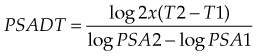Chapter 51 Prostate Cancer
Epidemiology and Etiology
Epdemiology
In 2010, it was estimated that 217,730 American men would be diagnosed with prostate cancer, accounting for 25% of all newly diagnosed cancers in men.1 It appears that one in six American men will develop a clinically recognized invasive prostate cancer during his lifetime. Ninety-one percent of cases were expected to be diagnosed of local or regional stage disease for which the 5-year relative survival rate approaches 100%. The age-adjusted annual incidence rate increased 6.4% per year between 1983 and 1989,2 with a 66% overall increase between the periods of 1975 to 1979 and 1987 to 1991.3 This change largely reflected the increased detection of localized, rather than regionally advanced or metastatic, prostate cancer cases.2,4 From 2001 to 2005 the incidence of newly diagnosed cases dropped 4.4% per year.5,6 This decrease in the incidence of prostate cancer may reflect stabilization related to PSA screening. Prostate cancer remains the second leading cause of cancer death in men, with 32,050 deaths being estimated in 2010. As with the incidence of newly diagnosed cancers there has been a significant drop in prostate cancer death rates from 1990 to 2005. The death rate from prostate cancer was 38.56/100,000 men in 1990 compared with 24.65/100,000 men in 2005 (p <.05), representing a 36% decline.1
The incidence of incidental (or latent) prostate cancer observed at autopsy examination and the incidence and prevalence of clinically manifest prostate cancer increase substantially with age.6–9 Information from the Surveillance, Epidemiology, and End Results (SEER) program indicates that approximately 1.8% of American men between ages 40 and 59 years had a clinical diagnosis of prostate cancer whereas 15% of men 60 to 79 years old would have prostate cancer by the time they attained this age. Although the prevalence of incidental carcinoma does not exhibit marked geographic variation, the regional disparity in the antemortem clinical expression of the disease and the cancer mortality rate of prostatic carcinoma is noteworthy.7,10 Men in North America, Australia/New Zealand, Western and Northern Europe, and the Caribbean have a much higher incidence of this disease than men in Asia and China.10
Etiology
Cohort and case-control studies are the two principal methods used to ascertain the etiology of cancer.11 Several reports describe various host-related and environmental factors that appear associated with prostate cancer (Table 51-1), but it is not yet understood which factors directly initiate or promote prostate carcinogenesis. As discussed by several authors,12,13 long-term androgen exposure, which is required for normal prostatic development and for cancer growth and maintenance, advanced age,1 race/ethnicity,14–16 and familial history of prostate cancer17,18 are presently viewed as the most likely risk factors. Other associations such as diet, body mass index, energy balance, and physical activity are becoming more suspect.19–23
Hormonal Factors
Although several lines of investigation suggest the importance of hormones in the promotion of prostate cancer, their mechanism of action and interactions are not clearly understood. Animal studies indicate that chronic testosterone exposure markedly enhances the effects of carcinogens on prostatic tissues.24 Furthermore, both benign prostatic hyperplasia and prostate cancer simultaneously develop under androgen stimulation, and some reports suggest that men with benign prostatic hyperplasia are at increased risk for development of prostate cancer.25,26 Various authors also report that men with prostate cancer have alterations in serum levels of androstenedione, dihydrotestosterone, sex hormone–binding globulin, or testosterone,27–30 which also was noted to vary according to race/ethnicity.29,30 Despite these associations, circulating androgens do not fully explain the development of prostate cancer and downstream effects in target tissue may be more relevant.31,32 Testosterone is the main circulating androgen, but it is converted to dihydrotestosterone in prostate and other peripheral tissues by 5α-reductase. Dihydrotestosterone binds the androgen receptor leading to nuclear translocation and activation of transcriptional and androgen responsive genes. The activity of 5α-reductase is different in various ethnic groups and may partially explain the variable incidence and aggressiveness of prostate cancers by race.30,33
Age
Age may be the single most important factor associated with prostate cancer, because most elderly men have histologic evidence of cancer in the prostate gland8 and the rate of clinical detection is a direct function of age.1 Although several causes may be invoked, such as senescence of the immune system, higher levels of oxidative stress, prolonged exposure to carcinogens, and impaired response to DNA damage, an exact explanation for this association is lacking.
Hereditary Factors
Twin studies suggest that heritable factors account for a proportion of prostate cancer risk.34 Several investigators observed familial clustering of prostate cancer and suggested that genetic constitution may increase susceptibility to the disease.17,18 It has been estimated that as many as 5% to 15% of prostate cancer cases are hereditary or familial35,36 and they are typically diagnosed at an earlier age than sporadic cases.35 Carter and associates describe an increased risk of the disease, a greater number of affected relatives, and an earlier age at onset with the familial type compared with the sporadic form.17 The age-adjusted risk of developing prostate cancer in the setting of a familial predisposition is approximately twice that of the general population but may be greater (i.e., 5- to 11-fold), depending on the number of affected first-degree relatives.17,37 A meta-analysis of 13 case-control and cohort studies determined a relative risk in first-degree relatives of 2.5 (95% CI 2.2 to 2.8).18 The highest risk was in relatives of cases diagnosed before 60 years of age. The risk of developing prostate cancer was 3.5-fold greater in men with two affected relatives.18 Candidate susceptibility genes such as RNASEL, ELAC2, and MSR1 have been identified in cases of heritable prostate cancer,36 whereas mutations in these genes are rare in the more common sporadic forms of the disease.38
Racial and Ethnic Variations
Prostate cancer incidence is characterized by marked racial/ethnic variation that is dependent on geographic factors.1 For example, a 30- to 50-fold difference in the incidence of the disease is observed between black men and native Asians.39 In the Health Professionals Follow-up Study, African-American race was strongly associated with a risk of developing prostate cancer.23 However, a complex interaction between race/ethnicity and environment exists. For example, the incidence of cancer in a particular racial/ethnic group (e.g., Japanese) tends to shift toward that of the population in the geographic area to which that group immigrates (e.g., the United States) as the group assimilates to its new environment and culture.40 Furthermore, certain racial/ethnic groups (e.g., blacks) present with more-advanced prostate cancer and appear to have a stage-adjusted outcome that is worse than for other members of the society (e.g., whites).41,42 Racial variations of hormonal, dietary, genetic, and perhaps socioeconomic factors may account for such findings,14,30,33,43,44 but definitive evidence in this regard is lacking, and further research is necessary to clarify the association between race/ethnicity and prostate cancer risk. There are racial differences in allelic frequencies of the microsatellites at the androgen receptor locus. African Americans have a high prevalence of short CAG microsatellite alleles and a low frequency of 16 GGC repeats compared with whites and Asians. Shorter CAG microsatellite alleles of the AR gene may promote prostate cell growth, resulting in a higher prostate cancer risk.32,45
Dietary Factors
The role of dietary factors in prostate cancer pathogenesis is difficult to discern because research in this area is confounded by complex interactions with other potential risk factors. There are striking racial/ethnicity and geographic variations in prostate cancer incidence that may have a dietary basis. In particular, a high-fat diet appears to correlate with prostate cancer incidence and mortality13,19,43,46,47 whereas ingestion of retinol,48 certain carotenoids (e.g., lycopene),13,49 phytoestrogens,50 and vitamin D may be protective.51 The influence of other vitamins (e.g., vitamin E) and minerals (e.g., zinc) is uncertain.13 In a large prospective cohort study of American men, Sinha reported an association of processed and red meat with the total prostate cancer incidence and the incidence of locally advanced disease.52 Other dietary factors such as α-linolenic acid are associated with increased risk of prostate cancer, whereas tomato-based products containing lycopene have been associated with reduced risk.23,53,54 It has been suggested that a diet high in fruit and vegetable intake is associated with a lower rate of prostate cancer.55 The Health Professionals Follow-up Study demonstrated an association for tomato sauce, the primary source of bioavailable lycopene, and a lower risk of prostate cancer.54
Some vitamins, minerals and other micronutrients have been implicated as having a protective role in prostate cancer. An association between low levels of ultraviolet light exposure and prostate cancer mortality rates led to the hypothesis that vitamin D insufficiency may play a role in prostate cancer risk.56 In contrast, the European Prospective Investigation into Cancer and Nutrition showed no significant association between circulating levels of 25-hydroxyvitamin D and risk of prostate cancer.57 Giovannucci58 hypothesized that high dairy and meat consumption increase risk of prostate cancer by lowering 1,25-dihydroxyvitamin D. Indeed, there may be important interactions with vitamin D and calcium that may influence the risk of developing prostate cancer. In the Health Professionals Follow-up Study, a high dietary calcium intake was associated with an increased risk of prostate cancer.23 Halthur and associates59 suggest that high serum levels of calcium in young overweight men may be a marker for a decreased risk of developing prostate cancer. The European Prospective Investigation into Cancer and Nutrition reported an increase risk of prostate cancer with dairy proteins and dairy calcium.21 The Nutritional Prevention of Cancer Study Group tested selenium supplementation as a preventative agent for a number of cancers. Clark and colleagues60 reported a 63% reduction in the incidence of prostate cancer with oral selenium supplementation. Similarly, a secondary analysis of the Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study showed a 32% reduction in the incidence of prostate cancer.61
Body Habitus
Although studies have failed to consistently show that obesity is related to the incidence of prostate cancer, a study of more than 400,000 men (Cancer Prevention Study II) showed that men with higher body mass index (weight vs. height) had an increased risk of prostate cancer death as compared with men with a lower body mass index.19,20 The mechanism through which obesity might cause prostate cancer or a higher mortality from prostate cancer is complex but is thought to be related to a state of hyperinsulinemia that leads to higher levels of insulin-like growth factor and available sex steroids (androgens).62 Different factors may be at play in the carcinogenesis of nonaggressive cancers and the evolution of disease to an aggressive form.63 Taller height was associated with in increased risk of fatal prostate cancer in the Health Professionals Follow-up Study.23 Height may be a surrogate for dietary and hormonal influences that may affect the risk of prostate cancer.
Other Lifestyle Factors
Research regarding other lifestyle factors such as tobacco use, alcohol consumption, physical activity level, sexual behavior, and sexually transmitted diseases has provided mixed findings with regard to the importance of these factors in prostate cancer pathogenesis.* Previous infection with Trichomonas vaginalis has been associated with an increased risk of aggressive prostate cancer.73 New evidence suggests that the xenotropic murine leukemia virus-related virus (XMRV) has been detected in a subset of human prostate cancers and it may play a role in tumorigenesis.74 Vigorous physical activity was associated with a lower risk of fatal prostate cancer in the Health Professionals Follow-up Study.23 In contrast, the European Prospective Investigation into Cancer and Nutrition study showed that only occupational and not leisure activity was associated with a reduction in prostate cancer risk.22 Although the results of these studies may be affected by the fact that several lifestyle factors may be simultaneously interacting and difficult to separate, nevertheless, this research increasingly suggests that there may be an effect for at least some of these etiologic factors.
Environmental Exposure
A link between environmental or occupational exposure to carcinogens and development of prostate cancer has also been explored, but establishing a correlation and identifying a causative agent(s) have been difficult. For example, laborers in heavy industry, rubber manufacturers, and newspaper printers may have a somewhat higher incidence of prostate cancer,75 but this association is weak, and its significance remains uncertain. Exposure to cadmium, as from cigarette smoke, nickel-cadmium batteries, or certain paints, has been implicated as a carcinogen, because cadmium workers have more aggressive prostate cancers and an increased cause-specific mortality.76 Although laboratory evidence would suggest a link between cadmium exposure and prostate cancer, a review of published epidemiologic studies suggests there is no valid connection.77 Nonetheless, a clear association between these factors, environmental or occupational exposure, and prostate cancer is conjectural, and further study is required before firm conclusions can be formed.
Vasectomy
Some investigations suggested that vasectomy increases androgen levels78 or produces an immunologic reaction79 that might promote prostate carcinogenesis. In addition, other studies indicate that vasectomy produces an approximate 1.6-fold increase in the risk of developing prostate cancer, which directly correlates with the interval from surgery.72,80–82 However, DerSimonian and associates83 concluded that these studies were inadequately designed and conducted, and John and colleagues84 and Sidney and colleagues85 could not confirm this association in large cohort studies. Furthermore, the National Institutes of Health determined that information regarding a putative association between vasectomy and prostate cancer was not convincing and that a causative relationship had not been established.86 Therefore vasectomy should not be considered a prostate cancer risk factor.
Precancerous Lesions
Of the two categories, PIN represents the putative precancerous end of the morphologic continuum of cellular proliferation and is more strongly associated with prostate cancer.87 PIN may appear hypoechoic on transrectal ultrasonography (TRUS), but biopsy is the definitive method with which to detect the condition. Two grades (low and high) are recognized, with high-grade PIN considered a precursor of invasive carcinoma.87,88 Studies suggest that PIN predates prostate cancer by 10 years or more, with low-grade PIN first emerging in the third decade of life.89 PIN is often found in proximity to carcinoma, and its presence warrants a search for invasive carcinoma.90 Davidson and associates91 noted that 35% of patients with PIN had prostate cancer identified on subsequent biopsy, which is consistent with the results of other studies.92 Results such as these indicate that PIN is a likely and strong risk factor for prostate cancer that warrants close surveillance and follow-up (e.g., 6-month intervals for 2 years and yearly intervals thereafter for life).93,94 Specific molecular findings associated with high-grade PIN may be able to predict which men may have invasive cancer on repeat biopsies. In a study of biopsy specimens expressing α-methylacyl-CoA racemase (AMACR), there was a 5.2 times greater incidence of cancer on repeat biopsy compared with high-grade PIN without AMACR positivity.95 AMACR overexpression was also more often identified in high-grade PIN adjacent to cancer than high-grade PIN lesions distant from cancer in prostatectomy specimens.96 PTOV1, a novel protein overexpressed in some high-grade PIN lesions, may predict for invasive cancer.97
Prevention and Early Detection
Prevention
Prostate cancer is an androgen-dependent tumor with a prolonged latency between initial malignant transformation and clinical expression, which are features well-suited to disease prevention efforts.7 Progression from tumor inception to invasive carcinoma often takes decades, allowing sufficient time for intervention.9 Chemoprevention strategies that use high-risk target populations, particularly those with premalignant lesions (e.g., high-grade PIN), have the greatest potential to identify promising agents in a time-efficient manner.98 The results of focused studies such as these can then be confirmed in large-scale trials applied to the general population. The ability to alter the hormonal environment of the host provides an excellent opportunity to interrupt the multistep process that results in clinical expression of the disease.7 Advances in our understanding of the process of carcinogenesis and the availability of promising new chemopreventive agents, including those producing reversible androgen deprivation, have the potential to favorably affect the morbidity and mortality of prostate cancer in the foreseeable future.
Intracellular 5α-reductase converts testosterone to dihydrotestosterone (DHT), the hormone responsible for prostate epithelial proliferation. DHT has greater affinity for the androgen receptor and is the primary agonist leading to prostate maintenance and growth. Three isoforms of 5α-reductase have been identified with various levels in different tissues. Type 1 is expressed in benign prostate hyperplasia, and its expression is greatly increased in prostate cancer, especially high-grade tumors.99 Type 2 5α-reductase expression is decreased in PIN and some early cancers but is increased in metastatic and recurrent prostate cancer.99 The role of type 3 5α-reductase is less defined. Competitive inhibitors of 5α-reductase (e.g., finasteride and dutasteride) suppress intraprostatic dihydrotestosterone to castrate levels. The Prostate Cancer Prevention (PCP) trial was initiated to test the efficacy of finasteride as a chemoprevention agent in men at low risk of having prostate cancer.100 This placebo-controlled phase III trial randomized 18,882 eligible men (age ≥55 years, normal digital rectal examination [DRE] and PSA levels <3 ng/mL) to finasteride (5 mg daily) or placebo for 7 years. There was a 25% reduction in the prevalence of prostate cancer over this 7-year period from 30.6% in the placebo group to 18.6% in the finasteride group.100 Of note, however, is that more aggressive tumors, with Gleason score 7 to 10, were more common in patients who took finasteride: 37% of all tumors and 6.4% of all men on the finasteride arm versus 22% of all tumors and 5.1% of all men on the placebo arm. The increased incidence of high-grade cancers seen in the PCP trial has been a topic of great debate. The investigators have argued that the increase in high-grade cancers is due to a detection bias related to the reduced volume of prostate tissue and therefore a greater ratio of cancer to benign tissue. Furthermore, there was no dose effect from the finasteride with no significant increase in worse cancers with higher cumulative doses of the drug.101 The PCP trial was not designed or powered to detect differences in cancer-specific survival (CSS) or overall survival (OS). Finasteride did reduce urinary symptoms compared with placebo, but there were also significantly more adverse sexual side effects.100 A reduced volume of ejaculate, erectile dysfunction, loss of libido, and gynecomastia were more common in the finasteride group (p <.001), but urinary urgency, frequency, retention, urinary tract infection, and prostatitis were less common with finasteride (p <.001).100
The Reduction by Dutasteride of Prostate Cancer Events (REDUCE) trial, a phase III study testing inhibition of 5α-reductase with dutasteride, has completed its accrual of patients.102 Unlike finasteride, which blocks 5α-reductase type 2, dutasteride blocks both types 1 and 2, suggesting it may be more effective at preventing the development of prostate cancer.99,103 Phase III trials evaluating dutasteride for the treatment of benign prostatic hyperplasia coincidentally showed a significant reduction in the incidence of prostate cancer.102,104 The REDUCE trial is a randomized trial of placebo versus dutasteride, 0.5 mg, administered daily to evaluate it as a chemopreventive agent for prostate cancer. This trial completed its accrual of 8000 subjects in 2004. Unlike the PCP trial, which enrolled men with a low risk of prostate cancer, the REDUCE trial sought patients with a higher risk of developing or being diagnosed with prostate cancer (e.g., PSA >2.5).102
The association of dietary consumption or serum levels of selenium and α-tocopherol and a low rate of prostate cancer have suggested that dietary supplements of these nutrients may protect men from the development of this disease.61 Despite this association, two prospective phase III clinical trials did not show a reduction in the incidence of prostate cancer with vitamin E or selenium supplementation. In the Selenium and Vitamin E Cancer Prevention Trial (SELECT) there was no significant reduction in prostate cancer incidence related to either selenium or vitamin E supplementation.105 SELECT enrolled 32,400 participants to a phase III randomized, double-blind, placebo-controlled trial with a 2 × 2 factorial design. The study was designed to determine the efficacy of selenium, vitamin E, or a combination of the two to prevent prostate cancer. Unfortunately, neither supplement alone nor in combination lowered the risk of prostate cancer in healthy men.105 The Physicians’ Health Study II enrolled 14,641 male physicians and randomized them to receive vitamin E and C supplements daily. Neither vitamin E nor vitamin C supplementation reduced the risk of prostate or other cancers.106
Early Detection
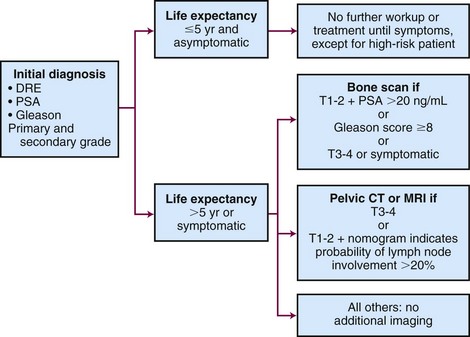
Considerable controversy surrounds the use of early detection programs for prostate cancer. Some argue that early detection efforts are too costly and will lead to the recognition of an increased number of clinically insignificant tumors, because autopsy studies demonstrate a high prevalence of incidental tumors in older men.9,10 Likewise, a study of prostate cancer discovered in organ donors found incidental prostate cancer in one third of men aged 60 to 69 and 46% in men older than age 70.107
Contributing further to the arguments against prostate cancer screening are the limited sensitivity and specificity of serum PSA level, DRE, and ultrasonography in diagnosing cancer. Although DRE has high specificity for prostate cancer, it has a low sensitivity profile and is not considered an effective detection tool on its own.108 In contemporary series,109 PSA testing with a threshold of 4.0 ng/mL has a sensitivity of only about 20%. Although the sensitivity of PSA testing could be improved by lowering the threshold value for all men, this would compromise the specificity and increase the detection of clinically insignificant cancers. Early detection strategies have also been criticized for exaggerated improvements in cancer-specific survival related to an early detection bias. In an early report the use of PSA resulted in a diagnostic lead time of approximately 5 years110,111 and longer in the detection of earlier-stage and lower-grade tumors.112 Data from the European Randomized Study of Screening for Prostate Cancer (ERSPC) and the SEER registry suggest the lead-time bias could range from 5.9 to 7.9 years.113 Included in the debate over prostate cancer screening are the risks of overdetection and overtreatment. Overdetection occurs when men are found to have disease that would never have remained silent and contributed no morbidity in their lifetime. Overtreatment occurs when an intervention plays no role in extending a patient’s life or preventing morbidity from the illness. The real challenge for clinicians involved in the management of prostate cancer is the identification of clinically significant disease.
Arguments in support of prostate cancer screening include the 36% reduction in prostate cancer deaths seen between 1990 and 2005.1 This trend began shortly after the introduction of PSA testing, and statistical models suggest that PSA testing contributed to this decline.114,115 Furthermore, PSA testing is responsible for the migration of prostate cancer diagnoses to earlier and more curable stages.1,116 These findings, as well as data from studies suggesting a survival benefit to treatment for early cancers, support a role for early detection and treatment. The Swedish randomized trial of surgery versus watchful waiting demonstrated an improvement in disease-specific and overall mortality in men undergoing radical prostatectomy (RP) for early-stage disease.117 In the United States, an observational cohort of 44,630 men from the SEER registry suggests a survival advantage with active treatment for low- and intermediate-risk prostate cancer in men aged 65 to 80 years.118
Early data from two large prospective randomized trials seeking to measure the benefit from prostate cancer screening contribute to this screening controversy.119,120 The U.S. Prostate, Lung, Colon and Ovary (PLCO) screening trial registered 76,693 men at 10 study centers to determine the impact of annual PSA determination and DRE on the cause-specific mortality for cancers in each of these organ sites.120 The primary exclusion criteria were a history of a PCLO cancer, current cancer therapy, and more than one PSA blood test in the 3 years prior to study enrollment. Subjects in the screening group were offered annual DRE for 4 years and annual PSA testing for 6 years. A PSA of more than 4.0 ng/mL or a DRE indicating nodularity or induration were considered suggestive of prostate cancer, and patients with these findings were advised to seek diagnostic evaluation. With a median follow-up of 11.5 years, although there were significantly more cancers diagnosed in the screened group there was no reduction in prostate cancer mortality.120 The ERSPC recruited 182,000 men between the ages of 50 and 74 years from seven European countries. The men were randomized to receive PSA screening once every 4 years or to a control group that did not get PSA screening. There was country to country variability in enrollment criteria and screening regimens, age of eligibility, case selection criteria, thresholds for PSA levels, and the inclusion of DRE in the screening assessments. PSA values as low as 3.0 ng/mL were considered abnormal and would prompt referral for further testing that differed between participating countries. Of 162,387 eligible men enrolled, 5,990 prostate cancers were diagnosed in the study group compared with 4307 (20% fewer) in the control group. With a median follow-up of 8.8 and 9.0 years in the screening and control groups, respectively, the adjusted rate ratio of prostate cancer death in the screened arm was 0.80 (95% confidence interval [CI], 0.65 to 0.98, p = .04).119 In the ERSPC trial the number of men who would need to be screened to prevent one prostate cancer death is 1,410. More importantly, 48 men would need to be treated to prevent each prostate cancer death.119
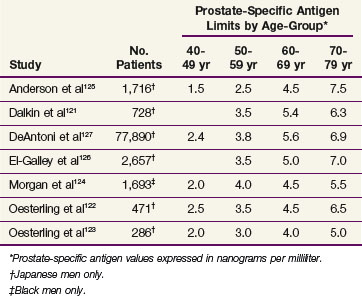
One criticism of published trials of PSA screening is the use of singular thresholds to prompt further diagnostic evaluation. Because serum PSA levels directly correlate with age in men without prostate cancer, several investigators sought to improve the diagnostic accuracy of PSA by defining the normal test range as a function of age and race121–127 (Table 51-2). In particular, DeAntoni and colleagues127 found that the mean PSA level was significantly different for successive decades of age and there was increased variability in PSA values with advancing age. It was this age-related variability that largely accounted for the phenomenon of age-specific reference ranges. El-Galley and associates126 studied the clinical impact of age-specific reference ranges in 2657 men who underwent prostatic biopsy. Analysis of the sensitivity, specificity, and positive predictive value profiles supported use of age-specific reference ranges because of increased detection sensitivity in younger men and increased specificity in older men.
TABLE 51-2 Upper Normal Age-Specific Limits for Prostate-Specific Antigen in Men without Prostate Cancer
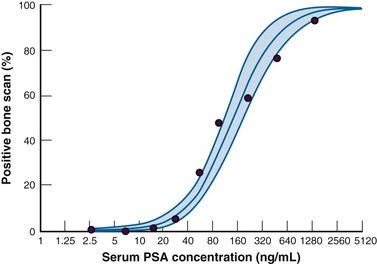
Measuring PSA velocity (PSAV), defined as the change in serum PSA over time, is another method to account for prostatic changes that occur during the aging process. In men who develop prostate cancer, an exponential increase in PSA values begins approximately 5 years before the diagnosis is established,128 and detecting a PSAV of 0.75 ng/mL/yr or more appears to be a sensitive means to distinguish these men from those without prostatic disease or those with benign prostatic hyperplasia.128–130 However, owing to interassay variability, only a PSA change exceeding +7.5% may be considered significant according to Kadmon and colleagues.131
Additional attempts to improve the screening accuracy of PSA measurements are based on the observation that serum PSA level depends on cancer volume, tumor differentiation, and the amount of benign prostatic tissue.132 To account for coexistent benign prostatic hyperplasia, the concept of PSA density (PSAD) was introduced by Benson and colleagues.133 PSAD is the total serum PSA value divided by the volume of the prostate gland, as determined by TRUS using the prolate ellipsoid formula (volume × length × width × height × 0.52). PSAD appears most useful for patients with a total serum PSA level in the range of 4 to 10 ng/mL, particularly when palpable prostatic abnormalities are absent. In this setting it is believed a PSAD of 0.15 ng/mL/cm3 or more best identifies men in whom prostatic biopsy should be considered.134,135 In other investigations, however, diagnostic accuracy was not enhanced by the PSAD compared with using the upper normal PSA concentration, defined as 4.0 ng/mL, as a cutoff point for early detection.136–138 Another PSA derivative, transition zone volume-adjusted PSA (PSAT) was introduced as an evolution in the PSAD concept for men with a serum PSA value in the indeterminate range (i.e., 4 to 10 ng/mL).139 PSAT is calculated by dividing the serum PSA value by the TRUS-determined transition zone volume and is based on the rationale that benign prostatic hyperplasia results exclusively from transition zone hyperplasia.
Because the proportion of PSA complexed to α1-antichymotrypsin is greater in patients with prostate cancer than in men with benign prostatic disease, the ratio of free-to-total (i.e., percent free) PSA will be lower in men with prostate cancer and may help discriminate between benign and malignant prostate conditions.140–144 Although the free-to-total PSA ratio can be applied to any serum PSA level, performing a free PSA determination improves the specificity for prostate cancer detection when the total serum PSA range is 3 to 10 ng/mL.142,145 To determine the optimal cutoff point that may warrant prostatic biopsy, various free-to-total PSA ratios were examined for their association with prostate cancer.144,145 A multicenter clinical performance study demonstrated that a free-to-total PSA ratio of less than 7% was highly suggestive of cancer whereas a free-to-total PSA ratio of above 25% was rarely associated with malignancy. In association with other study results, a diagnostic algorithm for the detection of early-stage prostate cancer based on the percent free PSA has been suggested.145 However, larger population-based trials must be conducted and the utility of prostate cancer screening must be ascertained before widespread application can be recommended.146
Screening Recommendations
The controversy in prostate cancer screening is evident when the current recommendations from various professional societies are reviewed. The American Cancer Society (ACS) previously recommended annual DRE and PSA testing in men older than 50 years of age with a life expectancy of at least 10 years.147 In 2009, after the publication of the interim results of the PLCO and ERSPC trials, the ACS no longer supports routine testing for prostate cancer. The ACS does support health care professionals discussing the potential benefits and limitations of early detection with an offer to test with annual PSA screening and DRE beginning at age 50 in men who are at average risk of prostate cancer with a life expectancy of more than 10 years.148 The American Urological Association (AUA)149 recommends PSA screening for well-informed men who wish to pursue early diagnosis beginning at age 50 and sooner for those men with a higher life-time risk (positive family history in a first-degree relative or African-American race). A baseline PSA value at age 40 above the median value (0.6 to 0.7 ng/mL) may identify a group of men with a significant risk of prostate cancer in the future.150,151 Based on the findings of the PLCO and ERSPC studies, the U.S. Preventative Services Task Force (USPSTF) concluded that for men younger than age 75 years the benefits of screening for prostate cancer are uncertain and the balance of benefits and harms cannot be determined. For men 75 years or older there is moderate certainty that the harms of screening for prostate cancer outweigh the benefits.152
Pathology and Pathways of Spread
Anatomy
The lymphatics of the prostate and the seminal vesicles were detailed through anatomic dissections described by Rouvière.153 Lymphatic drainage of the prostate originates in an extensive intraprostatic network that coalesces to form a subcapsular system and henceforth coalesces into a periprostatic lymphatic network and the four pedicles of the collecting trunks: the external iliac, hypogastric (or internal iliac), and posterior and inferior pedicles that terminate in the external iliac (including the obturator), presacral, and hypogastric lymph nodes, respectively. The lymphatics of the seminal vesicle originate in the mucous and muscular networks and give rise to the superficial plexus, which culminates in the lymph nodes of the external iliac system. Modern surgical series of extended pelvic lymph node dissections report involved lymph nodes commonly in the obturator fossa and external and internal iliac regions.154,155 These sites account for nearly 85% of lymph node metastases in prostate cancer.155 Another 15% of involved nodes can be identified in the presacral, paravesicular, and pararectal sites.155
Histology
The prostatic epithelium consists of three main histologic types: secretory, basal, and neuroendocrine cells. Despite having the lowest proliferative activity of the epithelial elements, secretory cells produce PSA, prostatic acid phosphatase (PAP), acid mucin, and other secretory products. The basal cells form a flattened layer of cells surmounting the basement membrane at the periphery of the glands. These cells possess the highest proliferative activity of the prostatic epithelium and are thought to act as stem or “reserve” cells that repopulate the secretory cell layer.156 Basal cells, selectively labeled with antibodies to high-molecular-weight keratin (e.g., 34βE12), are used to differentiate benign acinar processes from adenocarcinoma, which lacks a basal cell layer. Neuroendocrine cells are the least common epithelial cell type and are often not identified in routinely stained sections.
Pathology of Prostate Cancer
Prostatic Intraepithelial Neoplasia
PIN represents the putative precancerous end of the morphologic continuum of cellular proliferation within prostatic ducts, ductules, and acini.87 The histologic diagnosis requires both cytologic and architectural abnormalities, and lesions displaying some but not all such changes are considered atypical. PIN is designated as low grade or high grade, based on relative nuclear and nucleolar enlargement. The continuum culminating in high-grade PIN, and henceforth in early invasive cancer, is characterized by basal cell layer disruption, loss of markers of secretory differentiation, nuclear and nucleolar abnormalities, increased proliferative potential, and variations in DNA content (i.e., aneuploidy).157–159 Virtually all measures of phenotype and nuclear abnormality by computer-based image analysis reveal similarities between PIN and invasive cancer, in contrast to normal and hyperplastic epithelium.88 The recognition of PIN should serve as an indication for a thorough search for invasive cancer because of their close association.87
Occasionally, micropapillary or cribriform variants of PIN need to be differentiated from ductal adenocarcinoma. The glands in these ductal cancers are too crowded or have too many atypical cells that are negative for basal cell markers to be consistent with PIN.160
Histologic Appearance
The minimal criteria necessary to establish a diagnosis of prostate cancer in biopsy material are described by Algaba and associates.161 Approximately 99% of prostatic cancers are adenocarcinomas that have a microscopic appearance consisting of a proliferation of small acini with multiple patterns. Evaluation of small acinar proliferation of the prostate can be a diagnostic challenge, particularly when the suspicious focus is small. Diagnosis relies on a combination of architectural and cytologic findings that may be enhanced by ancillary studies such as immunohistochemistry. Architectural features include irregular glandular contours that deviate from the smoothly rounded contours of normal prostatic glands. The fact that glands are usually regular in shape is useful, because malignancies often exhibit an irregular and haphazard arrangement. Noting variations in gland size can also be of value, and comparison with adjacent normal prostatic glands may be useful. Atypical cytologic features are also important for the diagnosis of cancer, because nuclear and nucleolar enlargement is seen in the majority of cells and thus is suggestive.
The basal cell layer is critical to the diagnosis of adenocarcinoma, because an intact basal cell layer is present at the periphery of benign glands but entirely lacking in carcinoma. However, small foci of adenocarcinoma clustered around larger glands, which have an intact basal cell layer, may occasionally cause diagnostic difficulty, and it may be useful to employ monoclonal antibodies against high-molecular-weight keratin (HMWK) to evaluate the basal layer. α-Methylacyl-CoA racemase (AAMCR) is preferentially expressed in prostate cancer,162 and p63 is an important marker for basal cells163 that aids in the immunohistochemical diagnosis of prostate cancer. Other ancillary histologic features that may aid in diagnosis include acidic mucin in the acinar lumen, eosinophilic crystalloids, and microvascular invasion. Inflammation should be noted when evaluating small glandular proliferation, because reactive atypia may result or may be seen in the setting of prior RT, infarction, or other conditions.
Morphologic Variants
Several unusual morphologic variants (e.g., mucinous, prostatic duct, small cell, and transitional cell carcinomas) of prostatic carcinoma have been identified but account for less than 10% of cases. These tumors are usually associated with typical acinar adenocarcinoma and rarely occur in pure form. It is important to recognize the special variants and to understand the criteria that distinguish them from benign mimics. Although data are limited, the clinical behavior and prognostic significance of the morphologic variants may differ from typical prostatic adenocarcinoma.164
Grading
The Gleason system, based on a Veterans Administration study of more than 4000 patients, has become the global standard for grading prostate cancer (Fig. 51-1). The Gleason system is based on the degree of glandular differentiation and an overall pattern of tumor growth at relatively low microscopic magnification.165 Five patterns of growth are recognized and numbered in order of increasing aggressiveness. Because tumors may display variable histology, two patterns are recorded for each case: a primary or predominant pattern (Gleason 1 to 5) and a secondary or lesser pattern (Gleason 1 to 5). The Gleason score is the sum of the primary and secondary patterns and ranges from 2 to 10. If only one pattern is present, the primary and secondary patterns receive the same designation. Gleason166 noted that more than half of prostate cancers contain two or more patterns, and Aihara and colleagues167 found an average of 2.7 different Gleason grades in a series of prostatectomy specimens. Interobserver and intraobserver variability has been reported with the Gleason and other grading systems, and Gleason166 noted exact reproducibility of score in 50% of needle biopsies and a score of greater than 1 in 85% of cases.
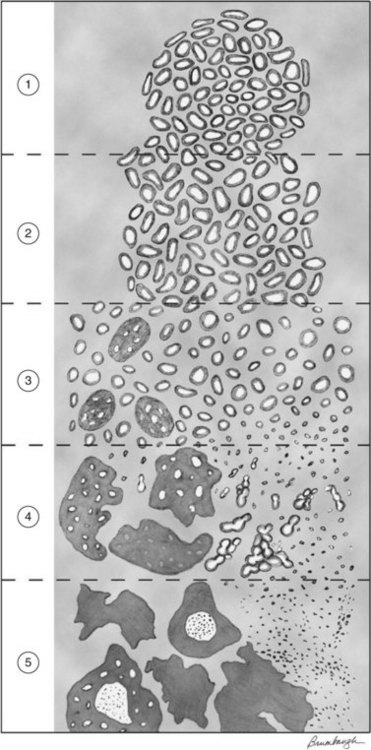
Figure 51-1 Schematic drawing of modified Gleason grading system.
From Epstein JI, et al: The 2005 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma, Am J Surg Pathol 29:1228-1242, 2005.
In 2005, the International Society of Urological Pathologists held a consensus conference to update the guidelines for Gleason scoring. The modifications were believed to be necessary owing to the evolving roles of PSA screening and TRUS-guided biopsy techniques on the diagnosis and identification of cancer in needle core specimens. For example, the Gleason scoring system predated the use of immunohistochemical staining for basal cells. Many of the grade 1 + 1 = 2 adenocarcinomas diagnosed in the past would now be considered adenosis owing to an intact basal layer. Furthermore, it was made clear that Gleason scores 3 or 4 should rarely be applied to TRUS-guided biopsies because these are rarely present in the peripheral zone but more typically found by transurethral resection of the prostate (TURP) including the transition zone. In addition, the finding that a cribriform pattern is more likely included as a Gleason grade 4 pattern has shifted some Gleason score 6 cases to Gleason score 7. These changes have resulted in a grade migration over the past decade that has paralleled the stage migration witnessed as a result of PSA screening.168
Although a correlation exists between the needle biopsy tumor grade and that found in the prostatectomy specimen, a higher grade (i.e., more poorly differentiated) is identified in approximately one third of cases.165,169,170 The correlation is strongest for the primary Gleason pattern, but the secondary pattern also provides useful predictive information, particularly when used to create the Gleason score.165 The incidences of undergrading and understaging are decreasing related to stage migration as well as the elimination of Gleason score 2 to 4 biopsy specimens.171 Whereas in the past downgrading of high-grade cancers was uncommon at RP, one third to half of high-grade cancers are now downgraded at surgery.172–176
Pathologic Staging
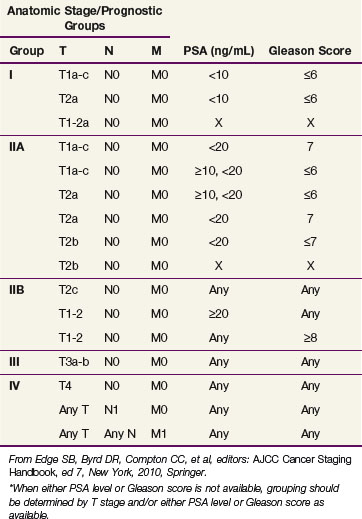
The criteria for assigning pathologic classification and stage were developed by the American Joint Commission on Cancer (AJCC)177 and the Union Internationale Contre le Cancer (UICC)178 (Tables 51-3 and 51-4). Currently, the seventh edition of the AJCC classification is in use.177 The details of handling, processing, and recording histologic findings of biopsy, radical prostatectomy, and nodal dissection tissue specimens are described in detail by the Association of Directors of Anatomic and Surgical Pathology.179 Thorough examination of RP specimens is critical for tumor classification and should allow accurate determination of pathologic stage, Gleason score, tumor volume, and surgical margin status. Pathologic stage is assessed by determining the presence or absence of extraprostatic extension (EPE), seminal vesicle invasion (SVI), and lymph node involvement (LNI).
TABLE 51-3 American Joint Commission on Cancer TNM Staging Classification
| Classification | Definition |
|---|---|
| Primary Tumor (T) | |
| Clinical | |
| Tx | Primary tumor cannot be assessed |
| T0 | No evidence of primary tumor |
| T1 | Clinically inapparent tumor neither palpable nor visible by imaging |
| T1a | Tumor incidental histologic finding in 5% or less of tissue resected |
| T1b | Tumor incidental histologic finding in more than 5% of tissue resected |
| T1c | Tumor identified by needle biopsy (e.g., because of elevated prostate-specific antigen level) |
| T2 | Tumor confined within prostate* |
| T2a | Tumor involves half of one lobe or less |
| T2b | Tumor involves more than half of one lobe but not both lobes |
| T2c | Tumor involves both lobes |
| T3 | Tumor extends through the prostate capsule† |
| T3a | Extracapsular extension (unilateral or bilateral) |
| T3b | Tumor invades seminal vesicle(s) |
| T4 | Tumor is fixed or invades adjacent structures other than seminal vesicles (e.g., external sphincter, rectum, bladder, levator muscles, and/or pelvic wall) |
| Pathologic (pT)‡ | |
| pT2 | Organ confined |
| pT2a | Unilateral, half of one side or less |
| pT2b | Unilateral, involving more than half of one side but not both sides |
| pT2c | Bilateral disease |
| pT3 | Extraprostatic extension |
| pT3a | Extraprostatic extension or microscopic invasion of bladder neck§ |
| pT3b | Seminal vesicle invasion |
| Regional Lymph Nodes (N) | |
| Clinical | |
| Nx | Regional lymph nodes were not assessed |
| N0 | No regional lymph node metastasis |
| N1 | Metastasis in regional lymph node(s) |
| Pathologic | |
| pNx | Regional nodes not sampled |
| pN0 | No positive regional nodes |
| pN1 | Metastases in regional node(s) |
| Distant Metastasis (M)‖ | |
| M0 | No distant metastasis |
| M1 | Distant metastasis |
| M1a | Nonregional lymph node(s) |
| M1b | Bone(s) |
| M1c | Other site(s) with or without bone disease |
* Tumor found in one or both lobes by needle biopsy, but not palpable or reliably visible by imaging, is classified as T1c.
† Invasion into the prostatic apex or into (but not beyond) the prostatic capsule is classified not as T3 but as T2.
‡ There is no pathologic T1 classification.
§ Positive surgical margin should be indicated by an R1 descriptor (residual microscopic disease).
‖ When more than one site of metastasis is present, the most advanced category is used. pM1c is most advanced.
From Edge SB, Byrd DR, Compton CC, et al, editors: AJCC Cancer Staging Handbook, ed 7, New York, 2010, Springer.
Extension of cancer beyond the edge or capsule of the prostate is diagnostic of EPE. The identification of tumor cells in fat is the only reliable way to diagnose EPE on needle biopsy specimens.179 In RP specimens, designation of EPE requires identification of tumor cells admixed or abutting fat or extending beyond the dense smooth muscle of the prostate to involve the loose connective tissue or less compact smooth muscle outside the prostate.179 Difficulty is occasionally encountered in the presence of a dense desmoplastic response in extraprostatic tissue, particularly in cases treated by androgen deprivation. Perineural invasion may exist in the absence of EPE. The anterior muscle is an uncommon site for EPE; this is observed only with large tumors involving the transition zone. Reporting EPE at the apex is challenging owing to the vague boundaries of the capsule in this region. Most patients (57% to 81%) with EPE have involved surgical margins,180 which predicts a worse prognosis.
SVI is a pathologic finding associated with an increased risk for disease relapse181,182 and occurs when tumor extends into the region where vas deferens and seminal vesicle converge at the prostatic base.183 Direct tumor growth from this location along the ejaculatory duct complex or through the prostatic capsule may result in SVI. This mechanism accounts for the observations that tumor volume is often greatest at the prostatic end of the seminal vesicle in the vicinity of the vas deferens,183 that involvement of both seminal vesicles is a common finding,182 and that isolated deposits of prostate cancer remote from the ejaculatory duct complex are observed in only one eighth of seminal vesicle specimens involved with tumor.182 Microscopic involvement of the bladder neck is not a pT4 designation. Patients with microscopic bladder neck invasion have a prognosis similar to those with SVI and should be designated as having stage pT3a disease.184
Postirradiation Histology
For 18 to 24 months after EBRT, and perhaps longer for interstitial implantation, needle biopsy of the prostate in the absence of clinical disease progression is of limited value owing to delayed and continuing tumor cell death.185 After this period, needle biopsy is associated with a lower level of diagnostic error that is minimized by obtaining multiple specimens.186–188 Histologic changes of prostatic radiation injury include acinar atrophy, shrinkage and distortion, marked cytologic abnormalities of the epithelium, basal cell hyperplasia, stromal fibrosis, and a decreased ratio of acini to stroma.185 Vascular sclerosis is also prominent and may involve small and large vessels. Although a definitive method to assess tumor viability after RT does not exist, PSA and PAP expression persists,189 suggesting that tumor cells capable of protein production retain the potential for cell division and spread.190,191 Keratin 34αE12 expression also persists and is often of value in separating treated adenocarcinoma from some of its mimics. Proliferative cell nuclear antigen (PCNA) may improve the sensitivity and specificity of a postirradiation biopsy. Negative PCNA staining in an otherwise positive biopsy predicts (83% to 97%) for eventual resolution of tumor, whereas positive PCNA staining is correlated with a local failure in 49% to 79% of cases.190 Grading of cancer after RT can be problematic. Squamous metaplasia is common, and the acini have nuclei that are large and hyperchromatic with conspicuous nucleoli. The tumor may show loss of architectural features suggestive of higher-grade disease. Cancer grading after RT has yielded conflicting results: some observers noted no difference in pretherapy grade or behavior,185,192 whereas others found increased grade.193 Therefore the consensus is that grading should not be relied on after RT.
Effects of Androgen Deprivation
Androgen deprivation of normal, hyperplastic, and dysplastic epithelial cells causes acceleration of programmed cell death (i.e., apoptosis), with fragmentation of tumor DNA, emergence of apoptotic bodies, and inhibition of cell growth. Furthermore, there is reduced nuclear size, loss of nucleoli, chromatin condensation, nuclear pyknosis, and cytoplasmic vacuolation.194,195 However, grading may be misleading and is to be avoided.195 PSA, PAP, and keratin 34αE12 immunohistochemistry are useful in identifying carcinoma, because PSA and PAP are retained and 34αE12 remains negative. Expression of neuroendocrine differentiation markers (e.g., chromogranin, neuron-specific enolase) remains unaltered, whereas proliferative activity, as measured by proliferating cell nuclear antigen immunoreactivity, is reduced after androgen deprivation.195 The PCP trial has provided important information about the impact of 5α-reductase inhibitors on the histologic appearance of prostate cancer. The initial report of the PCP trial suggested that the rate of high-grade tumors was increased with the use of finasteride. Subsequent reports suggest that the drug may have reduced the volume of benign prostate tissue and low-grade cancer, thereby increasing the likelihood of identifying higher-grade disease.196
Biologic Characteristics
Molecular Biology
Increasing focus is being given to the role of the androgen receptor (AR) in the tumorigenesis and prognosis of prostate cancer.197 Androgens, along with multiple co-regulatory factors, activate the AR, leading to transcription of AR target genes promoting the growth of normal and neoplastic prostate tissue. The ETS family of genes includes transcription factors that are involved in a variety of functions, including cellular differentiation and cell cycle control. Several ETS factors have been implicated in carcinogenesis through gene rearrangements. Tomlins and associates198 have identified recurrent gene fusions of two ETS transcription factors, ERG and ETV1, to the TMPRSS2 gene. In their initial series the TMPRSS2-ERG gene rearrangement was identified in 47% of clinically localized prostate cancers. TMPRSS2 is expressed in normal luminal epithelial and neoplastic prostate tissue and is strongly induced by androgen in androgen-sensitive prostate cell lines. The TMPRSS2-ERG fusion gene can be detected in a proportion of high-grade PIN lesions, and this molecular rearrangement may be an early event that precedes chromosome-level alterations in prostate carcinogenesis.199 The importance of the TMPRSS2-ERG fusion as an early event in the development of prostate cancer is supported by the homogeneous distribution of the fusion gene in a prostate cancer, the absence of the fusion in benign prostate tissue, and the association of TMPRSS2-ERG–positive cancers associated with the same fusion in high-grade PIN lesions.200 Mutation or changes in AR expression may account for the development of androgen-independent prostate cancer. ERG overexpression, mediated through TMPRSS2-ERG fusion, contributes to development of androgen independence through disruption of androgen receptor signaling. Recently, Yu and colleagues201 found that ERG inhibits AR expression and activity and induces repressive epigenetic programs that contribute to androgen resistance and cancer progression.
Predictive Factors
Pathologic Endpoints
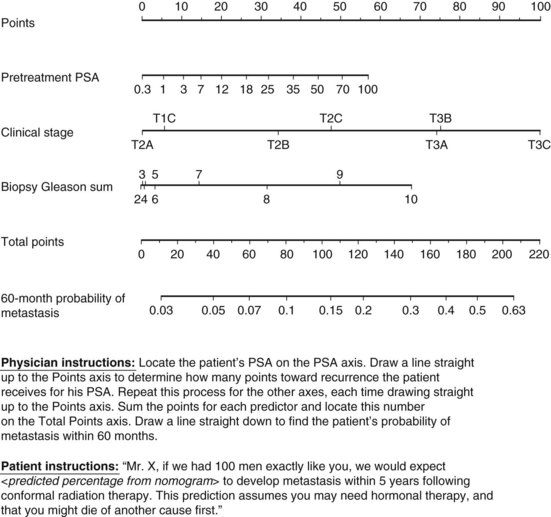
The therapeutic efficacy of RT and RP depends on the accurate determination of tumor burden and the risk of subclinical disease. Despite the clinical impression of disease confined to the prostate gland, occult tumor extension through the prostatic capsule or involvement of seminal vesicle(s) or regional lymph nodes is common at the time of diagnosis. Compared with the surgical-pathologic stage, the clinical staging of prostate cancer frequently underestimates disease extent, and many patients are “upstaged” after RP with pelvic node dissection.202 Among patients thought to have prostate-confined disease by clinical assessment, variable proportions of patients will be found to have extracapsular extension, SVI, or LNI at the time of prostatectomy with the likelihood of these findings correlating with pretreatment characteristics such as T stage, Gleason grade, and PSA level203 (Web-Only Table 51-1). Because these findings may have considerable importance for selection of an appropriate therapeutic strategy (e.g., radioactive seed implantation or RP vs. EBRT), several investigators studied the association between readily available pretherapy factors and the pathologic endpoints of EPE, SVI, and pelvic LNI.
WEB-ONLY TABLE 51-1 Nomograms Predicting Pathologic Stage of Prostate Cancer According to Clinical Stage (TNM), PSA Level, and Gleason Score

Extraprostatic Extension
EPE is a pathologic feature that is associated with a worse outcome after RP.204–208 Several pretherapy tumor-related factors are associated with EPE, and these include clinical tumor stage,202 pretherapy serum PSA value,202 prostatic biopsy tumor grade,202 microvessel density (i.e., neovascularity),209 and percent of biopsy containing cancer.209 Although any of these factors may be individually used to estimate the likelihood for EPE, the accuracy of such estimates is enhanced by combining those factors that independently contribute to the predictive model.202,203,210 The extent of EPE is associated with the risk of clinical failure. Epstein and associates206 reported significantly worse rates of progression with established extracapsular extension compared with pathology specimens that had just a few malignant glands outside the capsule. Wheeler and colleagues205 defined extensive EPE as tumor extending more than one high-power field outside the capsule on more than two separate sections. Both focal and extensive EPE were significantly associated with other adverse findings such as SVI and lymph node metastases as well as a lower progression-free probability (73% and 42%) compared with no progression in the absence of any capsular invasion.205
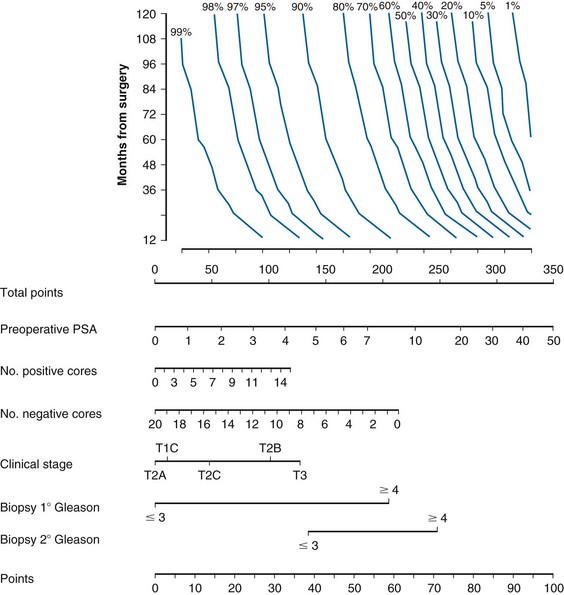
In the PSA era, Partin and colleagues211 were among the first to use multivariate statistical analysis to identify pretherapy variables associated with EPE in patients who underwent RP and demonstrated that clinical tumor stage, Gleason score of the diagnostic biopsy, and serum PSA level independently correlate with this finding. The combination of these factors was used to develop probability estimates, which were displayed as a nomogram and later incorporated into clinical practice guidelines for patient management. Kattan and associates210 conducted a separate validation study that confirmed that the nomogram discriminated well between men with organ-confined disease and those in whom the disease extended beyond the prostate gland. The updated Partin nomogram may be useful for predicting the probability of organ-confined status,203 but any estimation should consider the confidence of the prediction, as indicated by the associated 95% confidence intervals, for the combination of factors relevant to the new patient under consideration.
Seminal Vesicle Invasion
SVI is a pathologic finding associated with an increased risk for disease relapse.181,182,204 The adverse prognostic impact of SVI is exceeded only by lymph node metastases.212 Although TRUS with needle biopsy of the seminal vesicles213,214 may identify SVI, conventional CT imaging techniques are not considered reliable methods for evaluating disease extent before therapeutic intervention. Recent advances in MRI with endorectal coils, 3-T field strength, and imaging parameters such as diffusion weighting and dynamic contrast enhancement allow identification of SVI or EPE before treatment. Several investigators have correlated pretherapy tumor-related factors with SVI as a means to identify patients with a high likelihood for this condition.* These studies demonstrate that clinical tumor stage, tumor grade of the diagnostic biopsy specimen, percent cancer in the biopsy, and serum PSA level are associated with SVI in a statistically significant manner. MRI may improve the predictive power of the clinical parameters.216,217
Although serum PSA level was the best single predictor, Partin and colleagues202 noted that clinical tumor stage and Gleason score contributed to SVI risk estimation and so were among the first to combine these factors to develop nomograms for clinical use. Thereafter, Diaz and associates218 used this information to create a prediction equation. However, Kattan and colleagues210 applied the original SVI nomogram of Partin and colleagues202 to an independent dataset and concluded that the nomogram was suspect, particularly in the areas of high predicted probability, and ill suited to clinical application.
In response to Kattan and colleagues, Partin and colleagues219 performed a multi-institutional analysis in which updated nomograms were developed and validated. Although this provides useful information, patients were only assigned to the most advanced and mutually exclusive pathologic category based on the findings of the RP and pelvic lymph node dissection (PLND) specimens for the analysis. Furthermore, all these data are derived from patients selected for RP and may underestimate the true extent of disease in men not considered suitable for surgery. Although these nomograms may be appropriate for the surgical candidate, the risk of SVI may be underestimated with this approach, and caution is necessary in using the revised nomogram for other purposes (e.g., RT target volume definition).
In contrast, Pisansky and associates220 used a split-sample developmental and validation method based on a stepwise modeling process that identified clinical tumor stage (T1 to T2a, T2b to T2c, and T3a to T3b), diagnostic Gleason primary grade (1 to 2, 3, 4 to 5), and pretherapy serum PSA value as independent predictors. This methodology avoided the risk of constructing an overly optimistic model and allowed a reliable means to estimate the probability for SVI in the patient with newly diagnosed clinical stage T1 to T3b, N0/X prostate cancer.
Nodal Involvement
Clinical tumor stage, prostatic biopsy tumor grade, and serum PSA level are also associated with pelvic LNI, and a combination of these factors may be used to predict the probability of LNI in the patient with a new diagnosis of clinically localized prostate cancer.202,214,221,222 Partin and colleagues202,211 incorporated these factors in an LNI predictive nomogram. Kattan and associates210 were not able to validate the LNI nomogram in a separate group of patients and suggested that the nomograms were not broadly applicable. As a result, Partin and colleagues219 used a different mathematical method to analyze a larger and more diverse study population and provided revised nomograms to estimate the probability of LNI.202,203
In addition to the nomogram model to predict LNI, other investigators have used probability plots that combine the independent predictors of clinical tumor stage, Gleason primary grade, and serum PSA level.221 The split sample method was then used to validate these predictions, similar to the work related to the prediction of seminal vesicle involvement.220
Clinical Endpoints
Patient-Related Factors
Patient age had been identified as a prognostic factor in older series of patients managed with surgery, RT, or expectant management.223–227 These reports have offered conflicting results, suggesting that younger patients did better with expectant management in some series224,228 whereas older patients fared worse in others.223 Similarly, some series suggest a worse prognosis with surgery or RT for younger patients,225,227,229–231 whereas others do not.232–238 A meta-analysis of 34 studies with more than 27,000 patients did not show an association between age and outcome in the PSA era.239 Parker and associates239 speculated that PSA screening may have eliminated the impact of age on prognosis owing to less delay in diagnosis, improved measures of outcome, a screening-related age-specific lead-time bias, and an interaction between age and tumor grade.
Racial and ethnic disparities for the outcome of patients after diagnosis and treatment exist for many malignancies, including prostate cancer. African Americans are more likely to be diagnosed with advanced stage and higher-grade prostate cancer than whites or non–African-American minorities.1,138 The survival rate of African-American men is inferior to that of non-Hispanic white men with localized prostate cancer.42,138 The reason for these disparities is unclear and may be related to variations in genetics, nutrition, socioeconomic status, clinical and surgical staging procedures applied to different races, access to care, or inherent differences in cancer biology. SEER data have shown a difference in the rate of RP and surgical staging being performed in African-American men with localized prostate cancer.240 In a secondary analysis of Radiation Therapy Oncology Group (RTOG) clinical trial data in which eligibility and treatment guidelines were prescribed according to study specifications, Roach and colleagues241 reported that the observed inferior survival of black Americans was likely related to their presentation with more advanced disease and not due to inherent biologic differences in their cancer. In a review of Southwest Oncology Group (SWOG) trials,242 the survival of African-American men with advanced-stage prostate cancer was worse than all other patients, even when corrected for differences in socioeconomic status. Two other studies243,244 suggest that even when effects of age, presenting PSA value, Gleason score, and stage are controlled, a difference in progression-free survival persists. Moul and colleagues245 demonstrated differences in outcome in men treated with RP in an “equal access” medical system (i.e., the U.S. military). There is recent evidence for differences in presentation and outcome for Hispanic men with localized prostate cancer,246 but few to no reliable estimates exist for the Native American/Native Alaskan or Asian/Pacific Islander populations. Therefore it appears that some evidence suggestive of biologic differences in patient subgroups is emerging.
The presence of coexistent medical ailments (e.g., ischemic heart disease) in a patient with prostate cancer may have a significant impact on treatment decisions. These comorbidities may determine whether a patient is a candidate for an aggressive surgical intervention or whether the patient should be treated at all. These non–prostate cancer–related treatment selection criteria have a profound impact on outcome reporting that are unrelated to the effectiveness of any specific treatment strategy. Several instruments are available to quantify the severity of comorbidity. Albertsen and colleagues247 applied three comorbidity assessment instruments (i.e., the Kaplan-Feinstein and Charlson indices and the index of coexistent disease) to a cohort of men with localized prostate cancer to assess the impact of comorbidity on patient survival. Although the index of coexistent disease had a marginally better predictive value, each instrument was predictive of age-adjusted survival when corrected for the effect of Gleason score. The risk of death from causes other than prostate cancer (i.e., competing causes) strongly correlated with the severity of comorbidity, and a weak association with prostate cancer-related (i.e., cause-specific) mortality was also noted. The selection of a particular management approach based on overall health status has important implications in interpreting and comparing outcomes, because overall and, perhaps, cause-specific survival estimates will be influenced.248–251 Additional research is essential to quantify the confounding effect of comorbidity in the setting of prostate cancer outcomes reporting. A nomogram has been described that may allow estimation of life expectancy and aid physicians and patients in choosing treatment strategies.249
Tumor Stage
The anatomic extent of prostate cancer is an important tumor-related factor predictive of patient outcome. Studies consistently demonstrate the association of primary tumor stage with several endpoints in patients with localized disease. Cause-specific and overall survival are directly related to the extent of the primary tumor in both RT252–254 and expectant management224,255,256 series. The probability of local tumor control257 and the risk of clinical,253,258 metastatic,257,259 and biochemical relapse233,235,236,254 are influenced in a similar manner. Increasingly, tumor stage is combined with other prognostic factors (e.g., initial PSA, tumor grade) to define prognostic groups and report outcomes. Despite this, the tumor stage remains an important factor in selecting optimal local therapy.
Surgical Margins
Modifications to surgical technique have resulted in an improvement in the rate of positive margins with an open prostatectomy technique260; however, even with laparoscopic and robot-assisted procedures the rates of positive margins remain as high as 50% and 28%, respectively.261 In a systematic review of 37 comparative series, Ficarra and associates261 concluded that laparoscopic and robot-assisted laparoscopic prostatectomy had comparable positive margin rates as open prostatectomy. Positive margins are typically located at the apex (48%), rectal and lateral surfaces (24%), bladder neck (15%), and posterior pedicles (10%).180,262 The presence of a positive surgical margin increases the likelihood of biochemical recurrence, local recurrence, and the need for salvage therapies.263–265 In a series of 11,729 men undergoing RP between 1990 and 2006, Boorjian and associates263 reported positive surgical margins in 31% of cases. Patients with positive margins had a biochemical relapse free survival (RFS) of only 56% compared with 77% (p <.0001) and a local RFS of 89% compared with 95% (p <.0001) in patients with negative margins. The patients with positive surgical margins also had a significantly worse systemic progression-free survival (PFS), cancer-specific survival (CSS) and overall survival (OS). In a pooled series of 7816 patients from eight academic urology centers, the presence of a positive surgical margin increased the risk of biochemical recurrence by 3.7-fold.265
It has been suggested that an apical positive margin may actually represent an artifact in the processing of prostatectomy specimens and it may not carry as great a prognostic significance as margins involved elsewhere.266,267 Analyses of randomized trials evaluating the role of adjuvant RT failed to show any differences in outcome by site of surgical margin involvement.268,269
The extent of surgical margin involvement has been reported to carry prognostic significance. Van Oort and colleagues270 reported a higher rate of biochemical relapse in patients when the length of a positive surgical margin was more than 10 mm compared with smaller margins. In a series from the Mayo Clinic, Kausik and colleagues271 did not detect a difference in relapse rates in patients with EPE and one versus more than one positive margins. In a series of 2,468 patients, Lake and associates reported that even focally positive margins (≤3 mm) in patients with organ-confined disease conferred a significant decrease in disease-free survival (DFS).272 The 10-year DFS for patients with organ-confined disease was 84%, 64%, and 38% with negative, focally positive, and extensively positive margins (>3 mm), respectively (p <.0001).272 These data indicate strongly that the presence of a positive surgical margin has a profound impact on patient prognosis and that these patients should be considered for adjuvant therapy.
Nodal Involvement
Tumor spread to regional lymph nodes is associated with a particularly poor outcome after RT or surgical management alone.273–278 The prognostic significance of the number of involved nodes was identified in some.278–281 In a series of more than 10,000 patients treated with RP, Boorjian and colleagues278 reported that the risk of systemic cancer progression was threefold greater with one positive node and another twofold greater with two or more positive nodes. One positive node increased the risk of cancer-related death fourfold, and when two or more nodes were involved the risk was another twofold higher. Barzell and colleagues282 quantified nodal cancer volume and evaluated its association with disease outcome. Schmid and associates280 found that patients with micrometastasis (i.e., ≤5 mm in largest dimension) had significantly better PFS and OS compared with patients with more extensive nodal involvement. In contrast, Srignoli and colleagues283 found no difference in the risk of subsequent distant metastasis. Cheng and colleagues284 reported that the risk of distant metastasis in patients with regional nodal involvement was directly proportional to nodal cancer volume and that it was the best predictor of 5-year metastasis-free survival. Extranodal extension of disease, as well as Gleason score of the primary tumor, may also be significant predictors of CSS.285 In a series of 102 patients with pathologically involved lymph nodes at RP, Fleischmann and colleagues286 reported inferior outcome if extranodal extension was present, but this was strongly correlated to tumor volume and it was not an independent prognostic factor.
Tumor Grade
Long-term case series of EBRT,235,253,287 brachytherapy,288–290 RP,291–294 and expectant management224,255 uniformly identify tumor grade as a strong predictor of disease relapse and mortality in clinically localized prostate cancer. Studies consistently demonstrate that patients with a poorly differentiated tumor (i.e., grade 4-5 or Gleason score ≥7) have an increased risk of metastatic disease progression and reduced OS and disease-specific survival (DSS).255,257,295 For the patient with clinical T1 or T2 disease, tumor grade has greater predictive value than does the distinction between T1 and T2 categories.* The association of tumor grade with metastatic relapse235,257,258 and OS257 is preserved when pretherapy serum PSA level is considered. Although tumor grade is also associated with DFS and freedom from clinically evident disease relapse,225,235,296,297 its impact on local tumor control after EBRT is less certain, because some reports noted an association252,257 whereas others did not.296 PSA level (i.e., biochemical control) after local therapy is impacted by tumor grade. Multivariate analysis of pretherapy tumor-related factors in RT235,236,257 and surgical293,295,298 series confirms the significance of tumor grade as a predictor for biochemical relapse.
The relative amount of high-grade cancer in biopsy and surgical specimens predicts for clinical and biochemical outcomes.299,300 In patients with Gleason score 7, a primary component of Gleason grade 4 (4 + 3) has been associated with a higher rate of metastatic, biochemical, and clinical progression than a primary Gleason grade 3 component (3 + 4) in both surgical301–303 and RT series.304 However, whereas some series demonstrate that the presence of the higher primary Gleason grade is correlated with other adverse prognostic factors such as tumor volume, SVI, or positive surgical margins,303 it is not independently associated with a worse outcome in some surgical303 or RT series.305,306 Increasingly, it is being recognized that tertiary patterns of high-grade adenocarcinoma (Gleason grades 4 or 5) negatively impact prognosis.300,307–309 Patients with cancers with a Gleason score of 7 with any higher-grade tertiary pattern have a prognosis after RP that is indistinguishable from patients with a Gleason score of 8.307
Prostate-Specific Antigen
An abundant literature attests to the importance of the pretherapy serum PSA level as an independent and highly significant predictor of therapeutic outcome.† In general, the pretreatment PSA level is a measure of tumor volume, although one must also consider the relative PSA contribution from the normal prostate tissue and the fact that some tumors, especially the poorly differentiated ones, may not produce PSA commensurate with the tumor burden. In assessment of post-RT outcome, Landmann and Hunig312 were the first to draw attention to this association, which was confirmed by the detailed statistical analysis of larger study populations.‡ Furthermore, the prognostic significance of PSA is not limited to patients treated with RT, because similar observations appear in surgical reports.§
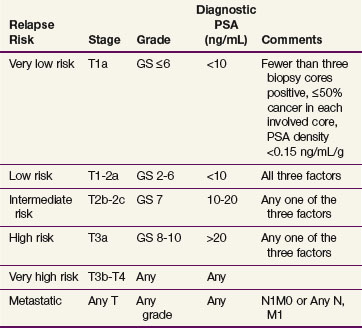
Pretreatment PSA is one of the strongest individual predictors of biochemical relapse.* The effect of pretreatment PSA on outcome increases in a continuous fashion.236,257 Zagars and colleagues257 described a relationship between pretherapy PSA and clinical and/or biochemical relapse after RT. In this series there was a significant difference in the risk of relapse for pretherapy PSA categories of 4.0 or less, 4.1 to 10.0, 10.1 to 20.0, and more than 20 ng/mL, which was 16%, 34%, 51%, and 89% at 6-year follow-up, respectively. D’Amico and associates318 described a risk grouping based on pretreatment PSA, Gleason score, and clinical stage. A slightly modified risk group category has been adopted by the National Comprehensive Center Network (NCCN) and is used for reporting outcomes and to assist in treatment decision making319 (Table 51-5). Most institutional and other large series now report outcomes by risk stratification group.
Although numerous studies relate pretherapy PSA level to biochemical relapse, reports using clinical endpoints are not as abundant.257,258,320 Nonetheless, Crook and colleagues321 affirmed that local tumor recurrence correlated with pretreatment PSA in patients in whom serial post-RT prostatic biopsies were performed. Zagars and associates257 reported a similar finding in a study population in whom post-RT prostatic biopsies were generally obtained to identify an otherwise unknown source for biochemical relapse. Studies with long enough follow-ups to document clinical disease relapse (local and distant) are now being reported and correlate clinical endpoints with pretreatment PSA levels.235,291,297
In an effort to improve the prognostic accuracy of pretherapy serum PSA, many investigators have examined derivatives of the serum PSA, including PSAD (a correction for benign prostate gland volume) or PSA kinetics (velocity of rise or doubling time). Zentner and associates320 evaluated PSAD, that is, the PSA value divided by prostatic volume, in patients treated with EBRT. Patients with a PSAD of 0.3 ng/mL/cm3 or less had a 100% DFS probability at 30 months, whereas the estimate for patients with a PSAD of more than 0.3 ng/cm3 was significantly worse (62%). Others have not shown this association, and PSAD does not appear to add significantly to the utility of serum PSA level as a predictor of outcome after EBRT, and it is not routinely used as a prognostic factor.322–324
The rate of change of the PSA over time has important diagnostic and prognostic implications. Carter and colleagues reviewed the outcomes of men participating in the Baltimore Longitudinal Study of Aging for up to 39 years. PSA velocity measured 10 to 15 years before a diagnosis of prostate cancer was correlated with prostate CSS 25 years later.325 The prostate CSS was 92% in men with a PSA velocity of 0.35 ng/mL/yr or less compared with 54% with a PSA velocity of more than 0.35 ng/mL/yr (p <.001). D’Amico and associates reported that the risk of prostate cancer death in men undergoing RP326 or EBRT327 for localized prostate cancer was higher if the PSA velocity was more than 2.0 ng/mL/yr.
A number of PSADT calculators are available for use on handheld computing devices or from websites (www.nomograms.org). Klotz and colleagues256 have proposed that PSADT be used as a factor in the selection of patients for active surveillance. A PSADT of 3 years or less is associated with an 8.5-fold greater risk of biochemical failure after definitive treatment than longer PSADT. Hanks and colleagues328 used PSADT as a pretherapy predictive factor in patients treated with EBRT and found that a PSADT of less than 12 months adversely affected therapeutic outcome. Most reports have focused on post-treatment PSADT to predict the clinical outcome of patients with biochemical relapse after RT237,296,329–333 or surgery.332–335 Although there is considerable individual variation, a local tumor recurrence tends to correlate with a longer PSADT (median, 12 to 14 months), whereas a shorter PSADT (median, 4 to 6 months) is observed for metastatic relapse.314,336 The post-treatment PSADT in patients who have failed treatment with RT is similar to that observed in surgical patients.337
After RT, residual prostatic epithelial cells may produce PSA under androgenic stimulation and serum levels of PSA may be detected even in the absence of prostate cancer.338 The post-RT nadir PSA (nPSA) level is considered to be a predictive variable similar to pretherapy factors.235,331,339–341 The nPSA correlates with pretherapy PSA level, tumor grade, and clinical tumor stage.339,342 Nonetheless, nPSA is an independent predictor associated with biochemical and/or clinical relapse,314,339,340,342,343 and with local and metastatic tumor control.320,331,340 Alcantara and associates344 reported a 10-year distant metastasis rate of 4% versus 19% in men with a 12-month nPSA of less than or equal to 2 ng/mL versus greater than 2 ng/mL (p <.0001). Zelefsky and colleagues340 reported the incidence of distant metastases was 10.3% and 17.5% at 10 years in men who had a 2-year nadir value of less than or equal to 1.5 ng/mL versus greater than 1.5 ng/m (p <.002). Although nadir level can be used to predict the relative likelihood of failure after radiation, there is no absolute level that can determine success or failure after this therapy. Kuban and colleagues235 in a multi-institutional study of 4839 patients treated by EBRT for prostate cancer demonstrated that progressively lower nadir levels were associated with incremental improvement in PSA disease-free survival.
PSA Failure Definitions after Local Therapy
PSA failure definitions after surgery or RT must use different criteria because of the inherent effect on the prostate by each of these modalities. Because the goal of RP is to remove all prostatic tissue, a detectable PSA level after surgery has been correlated with outcome. Although an undetectable PSA level is desirable, the possibility of residual benign glands capable of producing low levels of serum PSA has been suggested. The PSA level is very low, in the 0.1 to 0.4 ng/mL range, and is representative of a small amount of prostate tissue left behind.315,345,346 Although these levels have been correlated to postoperative relapse, further proof of progressive disease is evidenced by a subsequent rising PSA value. Cutpoints of 0.2 ng/mL and 0.4 ng/mL have been proposed to report biochemical failure after surgery. In each case, patients with PSA values greater than the cutpoint have higher rates of subsequent rising PSA or clinical failure.315,345,346 The AUA has recommended defining biochemical recurrence as an initial serum PSA greater than or equal to 0.2 ng/mL with a confirmatory PSA greater than 0.2 ng/mL.347 For irradiated patients, a rising PSA level is the criterion for relapse that is often used, because a very low target PSA value, especially soon after treatment, is not very specific in predicting clinical failure.348,349 This is because the prostate remains intact in irradiated patients and it takes a median of nearly 2 years for the PSA value to reach the nadir.350 Simultaneous with this gradual declining process, a PSA “bounce” can occur, mimicking failure because the “bounce” is a PSA rise but one secondary to a benign cause, likely prostatitis.351–353 Therefore the definition of PSA relapse after RT must take both of these factors into account to minimize false-positive failure reporting.
The American Society of Radiation Oncology (ASTRO) held a Consensus Conference in 1996 for the purpose of devising a PSA-based failure definition after EBRT that would standardize outcome reporting. The ASTRO definition for biochemical failure is characterized by three consecutive PSA rises with the time of failure backdated to halfway between the PSA nadir and the first PSA rise.354 After several additional years’ experience in the PSA era and more sophisticated statistical analysis, criticisms of the ASTRO definition were brought forward and alternative definitions that were more sensitive and specific were advocated.348,355 Problems with the original PSA definition included biases associated with back dating and the time necessary to collect three consecutive rises. The original ASTRO consensus also did not consider the use of neoadjuvant or adjuvant hormone therapy, nor did it include data from alternative radiation techniques such as permanent or temporary brachytherapy.
A second Consensus Conference sponsored by both ASTRO and RTOG was held in 2005 at which several investigators presented analyses with more long-term follow-up than was available at the first conference.356 A panel representing RT and surgical specialties as well as clinical trials groups formulated a revised consensus statement, commonly referred to as the “Phoenix” or nadir+2 definition. The revised definition states that a PSA rise of 2 ng/mL or more above the nadir PSA (defined as the lowest PSA achieved) is a biochemical failure. The date of failure is the date at which that PSA value is determined “at call” and not backdated.356 Patients not meeting these criteria but who undergo salvage therapy such as androgen deprivation, prostatectomy, brachytherapy, or cryosurgery are considered as having treatment failure at the time of a positive biopsy or when salvage therapy is initiated. Based on clinical outcomes reported by Kestin and colleagues,355 the original ASTRO definition had a sensitivity of 51%, specificity of 78%, positive predictive value of 31%, and negative predictive value of 88%. The Phoenix definition has a sensitivity of 64%, specificity of 78%, positive predictive value of 36%, and negative predictive value of 92%.356 Another important point stressed at both consensus conferences is the minimum follow-up necessary to use these definitions. The stated date of control should not be more than 2 years short of the median follow-up time. For example, to report a 5-year biochemical control rate, the median follow-up should be at least 7 years. The original ASTRO definition still has important utility because it can be used to compare results to prior publications. The consensus panel encouraged reporting results using both the Phoenix and ASTRO definitions.356
Predictive Models
Risk groupings are relatively easy to use and are based on a limited number of clinical variables that allow categorization of patients by their likelihood of recurrence. One of the most commonly used risk grouping was described by D’Amico and associates to estimate the biochemical outcome of men after RP, EBRT, or interstitial brachytherapy.318 Patients are classified into three categories based on their presenting PSA, Gleason score, and clinical stage: low risk (stage T1c or T2a or PSA ≤10 ng/mL or Gleason score ≤6), intermediate risk (stage T2b or Gleason score = 7 or PSA >10 and ≤20 ng/mL) or high risk (stage ≥T2c or PSA >20 ng/mL or Gleason score ≥8). The NCCN has adopted a similar classification for treatment recommendations except stages T2b to T2c are considered intermediate risk and stages T3a to T4 represent high risk.319 The University of California, San Francisco, group has described a risk grouping model that includes the percentage of positive biopsy cores and patient age. The Cancer of the Prostate Risk Assessment (CAPRA) score is calculated by assigning points to increasing risk factors. Unlike the groupings of D’Amico and associates, the PSA level is divided into five discrete categories. The CAPRA score may range from 0 to 10, although 0 to 1 and 7 to 10 groups are often combined. The CAPRA score has been validated as a useful tool to predict outcomes after RP.357 One problem with risk groupings is that each category may contain a heterogeneous population and continuous variables are converted into categorical variables, thereby removing the information about the actual value. It also assumes each variable carries equal weight in contributing to patient outcome. Probability tables have been used to estimate pathologic findings after RP and are commonly used in multidisciplinary settings to estimate the risks of extracapsular extension, SVI, or LNI. Partin and associates202 popularized the use of probability tables based on a series of more than 5000 men undergoing RP at Johns Hopkins University. The “Partin tables” combine serum PSA, clinical stage, and biopsy Gleason score to predict the pathologic stage in four groups: organ-confined disease, established extracapsular extension, SVI, or LNI. The outcomes are considered mutually exclusive so the tables tend to underestimate the risk of extracapsular extension or SVI because a significant proportion of patients with LNI will also have these findings.202,203 Nomograms are graphic calculation devices that incorporate at least two continuous or categorical variables. Risk points are determined by identifying the value of variables of interest on axes corresponding to a given prognostic factor. The risk points are then summed and used to estimate the combined effect of the predictors on the expected outcome. Nomograms often use Cox proportional hazards or modified logistic regression to correct for variable impact of incremental changes across a broad range of values to predict endpoints.358 Nomograms can be adapted to web-based or handheld personal data assistant applications to quickly and conveniently calculate predicted outcomes (see www.nomograms.org). Numerous nomograms have been developed to calculate the risk of positive biopsy results on screening examinations, estimate pathologic stage, and predict biochemical control before surgery,359 EBRT,360 or brachytherapy361 as well as the risk of prostate cancer progression after local therapy.362,363 Examples of nomograms for predicting biochemical DFS after RP and EBRT are presented in Figures 51-2 and 51-3.
Clinical Manifestations, Patient Evaluation, and Staging
Clinical Symptoms and Signs
Most early-stage prostate cancer patients are asymptomatic because most cancers arise in the peripheral zone of the gland. However, a tumor may arise in the transition zone or may extend from a peripheral location to encroach on the prostatic urethra and produce obstructive symptoms. The resulting symptoms, commonly referred to as prostatism, initially consist of urinary hesitancy, diminished force of stream, intermittency, and a sense of incomplete voiding. Thereafter, the bladder detrusor muscle may lose compliance and irritative symptoms such as urgency, frequency, nocturia, dysuria, and incontinence may develop. Although acute urinary retention may ensue, it is no more likely to occur in the patient with prostate cancer than in the general population.364,365 Indeed, the constellation of obstructive symptoms now referred to as lower urinary tract symptoms are common in the elderly male population366 and are often associated with benign prostatic hyperplasia or other mechanical or functional disorders of the urinary bladder. Hematuria, which may result from tumor involvement of the prostatic urethra or bladder trigone, and hematospermia are uncommon signs of prostate cancer and should prompt further investigation to identify their cause.
The pelvic, axial, and proximal regions of the appendicular skeleton are the most common sites of metastatic disease spread. Based on the distribution of uptake on radionuclide bone scan, the vertebral column (74%), ribs (70%), pelvis (60%), femora (44%), and shoulder girdle (41%) are the prevailing sites of involvement in patients with osseous metastases.367 Although approximately one third to one half of those with bone metastases may be asymptomatic, pain in these areas is commonly described or may be elicited through examination of the skeletal system. The extent of osseous metastasis and the presence of symptoms368 are associated with responsiveness to therapeutic intervention and survival. Pathologic fractures are uncommon but may affect the femora, humeri, and vertebral column. Extension of tumor into the epidural space or compression of nerve rootlets or peripheral nerves may cause neurologic deficits. Spinal cord compression is characterized by motor weakness, sensory changes, and bladder and bowel dysfunction. Its presence is considered an oncologic emergency that requires prompt recognition and treatment to avoid permanent disability.
Tumor involvement of pelvic or para-aortic lymph nodes is usually asymptomatic, and clinical signs, such as edema of the abdominal wall, genitalia, and lower extremities, are rarely apparent unless bulky nodal disease is present. Although metastases to other anatomic sites (e.g., adrenal glands, liver, lung, and skin) are described,369 symptoms or signs due to functional compromise are uncommon. Paraneoplastic syndromes, including Cushing syndrome, the syndrome of inappropriate antidiuretic hormone secretion, and abnormalities of serum calcium levels, and hematologic manifestations (e.g., anemia, disseminated intravascular coagulation) may also be present in the patient with advanced metastatic disease.370
History and Physical Examination
A complete physical examination is essential and may reveal metastatic disease or other clinically important conditions. Careful DRE of the prostate forms the cornerstone of the physical examination and is instrumental in tumor staging. The examination may be performed in the lithotomy, knee-chest, lateral prone (Sims), or standing position with a well-lubricated, gloved index finger. The examiner should measure the craniocaudad and transverse dimensions of the gland and determine its overall consistency, sensitivity, and mobility, as well as the presence and size of any firm or elevated areas. Typically, an area of prostate cancer is quite hard, may or may not be raised above the surface of the gland, and is surrounded by compressible prostatic tissue. However, an area of prostatic induration per se is not pathognomonic of cancer, as induration may be the result of prostatic calculi, infection, infarction, granulomatous prostatitis, or nodularity owing to benign prostatic hyperplasia. Although DRE is important in staging, it is not particularly accurate in screening. In a meta-analysis of 13 prostate cancer screening studies, the pooled sensitivity, specificity, and positive predictive value for DRE were 53.2%, 83.6%, and 17.8%, respectively.371 The lateral rectal sulci and the urogenital diaphragm are also examined to determine whether extraprostatic tumor extension is present. Although the normal seminal vesicles are too soft to be palpated as discrete structures, firmness extending superior to the prostate gland suggests seminal vesicle involvement, and further investigation may be warranted.
Staging
Whitmore372 is credited with developing the first widely accepted stage groupings for prostate cancer, and the general concepts set forth were later incorporated into the AUA system.373 Subsequently, the Organ Systems Coordinating Center of the National Cancer Institute initiated a process to align the Whitmore-based groupings and the TNM system.This and other efforts led to development of the AJCC TNM classification and stage groupings,374 which were unified with the UICC in 1987.178 This system retains the general organizational scheme of prior systems and conforms to the TNM rules applied to tumors originating in other sites.
The AJCC TNM classifications and the stage groupings for prostate cancer are shown in Tables 51-3 and 51-4, respectively. Clinical staging involves primary tumor assessment with DRE, complete general examination to determine disease extent, serum PSA determination, and diagnostic imaging (including TRUS). The regional lymph nodes are defined as those confined to the true pelvis (i.e., those lying below the bifurcation of the common iliac arteries) and include the obturator, external and internal iliac, and sacral lymph node groups. All information available before definitive therapy may be used for clinical staging. Pathologic staging requires histologic examination of the prostatoseminovesiculectomy and pelvic lymph node specimens; presence of EPE, SVI, and margin status, as well as the extent and location of involved lymph nodes should be specified.375
Diagnostic Imaging
Ultrasonography
TRUS is an important tool in prostate cancer diagnosis and may be used to identify abnormalities of the prostate gland and seminal vesicles for staging purposes. TRUS is performed by inserting a 5- to 7.5-MHz transducer in the rectum and imaging the prostate in the axial and sagittal planes. Although the typical ultrasonographic appearance of prostate cancer is an area of diminished echogenicity (hypoechoic) in the peripheral zone of the gland, between 14% and 29% of patients with prostate cancer have an isoechoic tumor that cannot be reliably distinguished from normal prostatic tissue.376–378 Furthermore, among prostate cancer screening participants with a hypoechoic finding on TRUS that prompts further evaluation, only 17% to 31% have a diagnosis of cancer established by needle biopsy of the prostate.376–378 Color or contrast-enhanced Doppler TRUS improves the sensitivity and specificity of prostate cancer detection.379
Several investigations address the ability of TRUS to detect EPE, which may affect tumor stage, by correlating ultrasound observations with the findings from histologic examination of the prostatoseminovesiculectomy specimen. In particular, the Radiology Diagnostic Oncology Group prospectively evaluated preoperative TRUS in 219 patients who underwent RP.380 Among 85 patients considered to have disease confined to the prostate gland, 43 (51%) had EPE, whereas 50 of 134 patients (37%) with suspected extraprostatic disease did not have this confirmed by histologic examination. Overall, the sensitivity and specificity of TRUS in identifying EPE were 66% and 46%, respectively, and the test accuracy was only 58%. Based on these results, these investigators concluded that TRUS is not sufficiently accurate to assist in staging early-stage prostate cancer, mainly because of its limitations in identifying microscopic extraprostatic spread of disease. In a series of 200 patients with clinical stage T3a disease, TRUS was not remarkably different than DRE in detecting EPE.381 Histopathologic examination of prostatectomy specimens confirmed unilateral stage T3a disease in 49.5% of cases detected by DRE and 55.7% detected by TRUS. In a prospective, multi-institutional study of 263 patients who underwent RP, preoperative clinical staging by TRUS and DRE was compared with pathologic staging.382 Overall, TRUS was not significantlybetter than DRE in predicting extracapsular extension.
Despite these limitations, tumor extension into the seminal vesicles may be appreciated by TRUS as echogenic abnormalities and by anterior displacement and enlargement of this structure. Terris and associates213 demonstrated the feasibility of obtaining ultrasound-guided preoperative bilateral seminal vesicle biopsies. Among 73 prostate cancer patients suitable for prostatectomy, biopsy showed tumor involvement adjacent to seminal epithelium (i.e., a pathognomonic finding) in eight patients; all of these instances were confirmed after examination of the surgical specimen. Eleven of 133 negative preoperative seminal vesicle biopsies showed involvement with cancer, resulting in a false-negative rate of 8%. TRUS-guided biopsy may be appropriate if predicted rates of SVI by preoperative nomograms are high and a treatment decision would change based on this finding.
Computed Tomography
CT has little value in assessing intraprostatic anatomic changes due to the primary tumor, because the neoplasm usually has the same attenuation as the normal prostate gland. In the presence of EPE, the periprostatic fat planes may become indistinct or there may be deformity of the bladder base, obliteration of the normal angle between the seminal vesicle(s) and the posterior aspect of the bladder, or asymmetric enlargement of a seminal vesicle. However, caution must be exerted in interpreting these images because they may be an artifact of biopsy or the plane between seminal vesicles and bladder may be obscured by rectal distention. Furthermore, EPE is often microscopic and cannot be detected with CT. This observation largely accounts for the low sensitivity of this procedure (24%), which limits its utility in staging the primary tumor.383
Diagnostic CT of the abdomen and pelvis for lymph node staging of newly diagnosed prostate cancer infrequently yields valuable information. CT can detect only abnormalities in lymph node size that may result from extensive tumor involvement. As a result, the diagnostic sensitivity of this procedure with respect to lymph node assessment is poor (~25%)383,384 and the routine use of this test is of little value in assignment of regional node stage in low-risk patients.385 For example, Huncharek and Muscat386 analyzed the CT results of 425 patients with newly diagnosed prostate cancer and found that only 12 patients (3%) had radiologic evidence of adenopathy. Because patients with adenopathy had higher serum PSA levels, these authors concluded that CT was not indicated in the patient with a PSA value under 20 ng/mL, wherein fewer than 1% had adenopathy. Other investigators noted a similar association between serum PSA level and radiologic and histologic findings and likewise concluded that CT for nodal assessment should be reserved for patients with higher (>20 ng/mL) PSA values.384,387 Fine-needle aspiration cytology may further enhance the specificity and accuracy of CT-based pelvic node evaluation, particularly in intermediate- and high-risk patients.388 The American College of Radiology (ACR) and the AUA restrict their recommendations on the use of abdominal and pelvic CT for staging to intermediate- and high-risk patients.149,389
Magnetic Resonance Imaging
Body-coil 1.5-T MRI for prostate cancer staging has been prospectively evaluated in a multi-institutional trial by the Radiology Diagnostic Oncology Group in the late 1980s.380 Based on a low sensitivity, specificity, and accuracy these researchers concluded that body-coil MRI was not an accurate method for prostate cancer staging. However, over the past decade technologic advances have allowed for higher MRI resolution and improved staging accuracy. Endorectal surface-coil MRI of the prostate has been shown to have superior spatial resolution and contrast than 1.5-T body coil images. In a prospective study of 82 patients, Futterer and colleagues390 reported that using an endorectal-pelvic phased-array coil significantly improved anatomic details, staging accuracy, and specificity. Local staging accuracy, sensitivity, and specificity using a pelvic phased-array coil alone were 59%, 54%, and 62%, respectively, whereas using a combined endorectal-pelvic phased-array coil, local staging accuracy, sensitivity, and specificity were 83%, 65%, and 98%, respectively. In addition, the simultaneous use of other clinical information in pretreatment nomograms enhances the value of MRI in predicting SVI and organ-confined disease.216 Therefore endorectal-coil MRI may be best applied in conjunction with the DRE, Gleason score, and pretherapy serum PSA findings in select patients with intermediate- or high-risk prostate cancer. High-field (3.0-T) MRI scanners are showing promise to further improve the sensitivity and specificity for the nonoperative staging of localized prostate cancer.391 In a prospective study of 32 patients undergoing 3.0-T endorectal MRI before RP the accuracy, sensitivity, and specificity of local staging were 94%, 88%, and 96%, respectively, for two experienced radiologists, and 81%, 50%, and 92%, respectively, for a less experienced radiologist.
Dynamic contrast-enhanced (DCE)-MRI may visualize neovasculature associated with prostate cancer. DCE-MRI shows earlier and more intense enhancement of prostate cancer than normal peripheral zone tissue. Padhani and colleagues392 have shown that DCE-MRI could reliably distinguish tumor from noncancerous prostate. In a prospective study of 32 patients, Bloch and associates393 demonstrated a mean sensitivity, specificity, positive predictive value, and negative predictive value of 86%, 95%, 90%, and 93%, respectively, for predicting extracapsular extension by two experienced readers. Although the value of DCE-MRI needs to be validated in larger multi-institutional studies, it appears to be a promising imaging tool for prostate cancer staging.
Magnetic resonance spectroscopy (MRS) can assess cellular metabolism and aid in distinguishing prostate cancer from benign intraglandular changes. Loss of a citrate peak and gain in the choline/creatine peak on MRS has been correlated with the presence of prostate cancer.394 Citrate levels in prostate adenocarcinoma appear to be grade dependent, with preliminary data suggesting that there is a strong linear relationship between a decrease in citrate levels and the elevation of choline with cancer aggressiveness.394 Although it has been hoped that MRS would improve the diagnostic accuracy and staging of prostate cancer, a clinical trial by the American College of Radiology Imaging Network (ACRIN) showed no benefit to MRS over standard MRI alone.395
As in CT, interpretation of pelvic images for lymph node metastases relies on lymph node size. Harisinghani and colleagues396 have described the intravenous iron oxide lymphotropic superparamagnetic nanoparticles in normal saline to enhance MRI of lymph nodes. Macrophages in lymph nodes involved with cancer do not take up the particles and provide contrast to detect metastases. For lymph nodes that were considered normal by standard MRI criteria, the sensitivity of detecting tumor by this method was 96%. Further trials are in progress using this promising technology. Pelvic MRI may also identify bone metastases and confirm the malignant nature of abnormalities identified on bone scintigraphy.
Nuclear Medicine Imaging
Bone Scintigraphy
Since the advent of serum PSA testing, Chybowski and associates397 were among the first to evaluate the utility of obtaining a routine bone scintiscan in the pretherapy evaluation of the patient with newly diagnosed prostate cancer. These investigators conducted a retrospective evaluation of 521 randomly selected patients and correlated clinical tumor stage, tumor grade of the diagnostic biopsy specimen, acid phosphatase, PAP, and PSA serum levels with bone scintiscan findings. The probability of a bone scintiscan showing metastatic disease was most strongly associated with and was directly proportional to the serum PSA level, as illustrated in Figure 51-4. Only 0.3% of patients with a pretherapy PSA of 20 ng/mL or lower had a positive bone scintiscan at presentation.397
In a study of 852 consecutive patients with newly diagnosed untreated prostate cancer with a pretherapy serum PSA level of less than 20 ng/mL, Oesterling and colleagues398 reported that only 7 patients (0.8%) had scintigraphic evidence of bone metastases, and five of these patients were symptomatic in the area corresponding to the bone scan abnormality. The observed rates of false-negative bone scan results were 0.5% and 0.8% for patients with serum PSA levels of 10 ng/mL or less and less than 20 ng/mL, respectively. On the basis of these observations, the authors concluded that a bone scan was not routinely required in the staging evaluation of the patient without skeletal discomfort if the pretherapy serum PSA level was 10 ng/mL or less. Furthermore, this investigation confirmed the finding that serum PSA was most closely associated with the bone scintigraphy results and that neither clinical tumor stage nor tumor grade improved the predictive accuracy of PSA alone. Oesterling and colleagues398 suggest that considerable economic savings could be realized if the use of a radionuclide bone scan were reserved for the symptomatic patient or for those with PSA levels above 10 ng/mL or (possibly) a high-grade tumor. Based on a similar retrospective analysis of 853 consecutive patients, Briganti and associates399 developed a classification and regression tree model that can be used to predict the frequency of a positive bone scintiscan. According to this tool, a staging bone scintiscan should be considered only for patients with a Gleason score greater than 7 or with a PSA greater than 10 ng/mL and palpable disease.
Radionuclide bone scintigraphy may also be of value in the post-therapeutic assessment of the patient with prostate cancer. The onset of skeletal symptoms, particularly in association with a rising serum PSA profile, should prompt radioscintigraphic investigation. However, post-therapeutic serum PSA monitoring is likely to identify tumor relapse before the occurrence of symptoms, because biochemical relapse may antedate development of bone scintigraphic abnormalities by several months. Freitas and colleagues400 used receiver operating characteristic curve analysis to identify a serum PSA threshold level at which a post-therapeutic bone scintiscan might be considered. This analysis indicated that few patients (1.5%) with a post-therapy PSA value of 8 ng/mL or less had a positive bone scintiscan, whereas 38% of patients with a PSA level greater than this value had a bone scintiscan indicative of osseous metastases. In a series of 239 patients evaluated with bone scintigraphy for biochemical relapse after RP, Dotan and colleagues401 reported an incidence of bone metastases of 4%, 36%, 50%, and 79% with trigger PSA values of 0 to 10, 10.1 to 20, 20.1 to 50, and above 50 ng/mL, respectively. In a multivariate analysis, PSA slope, PSA velocity, and trigger PSA were all significant factors for predicting a positive bone in the evaluation of biochemical failures after prostatectomy. The group developed a nomogram to predict the likelihood of a positive bone scintiscan using preoperative and pathologic tumor features, along with postoperative PSA kinetics.
Monoclonal Antibody Imaging
111In-labeled capromab pendetide (ProstaScint) is an antibody directed against a prostate-specific membrane antigen.402 The image performance of ProstaScint has been assessed in the pretherapy evaluation of patients with clinically localized prostate cancer who are considered at high risk for disease spread to regional nodes and in the post-therapy setting when occult residual or recurrent disease is suspected. Surgical series suggest the diagnostic accuracy in the 70% range.403,404 In a series of 255 men undergoing RP, Raj and associates405 reported that ProstaScint was able to differentiate between locally confined and metastatic disease, to detect soft tissue defects as small as 5 mm, and to have a sensitivity, specificity, and positive predictive value of 73%, 53%, and 89%, respectively. In the setting of a rising PSA value after prostatectomy, ProstaScint imaging has been used to evaluate patients for the presence of local or lymph node recurrence of disease after prostatectomy. The reported value of ProstaScint imaging has been inconsistent, with some investigators reporting improved patient selection and outcomes for salvage therapy of a presumed localized recurrence406,407 whereas others have reported no difference in outcome in patients with localized versus distant tracer uptake.408–410
Positron Emission Tomography
Positron emission tomography (PET) uses positron-emitting isotopes conjugated to compounds to detect physiologic or pathologic processes. The most common PET agent used to date is 18F-fluorodeoxyglucose (FDG), a glucose analogue that is taken up by up-regulated glucose transporters in many cancers. FDG-PET in prostate cancer has been disappointing, with poor sensitivity and specificity for staging the local and regional disease owing to poor uptake of FDG in slowly proliferating prostate cancer as well as urinary excretion causing bladder uptake to obscure pelvic disease.411,412 Newer PET agents of 11C-labeled methionine, acetate, or choline are showing promise in the staging of high-risk prostate cancers.413
Laboratory Evaluation
Prostate-Specific Antigen
PSA is a serine protease produced by normal prostatic acinar and ductal epithelial cells and by neoplastic cells of prostatic origin.404,414 It is detectable in the serum in a form immunologically identical to that purified from prostatic tissues and may be elevated in patients with benign (e.g., prostatic hyperplasia, prostatitis) or malignant disorders of the prostate gland.404 Although PSA exists in several forms in the serum, the PSA/α1-antichymotrypsin complex predominates415 whereas the free (noncomplexed) form of PSA is less abundant.140,415
Serum PSA levels may exhibit interassay (run-to-run) and physiologic (i.e., intraindividual) variation that must be considered in interpreting serial determinations. Physiologic variation refers to the difference in PSA levels observed when a second sample is obtained from the same patient within a few weeks of the initial sample. For physiologic variation, the ratio difference variation is significantly greater than the interassay variation, is inversely related to the PSA level, and may be as high as 0.298 (95% CI).416 This means, for example, that a serum PSA level of 4 ng/mL can increase to 5.2 ng/mL (4.0 × 1.298) and still remain in the range of physiologic variability.
The serum half-life of PSA is approximately 2.2 (±0.8) to 3.2 (±0.1) days,417 which means that several weeks are required for serum levels to return to baseline after prostatic manipulation or for a nadir value to be reached after prostatectomy. Although DRE of the prostate, prostatic massage, TRUS, ejaculation, and cystoscopy have an insignificant effect on the serum PSA level, prostate biopsy causes an immediate PSA elevation (median, 7.9 ng/mL) that requires several weeks to return to baseline.418–420 Transurethral resection of the prostate (TURP) also causes PSA elevation (median, 5.9 ng/mL), with a median of 17 days required to arrive at the new baseline value.420 Because the production and secretion of PSA are androgen dependent, androgen deprivation therapy often results in a striking reduction in the PSA level,421 which should be considered in interpreting serum PSA values in this setting.
As already stated, pretherapy serum PSA is an important prognostic factor in predicting outcome after treatment for prostate cancer and should play an important role in consideration of treatment options. In general, the higher the PSA level, the more likely that the tumor is outside the capsule and into the seminal vesicles or lymph nodes, which has major impact on the choice of therapy.235,257,360,422
Acid Phosphatase
Although also produced in nonprostatic tissues, the glandular epithelium of the prostate is the dominant source of acid phosphatase in humans. The prostate-specific form of this enzyme (i.e., PAP) is distinct from other isoenzymatic forms and is influenced by hormonal factors, and its expression is altered in the androgen-deprived state. Abnormally high serum acid phosphatase levels correlate with clinical tumor stage T3 to T4 disease, higher tumor grade, and pretherapy serum PSA.253 Since the introduction of assays for serum PSA, acid phosphatase testing has declined in frequency. Several clinical observations support the assertion that serum PAP determinations add little to patient evaluation in either the pretherapy or post-therapy setting. In patients with prostate cancer, serum PSA is at least 2 to 10 times more likely to be elevated than is serum PAP,314,423 and only 2% of patients with a normal serum PSA value will have an elevated serum PAP.424 Furthermore, fewer than 10% of patients treated for cure with RT have an elevated acid phosphatase level, whereas 80% to 90% will have an abnormal PSA value (i.e., >4.0 ng/mL).313,314
Staging Workup
The ACR389 and the NCCN have established guidelines for the pretherapy staging of patients with a recently established diagnosis of prostate cancer. The scheme proposed by the NCCN319 is displayed in Figure 51-5 and may be used for guidance. However, individual clinical circumstances may require departure from these guidelines, because independent medical judgment is necessary to meet needs unique to a particular situation.
Primary Therapy
Expectant Management
Expectant management is a clinical strategy in which immediate therapeutic intervention is not pursued when the diagnosis of prostate cancer is first established. With this approach, treatment is withheld until the time of disease progression or the development of symptoms. Expectant management is used throughout the world, and it is considered a satisfactory alternative to the use of primary RT or RP by its proponents. Under a program of watchful waiting, noncurative treatment would be offered at the time of progression and would consist primarily of hormonal therapy to induce tumor regression and alleviate symptoms.224,425,426 Increasingly, an alternative expectant management strategy, active surveillance, is replacing watchful waiting. Whereas the goal of watchful waiting is to avoid treatment, the goal of active surveillance is to individualize patient care and treat only those men who have a clinically significant cancer and avoid the risk of treatment-related morbidity in men whose cancers are unlikely to progress.
The rationale for expectant management is related to the natural history of the condition, the age and comorbidity of the incident population, the perceived need (or lack thereof) for therapeutic intervention, and health-related quality of life issues. PSA screening has resulted in an increased rate of prostate cancer “overdiagnosis.” Overdiagnosis refers to the lead-time bias in establishing a diagnosis of cancer that would otherwise not be detected during a man’s expected life span. Furthermore, incidental (or latent) prostate cancer is often found on autopsy examination of the prostate gland and, although particularly common in the elderly,9,10 is observed in as many as one third of men in their fourth and fifth decades.8 The key to a successful expectant management strategy is the proper selection of patients who have indolent disease and/or comorbidities that would impact their expected survival relative to the natural history of the prostate cancer.
Several studies demonstrate that many localized prostate cancers have an indolent course that may support the use of expectant management for this condition. In particular, the prognosis appears quite favorable for the patient with a small-volume, low-grade tumor identified incidental to TURP (stage T1a) for benign prostatic hyperplasia.427,428 However, the distinction between stage T1a and stage T1b is important, because the risk of disease progression and disease-specific mortality is highly dependent on tumor volume and grade.428–430
One of the largest studies of expectant management was reported by Chodak and colleagues,224 who collected patient and disease characteristics from several nonrandomized institutional series and from a population-based study to evaluate the outcome of expectant management in patients with localized prostate cancer. The expectant management approach in this study was watchful waiting with no attempt to implement curative local therapy in these patients. The investigators identified 10 studies published during the preceding decade and obtained original data on 828 patients from 6 of them. In this meta-analysis, multivariate regression analysis identified tumor grade as the most significant factor associated with DSS. Based on their observations, the authors concluded that localized prostate cancer is a steadily progressive disorder but that observation with delayed hormonal therapy is a reasonable approach for some men with low-grade disease.
Although single-institution series include patients selected for expectant management on the basis of advanced age or life-limiting comorbidity, population-based studies may minimize this potential source of bias. Johansson and colleagues425 described a group of 300 consecutive patients diagnosed with stage T1 to T2 prostate cancer at the Örebro Medical Centre, a hospital serving a geographically well-defined population. Among these patients, 223 patients with predominantly low-grade tumors were treated with exogenous estrogen or orchiectomy if symptomatic tumor progression occurred. By the time of last evaluation, EPE or metastatic disease had developed in 36% and 17%, respectively. Although the 15-year PFS (45%) and OS (21%) were rather low, the high 15-year DSS (79%) appeared consistent with the results of several single-institution investigations.431,432 However, in an update of this study, the prostate cancer mortality rate increased from 15/1000 person-years during the first 15 years to 44/1000 person-years beyond 15 years of follow-up.425 Although most cancers had an indolent course during the first 10 to 15 years after diagnosis, follow-up from 15 to 20 years revealed a substantial decrease in PFS (from 45% to 36%), survival without metastases (from 77% to 51%), and prostate CSS (from 79% to 54%). Therefore these findings support early curative therapy for patients with an estimated life expectancy of more than 15 years.
Other population-based studies from Sweden have not confirmed the high 10- to 15-year DSS rate observed in the series from the Örebro Medical Centre.425 For example, Aus and colleagues433 identified all patients with an antemortem diagnosis of nonmetastatic prostate cancer treated with primary hormonal (63%) or deferred (36%) therapy who died in Göteborg, Sweden, between 1988 and 1990. Among 301 such patients, the cause of death was related to prostate cancer in 149 (50%), and for those surviving at least 10 years, 63% died from this disease. The initial tumor stage and grade were associated with the prostate cancer–related death rate. Although only 10% of patients with stage T1a tumors died of prostate cancer, 47% to 53% of patients with stage T1b to T3 tumors and 70% of patients with stage T4 lesions died secondary to their malignant disease. Forty-three percent to 48% of patients with grade 1 and grade 2 tumors and 60% of patients with grade 3 lesions died of prostate cancer. Furthermore, 58% of patients underwent TURP, 22% underwent an upper urinary tract procedure (e.g., pyelostomy) due to urinary obstruction, and 35% received palliative irradiation.434 Norlén435 noted similar findings in an evaluation of 44,300 patients with prostate cancer, representing 99% of all newly diagnosed cases, reported to the Swedish Cancer Registry between 1960 and 1978. Survival rates were adjusted for mortality in the general population and were expressed as relative survival, which estimates the likelihood of dying of prostate cancer. Among patients with prostate cancer, the relative survival was 51%, 34%, and 17% after 5, 10, and 20 years of observation, respectively, representing an excess death rate of approximately 8% per year.
An important randomized study from Sweden comparing RP to watchful waiting has been reported.117 Although the effect of treatment in relative terms was substantial, a decrease in disease-specific mortality of approximately 50% with 8.2 years median follow-up, in absolute terms the impact was more modest. The disease-specific mortality decreased from 14.4% with watchful waiting to 8.6% with prostatectomy.117 Although after 5 years’ follow-up the overall mortality was similar, 7.8% for prostatectomy patients versus 9.8% for the watchful waiting group, at 10 years’ follow-up there was a significant difference (p = .04) in favor of the treated patients, 27% versus 32%, but still an absolute difference of only 5%. Furthermore, because the men in this study were largely recruited before widespread PSA screening, the lead time introduced with earlier detection may erode the benefit of definitive treatment in contemporary populations, especially those with less than a 10- to 15-year life expectancy.
Active Surveillance
Unlike watchful waiting, active surveillance strategies attempt to individualize the decision to recommend treatment based on a patient’s risk of cancer progression in his lifetime. The key to a successful active surveillance strategy is the accurate identification of patients with clinically insignificant cancer, a rational follow-up plan, and early intervention criteria. Epstein and colleagues436 introduced biopsy criteria to predict an insignificant cancer: clinical stage T1c, PSA density less than 0.15 ng/mL, no Gleason pattern 4 or 5, fewer than three positive cores, and less than 50% cancer per involved core. In an analysis of 237 nonpalpable (stage T1c) prostate cancer patients treated at Johns Hopkins University, the Epstein criteria predicted for organ-confined disease in 91.6% of cases.437 The other 8.4% who fulfilled the Epstein criteria had evidence of extraprostatic disease. In a European validation study of the Epstein criteria, Jeldres and colleagues438 examined a cohort of 366 patients. In that series, 20% of patients who fulfilled the Epstein criteria had unfavorable findings at RP, such as Gleason score 7 or non–organ-confined disease.438 Consequently, as many as 20% to 25% of patients predicted to have insignificant cancer by the Epstein criteria may be expected to have non–organ-confined disease.439 The risk of missing a clinically significant cancer and avoiding necessary treatment in a potentially curative patient is the primary reason that active surveillance has not been more broadly adapted.
To accurately identify truly clinically insignificant cancers, many active surveillance series have included additional selection factors to the Epstein criteria. These have included an absolute PSA value less than 10 ng/mL440,441 or less than 15 ng/mL442,443 and/or fewer than 33% positive cores.440 Although clinical validation has not been established, Shukla-Dave and associates444 have reported the utility of incorporating MRI and MRS into nomograms to predict for clinical significant disease. The receiver operating characteristic area under the curve for the nomogram was 0.54 compared with only 0.726 for the best clinical nomogram that did not include MRI data (p <.0001).
While on active surveillance, clear criteria need to be described for patient follow-up. All published series to date include repeated DRE, determination of PSA level, and repeated prostate biopsies. The interval for DRE varies from every 3 to 6 months for the first 2 years to every 6 months after 2 years. PSA testing frequency ranges from every 1 to 3 months for the first 1 to 2 years and then every 6 months thereafter. Recommended biopsy intervals vary from every 6 or 12 months during the first year, then annually or biannually thereafter. Likewise, the indications for treatment should be well defined before advising an active surveillance strategy. Common criteria for implementing therapy include a change in PSA kinetics, such as an increase in PSA velocity or a decrease in the PSA doubling time to less than 2 or 3 years.256 Clinical progression of a palpable or ultrasound-detected nodule and/or a rebiopsy pathology with any Gleason pattern 4 or 5 with more than two cores involved or more than 50% of any core involved with prostate cancer is also used as a trigger to implement treatment.
Several large institutional series have reported excellent outcomes with active surveillance strategies. The largest and most mature prospective series to date is that of the Princess Margaret Hospital with 450 patients and a median follow-up of 6.8 years.256 The criteria for active surveillance initially included all favorable-risk patients (i.e., Gleason score ≤6, PSA ≤10 ng/mL), and for patients older than 70 years a PSA level up to 15 ng/mL or Gleason scores up to 3 + 4 were allowed. After 2000 the study strictly limited enrollment to favorable-risk patients. The criteria for implementing treatment were PSADT of less than 3 years, histologic upgrading and repeat biopsy, and/or clinical progression on DRE. Initially, a PSADT of less than 2 years was used but this was increased to 3 years when it became apparent that only l0% of patients were identified as higher risk, necessitating treatment. Patients had a PSA test every 3 months for the first 2 years and every 6 months thereafter, as long as the PSA value was stable. A repeat biopsy was performed at 6 to 12 months after enrollment and repeated every 3 to 4 years until the patient reached the age of 80 years. Overall, 83% of patients had a Gleason score 6 or less and 13% had a Gleason score 3 + 4 = 7 at study entry. The 10-year prostate cancer–free survival rate was 97.2%. Overall, 30% of patients had evidence of progression to higher-risk categories and were offered therapy. Of 117 patients treated, the PSA failure rate from treatment was 50%, but this represented only 13% of the entire study cohort.256
From the Royal Marsden Hospital, van As and colleagues443 reported the results of 326 men managed with active surveillance. Inclusion criteria for their study were clinical stage T1 to T2a, Gleason score less than 7 (3 + 4), PSA less than 15 ng/mL, and less than 50% of biopsy cores positive. Treatment criteria included PSA velocity of greater than 1 ng/mL per year, Gleason score greater than or equal to 4 + 3, or greater than or equal to 50% cancer involvement in any involved core on repeat biopsy. With a median follow-up of 22 months, these researchers have reported no prostate cancer deaths and 20% of their patients have moved to active treatment.
Dall’era and colleagues440 reported the University of California, San Francisco, experience of 321 men selected as having PSA less than or equal to 10 ng/mL, no Gleason pattern 4 or 5, clinical stage T2a or lower, PSAD of less than 0.75 ng/mL, and fewer than 33% positive cores involved with cancer. Patients were offered treatment if they had a repeat biopsy Gleason score greater than or equal to 7 or any increase in cancer volume on repeat biopsy or a rising PSA value. With a median follow-up of 3.6 years, 37% of patients met at least one criterion for treatment. Overall, 38% had higher-grade cancer on repeat biopsy and 26% had a PSA velocity of more than 0.75 ng/mL. Seventy-eight of the 321 men (24%) received treatment, and there have been no prostate cancer deaths reported to date. These investigators reported that 13% of patients chose therapy despite no evidence of disease progression.
The Johns Hopkins University experience of 407 men with a median age of 65.7 years has been reported by Carter and colleagues.445 All patients met the Epstein criteria for clinically insignificant cancers. With a median follow-up of 3.4 years, 59% remain on active surveillance and 25% underwent active treatment with curative intent. No patient has died of prostate cancer in this series.
Several single-institution studies suggest a role for active surveillance in the management of men with low-risk and low-volume prostate cancer. The optimal selection criteria for active surveillance remain to be defined, as well as the frequency of follow-up examinations, such as DRE, PSA testing, and repeat biopsy. The criteria for which to initiate active therapy vary from series to series, and the long-term impact on prostate CSS and quality of life remains to be determined. It is worth noting that men often experience anxiety due to their diagnosis and fear of disease progression. A randomized clinical trial from Sweden comparing treatment to watchful waiting showed no difference in quality of life in men at 5 years. Worry, depression, and anxiety were equally common in each group.446
Increasingly, expectant management strategies are being endorsed by oncologic specialty societies and networks. The NCCN strongly recommends active surveillance for men with a short life expectancy.319 The NCCN cites the observation by Lu-Yao and associates447 that the prostate cancer–specific death rate for men on an active surveillance strategy has dropped by 74% in the decade between 1992 and 2002 largely owing to earlier diagnosis and the identification of more indolent cancers through PSA screening. The AUA guidelines448 and the ACS449 both recommend that patients be informed of active surveillance as an alternative to a curative intervention. Currently, three randomized clinical trials are underway to evaluate the role of active surveillance relative to treatment. The Standard Treatment Against Restrictive Treatment (START)450 trial in North America, the Prostate Cancer Research Interactive: Active Surveillance (PRIAS)441 trial in Europe and North America, and the PROstate TEsting for Cancer and Treatment (PROTECT)451 trial in the United Kingdom are actively recruiting patients (START, PRIAS) or are currently in follow-up (PROTECT). These studies, along with the U.S.-based Prostate Cancer Intervention Versus Observation Trial (PIVOT) will help bring clarity to the issues surrounding expectant management and early-stage prostate cancer.452