. Circulation. 2000;101:510-515.
Burnett D, Abi-Samra F, Vacek JL. Use of intravenous adenosine as a noninvasive diagnostic test for sick sinus syndrome. Am Heart J. 1999;137:435-438.
Camm AJ, Garratt CJ. Adenosine and supraventricular tachycardia. N Engl J Med. 1991;325:1621-1629.
Cheng A, Dalal D, Fetics BJ, et al. Ibutilide-induced changes in the temporal lability of ventricular repolarization in patients with and without structural heart disease. J Cardiovasc Electrophysiol. 2009;20:873-879.
Kaab S, Hinterseer M, Nabauer M, Steinbeck G. Sotalol testing unmasks altered repolarization in patients with suspected acquired long-QT-syndrome—a case-control pilot study using I.V. sotalol. Eur Heart J. 2003;24:649-657.
Mehta D, Wafa S, Ward DE, Camm AJ. Relative efficacy of various physical manoeuvres in the termination of junctional tachycardia. Lancet. 1988;1:1181-1185.
Napolitano C, Priori SG. Diagnosis and treatment of catecholaminergic polymorphic ventricular tachycardia. Heart Rhythm. 2007;4:675-678.
Noda T, Takaki H, Kurita T, et al. Gene-specific response of dynamic ventricular repolarization to sympathetic stimulation in LQT1, LQT2 and LQT3 forms of congenital long QT syndrome. Eur Heart J. 2002;23:975-983.
Oral H, Crawford T, Frederick M, et al. Inducibility of paroxysmal atrial fibrillation by isoproterenol and its relation to the mode of onset of atrial fibrillation. J Cardiovasc Electrophysiol. 2008;19:466-470.
Takenaka K, Ai T, Shimizu W, et al. Exercise stress test amplifies genotype-phenotype correlation in the LQT1 and LQT2 forms of the long-QT syndrome. Circulation. 2003;107:838-844.
Viskin S, Postema PG, Bhuiyan ZA, et al. The response of the QT interval to the brief tachycardia provoked by standing: A bedside test for diagnosing long QT syndrome. J Am Coll Cardiol. 2010;55:1955-1961.
Viskin S, Rosso R, Rogowski O, et al. Provocation of sudden heart rate oscillation with adenosine exposes abnormal QT responses in patients with long QT syndrome: A bedside test for diagnosing long QT syndrome. Eur Heart J. 2006;27:469-475.
1 James TN. Cardiac innervation: Anatomic and pharmacologic relations. Bull N Y Acad Med. 1967;43:1041-1086.
2 Brembilla-Perrot B. Pharmacological testing in the diagnosis of arrhythmias. Minerva Cardioangiol. 2010;58:505-517.
3 Parry SW, Nath S, Bourke JP, et al. Adenosine test in the diagnosis of unexplained syncope: Marker of conducting tissue disease or neurally mediated syncope? Eur Heart J. 2006;27:1396-1400.
4 Camm AJ, Garratt CJ. Adenosine and supraventricular tachycardia. N Engl J Med. 1991;325:1621-1629.
5 Lerman BB, Belardinelli L. Cardiac electrophysiology of adenosine. Basic and clinical concepts. Circulation. 1991;83:1499-1509.
6 Brignole M, Gaggioli G, Menozzi C, et al. Adenosine-induced atrioventricular block in patients with unexplained syncope: The diagnostic value of ATP testing. Circulation. 1997;96:3921-3927.
7 Donateo P, Brignole M, Menozzi C, et al. Mechanism of syncope in patients with positive adenosine triphosphate tests. J Am Coll Cardiol. 2003;41:93-98.
8 Fragakis N, Iliadis I, Sidopoulos E, et al. The value of adenosine test in the diagnosis of sick sinus syndrome: Susceptibility of sinus and atrioventricular node to adenosine in patients with sick sinus syndrome and unexplained syncope. Europace. 2007;9:559-562.
9 Parry SW, Reeve P, Lawson J, et al. The Newcastle protocols 2008: An update on head-up tilt table testing and the management of vasovagal syncope and related disorders. Heart. 2009;95:416-420.
10 Sullivan RM, Olshansky B. Carotid sinus hypersensitivity: Disease state or clinical sign of aging? The need for hard endpoints. Europace. 2010;12:1516-1517.
11 Tan MP, Kenny RA, Chadwick TJ, et al. Carotid sinus hypersensitivity: Disease state or clinical sign of ageing? Insights from a controlled study of autonomic function in symptomatic and asymptomatic subjects. Europace. 2010;12:1630-1636.
12 Bruce RA. Exercise testing of patients with coronary heart disease. Principles and normal standards for evaluation. Ann Clin Res. 1971;3:323-332.
13 Flammang D, Pelleg A, Benditt DG. The adenosine triphospate (ATP) test for evaluation of syncope of unknown origin. J Cardiovasc Electrophysiol. 2005;16:1388-1389.
14 Flammang D, Erickson M, McCarville S, et al. Contribution of head-up tilt testing and ATP testing in assessing the mechanisms of vasovagal syndrome: Preliminary results and potential therapeutic implications. Circulation. 1999;99:2427-2433.
15 Parry SW, Chadwick T, Gray JC, et al. The intravenous adenosine test: A new test for the identification of bradycardia pacing indications? A pilot study in subjects with bradycardia pacing indications, vasovagal syncope and controls. QJM. 2009;102:461-468.
16 Brignole M, Gaggioli G, Menozzi C, et al. Clinical features of adenosine sensitive syncope and tilt induced vasovagal syncope. Heart. 2000;83:24-28.
17 Moya A, Sutton R, Ammirati F, et al. Guidelines for the diagnosis and management of syncope (version 2009). Eur Heart J. 2009;30:2631-2671.
18 Dobrzynski H, Boyett MR, Anderson RH. New insights into pacemaker activity: Promoting understanding of sick sinus syndrome. Circulation. 2007;115:1921-1932.
19 Jordan JL, Yamaguchi I, Mandel WJ. Studies on the mechanism of sinus node dysfunction in the sick sinus syndrome. Circulation. 1978;57:217-223.
20 Burnett D, Abi-Samra F, Vacek JL. Use of intravenous adenosine as a noninvasive diagnostic test for sick sinus syndrome. Am Heart J. 1999;137:435-438.
21 Vavetsi S, Nikolaou N, Tsarouhas K, et al. Consecutive administration of atropine and isoproterenol for the evaluation of asymptomatic sinus bradycardia. Europace. 2008;10:1176-1181.
22 Elvas L, Gursoy S, Brugada J, et al. Atrioventricular nodal reentrant tachycardia: A review. Can J Cardiol. 1994;10:342-348.
23 Curtis AB, Belardinelli L, Woodard DA, Brown CS, Conti JB. Induction of atrioventricular node reentrant tachycardia with adenosine: Differential effect of adenosine on fast and slow atrioventricular node pathways. J Am Coll Cardiol. 1997;30:1778-1784.
24 Tebbenjohanns J, Niehaus M, Korte T, Drexler H. Noninvasive diagnosis in patients with undocumented tachycardias: Value of the adenosine test to predict AV nodal reentrant tachycardia. J Cardiovasc Electrophysiol. 1999;10:916-923.
25 Domanovits H, Laske H, Stark G, et al. Adenosine for the management of patients with tachycardias—a new protocol. Eur Heart J. 1994;15:589-593.
26 Viskin S, Fish R, Glick A, et al. The adenosine triphosphate test: A bedside diagnostic tool for identifying the mechanism of supraventricular tachycardia in patients with palpitations. J Am Coll Cardiol. 2001;38:173-177.
27 Belhassen B, Fish R, Eldar M, Glick A, Glikson M, Viskin S. Simplified “ATP test” for noninvasive diagnosis of dual AV nodal physiology and assessment of results of slow pathway ablation in patients with AV nodal reentrant tachycardia. J Cardiovasc Electrophysiol. 2000;11:255-261.
28 Belhassen B, Shoshani D, Laniado S. Unmasking of ventricular preexcitation by adenosine triphosphate: Its usefulness in the assessment of ajmaline test. Am Heart J. 1989;118:634-636.
29 Garratt CJ, Antoniou A, Griffith MJ, et al. Use of intravenous adenosine in sinus rhythm as a diagnostic test for latent preexcitation. Am J Cardiol. 1990;65:868-873.
30 Faulds D, Chrisp P, Buckley MM. Adenosine. An evaluation of its use in cardiac diagnostic procedures, and in the treatment of paroxysmal supraventricular tachycardia. Drugs. 1991;41:596-624.
31 Mehta D, Wafa S, Ward DE, Camm AJ. Relative efficacy of various physical manoeuvres in the termination of junctional tachycardia. Lancet. 1988;1:1181-1185.
32 Tebbenjohanns J, Schumacher B, Pfeiffer D, et al. Dose and rate-dependent effects of adenosine on atrial action potential duration in humans. J Interv Cardiol Electrophysiol. 1997;1:33-37.
33 Mallet ML. Proarrhythmic effects of adenosine: A review of the literature. Emerg Med J. 2004;21:408-410.
34 Oral H, Crawford T, Frederick M, et al. Inducibility of paroxysmal atrial fibrillation by isoproterenol and its relation to the mode of onset of atrial fibrillation. J Cardiovasc Electrophysiol. 2008;19:466-470.
35 Bettoni M, Zimmermann M. Autonomic tone variations before the onset of paroxysmal atrial fibrillation. Circulation. 2002;105:2753-2759.
36 Crawford T, Chugh A, Good E, et al. Clinical value of noninducibility by high-dose isoproterenol versus rapid atrial pacing after catheter ablation of paroxysmal atrial fibrillation. J Cardiovasc Electrophysiol. 2010;21:13-20.
37 Ninomiya Y, Iriki Y, Ishida S, et al. Usefulness of adenosine triphosphate with a sufficient observation period for detecting reconduction after pulmonary vein isolation. Pacing Clin Electrophysiol. 2009;32:1307-1312.
38 Arentz T, Macle L, Kalusche D, et al. “Dormant” pulmonary vein conduction revealed by adenosine after ostial radiofrequency catheter ablation. J Cardiovasc Electrophysiol. 2004;15:1041-1047.
39 Datino T, Macle L, Qi XY, et al. Mechanisms by which adenosine restores conduction in dormant canine pulmonary veins. Circulation. 2010;121:963-972.
40 Freedman RA, Swerdlow CD, Echt DS, et al. Facilitation of ventricular tachyarrhythmia induction by isoproterenol. Am J Cardiol. 1984;54:765-770.
41 Naccarelli GV, Prystowsky EN, Jackman WM, et al. Role of electrophysiologic testing in managing patients who have ventricular tachycardia unrelated to coronary artery disease. Am J Cardiol. 1982;50:165-171.
42 Reddy CP, Gettes LS. Use of isoproterenol as an aid to electric induction of chronic recurrent ventricular tachycardia. Am J Cardiol. 1979;44:705-713.
43 Saenen JB, Vrints CJ. Molecular aspects of the congenital and acquired long QT syndrome: Clinical implications. J Mol Cell Cardiol. 2008;44:633-646.
44 Moss AJ, Schwartz PJ. 25th anniversary of the International Long-QT Syndrome Registry: An ongoing quest to uncover the secrets of long-QT syndrome. Circulation. 2005;111:1199-1201.
45 Allan WC, Timothy K, Vincent GM, et al. Long QT syndrome in children: The value of rate corrected QT interval and DNA analysis as screening tests in the general population. J Med Screen. 2001;8:173-177.
46 Hofman N, Wilde AA, Kaab S, et al. Diagnostic criteria for congenital long QT syndrome in the era of molecular genetics: Do we need a scoring system? Eur Heart J. 2007;28:575-580.
47 Vincent GM, Timothy KW, Leppert M, Keating M. The spectrum of symptoms and QT intervals in carriers of the gene for the long-QT syndrome. N Engl J Med. 1992;327:846-852.
48 Goldenberg I, Horr S, Moss AJ, et al. Risk for life-threatening cardiac events in patients with genotype-confirmed long-QT syndrome and normal-range corrected QT intervals. J Am Coll Cardiol. 2011;57:51-59.
49 Priori SG, Schwartz PJ, Napolitano C, et al. Risk stratification in the long-QT syndrome. N Engl J Med. 2003;348:1866-1874.
50 Schechter E, Freeman CC, Lazzara R. Afterdepolarizations as a mechanism for the long QT syndrome: Electrophysiologic studies of a case. J Am Coll Cardiol. 1984;3:1556-1561.
51 Ackerman MJ, Khositseth A, Tester DJ, et al. Epinephrine-induced QT interval prolongation: A gene-specific paradoxical response in congenital long QT syndrome. Mayo Clin Proc. 2002;77:413-421.
52 Vyas H, Hejlik J, Ackerman MJ. Epinephrine QT stress testing in the evaluation of congenital long-QT syndrome: Diagnostic accuracy of the paradoxical QT response. Circulation. 2006;113:1385-1392.
53 Noda T, Takaki H, Kurita T, et al. Gene-specific response of dynamic ventricular repolarization to sympathetic stimulation in LQT1, LQT2 and LQT3 forms of congenital long QT syndrome. Eur Heart J. 2002;23:975-983.
54 Khositseth A, Hejlik J, Shen WK, Ackerman MJ. Epinephrine-induced T-wave notching in congenital long QT syndrome. Heart Rhythm. 2005;2:141-146.
55 Roden DM, George ALJr. The cardiac ion channels: Relevance to management of arrhythmias. Annu Rev Med. 1996;47:135-148.
56 Vatta M, Li H, Towbin JA. Molecular biology of arrhythmic syndromes. Curr Opin Cardiol. 2000;15:12-22.
57 Kass RS, Moss AJ. Long QT syndrome: Novel insights into the mechanisms of cardiac arrhythmias. J Clin Invest. 2003;112:810-815.
58 Shimizu W, Antzelevitch C. Differential effects of beta-adrenergic agonists and antagonists in LQTt1, LQT2 and LQT3 models of the long QT syndrome. J Am Coll Cardiol. 2000;35:778-786.
59 Shimizu W, Noda T, Takaki H, et al. Diagnostic value of epinephrine test for genotyping LQT1, LQT2, and LQT3 forms of congenital long QT syndrome. Heart Rhythm. 2004;1:276-283.
60 Viskin S, Alla SR, Barron HV, et al. Mode of onset of torsade de pointes in congenital long QT syndrome. J Am Coll Cardiol. 1996;28:1262-1268.
61 Viskin S, Rosso R, Rogowski O, et al. Provocation of sudden heart rate oscillation with adenosine exposes abnormal QT responses in patients with long QT syndrome: A bedside test for diagnosing long QT syndrome. Eur Heart J. 2006;27:469-475.
62 Roden DM. Taking the “idio” out of “idiosyncratic”: Predicting torsades de pointes. Pacing Clin Electrophysiol. 1998;21:1029-1034.
63 Roden DM, Spooner PM. Inherited long QT syndromes: A paradigm for understanding arrhythmogenesis. J Cardiovasc Electrophysiol. 1999;10:1664-1683.
64 Kaab S, Hinterseer M, Nabauer M, Steinbeck G. Sotalol testing unmasks altered repolarization in patients with suspected acquired long-QT-syndrome—a case-control pilot study using I.V. sotalol. Eur Heart J. 2003;24:649-657.
65 Cheng A, Dalal D, Fetics BJ, et al. Ibutilide-induced changes in the temporal lability of ventricular repolarization in patients with and without structural heart disease. J Cardiovasc Electrophysiol. 2009;20:873-879.
66 Abildskov JA, Lux RL. Cycle-length effects on the initiation of simulated torsade de pointes. J Electrocardiol. 1994;27:1-9.
67 Takenaka K, Ai T, Shimizu W, et al. Exercise stress test amplifies genotype-phenotype correlation in the LQT1 and LQT2 forms of the long-QT syndrome. Circulation. 2003;107:838-844.
68 Wong JA, Gula LJ, Klein GJ, et al. Utility of treadmill testing in identification and genotype prediction in long-QT syndrome. Circ Arrhythm Electrophysiol. 2010;3:120-125.
69 Viskin S, Postema PG, Bhuiyan ZA, et al. The response of the QT interval to the brief tachycardia provoked by standing: A bedside test for diagnosing long QT syndrome. J Am Coll Cardiol. 2010;55:1955-1961.
70 Brugada P, Brugada J. Right bundle branch block, persistent ST segment elevation and sudden cardiac death: A distinct clinical and electrocardiographic syndrome. A multicenter report. J Am Coll Cardiol. 1992;20:1391-1396.
71 Antzelevitch C, Brugada P, Borggrefe M, et al. Brugada syndrome: Report of the second consensus conference. Heart Rhythm. 2005;2:429-440.
72 Brugada R, Brugada J, Antzelevitch C, et al. Sodium channel blockers identify risk for sudden death in patients with ST-segment elevation and right bundle branch block but structurally normal hearts. Circulation. 2000;101:510-515.
73 Meregalli PG, Ruijter JM, Hofman N, et al. Diagnostic value of flecainide testing in unmasking SCN5A-related Brugada syndrome. J Cardiovasc Electrophysiol. 2006;17:857-864.
74 Rolf S, Bruns HJ, Wichter T, et al. The ajmaline challenge in Brugada syndrome: Diagnostic impact, safety, and recommended protocol. Eur Heart J. 2003;24:1104-1112.
75 Priori SG, Napolitano C, Memmi M, et al. Clinical and molecular characterization of patients with catecholaminergic polymorphic ventricular tachycardia. Circulation. 2002;106:69-74.
76 Napolitano C, Priori SG. Diagnosis and treatment of catecholaminergic polymorphic ventricular tachycardia. Heart Rhythm. 2007;4:675-678.
77 Beckmann BM, Wilde AA, Kaab S. Dual inheritance of sudden death from cardiovascular causes. N Engl J Med. 2008;358:2077-2078.
78 Krahn AD, Gollob M, Yee R, et al. Diagnosis of unexplained cardiac arrest: Role of adrenaline and procainamide infusion. Circulation. 2005;112:2228-2234.
79 Brunetti ND, De Gennaro L, Gaglione A, Di Biase M. An unusual catecholaminergic polymorphic ventricular tachycardia not inducible at stress test. Int J Cardiol. Mar 11, 2011. [Epub ahead of print]
80 Sumitomo N, Harada K, Nagashima M, et al. Catecholaminergic polymorphic ventricular tachycardia: Electrocardiographic characteristics and optimal therapeutic strategies to prevent sudden death. Heart. 2003;89:66-70.
81 Francis J, Sankar V, Nair VK, Priori SG. Catecholaminergic polymorphic ventricular tachycardia. Heart Rhythm. 2005;2:550-554.
82 Fisher JD, Krikler D, Hallidie-Smith KA. Familial polymorphic ventricular arrhythmias: A quarter century of successful medical treatment based on serial exercise-pharmacologic testing. J Am Coll Cardiol. 1999;34:2015-2022.
83 Rosso R, Kalman JM, Rogowski O, et al. Calcium channel blockers and beta-blockers versus beta-blockers alone for preventing exercise-induced arrhythmias in catecholaminergic polymorphic ventricular tachycardia. Heart Rhythm. 2007;4:1149-1154.
84 Hoffmann E, Reithmann C, Neuser H, et al. Repetitive monomorphic ventricular tachycardia (Gallavardin type): Clinical and electrophysiological characteristics in 20 patients [translation]. Z Kardiol. 1998;87:353-363.