CHAPTER 61 Proliferative diabetic retinopathy
Management of proliferative diabetic retinopathy is guided by the findings and recommendations of the Diabetic Retinopathy Study (DRS)1–3, Early Treatment Diabetic Retinopathy Study (ETDRS)4, Diabetic Retinopathy Vitrectomy Study (DRVS)5,6 and the Diabetes Control and Complication Trial (DCCT)7. The combination of successful results achieved with the DRS, ETDRS, DRVS, and DCCT have clearly established the indications, techniques, and benefits of photocoagulation and pars plana vitreous surgery, and intensive control of blood sugars for people with diabetes. Advances in anti-angiogenic therapies have the potential to influence the way we care for patients with proliferative diabetic retinopathy in the future.
Clinical features
The severity of diabetic retinopathy is strongly related to the duration of diabetes: the prevalence of proliferative diabetic retinopathy ranges from 0% in individuals with fewer than 5 years of diabetes to as high as 50% in individuals with 20 or more years of diabetes8. In addition to duration of diabetes, other factors are influential in the development and progression of proliferative diabetic retinopathy. These include puberty, pregnancy, poor metabolic control, hypertension, hypercholesterolemia, renal disease, and intraocular surgery. Recent reports of lower rates of proliferative diabetic retinopathy may reflect improvements in primary care for diabetes and these other systemic associations9,10.
Proliferative diabetic retinopathy is characterized by the presence of newly formed blood vessels arising from the retina or optic disc. The risk of proliferative diabetic retinopathy is related to the extent of ischemia within the retina11,12. As the retina becomes progressively more ischemic, a cascade of events occurs that ultimately results in the proliferation of abnormal blood vessels; vascular endothelial growth factor (VEGF) plays a significant role in this process13. Clinically, the most important findings in predicting progression to proliferative diabetic retinopathy include intraretinal hemorrhages and microaneurysms, venous beading, and intraretinal microvascular abnormalities (IRMA)14. The ‘four-two-one rule’ is helpful in assessing the severity of diabetic retinopathy and predicting the progression to proliferative retinopathy. Severe non-proliferative diabetic retinopathy is defined as the presence of either four quadrants of dot/blot hemorrhages or microaneurysms, two quadrants of venous beading, or one quadrant of IRMA. In eyes with severe non-proliferative diabetic retinopathy, approximately 50% will progress to proliferative diabetic retinopathy within 1 to 3 years.
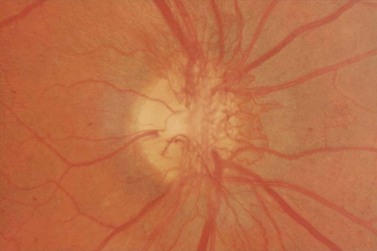
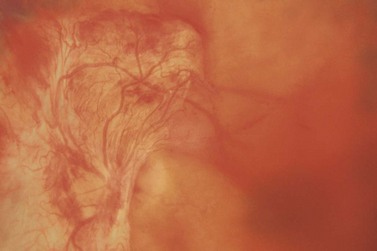
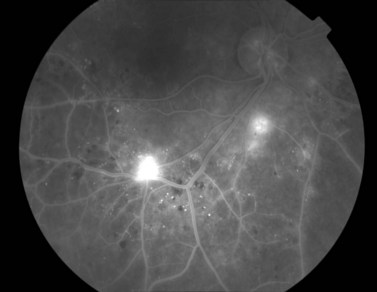
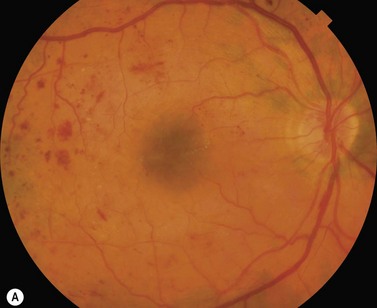
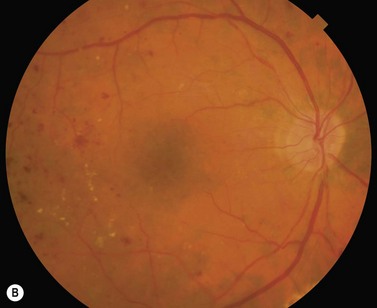
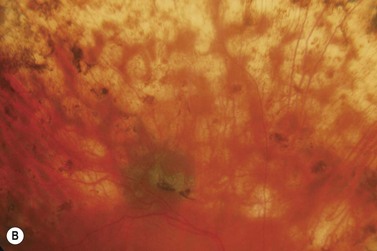
Most eyes with neovascularization will have neovascularization on the disc itself or within one disc diameter of the disc (NVD). Neovascularization of the disc appears as fine lacy vessels lying on the surface of the disc or bridging the physiologic cup (Fig. 61.1). As the NVD progresses, the vessels become more prominent and extend into the vitreous cavity or along the vascular arcades; the new vessels may be associated with significant fibrous proliferation (Fig. 61.2). Neovascularization elsewhere (NVE) is defined as new vessels located at retinal sites other than on or within one disc diameter of the optic disc. Neovascularization elsewhere is usually associated with the retinal veins and arteriovenous crossing sites between areas of perfused and non-perfused retina (Fig. 61.3).
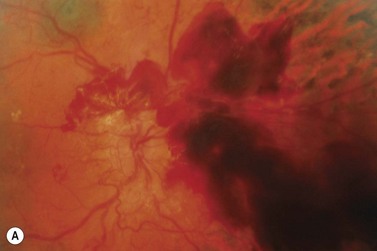
The vitreous plays a critical role in the development and progression of proliferative diabetic retinopathy15. It appears that the posterior vitreous surface provides a ‘scaffold’ for the progression of neovascularization. As the posterior vitreous detachment progresses, there is increased traction on the fibrovascular tissue. This may result in recurrent preretinal/vitreous hemorrhage or traction retinal detachment, or both.
Major diabetic retinopathy studies
The DRS and ETDRS provide clear guidelines for the management of proliferative diabetic retinopathy. Definitions used by the DRS and ETDRS are listed in Table 61.1.
Table 61.1 Diabetic Retinopathy Study (DRS) and Early Treatment Diabetic Retinopathy Study (ETDRS) definitions
| Moderate visual loss | Loss of 15 or more letters on the ETDRS reading chart (equivalent to a doubling of the initial visual angle, e.g., 20/20 to 20/40 or 20/50 to 20/100) |
| Severe visual loss | Visual acuity less than 5/200 on two consecutive follow-up visits scheduled at 4-month intervals |
| Severe non-proliferative retinopathy | Any one of the following: |
| Hemorrhages and/or microaneurysms in four quadrants | |
| Venous beading in two quadrants | |
| Intraretinal microvascular abnormalities (IRMA) in one quadrant | |
| Early proliferative retinopathy | Proliferative retinopathy without DRS high risk characteristics |
| Less severe retinopathy | Mild or moderate non-proliferative retinopathy |
| More severe retinopathy | Severe non-proliferative or early proliferative retinopathy |
| High risk proliferative diabetic retinopathy | At least three of the following risk factors: |
| New vessels present | |
| New vessels located on or within 1 disc diameter of the disc (NVD) | |
| Vitreous and/or preretinal hemorrhage | |
| Moderate to severe new vessels | |
NVD greater than standard photo 10a (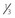 disc area) (Fig. 61.2) disc area) (Fig. 61.2) |
|
In absence of NVD, neovascularization elsewhere (NVE) greater than 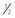 disc area disc area |
Diabetic retinopathy study
Does photocoagulation surgery reduce the risk of severe visual loss in diabetic retinopathy? The DRS evaluated individuals with proliferative diabetic retinopathy in at least one eye or severe non-proliferative diabetic retinopathy in both eyes1,2. One eye was randomly selected for photocoagulation, while the other eye was observed. Extensive scatter panretinal photocoagulation (PRP) and focal laser of new vessels on the surface of the retina was performed using either argon laser or xenon arc. Typical argon laser parameters included: 800–1600 burns, 500 μm spot size, 0.1 second duration, and intensity to obtain definite whitening.
The DRS identified four retinopathy risk factors for severe visual loss in diabetic retinopathy: new vessels present, new vessels located on or within one disc diameter of the disc, vitreous or preretinal hemorrhage, and moderate to severe new vessels (NVD greater than one-third disc area or, in the absence of NVD, NVE greater than one half the disc area)3. The risk of severe visual loss increased progressively as more risk factors were added: the risk of severe visual loss remained relatively low with two or fewer risk factors (roughly 5% at 2 years) but increased considerably with three or more risk factors (roughly 25% at 2 years). Therefore, the presence of three or four of these retinopathy risk factors indicates ‘high risk’ for severe visual loss.
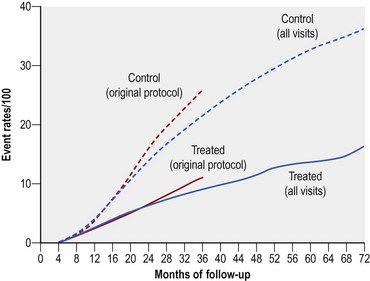
Extensive PRP reduced the risk of severe visual loss by greater than 50% regardless of the baseline severity of retinopathy (Fig. 61.4). After 2 years, severe visual loss occurred in 16.3% of all untreated eyes compared to only 5.3% of eyes that received xenon arc and 7.4% of eyes treated with argon laser.
Early treatment diabetic retinopathy study
When, during the course of diabetic retinopathy, is it most appropriate to initiate photocoagulation therapy? The ETDRS enrolled patients with mild to severe non-proliferative diabetic retinopathy or early proliferative retinopathy without high risk characteristics in both eyes4. One eye of each patient was assigned randomly to early full or mild PRP and the other to deferral. For eyes assigned to deferral, full PRP was performed immediately if high risk proliferative diabetic retinopathy developed. Eyes with macular edema were also randomized to immediate or delayed focal laser surgery.
Performing panretinal photocoagulation
Eyes with high risk proliferative diabetic retinopathy are at significant risk for severe visual loss and should undergo PRP without delay. Recall that high risk proliferative diabetic retinopathy is defined as the presence of three or four of the following risk factors: new vessels present, new vessels on the disc, preretinal or vitreous hemorrhage, and moderate to severe new vessels (new vessels on the disc greater than one third disc area or new vessels elsewhere greater than one half disc area)3. The presence of three or four high risk characteristics translates into essentially three clinical situations. These include moderate to severe neovascularization on the disc in the presence or absence of preretinal or vitreous hemorrhage, less severe neovascularization on the disc associated with preretinal or vitreous hemorrhage, or moderate to severe neovascularization elsewhere associated with preretinal or vitreous hemorrhage. Iris neovascularization is another indication for prompt PRP, whether or not high risk characteristics are present.
Panretinal photocoagulation may be performed in one or multiple sessions. Eyes undergoing treatment in one session are more likely to have transient adverse effects than eyes treated in multiple sessions16. These adverse effects include exudative retinal detachment, choroidal detachment, and angle closure. However, the long-term beneficial effects of laser are equal whether laser is administered in one or multiple sessions. In eyes with macular edema, extensive fibrovascular proliferations, or traction retinal detachments, it is advisable to perform PRP in two or more sessions separated by 2 to 3 weeks.
The type or wavelength of laser may vary. Studies have demonstrated similar efficacies of argon, krypton, diode, and dye lasers17–20. In cases of dense cataract or vitreous hemorrhage, the red wavelength may be more useful.
Laser burns are spaced one-half to one spot-width apart (Fig. 61.5). Peripheral PRP is advocated to reduce the risk of visual loss, exacerbation or development of macular edema, and extensive visual field loss21. The posterior fundus is demarcated with two to three rows of laser to reduce the risk of extension into the macula or optic disc. The burns are placed one disc diameter from the optic disc and the major temporal vascular arcades. Special care must be used when demarcating the posterior extent of treatment of the temporal retina. Generally, laser should not extend posterior to an imaginary line drawn at two fovea-disc diameters from the optic disc. Panretinal photocoagulation is then extended peripherally to the equator and beyond.
Regression of proliferative diabetic retinopathy
The efficacy of PRP is related to the induced regression of retinopathy risk factors (Fig. 61.6)22,23. Regression of high risk characteristics may take several forms: complete regression of new vessels, marked reduction in the severity of new vessels, or resolution of preretinal and vitreous hemorrhage.
A subset of eyes fail to show regression of retinopathy risk factors despite standard PRP; failure to demonstrate regression of high risk characteristics may be associated with a poorer prognosis22–25. Supplemental laser may induce regression of high risk retinopathy in eyes that fail to respond to initial therapy24–29. The DRS and the ETDRS did not evaluate the efficacy of supplemental PRP in eyes that fail to respond to standard therapy. However, the DRS showed that the risk of severe visual loss decreased with an increasing amount of initial treatment as measured by treatment density in the post-treatment photographs30.
In addition to regression of retinopathy risk factors, manifest changes in the retinal vascular system are associated with a favorable response to laser treatment. There is a significant increase in the venous caliber as the stage of diabetic retinopathy progresses31. Panretinal photocoagulation has been shown to reduce retinal blood flow and retinal vessel diameter32–35. The diameter of the retinal vessels is correlated to the amount of regression of proliferative diabetic retinopathy36,37.
How does panretinal photocoagulation work?
Photocoagulation destroys the photoreceptor–retinal pigment epithelial (RPE) complex and produces significant thinning of the outer retina38. By decreasing the oxygen consumption at the photoreceptor–RPE complex, more oxygen is available to diffuse into the inner retina and vitreous38–41. Enhanced oxygen diffusion into the inner retina and vitreous reduces inner retina ischemia and the stimulus for neovascularization.
VEGF is an angiogenic molecule whose expression is markedly induced by retinal hypoxia42. Vitreous VEGF levels are significantly higher in eyes with proliferative diabetic retinopathy than in eyes without proliferative diabetic retinopathy43. VEGF concentrations in vitreous fluid decline significantly after successful PRP, suggesting that PRP reduces retinal ischemia and the hypoxia-induced expression of VEGF44.
In addition, photocoagulation may inhibit neovascularization by inducing changes in the RPE. Retinal pigment epithelial cells have the ability to inhibit vascular endothelial cell proliferation and induce regression of new blood vessels45. Photocoagulation of RPE cells may induce changes in the production of growth factors. In particular, it appears that photocoagulated RPE cells may release potential inhibitors of neovascularization46.
Complications of panretinal photocoagulation
Laser photocoagulation effectively reduces the risk of severe visual loss in eyes with high risk proliferative diabetic retinopathy. However, adverse effects may occur (Box 61.1).
Subjective changes in vision after PRP include blurred vision, decreased accommodation, difficulty adjusting to changing levels of illumination, color vision abnormalities, constriction of peripheral visual fields, and poor night vision47. Adverse effects to the cornea include corneal burns, erosions, and superficial punctate keratitis48,49.
Panretinal photocoagulation may affect intraocular pressure. Blondeau and associates noted transient intraocular pressure elevations soon after treatment, which resolved within several hours50. Conversely, transient decreases in intraocular pressure may occur51,52. Elevation of intraocular pressure may be associated with anterior chamber shallowing and frank angle closure glaucoma.
Iris and pupillary abnormalities include pupillary border hyperpigmentation or atrophy, posterior synechiae, reduced pupillary reactivity, mydriasis, miosis, accommodative palsy, light-near dissociation, and sector palsies of the iris sphincter53.
Stable, non-progressive lens opacities may occur after laser treatment54,55.
Posterior segment complications of laser surgery include inadvertent macular/foveal burns, exacerbation or development of macular edema, exudative macular detachment, ciliochoroidal effusion, hemorrhage, rupture of RPE/Bruch’s membrane, cilioretinal and choriovitreal neovascularization, extension of tractional retinal detachment, and optic neuropathy4,56–68.
Retrobulbar anesthesia may be employed safely to reduce the discomfort of PRP69,70. Risks of retrobulbar anesthesia include penetration or perforation of the globe, retrobulbar hemorrhage, central retinal artery or central retinal vein occlusion, optic neuropathy, Purtscher’s retinopathy, central nervous system depression, or even respiratory arrest.
Intravitreal medications
As more information is uncovered about the underlying mechanisms of diabetic retinopathy, novel treatment strategies are being explored. A variety of anti-VEGF agents, including intravitreal bevacizumab, ranibizumab, and pegaptanib have been used as alternative or adjunctive therapy for proliferative diabetic retinopathy, vitreous hemorrhage, and neovascular glaucoma. Preliminary studies suggest these agents may be effective alternative treatment strategies alone or in combination with standard therapeutic methods for vision stabilization or improvement71,72. Further randomized clinical trials are needed.
Cryosurgery
Peripheral retina ablation with cryosurgery may be useful in proliferative diabetic retinopathy when media opacities such as severe cataract or vitreous hemorrhage prevent PRP, or in individuals who fail to demonstrate regression despite extensive laser surgery73–78. This method of surgery may be particularly helpful for individuals who are not candidates for pars plana vitrectomy with intraoperative PRP.
Pars plana vitrectomy
Pars plana vitrectomy is an important surgical option for complicated proliferative diabetic retinopathy (Fig. 61.7). Indications include non-clearing vitreous hemorrhage, retinal detachment, premacular fibrosis, macular heterotopia, proliferative diabetic retinopathy/iris neovascularization unresponsive to conventional therapy, hemolytic glaucoma, and dense cataract impeding photocoagulation. The technique of vitrectomy is described in other chapters on vitreoretinal surgery.
1 Diabetic Retinopathy Study Research Group. Preliminary report on effects of photocoagulation therapy. Am J Ophthalmol. 1976;81:383-396.
2 The Diabetic Retinopathy Study Research Group. Photocoagulation treatment of proliferative diabetic retinopathy. Clinical application of Diabetic Retinopathy Study (DRS) findings. DRS Report Number 8. Ophthalmology. 1981;88:583-600.
3 Diabetic Retinopathy Study Research Group. Four risk factors for severe visual loss in diabetic retinopathy. The third report from the Diabetic Retinopathy Study. Arch Ophthalmol. 1979;97:654-655.
4 Early Treatment Diabetic Retinopathy Study Research Group. Early photocoagulation for diabetic retinopathy. ETDRS Report Number 9. Ophthalmology. 1991;98:766-785.
5 Diabetic Retinopathy Vitrectomy Study Research Group (Appended). Early vitrectomy for severe vitreous hemorrhage in diabetic retinopathy. Arch Ophthalmol. 1985;103:1644-1652.
6 The Diabetic Retinopathy Vitrectomy Study Research Group. Early vitrectomy for severe proliferative diabetic retinopathy in eyes with useful vision. Results of a randomized trial – Diabetic Retinopathy Vitrectomy Study Report 3. Ophthalmology. 1988;95:1307-1320.
7 The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977-986.
8 Klein R. The epidemiology of diabetic retinopathy. Findings from the Wisconsin epidemiologic study of diabetic retinopathy. Int Ophthalmol Clin. 1987;27:230-238.
9 Klein R, Knudtson MD, Lee KE, et al. The Wisconsin Epidemiologic Study of Diabetic Retinopathy. XXII the twenty-five-year progression of retinopathy in persons with type 1 diabetes. Ophthalmology. 2008;115:1859-1868.
10 Sloan FA, Belsky D, Ruiz DJr, et al. Changes in incidence of diabetes mellitus-related eye disease among US elderly persons, 1994–2005. Arch Ophthalmol.. 2008;126:1548-1553.
11 Shimizu K, Kobayashi Y, Muraoka K. Midperipheral fundus involvement in diabetic retinopathy. Ophthalmology. 1981;88:601-612.
12 Early Treatment Diabetic Retinopathy Study Research Group. Fluorescein angiographic risk factors for progression of diabetic retinopathy. ETDRS Report 12. Ophthalmology. 1991;98:834-840.
13 Aiello LP. Angiogenic pathways in diabetic retinopathy. N Engl J Med. 2005;353:839-841.
14 Early Treatment Diabetic Retinopathy Study Research Group. Fundus photographic risk factors for progression of diabetic retinopathy. ETDRS Report 12. Ophthalmology. 1991;98:823-833.
15 Davis MD. Vitreous contraction in proliferative diabetic retinopathy. Arch Ophthalmol. 1965;74:741-751.
16 Doft BH, Blankenship GW. Single versus multiple treatment sessions of argon laser panretinal photocoagulation for proliferative diabetic retinopathy. Ophthalmology. 1982;89:772-779.
17 The Krypton Argon Regression Neovascularization Study Research Group. Randomized comparison of krypton versus argon scatter photocoagulation for diabetic disc neovascularization. The Krypton Argon Regression Neovascularization Study Report 1. Ophthalmology. 1993;100:1655-1664.
18 Blankenship GW, Gerke E, Batle JF. Red krypton and blue–green argon laser diabetic panretinal photocoagulation. Graefe’s Arch Clin Exp Ophthalmol. 1989;227:364-368.
19 Bandello F, Brancato R, Trabucchi G, et al. Diode versus argon–green laser panretinal photocoagulation in proliferative diabetic retinopathy. A randomized study in 44 eyes with a long follow-up time. Graefe’s Arch Clin Exp Ophthalmol. 1993;231:491-494.
20 Seiberth V, Schatanek S, Alexandridis E. Panretinal photocoagulation in diabetic retinopathy. Argon versus dye laser coagulation. Graefe’s Arch Clin Exp Ophthalmol. 1993;231:318-322.
21 Blankenship GW. A clinical comparison of central and peripheral arson laser panretinal photocoagulation for proliferative diabetic retinopathy. Ophthalmology. 1988;95:170-177.
22 Doft BH, Blankenship GW. Retinopathy risk factor regression after laser panretinal photocoagulation for proliferative diabetic retinopathy. Ophthalmology. 1984;91:1453-1457.
23 Vander JF, Duker JS, Benson WE, et al. Long-term stability and visual outcome after favorable initial response of proliferative diabetic retinopathy to panretinal photocoagulation. Ophthalmology. 1991;98:1575-1579.
24 Vine AK. The efficacy of additional argon laser photocoagulation for persistent, severe proliferative diabetic retinopathy. Ophthalmology. 1985;92:1532-1537.
25 Doft BH, Metz DJ, Kelsey SF. Augmentation laser for proliferative diabetic retinopathy that fails to respond to initial panretinal photocoagulation. Ophthalmology. 1992;99:1728-1735.
26 Rogell GD. Incremental panretinal photocoagulation. Results in treating proliferative diabetic retinopathy. Retina. 1983;3:308-311.
27 Theodossiadis GP, Ismiridis KJ. Time span of regression or disappearance of optic disk neovascularization in proliferative diabetic retinopathy after panretinal argon laser photocoagulation and results over a two-year follow-up. Fortschr Ophthalmol. 1987;84:465-467.
28 Reddy VM, Zamora RL, Olk RJ. Quantitation of retinal ablation in proliferative diabetic retinopathy. Am J Ophthalmol. 1995;119:760-766.
29 Aylward GW, Pearson RV, Jagger JD, et al. Extensive argon laser photocoagulation in the treatment of proliferative diabetic retinopathy. Br J Ophthalmol. 1989;73:197-201.
30 Kaufman SC, Ferris FL, Seigel DG, et al. Factors associated with the visual outcome after photocoagulation for diabetic retinopathy. Diabetic Retinopathy Study Report 13. Invest Ophthalmol Vis Sci. 1989;30:23-28.
31 Yoshida A, Feke GT, Morales-Stoppello J, et al. Retinal blood flow alterations during progression of diabetic retinopathy. Arch Ophthalmol. 1983;101:225-227.
32 Feke GT, Green GJ, Goger DG, et al. Laser Doppler measurements of the effect of panretinal photocoagulation on retinal blood flow. Ophthalmology. 1982;89:757-762.
33 Oswald B, Oswald VH, Jutte A, et al. Measurement of flow – Physiologic parameters of retinal blood circulation in type 1 and 2 diabetics before and after photocoagulation. Graefe’s Arch Clin Exp Ophthalmol. 1985;223:154-157.
34 Grunwald JE, Riva CE, Brucker AJ, et al. Effect of panretinal photocoagulation on retinal blood flow in proliferative diabetic retinopathy. Ophthalmology. 1986;93:590-595.
35 Fujio N, Feke GT, Goger DG, et al. Regional retinal blood flow reduction following half fundus photocoagulation treatment. Br J Ophthalmol. 1994;78:335-338.
36 Wilson CA, Stefansson E, Clombers L, et al. Optic disk neovascularization and retinal vessel diameter in diabetic retinopathy. Am J Ophthalmol. 1988;106:131-134.
37 Grunwald JE, Brucker AJ, Petrig BL, et al. Retinal blood flow regulation and the clinical response to panretinal photocoagulation in proliferative diabetic retinopathy. Ophthalmology. 1989;96:1518-1522.
38 Molnar I, Poitry S, Tsacopoulas M, et al. Effect of laser photocoagulation on oxygenation of the retina in miniature pigs. Invest Ophthalmol Vis Sci. 1985;26:1410-1414.
39 Alder VA, Cringle SJ, Brown M. The effect of regional retinal photocoagulation on vitreal oxygen tension. Invest Ophthalmol Vis Sci. 1987;28:1078-1085.
40 Stefansson E, Machemer R, deJuan E, et al. Retinal oxygenation and laser treatment in patients with diabetic retinopathy. Am J Ophthalmol. 1992;113:36-38.
41 Novack RL, Stefansson E, Hatchell DL. The effect of photocoagulation on the oxygenation and ultrastructure of avascular retina. Exp Eye Res. 1990;50:289-296.
42 Pe’er J, Shweiki D, Itin A, et al. Hypoxia-induced expression of vascular endothelial growth factor by retinal cells is a common factor in neovascularizing ocular diseases. Lab Invest. 1995;72:638-645.
43 Adamis AP, Miller JW, Bernal MT, et al. Increased vascular endothelial growth factor levels in the vitreous of eyes with proliferative diabetic retinopathy. Am J Ophthalmol. 1994;118:445-450.
44 Aiello LP, Avery RL, Arrigg PG. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med. 1994;331:1480-1487.
45 Glaser BM, Campochiaro PA, Davis JL, Sato M. Retinal pigment epithelial cells release and inhibitor of neovascularization. Arch Ophthalmol. 1985;103:1870-1875.
46 Yoshimura N, Matsumoto M, Shimizu H. Photocoagulated human retinal pigment epithelial cells produce an inhibitor of vascular endothelial cell proliferation. Invest Ophthalmol Vis Sci. 1995;36:1686-1691.
47 Russell PW, Sekuler R, Fetkenhour C. Visual function after pan-retinal photocoagulation. A survey. Diabetes Care. 1985;8:57-63.
48 Kanski JJ. Anterior segment complications of retinal photocoagulation. Am J Ophthalmol. 1975;79:424-427.
49 Little HL. Complications of argon laser photocoagulation. A five-year study. Int Ophthalmol Clin. 1976;16:145-159.
50 Blondeau P, Pavan PR, Phelps CD. Acute pressure elevation following panretinal photocoagulation. Arch Ophthalmol. 1981;99:1239-1241.
51 Schiodte SN. A pressure-lowering effect of retinal xenon photocoagulation in normotensive diabetic eyes. Acta Ophthalmol. 1980;58:369-376.
52 Schiodte SN. Changes in eye tension after panretinal xenon arc and argon laser photocoagulation in normotensive diabetic eyes. Acta Ophthalmol. 1982;60:692-700.
53 Lobes LA, Bourgon P. Pupillary abnormalities induced by argon laser photocoagulation. Ophthalmology. 1985;92:234-236.
54 Lankhanpal V, Schocket SS, Richards RD, et al. Photocoagulation-induced lens opacity. Arch Ophthalmol. 1982;100:1068-1070.
55 McCanna P, Chandra SR, Stevens TS, et al. Argon laser-induced cataract as a complication of retinal photocoagulation. Arch Ophthalmol. 1982;100:1071-1073.
56 Ferris FL, Podgor MJ, Davis MD. The DRS Research Group. Macular edema in diabetic retinopathy study patients. DRS Report 12. Ophthalmology. 1987;94:754-760.
57 Francois J, Cambie E. Further vision deterioration after argon laser photocoagulation in diabetic retinopathy. Ophthalmologica. 1976;173:28-39.
58 McDonald HR, Schatz H. Visual loss following panretinal photocoagulation for proliferative diabetic retinopathy. Ophthalmology. 1985;92:388-393.
59 Seiberth V, Alexandridis E, Feng W. Function of the diabetic retina after panretinal argon laser coagulation. Graefe’s. Arch Clin Exp Ophthalmol. 1987;225:385-390.
60 Kleiner RC, Elman MJ, Murphy RP, et al. Transient severe visual loss after panretinal photocoagulation. Am J Ophthalmol. 1988;106:298-306.
61 Elliott A, Flanagan D. Macular detachment following laser treatment for proliferative diabetic retinopathy. Graefe’s Arch Clin Exp Ophthalmol. 1990;228:438-441.
62 Benson WE, Townsend RE, Pheasant TR. Choriovitreal and subretinal proliferations. Complications of photocoagulation. Ophthalmology. 1979;86:283-289.
63 Chandra SR, Bresnick GH, Davis MD, et al. Choroidovitreal neovascular ingrowth after photocoagulation for proliferative diabetic retinopathy. Arch Ophthalmol. 1980;98:1593-1599.
64 Wallow I, Johns K, Barry P, et al. Chorioretinal and choriovitreal neovascularization after photocoagulation for proliferative diabetic retinopathy. A clinicopathologic correlation. Ophthalmology. 1985;92:523-532.
65 Diabetic Retinopathy Study Research Group. Photocoagulation treatment of proliferative diabetic retinopathy. Relationship of adverse treatment effects to retinopathy severity. Diabetic Retinopathy Study Report Number 5. Dev Ophthalmol. 1981;2:248-261.
66 D’Amico DJ. Diabetic traction retinal detachments threatening the fovea and panretinal argon laser photocoagulation. Semin Ophthalmol. 1991;6:11-17.
67 Wade EC, Blankenship GW. The effect of short versus long exposure times of argon laser panretinal photocoagulation on proliferative diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 1990;228:226-231.
68 Swartz M, Apple DJ, Creel D. Sudden severe visual loss associated with peripapillary burns during panretinal argon photocoagulation. Br J Ophthalmol. 1983;67:517-519.
69 Smith JL. Retrobulbar bupivacaine can cause respiratory arrest. Ann Ophthalmol. 1982;14:1005-1006.
70 Hay A, Flynn HW, Hoffman JI, et al. Needle penetration of the globe during retrobulbar and peribulbar injections. Ophthalmology. 1991;98:1017-1024.
71 Jardeleza MS, Miller JW. Review of anti-VEGF therapy in proliferative diabetic retinopathy. Semin Ophthalmol. 2009;24:87-92.
72 Schwartz SG, Flynn HWJr, Scott IU. Pharmacotherapy for diabetic retinopathy. Expert Opin Pharmacother. 2009;10:1123-1131.
73 Schimek RA, Spencer R. Cryopexy treatment of proliferative diabetic retinopathy. Retinal cryoablation in patients with severe vitreous hemorrhage. Arch Ophthalmol. 1979;97:1276-1280.
74 Hilton GF. Panretinal cryotherapy for diabetic rubeosis. Arch Ophthalmol. 1979;97:776-779.
75 Daily MJ, Gieser RG. Treatment of proliferative diabetic retinopathy with panretinal cryotherapy. Ophthalmic Surg. 1984;15:741-745.
76 Segato T, Piermarocchi S, Midena E, et al. Retinal cryotherapy in the management of proliferative diabetic retinopathy. Am J Ophthalmol. 1984;98:240-241.
77 Mosier MA, Del Piero E, Gheewala SM. Anterior retinal cryotherapy in diabetic hemorrhage. Am J Ophthalmol. 1985;100:440-444.
78 Benedett R, Olk RJ, Arribas NP, et al. Transconjunctival anterior retinal cryotherapy for proliferative diabetic retinopathy. Ophthalmology. 1987;94:612-619.