CHAPTER 54 Proton Radiation Therapy for Meningiomas
PHYSICAL PROPERTIES OF PROTON RADIATION AND THE CLINICAL IMPLICATIONS
Physical Properties of Proton Radiation
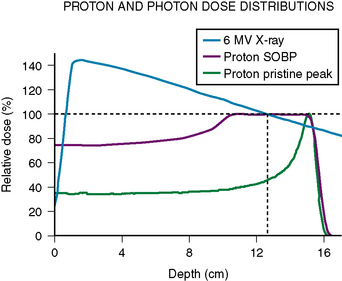
The primary difference between protons and photons in clinical application is the greater ability of proton beams to conform to the target and thereby decrease unnecessary radiation exposure to surrounding nontarget normal tissues. Protons have finite path lengths. As they decelerate in speed within tissues, they transfer their energy. The greatest deposition of energy is within the last few millimeters of their path length. This final large release of energy is known as the Bragg peak (Fig. 54-1). The path length of a proton beam within a given tissue increases with the beam energy. Multiple proton beam energies can be used to create a spread out Bragg peak (SOBP) to irradiate the entire target to a relatively uniform dose. Because proton beams have a finite travel distance, there is essentially no radiation delivered downstream to where the proton stops. In contrast, photons are partially attenuated by the matter with which they pass through but they do not have a finite path length. To achieve an adequate dose delivery to clinical targets, photon beams deposit both a higher amount of energy upstream to the target and continue to deliver a significant amount of energy downstream to the target. Typically multiple radiation beams from different orientations are used to maintain an adequately low-dose deposition to the normal tissues between the skin surface and target. Overlapping radiation beams within the target achieves the higher and therapeutic dose desired.
The increased conformality of protons in dose distribution has two primary benefits. It may reduce the risk of adverse effects derived from normal tissue exposure to ionizing radiation. This is the major advantage of protons versus photons for patients with benign meningiomas. Although there is still energy deposited between tissue entry and target, the elimination of all downstream radiation exposure significantly reduces the volume of normal tissues irradiated. This also allows for the selection on many more beam arrangements in proton therapy because downstream irradiation of radiation-sensitive structures is not a concern. When treating irregularly shaped targets such as skull-base meningiomas, proton radiation has both the benefit of diverse beam arrangements and minimization of irradiation of neighboring tissues such as the brain and eyes. Dosimetric studies comparing modern photon-based radiation therapies with proton radiation find that no more than three proton beams are required for meningiomas plans whereas five or six photon beams are required for optimization of each technique.1 Proton planning is associated with lower volume of normal tissues exposed to low-dose excess radiation. A second study comparing proton and photon planning of intracranial tumors including five cases of meningiomas also found superior conformality with protons.2 Although these studies are purely planning studies, it is expected that they will translate into reduction of potential late effects of normal tissue irradiation.
A second important feature of proton therapy is its ability to deliver higher doses than photons while maintaining an equivalent risk of normal tissue injury probability. This is an important concept for managing patients with atypical and malignant meningiomas. It is a direct consequence of decrease in normal tissue volume irradiated and dose delivered to these tissues. With proton radiation, the target dose can be increased while the surrounding normal tissue risk of complication can be maintained equivalent to or less than alternatively achieved with photon radiation. In diseases in which treatment efficacy was limited by insufficient dose, proton radiation may increase the effectiveness of radiotherapy as a treatment modality. As mentioned earlier, in the setting of management of atypical or malignant histologies of meningiomas where doses in excess of 60 Gy may be beneficial to improving local control, the both improved local control and reduction of normal tissue injury can be expected with the use of proton radiation.3
PREPARATION AND PLANNING FOR PROTON THERAPY
Treatment Planning and Delivery for Proton Radiation Therapy
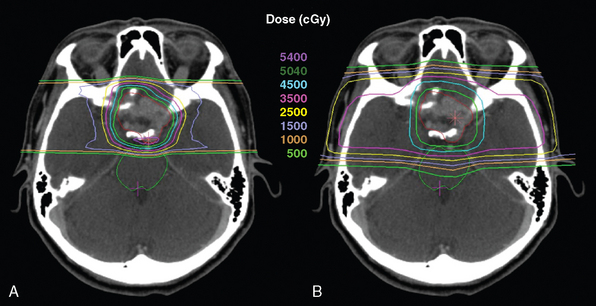
Proton radiation treatment planning is similar to that of other radiation therapy planning in that it is CT-based. Targets and critical structures are outlined. Between one and six optimized beams are used, depending on the geometry of target and normal tissues. Careful port design should maximize sharp lateral and distal dose fall-off by avoiding beams directed tangential to bony ridges or through heterogeneous areas such as the mastoids, sinuses, and auditory canals. Field specific collimation and distal range compensation are used to provide conformal beam shaping. Various ranges of beam energies are superimposed to create the SOBP. Dose distribution is typically far more uniform regardless of target dimensions as compared to photon-based treatments for either fractionated or stereotactic therapies. Frequently excellent sparing of irradiation of surrounding normal structures can be achieved (Fig. 54-2). Daily high precision treatment alignment is achieved with on-board diagnostic imaging. Anatomic bony landmarks are used for alignment. Skull fiducial markers provide added assurance of this accuracy. Verification imaging is performed prior to each treated field.
CLINICAL EXPERIENCE WITH PROTON RADIATION THERAPY
Fractionated Proton Radiation Therapy
Limited experience with fractionated proton radiation in the treatment of meningiomas suggests equivalence in tumor control as compared to other forms of radiotherapy. A report from the National Accelerator Center on the efficacy and toxicities of proton radiation therapy included five cases of fractionated stereotactic radiotherapy (54.1–61.6 GyE in 16–28 fractions) in the management of skull-base meningiomas.4 The mean follow-up period was 40 months. Local control was maintained in all patients and provides some preliminary data in support of its use. In regards to morbidity, no patient experienced an acute toxicity but one patient developed a decrement in short-term memory.
At the Paul Scherrer Institute (PSI) in Switzerland, a novel form of proton radiation delivery using a scanning beam has been developed with the goal of achieving greater conformal radiation delivery. This spot-scanning beam technology has bean applied to the treatment of 16 patients with intracranial meningiomas.5 These patients were otherwise treated in typical fractionated radiotherapy fashion with a median dose of 56 GyE. Local control at 3 years was 92%. Treatment-related adverse effects were found in 3 of 16 patients and included cases of retinopathy, optic neuropathy, and brain necrosis. The authors note that all sequelae occurred when the respective normal tissues were irradiated to higher doses than currently employed normal tissue dose constraints. Thus, this data served to support both the equivalent efficacy of proton radiation to photon therapies and to reaffirm currently accepted dose constraints of normal neurologic tissues.
Hypofractionated Proton Radiation Therapy
Experience with hypofractionated proton radiation is limited. The largest reported experience from the Svedberg Laboratory in Uppsala, Sweden describes the treatment results of 19 patients with meningiomas.6 With the exception of one patient, all cases were skull-base tumors. Patients were treated with 24 GyE divided in four daily fractions. No failures and no neurologic injury have occurred yet with a minimum follow-up of at least 36 months. Although this follow-up time is relatively short, this study suggests that hypofractionated treatments might be an excellent treatment option offering greater patient convenience as compared to standard fractionated therapy. The experience at the National Accelerator Center also included the treatment of 18 patients treated with hypofractionated stereotactic radiotherapy (3 fractions, mean dose 20.3 GyE).4 Sixteen (89%) patients remained at least clinically stable whereas two patients showed tumor progression. Thus, control rates by hypofractionated proton radiation were comparable to photon-based methods. Two patients experienced temporary cranial nerve dysfunction. Two other patients developed late neurologic toxicities of partial hearing loss and temporal lobe epilepsy.
Combined Photon-Proton Therapy
Combined modality radiotherapy with both photons and protons has been used successfully and may maximize the number of patients able to benefit from the limited resources of proton therapy. In these regimens, only a portion of the radiation fractions is delivered with protons and the remainder is given by photons. Among a French report of 51 patients with presumed benign meningiomas of the skull base treated with a combined proton-photon approach to a median dose of 60.6 GyE, the 4-year local control rate was 98%.7 No significant adverse effect was reported at a median follow-up of 21 months. Longer follow-up will be needed to truly assess the potential late effects of therapy. A second mixed photon-proton series of 46 patients with subtotally resected or recurrent meningiomas reported a recurrence free survival rate at 5 and 10 years of 100% and 88%, respectively.8 Median dose was 59.0 GyE with a range of 53.1 to 74.1 GyE. Among survivors, the treatment toxicity rate was 20% at both 5 and 10 years and was described as a result of accepted high dose constraints to the normal tissues. All treatment-related morbidity occurred as a result of normal tissue irradiation to doses exceeding commonly recognized neural tissue dose tolerances.
Proton Stereotactic Radiosurgery
Proton radiosurgery is a relatively new application of proton radiation. Similar to that of photon-based methods such as linear accelerator or Gamma Knife® radiosurgery, treatment is delivered in a single setting. Dosimetrically, proton radiosurgery differs from both linear accelerator and Gamma Knife radiosurgery in that the dose is highly uniform within the treatment target. The uniform dose has less areas of both underdosing and overdosing. This may lead to improve local control and decrease normal tissue injury, respectively. The experience of treatment of 44 benign meningiomas at MGH with proton radiosurgery has thus far shown a three year local control rate of 91%.9 Median dose delivered was 13 GyE (range 10–15 GyE). Transient neurologic symptoms that may have been attributable to treatment included seizures in two patients, hydrocephalus requiring shunting in one patient, and facial pain in one patient. An additional patient developed hypopituitarism from direct pituitary irradiation. Although this is the only available proton radiosurgery data currently available, the preliminary results are promising for an effective, safe, and patient friendly modality of managing small meningiomas.
Proton Therapy in Atypical and Malignant Meningiomas
Atypical and malignant meningiomas represent a subset of meningiomas that may benefit from multiple properties of proton radiation. As with benign meningiomas, a reduction of radiation toxicity may be achieved as a result of increased dose conformality that minimizes unnecessary normal tissue radiation exposure. In addition, an improved tumor control rate may be feasible with the delivery of higher radiation doses without increase of the morbidity rate. In a series from Massachusetts General Hospital, 31 patients with atypical or malignant skull-base meningiomas were treated with either photons alone (15 patients) or mixed photon–proton radiation therapy (16 patients).3 With mean doses of 62 GyE for atypical meningiomas and 58 GyE for malignant meningiomas, the local control rates at 5 years were 38% and 52%, respectively. Improved local control correlated with doses greater than or equal to 60 GyE and with the use of proton radiation. This may reflect the benefit of greater dose homogeneity with proton radiation which decreases the probability of spots of underdosing which is more common with photon therapy alone. Among the atypical meningiomas cases, those treated to greater than or equal to 60 GyE achieved a 5-year local control of 90% as compared to 0% for those receiving less than 60 GyE. Similarly among the malignant meningioma patients, 5-year local control following greater than or equal to 60 GyE was 83% as compared to 14% for lower doses. Thus, local control of atypical and malignant meningiomas was improved with higher doses. The cost of using higher doses was a 9% late complication rate (with ≥59 Gy). Proton radiation may be a mechanism of achieving necessary higher doses while minimizing acceptable rates of normal tissue injury.
RADIATION TOXICITY
Limitations in radiation therapy efficacy are not because of dose constraints of tumors but rather of the surrounding normal tissues. It is imperative to understand the effects and tolerance of tissues to radiation to optimize use of technological advancements in radiation delivery. Radiation-related injury of the CNS can be significantly debilitating if not life threatening and thus treatment must be administered with extreme care and consideration. Radiation adverse effects are typically not defined to occur by absolute thresholds but rather arise on a spectrum of probabilities influenced by total radiation dose, dose per fraction, time between fractions, overall treatment time, radiosensitivity and volume of tissues treated, health of the normal tissues prior to irradiation, general health of the patient, individual genetic predisposition, amongst perhaps additional factors. There are physical radiation properties such as the relative biological effectiveness (RBE) of the radiation source that must be accounted for in determining treatment dose. With proton radiation, there is also concern of variable RBE by location along the beam path.10
[1] Baumert B.G., Norton I.A., Lomax A.J., et al. Dose conformation of intensity-modulated stereotactic photon beams, proton beams, and intensity-modulated proton beams for intracranial lesions. Int J Radiat Oncol Biol Phys. 2004;60:1314.
[2] Bolsi A., Fogliata A., Cozzi L. Radiotherapy of small intracranial tumours with different advanced techniques using photon and proton beams: a treatment planning study. Radiother Oncol. 2003;68:1.
[3] Hug E.B., DeVries A., Thornton A.F., et al. Management of atypical and malignant meningiomas: role of high-dose, 3D-conformal radiation therapy. J Neurooncol. 2000;48:151.
[4] Vernimmen F.J., Harris J.K., Wilson J.A., et al. Stereotactic proton beam therapy of skull base meningiomas. Int J Radiat Oncol Biol Phys. 2001;49:99.
[5] Weber D.C., Lomax A.J., Peter Rutz H., et al. Spot-scanning proton radiation therapy for recurrent, residual or untreated intracranial meningiomas. Radiother Oncol. 2004;71:251.
[6] Gudjonsson O., Blomquist E., Nyberg G., et al. Stereotactic irradiation of skull base meningiomas with high energy protons. Acta Neurochi (Wien). 1999;141:933.
[7] Noël G., Bollet M.A., Calugaru V., et al. Functional outcome of patients with benign meningioma treated by 3D conformal irradiation with a combination of photons and protons. Int J Radiat Oncol Biol Phys. 2005;62:1412.
[8] Wenkel E., Thornton A.F., Finkelstein D., et al. Benign meningioma: partially resected, biopsied, and recurrent intracranial tumors treated with combined proton and photon radiotherapy. Int J Radiat Oncol Biol Phys. 2000;48:1363.
[9] Halasz L.M., Bussiere M.M., Niemierko A., et al. Outcomes of patients with benign meningiomas treated with proton beam stereotactic radiosurgery. To be presented at the American Society for Therapeutic Radiology and Oncology 50th Annual Meeting, September 2008.
[10] Paganetti H., Niemierko A., Ancukiewicz M., et al. Relative biological effectiveness (RBE) values for proton beam therapy. Int J Radiat Oncol Biol Phys. 2002;53:407.