Chapter 19 Prolactinomas
Prolactinomas are the most common type of functioning pituitary tumors, accounting for approximately 50% to 60% of functioning pituitary adenomas.1,2 These prolactin-secreting adenomas are the second most common type of pituitary tumor overall after nonfunctioning adenomas and represent 30% to 40% of all pituitary tumors.3,4 Their prevalence is generally thought to be up to 100 cases per 1 million people,5 and one meta-analysis of the literature estimated a much higher prevalence rate of approximately 17% of the population, with a third of tumors staining positive for prolactin.6
The objectives for treatment of hyperprolactinemia due to prolactinomas are normalization of the hyperprolactinemic state, preservation of residual pituitary function, reduction of tumor mass, and prevention of disease recurrence. Pharmacologic therapy with dopamine agonists remains the mainstay of treatment;7–10 however, surgical removal of prolactinomas remains an important treatment in those patients who cannot tolerate or are resistant to medical therapy. Surgery may play a curative role in some cases of microprolactinomas.1,11,12 The current surgical management strategies for patients with prolactinoma are discussed in this chapter.
Clinical Presentation
Patients with prolactinomas can present with clinical manifestations of hyperprolactinemia, endocrine dysfunction, local mass effect, or pituitary apoplexy (Table 19-1). The biologic effects of hyperprolactinemia predominantly affect the gonadal axis and breast tissue. The primary effects of prolactin are to stimulate lactation, but this hormone can also promote deleterious effects on the gonadal axis. Excessive prolactin centrally inhibits hypothalamic production of gonadotropin-releasing hormone and subsequently inhibits secretion of luteinizing hormone and follicle-stimulating hormone, resulting in infertility and hypogonadism.13,14 Clinical presentation appears to differ between males and females.
| Hyperprolactinemia |
| Amenorrhea (females) |
| Galactorrhea (females) |
| Gonadal dysfunction |
| Infertility |
| Decreased libido |
| Impotence (males) |
| Osteoporosis |
| Delayed puberty (adolescents) |
| Mass Effect |
| Headaches |
| Visual acuity and field loss |
| Diplopia |
| Ophthalmoplegia |
| Facial numbness |
| Facial pain |
| Hypothalamic impairment |
| Hydrocephalus |
| Hypopituitarism |
| Pituitary apoplexy |
In premenopausal women, the most common presentation includes galactorrhea, amenorrhea, and infertility. Although prolactinomas are equally distributed at autopsy, women are four times more likely to develop symptoms than men. Because of these readily identifiable symptoms, women generally present earlier in the course of the disease and have smaller tumors at the time of diagnosis. Hyperprolactinemia may not be detected until after discontinuation of an oral contraceptive.15 Approximately 5% of women with primary amenorrhea and 25% of women with secondary amenorrhea (excluding pregnancy) are found to have a prolactinoma,16 and this incidence rises to 70% to 80% when galactorrhea accompanies amenorrhea.17 Galactorrhea is present in 50% to 80% of women with hyperprolactinemia.18–20 In men harboring prolactinomas, hypogonadal manifestations of decreased libido and impotence are often attributed to aging or functional causes rather than hyperprolactinemia, delaying detection until the tumor becomes large (mostly macroprolactinomas) and causes local mass effect on neighboring structures. Compression on the optic chiasm, cavernous sinus, and pituitary gland can result in symptoms of visual loss (visual acuity and/or visual field loss), cranial nerve dysfunction resulting in ophthalmoplegia, and/or hypopituitarism, respectively.21 Galactorrhea and gynecomastia are extremely rare in men. About 2% of all men with impotence have a prolactinoma.16 Low testosterone levels can result from either hyperprolactinemia or hypopituitarism secondary to mass effect on the normal pituitary gland.
In young individuals, prolactinomas are the most common type of pituitary adenoma overall, particularly in adolescents older than 12 years of age.22,23 Prolactinomas are more common in girls and present with primary amenorrhea. Boys tend to have much larger tumors and higher preoperative prolactin levels than girls. They can also present with gynecomastia and hypogonadism and tend to have neurologic signs of mass effect.23 Growth retardation and short stature can be common presentations because growth hormone is the first hormone to undergo hyposecretion in pituitary adenomas.22 The signs and symptoms of hypogonadism in prepubescent children and post-menopausal patients, however, are clinically absent.24–26 Adolescent children can present with delay or failure of sexual/reproductive development.21,27–30
Persistent gonadal dysfunction resulting in estrogen or testosterone deficiency from prolonged hyperprolactinemia that is left untreated can result in premature osteoporosis in patients of either sex.17,31–34 These important but often overlooked effects of hyperprolactinemia are additional arguments for treating patients who may not be concerned about sexual dysfunction or fertility. Hyperprolactinemia-induced osteopenia is progressive and correlates with the duration of hypogonadism.35 If treatment is undertaken, normalization of hyperprolactinemia can impede further bone loss; however, although bone density increases to a certain extent, it may not necessarily return to normal baseline values.33,36–38
Tumor compression on neighboring structures can result in symptoms from mass effect such as visual acuity and visual field deficits; cranial nerve palsies resulting in diplopia, ophthalmoplegia, and facial numbness; or impedance of cerebrospinal fluid flow resulting in obstructive hydrocephalus. Compression of the normal pituitary gland can result in hypopituitarism, namely hypocortisolism and hypothyroidism. Pituitary apoplexy from an acute hemorrhage and/or infarction into a prolactinoma can cause rapid enlargement of the tumor, resulting in hypopituitarism and acute compression of the sellar and parasellar structures.39,40
Diagnosis
The diagnosis of a prolactin-secreting adenoma is determined by elevation of serum prolactin with radiographic evidence of a pituitary adenoma on magnetic resonance (MR) imaging of the pituitary region with and without gadolinium enhancement.41 Interpreting serum prolactin levels in conjunction with radiographic findings is important in making the correct diagnosis of a prolactinoma to ensure proper treatment. Serum prolactin levels generally correlate with tumor size: Levels from 100 to 250 ng/ml often signal a microprolactinoma (<10 mm), whereas levels greater than 250 ng/ml generally reflect a macroprolactinoma (>10 mm).4,42 Extremely elevated serum prolactin levels that are greater than 1000 ng/ml may correlate with a macroprolactinoma that has invaded the cavernous sinus. Macroadenomas associated with a mildly elevated prolactin level, roughly 50 to 125 ng/ml, can be attributed to the stalk-section effect from a nonfunctioning pituitary adenoma. This is because prolactin is under tonic inhibition from the hypothalamus, and lesions or compression of the pituitary stalk may interfere with this inhibition, resulting in mild elevation of prolactin. However, it is important to rule out the “hook effect” in cases of giant and invasive macroprolactinomas in which the serum prolactin level is falsely low (25 to 150 ng/ml). This is due to excessive serum prolactin saturating the binding sites of the two-site (monoclonal “sandwich”) technique resulting in falsely normal levels of prolactin. Subsequent dilutional testing of prolactin samples can counteract this assay phenomenon and prevent incorrect diagnosis.43–46
The diagnosis of prolactinoma requires that other causes of hyperprolactinemic states (either physiologic or pathologic) and other mass lesions in the sellar and parasellar region are ruled out (Table 19-2).13,14,42 Most cases of hyperprolactinemia can be ruled out on the basis of the history and physical examination, a pregnancy test, and thyroid and renal function tests. MR imaging with gadolinium enhancement should then be obtained to confirm the diagnosis of a prolactinoma. Other pituitary-related serum hormone levels (growth hormone, insulin-like growth factor-1, fasting AM cortisol, adrenocorticotrophic hormone, luteinizing hormone, follicular stimulating hormone, sex hormones, and thyroid function tests) can be used to test anterior pituitary function in all patients with a radiographically confirmed pituitary adenoma.
| Physiologic |
| Exercise |
| Stress |
| Pregnancy |
| Breast feeding (suckling reflex) |
| Pharmacologic |
| Antidepressants (tricyclic, MAO inhibitors, SSRIs) |
| Antihypertensives (a-methyldopa, reserpine, verapamil) |
| Neuroleptics (phenothiazines, haloperidol) |
| Metoclopramide |
| H-2 blockers |
| Sellar/Parasellar Lesions |
| Prolactinomas |
| Nonfunctioning pituitary macroadenomas with “stalk effect” |
| Craniopharyngiomas |
| Rathke cleft cysts |
| Meningiomas |
| Germinomas |
| Sarcoidosis |
| Lymphocytic hypophysitis |
| Histiocytosis X |
| Metastasis |
| Primary empty sella syndrome |
| Other Disease States |
| Ectopic secretion of prolactin |
| Primary hypothyroidism |
| Hypothalamic dysfunction |
| Chronic renal failure |
| Cirrhosis |
| Chest wall lesions (trauma, surgery, herpes zoster) |
| Seizures |
Medical Treatment
The primary goals of treatment for prolactinomas are to normalize hyperprolactinemia and its clinical sequelae, restore fertility, relieve tumor mass effect, preserve residual pituitary function, and prevent disease recurrence or progression.41 For smaller tumors, such as microprolactinomas (tumors <10 mm), removal of tumor mass is less concerning since these are usually not large enough to produce symptoms related to mass effect. Studies have demonstrated a lack of tumor growth in the vast majority of patients with microprolactinomas, which may remain unchanged throughout the patient’s life.47–49 Thus, in a patient with a microprolactinoma with only mild elevation of prolactin who has normal anterior pituitary function and no desire for pregnancy, observation can be a reasonable option.4
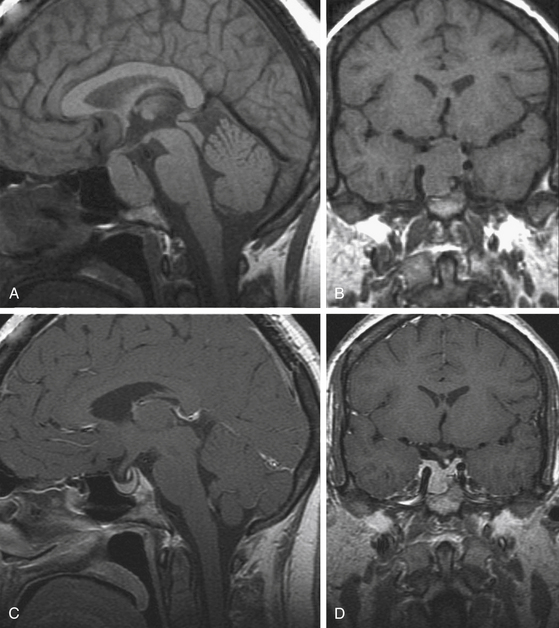
The first-line of treatment for prolactinomas is pharmacological intervention with dopamine receptor agonists, which bind to D2 receptors on lactotrophs to inhibit prolactin synthesis and release and reduce tumor volume.50,51 The agents that are currently approved for use in the United States are bromocriptine and cabergoline. Bromocriptine is very effective in normalizing prolactin levels in more than 90% of patients, significant reducing tumor mass in approximately 85% of patients,7,52–54 and restoring gonadal and anterior pituitary functions in over 80% of patients. Most female patients resume menstruation within 6 months of initiating therapy. Tumor shrinkage can occur rapidly within several days, decompressing the visual apparatus in patients with macroprolactinomas who present with visual deficits (Fig. 19-1).16,52,55 About 5% to 10% of patients may not be able to tolerate side effects of bromocriptine, including dizziness, nausea, arrhythmias, gastrointestinal discomfort, and orthostatic hypotension. About 10% to 25% patients are partially or totally resistant to bromocriptine.56–58
More recently, cabergoline has become the preferred first-line agent.59 Cabergoline is associated with less frequent and less severe side effects and is easier to administer than bromocriptine, although it is more expensive.60 In one study, cabergoline normalized serum prolactin in 84% of bromocriptine-intolerant patients and in 70% of bromocriptine-resistant patients.61 Cabergoline has been shown to be more effective in shrinking macroprolactinomas in naive patients than in patients pretreated with other dopamine agonists.62
After normalization of serum prolactin levels has been sustained for at least 2 years with adequate reduction and stabilization of tumor size, dopamine agonist therapy can be tapered to lower doses that continue to control hyperprolactinemia and tumor growth. In general, dopamine agonist therapy must be continued for life, and cessation of therapy usually results in recurrent hyperprolactinemia and tumor enlargement.63–65 However, in one study, Colao et al.5 reported sustained normalization of prolactin levels in 69% of patients with microprolactinomas and in 64% of macroprolactinomas without evidence of new tumor growth. Although there was no evidence of tumor recurrence in the face of recurrent hyperprolactinemia, the follow-up was relatively short and probably insufficient to detect delayed tumor recurrence.66 Given the above data, we currently continue all patients on cabergoline for 2 to 3 years. If there is documentation of tumor disappearance and normalization of prolactin levels, we will discontinue medication and monitor the patient with twice-yearly review of prolactin levels and annual MR imaging scans. Recurrence detected by increasing prolactin levels would then indicate the need for repeat treatment with dopamine agonists.
It should be emphasized that primary resistance to dopamine agonist therapy occurs in 10% to 15% of prolactinomas67 and secondary resistance may also rarely occur.68 Thus, all patients treated with dopamine agonists should undergo serial prolactin measurements and yearly MR imaging surveillance studies.
In patients desiring pregnancy, bromocriptine is the drug of choice because of its safety record.69,70 The experience with cabergoline is more limited, and it is not used as a primary therapy for infertility, although some reports state that it does not increase the risk of teratogenesis.71,72 Once menses is restored, normal conception and pregnancy may follow. If a menstrual cycle has been missed, a pregnancy test should be obtained and bromocriptine or cabergoline use should be discontinued immediately.18 If the patient develops symptomatic tumor enlargement during pregnancy, bromocriptine can be safely initiated (Fig. 19-1).73–76 There does not appear to be an increased risk of congenital anomalies or spontaneous abortions with use of bromocriptine in this manner.77 Alternatively, surgical debulking compression may also be an option if the patient does not respond to medical therapy. Patients with pre-existing macroprolactinomas tend to have a higher risk of symptomatic tumor enlargement (15%–35%)77 than those with microprolactinomas (0.5%–1%).3,55
Surgical Treatment
Indications
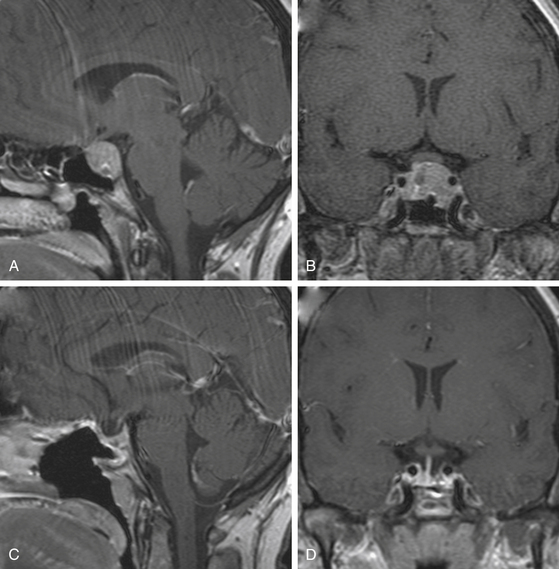
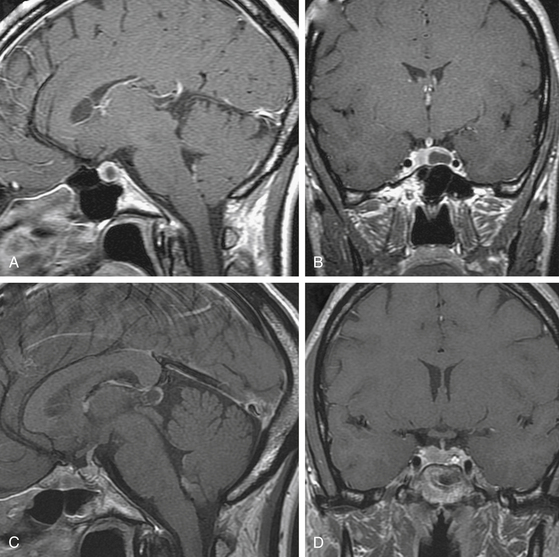
The efficacy and success of dopamine agonist therapy has limited the indications of surgical therapy for prolactinomas. Furthermore, surgery is rarely curative in patients with macroprolactinomas and so should be reserved for patients who cannot tolerate the side effects of dopamine agonists or for whom medical therapy is ineffective (those with persistent hyperprolactinemia, progressive tumor enlargement, and persistent tumor mass effect despite maximal medical therapy) (Fig. 19-2).11,78–80 Surgery may also be an option for treatment of microprolactinomas in patients who do not wish or cannot afford to be on life-long medical therapy (Fig. 19-3).1,12,66,81,82 For patients whose tumors are more likely to be cured surgically (microprolactinomas or tumors with preoperative prolactin <200 ng/ml) and who desire fertility, surgery can be considered to limit the need for long-term medical therapy, and in some instances may be less costly.66 Surgery may also be indicated in patients who are dependent on anti-psychotic medications, because dopamine agonists can precipitate psychotic episodes.83 Surgical resection should also be considered if impaired visual function or cranial nerve palsies are not immediately responsive to medical treatment, especially in cases of pituitary apoplexy, and in patients who present with a spontaneous cerebrospinal fluid leak after tumor shrinkage with dopamine agonist therapy.4
Surgical Approaches and Outcomes
The trans-sphenoidal approach, either microscopic or endoscopic, is the preferred surgical route and is associated with low rates of morbidity and mortality.84,85 The extended trans-sphenoidal approach may be used in some cases where the tumor is located beyond the confines of the sella.86–89 Even in giant prolactinomas, a trans-sphenoidal approach should be considered first; however, a transcranial approach should be considered if there is extensive tumor extension lateral into the sylvian fissure.80,89 The details of the trans-sphenoidal and transcranial approaches are well described elsewhere.89–91
Surgical outcomes appear to be correlated with tumor size and preoperative serum prolactin level.4,11 In a large series of 489 prolactinomas surgically removed using the trans-sphenoidal approach, the overall remission rate was 42%.4 For microadenomas, the remission rate was 82% if the preoperative prolactin level was less than 200 ng/ml and 50% if the level was greater than 200 ng/ml. For macroadenomas, the remission rate ranged from 15% to 52% and was 0% for giant adenomas. In the series by Amar et al.,11 the immediate postoperative biochemical remission rate for microadenomas was 91%, which remained stable at 5 years after surgery. For macroadenomas, the remission rate immediately after surgery was 69%, which dropped to 33% at 5 years. The remission rates were also higher in patients with preoperative prolactin levels less than 200 ng/ml (86%) than in those with levels above 200 ng/ml (45%). Given this fact, we are reluctant to offer surgical treatment for microprolactinomas associated with a prolactin level of greater than 200 ng/ml in the absence of another indication (failure of medical therapy, intolerance to medical therapy, etc.).
Immediate postoperative serum prolactin levels have been used as potential predictors of biochemical cure. In a series of 222 patients who underwent trans-sphenoidal surgery for prolactinomas, fasting morning serum prolactin levels obtained on the first postoperative day that were less than 10 ng/ml predicted a 100% cure rate in microprolactinomas and a 93% cure rate in macroprolactinomas. In patients who had a postoperative level between 10 and 20 ng/ml, 100% of microprolactinomas and 0% of macroprolactinomas achieved biochemical remission.11
Although surgery may not be curative in most macroprolactinomas, surgical debulking has been used as a cytoreductive strategy to lower the dosage and increase the responsiveness of dopamine agonist therapy.3,55 In patients with giant and invasive prolactinomas, pretreatment with bromocriptine resulting in significant tumor reduction may improve the cure rate with subsequent surgery.53 However, Landolt92 has reported that prior long-term treatment with dopamine agonists alters the tumor consistency by making the tumor more fibrous, preventing complete resection. We do not offer surgery to patients with macroprolactinomas who have not already tried medical therapy. The current indications used for surgery in these cases include lack of response to medication, development of resistance to medical therapy, intolerance to medical therapy, or cerebrospinal fluid leak after shrinkage of large tumor.
Surgery for Microprolactinomas
Trans-sphenoidal removal of microprolactinomas by an experienced pituitary surgeon can be considered in patients who do not wish to be on life-long medical therapy (Fig. 19-3).1,11,12,81 The choice of trans-sphenoidal surgery for microprolactinomas should take into account the size and location of the tumor, the preoperative serum prolactin level, the age of the patient, the desire for restoration of fertility, the efficacy and tolerability of the dopamine agonists, the patient’s desire to be free of long-term medical therapy, and the experience of the surgeon. Surgery should not be considered unless a complete removal with biochemical cure of the microprolactinoma is an expected outcome. The presence of a symptomatic microprolactinoma, especially in a young patient, should remain an indication for microsurgical or endoscopic trans-sphenoidal resection.
In patients with microprolactinomas with serum prolactin levels below 200 ng/ml, trans-sphenoidal surgery performed by experienced pituitary surgeons at high-volume centers offers over a 90% chance of biochemical and oncologic cure11,12,93,94 with minimal risks of morbidity and mortality of less than 1%.85,88 Amar et al.11 achieved a cure rate of 91% in patients with microprolactinomas. Similarly, although Tyrrell et al.78 found that women with preoperative prolactin levels above 200 ng/ml and larger, more invasive prolactinomas had poorer outcomes (37%–41% cure rate), long-term remission was achieved in patients with microadenomas and noninvasive macroadenomas (moderate suprasellar extension and focal sphenoid sinus invasion). The continued evolution of endoscopic approaches for tumor resection, which are somewhat less invasive, may reduce the morbidity rate of the surgical approach even further.95 The financial cost of treatment over a 10-year period is similar in uncomplicated surgical cases to that of long-term dopamine agonist therapy.96
Pituitary Apoplexy
Although most surgeons recommend emergent trans-sphenoidal decompression and administration of glucocorticoids for patients who present with pituitary apoplexy,39,40,97–100 some have reported excellent results with dopamine agonist therapy in patients with pituitary apoplexy in prolactinomas.39,101 In the absence of visual deficits, an initial trial of dopamine agonist therapy in conjunction with glucocorticoids may be considered. However, the clinician should be prepared for urgent or emergent trans-sphenoidal surgery if visual loss is severe at presentation or if visual loss does not improve after dopamine agonist therapy.
Stereotactic Radiosurgery
Stereotactic radiosurgery is an important modality in the armamentarium of treatment for prolactinomas as a secondary treatment after failed trans-sphenoidal surgery or failed medical therapy or as a primary treatment for prolactinomas in patients who are reluctant to undergo surgical resection but cannot tolerate medical therapy.102–109 Current technology allows high-resolution targeting and dose planning with excellent accuracy. The preliminary data regarding tumor control and normalization of functioning pituitary adenomas after radiosurgery appear favorable. The proximity of the pituitary gland to the region of radiosurgical treatment, however, may carry the risk of hypopituitarism. Longer follow-up is necessary to assess the likelihood of this complication.
Landolt and Lomax107 reported a 25% rate of normalization of hyperprolactinemia after gamma knife radiosurgery for residual prolactinoma after failed medical or surgical therapy. Eleven patients experienced improvement of prolactin levels decreased by at least 20%, while the treatment failed in four patients who were receiving dopamine agonist therapy at the time of radiosurgical treatment. Pollock et al.,110 showed that absence of hormone-suppressive medications at the time of radiosurgery correlated with an endocrine cure, supporting the theory by Landolt and Lomax that dopamine agonist therapy may offer some radioprotective effect.107 Pan et al.109 reported that tumor growth was controlled in all but two of 128 patients with greater than 2-year follow-up. Biochemical cure was achieved in 52% of patients and improvement in 28%.
Castinetti et al.111 reported a 46.6% normalization rate of hyperprolactinemia after radiosurgery in 15 patients with a mean follow-up of 96 months. Mean time to remission was approximately 24 to 28 months. Those that achieved remission had a smaller mean tumor volume and lower mean initial prolactin level than those that remained uncured. This raises the possible role of surgical or chemical debulking prior to radiosurgery to decrease the target size to achieve better radiosurgical results. Radiation-induced hypopituitarism was observed in 23% after a mean time of 48 to 96 months.
In another study of 35 prolactinomas treated with gamma knife radiosurgery, normoprolactinemia was achieved in 37.1% who discontinued dopamine agonists after radiosurgery, and in 42.9% who continued medical therapy after radiosurgery.112 Median time to normalization was 96 months which suggests that the effects of treatment acts slowly and requires long-term follow-up.
Pituitary Transposition (Hypophysopexy)
Surgical debulking with postoperative stereotactic radiosurgery of residual cavernous sinus tumor may provide another treatment option in patients with macroprolactinomas with cavernous sinus invasion that are refractory to medical therapy. To protect the pituitary gland during stereotactic radiosurgery, we perform a technique for pituitary gland transposition (hypophysopexy) after the tumor is adequately debulked.113,114 This technique involves transposing the normal pituitary gland away from the cavernous sinus tumor and interposing a fat graft between the normal gland and the tumor in the cavernous sinus to increase the distance between the normal pituitary gland and residual tumor to facilitate radiosurgical treatment of the tumor, thereby reducing the effective biological dose to the normal pituitary gland. The goal of this reduction is to decrease the likelihood of the patient developing hypopituitarism.113,114 Long-term results indicate a very low incidence of new hypopituitarism following the use of this technique (WT Couldwell, in prep.).
Amar A.P., Couldwell W.T., Chen J.C., et al. Predictive value of serum prolactin levels measured immediately after transsphenoidal surgery. J Neurosurg. 2002;97:307-314.
Barkan A.L., Chandler W.F. Giant pituitary prolactinoma with falsely low serum prolactin: the pitfall of the “high-dose hook effect”: Case report. Neurosurgery. 1998;42:913-915. discussion 915–916
Colao A., Annunziato L., Lombardi G. Treatment of prolactinomas. Ann Med. 1998;30:452-459.
Colao A., Di Sarno A., Cappabianca P., et al. Withdrawal of long-term cabergoline therapy for tumoral and nontumoral hyperprolactinemia. N Engl J Med. 2003;349:2023-2033.
Colao A., Di Sarno A., Landi M.L., et al. Macroprolactinoma shrinkage during cabergoline treatment is greater in naive patients than in patients pretreated with other dopamine agonists: a prospective study in 110 patients. J Clin Endocrinol Metab. 2000;85:2247-2252.
Colao A., Di Sarno A., Sarnacchiaro F., et al. Prolactinomas resistant to standard dopamine agonists respond to chronic cabergoline treatment. J Clin Endocrinol Metab. 1997;82:876-883.
Colao A., Loche S., Cappa M., et al. Prolactinomas in children and adolescents. Clinical presentation and long-term follow-up. J Clin Endocrinol Metab. 1998;83:2777-2780.
Comtois R., Robert F., Hardy J. Immunoradiometric assays may miss high prolactin levels. Ann Intern Med. 1993;119:173.
Couldwell W.T., Rovit R.L., Weiss M.H. Role of surgery in the treatment of microprolactinomas. Neurosurg Clin North Am. 2003;14:89-92. vii
Couldwell W.T., Weiss M.H. Medical and surgical management of microprolactinoma. Pituitary. 2004;7:31-32.
Landolt A.M., Lomax N. Gamma knife radiosurgery for prolactinomas. J Neurosurg. 2000;93(suppl 3):14-18.
Liu J.K., Couldwell W.T. Contemporary management of prolactinomas. Neurosurg Focus. 2004;16:E2.
Molitch M.E. Medical management of prolactin-secreting pituitary adenomas. Pituitary. 2002;5:55-65.
Molitch M.E. Management of prolactinomas during pregnancy. J Reprod Med. 1999;44:1121-1126.
Molitch M.E. Diagnosis and treatment of prolactinomas. Adv Intern Med. 1999;44:117-153.
Nomikos P., Buchfelder M., Fahlbusch R. Current management of prolactinomas. J Neurooncol. 2001;54:139-150.
Schlechte J.A. Clinical practice. Prolactinoma. N Engl J Med. 2003;349:2035-2041.
Shrivastava R.K., Arginteanu M.S., King W.A., et al. Giant prolactinomas: Clinical management and long-term follow-up. J Neurosurg. 2002;97:299-306.
Thorner M.O., Perryman R.L., Rogol A.D., et al. Rapid changes of prolactinoma volume after withdrawal and reinstitution of bromocriptine. J Clin Endocrinol Metab. 1981;53:480-483.
Turner T.H.E., Adams C.B., Wass J.A. Trans-sphenoidal surgery for microprolactinoma: an acceptable alternative to dopamine agonists? Eur J Endocrinol. 1999;140:43-47.
Tyrrell J.B., Lamborn K.R., Hannegan L.T., et al. Transsphenoidal microsurgical therapy of prolactinomas: initial outcomes and long-term results. Neurosurgery. 1999;44:254-261. discussion 261–263
Vance M.L., Thorner M.O. Prolactinomas. Endocrinol Metab Clin North Am. 1987;16:731-753.
Wang M.Y., Weiss M.H. Is there a role for surgery for microprolactinomas? Semin Neurosurg. 2001;12:289-294.
Weiss M.H., Teal J., Gott P., et al. Natural history of microprolactinomas: six-year follow-up. Neurosurgery. 1983;12:180-183.
Weiss M.H., Wycoff R.R., Yadley R., et al. Bromocriptine treatment of prolactin-secreting tumors: Surgical implications. Neurosurgery. 1983;12:640-642.
1. Wang M.Y., Weiss M.H. Is there a role for surgery for microprolactinomas? Semin Neurosurg. 2001;12:289-294.
2. Colao A. Pituitary tumours: the prolactinoma. Best Pract Res Clin Endocrinol Metab. 2009;23:575-596.
3. Vance M.L., Thorner M.O. Prolactinomas. Endocrinol Metab Clin North Am. 1987;16:731-753.
4. Nomikos P., Buchfelder M., Fahlbusch R. Current management of prolactinomas. J Neurooncol. 2001;54:139-150.
5. Colao A., Di Sarno A., Cappabianca P., et al. Withdrawal of long-term cabergoline therapy for tumoral and nontumoral hyperprolactinemia. N Engl J Med. 2003;349:2023-2033.
6. Ezzat S., Asa S.L., Couldwell W.T., et al. The prevalence of pituitary adenomas: a systematic review. Cancer. 2004;101:613-619.
7. Bevan J.S., Webster J., Burke C.W., et al. Dopamine agonists and pituitary tumor shrinkage. Endocr Rev. 1992;13:220-240.
8. Vance M.L., Evans W.S., Thorner M.O. Drugs five years later. Bromocriptine. Ann Intern Med. 1984;100:78-91.
9. Molitch M.E. Medical management of prolactin-secreting pituitary adenomas. Pituitary. 2002;5:55-65.
10. Molitch M.E. Medical treatment of prolactinomas. Endocrinol Metab Clin North Am. 1999;28:143-169. vii
11. Amar A.P., Couldwell W.T., Chen J.C., et al. Predictive value of serum prolactin levels measured immediately after transsphenoidal surgery. J Neurosurg. 2002;97:307-314.
12. Couldwell W.T., Rovit R.L., Weiss M.H. Role of surgery in the treatment of microprolactinomas. Neurosurg Clin North Am. 2003;14:89-92. vii
13. Blackwell Hyperprolactinemia R.E. Evaluation and management. Endocrinol Metab Clin North Am. 1992;21:105-124.
14. Biller B.M. Diagnostic evaluation of hyperprolactinemia. J Reprod Med. 1999;44:1095-1099.
15. Pituitary Adenoma Study Group. Pituitary adenomas and oral contraceptives: a multicenter case-control study. Fertil Steril. 1983;39:753-760.
16. Weiss M. Pituitary tumors: an endocrinological and neurosurgical challenge. Clin Neurosurg. 1992;39:114-122.
17. Franks S., Murray M.A., Jequier A.M., et al. Incidence and significance of hyperprolactinaemia in women with amenorrhea. Clin Endocrinol (Oxf). 1975;4:597-607.
18. Schlechte J.A. Clinical practice. Prolactinoma. N Engl J Med. 2003;349:2035-2041.
19. Schlechte J., Sherman B., Halmi N., et al. Prolactin-secreting pituitary tumors in amenorrheic women: a comprehensive study. Endocr Rev. 1980;1:295-308.
20. Touraine P., Plu-Bureau G., Beji C., et al. Long-term follow-up of 246 hyperprolactinemic patients. Acta Obstet Gynecol Scand. 2001;80:162-168.
21. Carter J.N., Tyson J.E., Tolis G., et al. Prolactin-screening tumors and hypogonadism in 22 men. N Engl J Med. 1978;299:847-852.
22. Kunwar S., Wilson C.B. Pediatric pituitary adenomas. J Clin Endocrinol Metab. 1999;84:4385-4389.
23. Mindermann T., Wilson C.B. Pediatric pituitary adenomas. Neurosurgery. 1995;36:259-268. discussion 69
24. Dyer E.H., Civit T., Visot A., et al. Transsphenoidal surgery for pituitary adenomas in children. Neurosurgery. 1994;34:207-212. discussion 12
25. Maira G., Anile C. Pituitary adenomas in childhood and adolescence. Can J Neurol Sci. 1990;17:83-87.
26. Shringi M.S., Vaidya R.A., Gogate J. Prolactin producing pituitary tumors in postmenopausal patients—‟infrequent or infrequently recognized”. Fertil Steril. 1998;69:603-605.
27. Artese R., D’Osvaldo D.H., Molocznik I., et al. Pituitary tumors in adolescent patients. Neurol Res. 1998;20:415-417.
28. Colao A., Loche S., Cappa M., et al. Prolactinomas in children and adolescents. Clinical presentation and long-term follow-up. J Clin Endocrinol Metab. 1998;83:2777-2780.
29. Duntas L.H. Prolactinomas in children and adolescents—consequences in adult life. J Pediatr Endocrinol Metab. 2001;14(suppl 5):1227-1232. discussion 61–2
30. Minniti G., Jaffrain-Rea M.L., Ferretti E., et al. Macroprolactinomas as cause of delayed puberty. A report of two cases and effects of medical therapy. Minerva Endocrinol. 1996;21:67-71.
31. Jacobs H.S., Franks S., Murray M.A., et al. Clinical and endocrine features of hyperprolactinaemic amenorrhoea. Clin Endocrinol (Oxf). 1976;5:439-454.
32. Greenspan S.L., Oppenheim D.S., Klibanski A. Importance of gonadal steroids to bone mass in men with hyperprolactinemic hypogonadism. Ann Intern Med. 1989;110:526-531.
33. Klibanski A., Greenspan S.L. Increase in bone mass after treatment of hyperprolactinemic amenorrhea. N Engl J Med. 1986;315:542-546.
34. Vartej P., Poiana C., Vartej I. Effects of hyperprolactinemia on osteoporotic fracture risk in premenopausal women. Gynecol Endocrinol. 2001;15:43-47.
35. Biller B.M., Baum H.B., Rosenthal D.I., et al. Progressive trabecular osteopenia in women with hyperprolactinemic amenorrhea. J Clin Endocrinol Metab. 1992;75:692-697.
36. Di Somma C., Colao A., Di Sarno A., et al. Bone marker and bone density responses to dopamine agonist therapy in hyperprolactinemic males. J Clin Endocrinol Metab. 1998;83:807-813.
37. Klibanski A., Biller B.M., Rosenthal D.I., et al. Effects of prolactin and estrogen deficiency in amenorrheic bone loss. J Clin Endocrinol Metab. 1988;67:124-130.
38. Schlechte J., el-Khoury G., Kathol M., et al. Forearm and vertebral bone mineral in treated and untreated hyperprolactinemic amenorrhea. J Clin Endocrinol Metab. 1987;64:1021-1026.
39. Liu J.K., Couldwell W.T. Pituitary apoplexy: diagnosis and management. Contemp Neurosurg. 2003;25:1-6.
40. Liu J.K., Rovit R.L., Couldwell W.T. Pituitary apoplexy. Semin Neurosurg. 2001;12:315-320.
41. Liu J.K., Couldwell W.T. Contemporary management of prolactinomas. Neurosurg Focus. 2004;16(4):E2.
42. Molitch M.E. Diagnosis and treatment of prolactinomas. Adv Intern Med. 1999;44:117-153.
43. Comtois R., Robert F., Hardy J. Immunoradiometric assays may miss high prolactin levels. Ann Intern Med. 1993;119:173.
44. Barkan A.L., Chandler W.F. Giant pituitary prolactinoma with falsely low serum prolactin: the pitfall of the ‟high-dose hook effect”: Case report. Neurosurgery. 1998;42:913-915. discussion 5–6
45. Frieze T.W., Mong D.P., Koops M.K. ‟Hook effect” in prolactinomas: Case report and review of literature. Endocr Pract. 2002;8:296-303.
46. St-Jean E., Blain F., Comtois R. High prolactin levels may be missed by immunoradiometric assay in patients with macroprolactinomas. Clin Endocrinol (Oxf). 1996;44:305-309.
47. March C.M., Kletzky O.A., Davajan V., et al. Longitudinal evaluation of patients with untreated prolactin-secreting pituitary adenomas. Am J Obstet Gynecol. 1981;139:835-844.
48. Schlechte J., Dolan K., Sherman B., et al. The natural history of untreated hyperprolactinemia: a prospective analysis. J Clin Endocrinol Metab. 1989;68:412-418.
49. Weiss M.H., Teal J., Gott P., et al. Natural history of microprolactinomas: six-year follow-up. Neurosurgery. 1983;12:180-183.
50. Colao A., Annunziato L., Lombardi G. Treatment of prolactinomas. Ann Med. 1998;30:452-459.
51. Colao A., di Sarno A., Pivonello R., et al. Dopamine receptor agonists for treating prolactinomas. Exp Opin Investig Drugs. 2002;11:787-800.
52. Cunnah D., Besser M. Management of prolactinomas. Clin Endocrinol (Oxf). 1991;34:231-235.
53. Weiss M.H., Wycoff R.R., Yadley R., et al. Bromocriptine treatment of prolactin-secreting tumors: surgical implications. Neurosurgery. 1983;12:640-642.
54. Ciccarelli E., Camanni F. Diagnosis and drug therapy of prolactinoma. Drugs. 1996;51:954-965.
55. Thorner M.O. Prolactinoma. West J Med. 1983;139:703-705.
56. Brue T., Pellegrini I., Priou A., et al. Prolactinomas and resistance to dopamine agonists. Horm Res. 1992;38:84-89.
57. Caccavelli L., Feron F., Morange I., et al. Decreased expression of the two D2 dopamine receptor isoforms in bromocriptine-resistant prolactinomas. Neuroendocrinology. 1994;60:314-322.
58. Pellegrini I., Rasolonjanahary R., Gunz G., et al. Resistance to bromocriptine in prolactinomas. J Clin Endocrinol Metab. 1989;69:500-509.
59. Colao A., Di Sarno A., Sarnacchiaro F., et al. Prolactinomas resistant to standard dopamine agonists respond to chronic cabergoline treatment. J Clin Endocrinol Metab. 1997;82:876-883.
60. Couldwell W.T., Weiss M.H. Medical and surgical management of microprolactinoma. Pituitary. 2004;7:31-32.
61. Verhelst J.A. Toward the establishment of a clinical prediction rule for response of prolactinomas to cabergoline. J Clin Endocrinol Metab. 1999;84:4747.
62. Colao A., Di Sarno A., Landi M.L., et al. Macroprolactinoma shrinkage during cabergoline treatment is greater in naive patients than in patients pretreated with other dopamine agonists: a prospective study in 110 patients. J Clin Endocrinol Metab. 2000;85:2247-2252.
63. Thorner M.O., Perryman R.L., Rogol A.D., et al. Rapid changes of prolactinoma volume after withdrawal and reinstitution of bromocriptine. J Clin Endocrinol Metab. 1981;53:480-483.
64. Zarate A., Canales E.S., Cano C., et al. Follow-up of patients with prolactinomas after discontinuation of long-term therapy with bromocriptine. Acta Endocrinol (Copenh). 1983;104:139-142.
65. Orrego J.J., Chandler W.F., Barkan A.L. Rapid re-expansion of a macroprolactinoma after early discontinuation of bromocriptine. Pituitary. 2000;3:189-192.
66. Couldwell W.T., Weiss M.H., Laws E.R.Jr. Prolactinomas. N Engl J Med. 2004;350:1054-1057. author reply 1054-1057
67. Kars M., Pereira A.M., Smit J.W., et al. Long-term outcome of patients with macroprolactinomas initially treated with dopamine agonists. Eur J Intern Med. 2009;20:387-393.
68. Behan L.A., Draman M.S., Moran C., et al. Secondary resistance to cabergoline therapy in a macroprolactinoma: a case report and literature review. Pituitary. 2009.
69. Rasmussen C., Bergh T., Nillius S.J., et al. Return of menstruation and normalization of prolactin in hyperprolactinemic women with bromocriptine-induced pregnancy. Fertil Steril. 1985;44:31-34.
70. Molitch M.E. Management of prolactinomas during pregnancy. J Reprod Med. 1999;44:1121-1126.
71. Robert E., Musatti L., Piscitelli G., et al. Pregnancy outcome after treatment with the ergot derivative, cabergoline. Reprod Toxicol. 1996;10:333-337.
72. Ricci E., Parazzini F., Motta T., et al. Pregnancy outcome after cabergoline treatment in early weeks of gestation. Reprod Toxicol. 2002;16:791-793.
73. Canales E.S., Garcia I.C., Ruiz J.E., et al. Bromocriptine as prophylactic therapy in prolactinoma during pregnancy. Fertil Steril. 1981;36:524-526.
74. van Roon E., van der Vijver J.C., Gerretsen G., et al. Rapid regression of a suprasellar extending prolactinoma after bromocriptine treatment during pregnancy. Fertil Steril. 1981;36:173-177.
75. Konopka P., Raymond J.P., Merceron R.E., et al. Continuous administration of bromocriptine in the prevention of neurological complications in pregnant women with prolactinomas. Am J Obstet Gynecol. 1983;146:935-938.
76. Bergh T., Nillius S.J., Enoksson P., et al. Bromocriptine-induced regression of a suprasellar extending prolactinoma during pregnancy. J Endocrinol Invest. 1984;7:133-136.
77. Molitch M.E. Pregnancy and the hyperprolactinemic woman. N Engl J Med. 1985;312:1364-1370.
78. Tyrrell J.B., Lamborn K.R., Hannegan L.T., et al. Transsphenoidal microsurgical therapy of prolactinomas: initial outcomes and long-term results. Neurosurgery. 1999;44:254-261. discussion 61–3
79. Wilson C.B. Role of surgery in the management of pituitary tumors. Neurosurg Clin North Am. 1990;1:139-159.
80. Shrivastava R.K., Arginteanu M.S., King W.A., et al. Giant prolactinomas: clinical management and long-term follow up. J Neurosurg. 2002;97:299-306.
81. Wolfsberger S., Czech T., Vierhapper H., et al. Microprolactinomas in males treated by transsphenoidal surgery. Acta Neurochir (Wien). 2003;145:935-940. discussion 40–1
82. Liuzzi A., Oppizzi G. Microprolactinomas: why requiem for surgery? J Endocrinol Invest. 1996;19:196-198.
83. Peter S.A., Autz A., Jean-Simon M.L. Bromocriptine-induced schizophrenia. J Natl Med Assoc. 1993;85:700-701.
84. Liu J.K., Orlandi R.R., Apfelbaum R.I., et al. Novel closure technique for the endonasal transsphenoidal approach. Technical note. J Neurosurg. 2004;100:161-164.
85. Ciric I., Ragin A., Baumgartner C., et al. Complications of transsphenoidal surgery: results of a national survey, review of the literature, and personal experience. Neurosurgery. 1997;40:225-236. discussion 36–7
86. Jane J.A.Jr, Thapar K., Kaptain G.J., et al. Pituitary surgery: transsphenoidal approach. Neurosurgery. 2002;51:435-442. discussion 42–4
87. Kaptain G.J., Vincent D.A., Sheehan J.P., et al. Transsphenoidal approaches for the extracapsular resection of midline suprasellar and anterior cranial base lesions. Neurosurgery. 2001;49:94-100. discussion -1
88. Liu J.K., Das K., Weiss M.H., et al. The history and evolution of transsphenoidal surgery. J Neurosurg. 2001;95:1083-1096.
89. Liu J.K., Weiss M.H., Couldwell W.T. Surgical approaches to pituitary tumors. Neurosurg Clin North Am. 2003;14:93-107.
90. Day J.D. Surgical approaches to suprasellar and parasellar tumors. Neurosurg Clin North Am. 2003;14(6):109-122.
91. Jane J.A.Jr, Han J., Prevedello D.M., et al. Perspectives on endoscopic transsphenoidal surgery. Neurosurg Focus. 2005;19(6):E2.
92. Landolt A.M. Surgical treatment of pituitary prolactinomas: postoperative prolactin and fertility in seventy patients. Fertil Steril. 1981;35:620-625.
93. Jane J.A.Jr, Laws E.R.Jr. The surgical management of pituitary adenomas in a series of 3,093 patients. J Am Coll Surg. 2001;193:651-659.
94. Randall R.V., Laws E.R.Jr, Abboud C.F., et al. Transsphenoidal microsurgical treatment of prolactin-producing pituitary adenomas. Results in 100 patients. Mayo Clin Proc. 1983;58:108-121.
95. Jho H.D., Carrau R.L. Endoscopic endonasal transsphenoidal surgery: experience with 50 patients. J Neurosurg. 1997;87:44-51.
96. Turner H.E., Adams C.B., Wass J.A. Trans-sphenoidal surgery for microprolactinoma: an acceptable alternative to dopamine agonists? Eur J Endocrinol. 1999;140:43-47.
97. Arnesen M., Scheithauer B.W. Aggressive small cell tumor of the skull base. Ultrastruct Pathol. 1994;18:191-197.
98. Bills D.C., Meyer F.B., Laws E.R.Jr, et al. A retrospective analysis of pituitary apoplexy. Neurosurgery. 1993;33:602-608. discussion 8–9
99. Ebersold M.J., Laws E.R.Jr, Scheithauer B.W., et al. Pituitary apoplexy treated by transsphenoidal surgery. A clinicopathological and immunocytochemical study. J Neurosurg. 1983;58:315-320.
100. Laws E.R.Jr, Ebersold M.J. Pituitary apoplexy—an endocrine emergency. World J Surg. 1982;6:686-688.
101. Brisman M.H., Katz G., Post K.D. Symptoms of pituitary apoplexy rapidly reversed with bromocriptine. Case report. J Neurosurg. 1996;85:1153-1155.
102. Shin M. Gamma knife radiosurgery for pituitary adenoma. Biomed Pharmacother. 2002;56(suppl 1):178s-181s.
103. Ganz J.C. Gamma Knife treatment of pituitary adenomas. Stereotact Funct Neurosurg. 1995;64(suppl 1):3-10.
104. Ganz J.C., Backlund E.O., Thorsen F.A. The effects of gamma knife surgery of pituitary adenomas on tumor growth and endocrinopathies. Stereotact Funct Neurosurg. 1993;61(suppl 1):30-37.
105. Kim M.S., Lee S.I., Sim J.H. Gamma knife radiosurgery for functioning pituitary microadenoma. Stereotact Funct Neurosurg. 1999;72(suppl 1):119-124.
106. Kim S.H., Huh R., Chang J.W., et al. Gamma knife radiosurgery for functioning pituitary adenomas. Stereotact Funct Neurosurg. 1999;72(suppl 1):101-110.
107. Landolt A.M., Lomax N. Gamma knife radiosurgery for prolactinomas. J Neurosurg. 2000;93(suppl 3):14-18.
108. Pan L., Zhang N., Wang E., et al. Pituitary adenomas: The effect of gamma knife radiosurgery on tumor growth and endocrinopathies. Stereotact Funct Neurosurg. 1998;70(suppl 1):119-126.
109. Pan L., Zhang N., Wang E.M., et al. Gamma knife radiosurgery as a primary treatment for prolactinomas. J Neurosurg. 2000;93(suppl 3):10-13.
110. Pollock B.E., Nippoldt T.B., Stafford S.L., et al. Results of stereotactic radiosurgery in patients with hormone-producing pituitary adenomas: factors associated with endocrine normalization. J Neurosurg. 2002;97:525-530.
111. Castinetti F., Nagai M., Morange I., et al. Long-term results of stereotactic radiosurgery in secretory pituitary adenomas. J Clin Endocrinol Metab. 2009;94:3400-3407.
112. Jezkova J., Hana V., Krsek M., et al. Use of the Leksell gamma knife in the treatment of prolactinoma patients. Clin Endocrinol (Oxf). 2009;70:732-741.
113. Couldwell W.T., Rosenow J.M., Rovit R.L., et al. Hypophysopexy technique for radiosurgical treatment of cavernous sinus pituitary adenoma. Pituitary. 2002;5:169-173.
114. Liu J.K., Schmidt M.H., MacDonald J.D., et al. Hypophysial transposition (hypophysopexy) for radiosurgical treatment of pituitary tumors involving the cavernous sinus: technical note. Neurosurg Focus. 14(5), 2003. Article 11