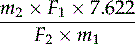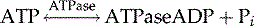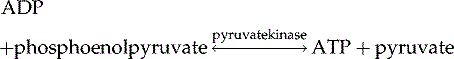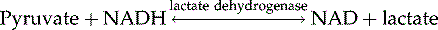Chapter 9 Production, standardization and quality control
• pure compounds, which are often isolated from botanical drugs (and which are not considered to be herbal medicines)
• traditionally used medicinal plants, loose or in teabags to form infusions, including ‘instant teas’, and tinctures, ethanolic extracts, essential oils, fatty acids and dried extracts
• cut or powdered botanical crude drugs (generally simply called crude drug throughout the text), used as such (i.e. unprocessed)
• non-standardized extracts, with varying information about quality and, consequently, sometimes uncertain information about clinical efficacy and pharmacological effects
• standardized extracts, generally with relatively well-established clinical and pharmacological profiles.
Here a very short overview is given of the whole process, from the agricultural production of materials collected from the wild, to the processing and production of the pharmaceutical product or health-food supplement. A more detailed discussion is beyond the scope of this introduction, but can be found, for example, in Evans (2009).
In all cases, the basis for drug production is the botanical drug (see Chapter 2), which can be defined as:
 dried parts of entire plants, plants organs, or parts of plant organs for use as medicines, aromatics and spices, or as excipients used in the production of pharmaceuticals. A typical example is the flower of camomile (Matricaria recutita)
dried parts of entire plants, plants organs, or parts of plant organs for use as medicines, aromatics and spices, or as excipients used in the production of pharmaceuticals. A typical example is the flower of camomile (Matricaria recutita)
 Isolated products obtained directly from plants but which no longer have an organ structure, such as essential and fatty oils, balsams, etc. An example is the exudate of the leaves of aloe [Aloe vera (L.) Burm. f., or Aloe barbadensis Mill.], obtained by cutting the fleshy leaves and collecting the resulting liquid, which is called ‘aloes’ when dried.
Isolated products obtained directly from plants but which no longer have an organ structure, such as essential and fatty oils, balsams, etc. An example is the exudate of the leaves of aloe [Aloe vera (L.) Burm. f., or Aloe barbadensis Mill.], obtained by cutting the fleshy leaves and collecting the resulting liquid, which is called ‘aloes’ when dried.
Biological resources and conservation
• Problems in obtaining a consistent quality of product, including the risk of adulteration
• Over-exploitation of native populations of certain plant species.
More than half of all medicinally used species are still collected from the wild, including the less frequently used species. An example of an over-exploited resource collected from the wild has been discussed in detail by Lange (2000). Pheasant’s eye (false hellebore or Adonis vernalis L.) is a native of Southern and Central Europe and is used there for cases of minor cardiac arrhythmias. The plant is threatened not only by its pharmaceutical use (as a phytomedicine as well as a homoeopathic remedy), but also by its use as an ornamental plant and a dye source. Exploitation of Adonis vernalis affects many south-eastern European countries, including Hungary, Romania and the Ukraine. Importantly, detrimental (unsustainable) harvesting techniques are still used and there is a constant risk that the exploited biomass exceeds the sustainable levels and that techniques are used which harm the population severely (Lange 2000).
Agricultural and biotechnological production
• temperature and annual course of temperature
• rainfall (if it is not possible to irrigate the fields)
• soil characteristics and quality (edaphic factors)
Moisture levels
All drugs are at risk of decaying if the humidity in the drug material exceeds 15%. Improperly stored botanical drugs have a musty smell and often change colour (green material turning yellow or brown). However, different levels of moisture are acceptable for each drug. For example, the moisture contents given in Table 9.1 are considered to be within the normal range and do not pose any problems for these drugs.
Table 9.1 Acceptable moisture content for storage of some botanical drugs (after Franz 1999)
| Botanical drug | Moisture content (%) |
|---|---|
| Chamomile flower (Matricariae flos, from Matricaria recutita L.) | 8–10 |
| Linseed (Lini semen from Linum usitatissimum L.) | 5–9 |
| Digitalis leaf (Digitalis lanatae folium, from Digitalis lanata Ehrh.) | 8–12 |
| Frangula bark (Frangulae cortex, from Rhamnus frangula L, syn. Frangula alnus Mill.) | 5–8 |
| Thyme herb (Thymi herba, from Thymus vulgaris L.) | 8–11 |
| Gentian rootstock (Gentianae radix, from Gentiana lutea L.) | 8–15 |
| Fennel fruit (Foeniculi fructus, from Foeniculum vulgare Mill. subsp. vulgare) | 6–12 |
Pesticides
Pesticides are commonly used to prevent the infestation of the botanical material with large amounts of unwanted species of plants, insects or animals. These may cause harm or otherwise interfere with the production, storage, processing, transport and marketing of botanical drugs. Acceptable limits have been defined in the Eur. Ph. (Chapter 2.8.13). There is also a detailed description of the sampling methods and the relevant qualitative and quantitative methods. Importation of herbs from countries with less restrictive legislation on pesticides means that this material should be checked very carefully. Examples of relevant limits for some important pesticides are given in Table 9.2.
Table 9.2 Limits of some important pesticides allowed in botanical material
| Pesticide | Concentration limit (mg/kg) |
|---|---|
| Aldrin and dieldrin (sum of) | 0.05 |
| Chlordane (sum of cis-, trans– and oxychlordane) | 0.05 |
| DDT (sum of various related compounds) | 1.0 |
| Endrin | 0.05 |
| Fonofos | 0.05 |
| Malathion | 1.0 |
| Parathion | 0.5 |
| Permethrin | 1.0 |
| Pyrethrins (sum of) | 3.0 |
Source: Eur. Ph. 2002.
In vitro cultivation
• as a starting point for investigating the biochemical basis of the biosynthesis of natural products and for breeding new varieties/strains
• as a basis for vegetative propagation of plants to be used as a phytomedicine or for isolating a pure natural product, especially if a consistent quality of the strains or a fungi- or virus-free culture is required. For example, high-yielding strains of Catharanthus and Dioscorea may be required, or the development and maintenance of pyrrolizidine-free strains of common comfrey (Symphytum officinale L.) and coltsfoot (Tussilago farfara L.), or virus-free Digitalis lanata Ehrh. cultures
• for the semi-synthetic production of some natural products (e.g. production of digoxin from digitoxin)
• for the direct synthesis of a medicinal natural product, although this is only rarely achieved by this method. Today, this process is restricted to the production of paclitaxel and its precursors (first isolated from the Pacific yew, Taxus brevifolia Nutt.) and shikonin (a dye and medicinal product used in Asian medicine from Lithospermum erythrorhizon Sieb. & Zucc.).
The Pacific yew as an example
The Pacific yew (Taxus brevifolia Nutt., Taxaceae) is a botanical drug which exemplifies all the various approaches for producing a medicinally used natural compound. In 1962 several samples of Taxus brevifolia Nutt. were collected at random for the National Cancer Institute (NCI) and the US Department of Agriculture. These samples were included in a large screening programme at the NCI. A potent cytotoxic effect was documented in one in vitro system. After a lengthy development process, clinical studies started 13 years later in 1984. It took a further 10 years before paclitaxel was approved for the treatment of anthracycline-resistant metastasizing mammary carcinomas. In the meantime the compound had been licensed for a variety of other cancers and semi-synthetic derivatives produced such as docetxel, which are also now employed (see also Chapter 8).
• Taxus brevifolia is a very slow-growing species, which produces the active ingredients only in very small amounts. Since paclitaxel was for many years isolated from the bark, trees had to be felled in order to obtain it. The requirement for paclitaxel increased dramatically with progress in the clinical development of the drug in the mid-1970s (the amount required to meet the annual therapeutic requirements of patients with ovarian cancer in the USA was estimated to be 15–20 kg). If other cancers common in the USA were to be treated with this compound, around 200–300 kg of the pure compound would have been required per year. This amount can be isolated from approximately 145,000 tons of bark. Collecting such amounts would have been completely unsustainable and would have resulted in the extinction of the species within a few years.
• In the 1990s the semi-synthetic production of paclitaxel from natural products in other Taxus spp. (10-desacetylbaccatin II isolated from the European yew, T. baccata) allowed for the production of large amounts of paclitaxel. Up to this point, a conflict of interest between conservation and medicinal use was unavoidable.
• Large-scale production using cell-culture techniques is now feasible and has been approved by the FDA. Today, most of the paclitaxel required is produced using fermentation technology (Goodman & Walsh 2001).
Drug preparation and extraction
There are many different types of drug preparations, including:
• fresh plant material, used popularly as an infusion or decoction
• dried and cut drug material, often used in industrial production
• dried and powdered drug material, commonly used as an infusion or decoction. If such material is to be used pharmaceutically it must comply with standards as defined in the monograph for the specific botanical drug. If no such monograph exists the material has to comply with the general monograph for herbal drugs (Eur. Ph. 2002, 01/2002-1433)
• extraction and subsequent bulk production of pure natural products (e.g. morphine, digoxin, digitoxin, camptothecin) or a mixture of closely related ones (e.g. sennosides from Senna, aescin from horse chestnut, quillaia saponins from soapbark) using validated, standard phytochemical techniques (chromatography, partitioning between solvents of differing polarity, precipitation, etc.)
• unstandardized tinctures are hydroalcoholic (or alcoholic) extracts of crude drug material used as a liquid botanical drug
• an extract prepared from dried drug material using defined solvent systems is processed into a variety of pharmaceutical products (e.g. tablets for crude extracts). Such extracts are often characterized by the drug:solvent ratio, which gives the relationship of the volume of solvent to the amount of drug extracted (e.g. 1:10) (see p. 154). In many high-quality products, these extracts are ‘standardized’ by mixing high- and low-yield material. By ‘spiking’ the extract with an enriched extract, a ‘modified’ extract with a defined range of active natural products is obtained (e.g. dry aloe extracts standardized to 19.0–21.0% of hydroxyanthracene derivatives calculated as barbaloin)
• a particularly interesting case is that of the so-called ‘special extracts’. A special extract is prepared by first extracting the drug with a defined solvent system and then processing the extract so that a well-defined extract with specific ranges of ingredients is obtained. These extracts have a significantly reduced percentage of unwanted compounds, and an increased percentage of compounds that contribute significantly to the pharmacological activity and clinical effectiveness. In the case of ginkgo leaves (Ginkgo folium, Ginkgo biloba L.), for example, the desired natural products include the flavone glycosides (16–26%) and the terpene lactones (5–7%); whereas the polyphenols, polysaccharides, and especially the ginkgolic acids, are less desirable constituents (for details see p. 158–159 and 249)
• there are several special methods of extraction; for example, the cold pressed extract of the rootstock of Echinacea species is developed into an immunostimulant product. The fresh rootstock is used for this and the sap is processed into a commercial botanical pharmaceutical. For material to be used pharmaceutically, the process must be validated.
Effect of preparation methods on content
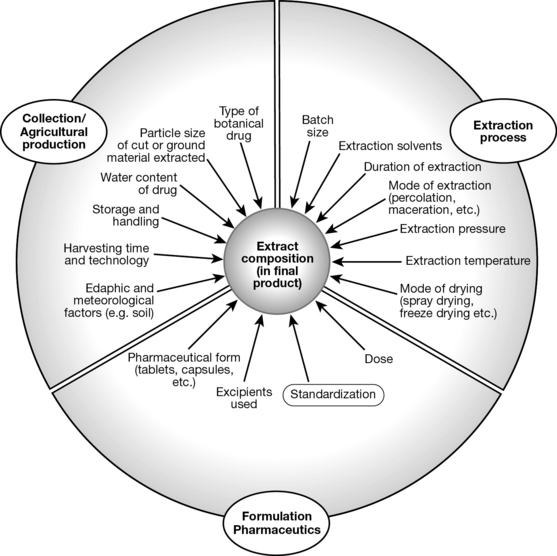
Different methods of preparing botanical material and subsequent extraction result in extracts with differing composition and different concentrations of active (as well as undesired) ingredients. A wide range of factors both in relation to the production of the botanical starting material (the botanical drug) and its processing, extraction and formulation have an impact on the chemical composition and thus the pharmacological activity of a phytotherapeutic preparation (Fig. 9.1). Strictly speaking, for an assessment of the pharmacological effect and clinical effectiveness of a botanical drug, precise data on the composition of the extract are needed. Just as importantly, pharmacological or clinical data on two products can only be compared meaningfully if the composition of the extracts is known. This implies, for example, that a meta-analysis of clinical studies is only feasible if the botanical drug materials used are similar and the resulting extracts have a comparable composition, a consideration often omitted by authors of such studies.
Quality control and standardization
Quality control: general procedures
• the quality of the botanical material used, which in turn is influenced by a multitude of biogenic (e.g. infections with fungi) and climatic factors, and also includes the risk of contamination with heavy metals, pesticides, herbicides and the like (see above)
• the adequate processing of the fresh material, including drying, transportation and storage
• the use of appropriate and reproducible extraction techniques
• storage under appropriate conditions (generally dry, cool, in the dark)
• use of the material only within the generally accepted shelf-life of the botanical drug.
Specifically, quality control needs to assure:
• the correct botanical identity of the drug (i.e. the correct species and plant part) in an appropriate quality (time of collection, age)
• the purity of the material used (i.e. that other botanical drugs are only present in minimal amounts)
• contaminants such as insects, mites, bacteria, fungi, heavy metals, herbicides, fungicides, pesticides, insecticides and any other toxins are below the legal (e.g. Eur. Ph.) threshold
• that the required level of active compounds (only if a minimum level of these natural products is defined) or a defined level of biological activity (if the drug is characterized biologically or pharmacologically) is reached.
• Title (English name, Latin name used in international trade)
• Definition of the drug (plant part to be used; whether it is fresh, dried, cut or powdered, and possibly also specifying constituents typical for the drug, with minimal amounts required)
• Characteristics: i.e. organoleptic or other properties of the drug (smell, colour, other similar characteristics; taste is rarely included in the Eur. Ph. for reasons of safety)
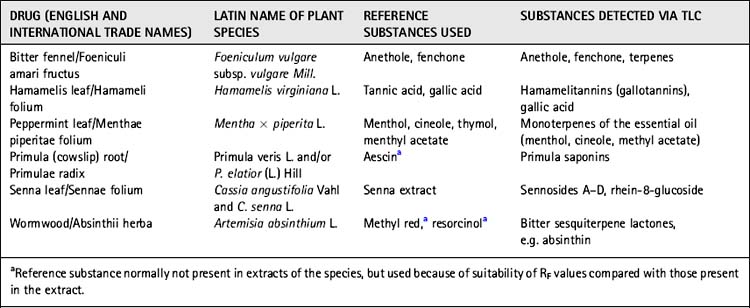
• Identification (macroscopic and microscopic description, and in some cases thin-layer chromatographic (TLC) characteristics; see Table 9.3)
• Tests for purity (providing data on maximum amounts of foreign matter, i.e. non-acceptable substances, loss on drying, ash)
• Required level of biologically active or lead compounds
• Storage (general information about required forms of storage).
Pharmaceutical drugs have to comply with all the characteristics as they are defined in such a monograph, and material that does not comply must be rejected. In many cases, TLC methods for quality control are included, such as those given in Table 9.3.
Botanical (classical pharmacognostical) methods
One of the main tools for analysing botanical material is the microscope. Since botanical drugs have characteristic features, these can easily be used to establish the botanical identity and quality of a drug (see also Chapter 3).
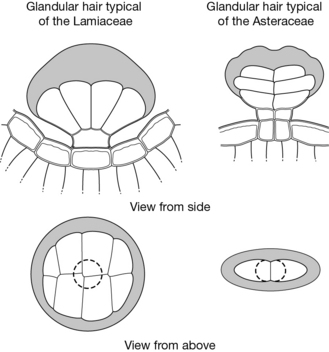
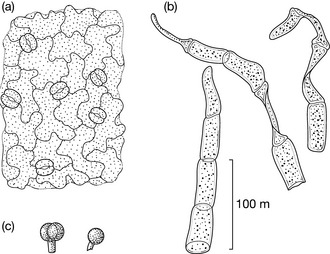
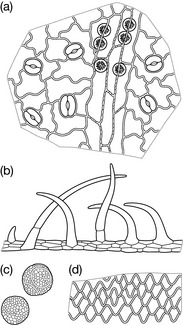
Another typical example is that of the glandular hairs, which in many species contain the essential oil. They are a very useful diagnostic feature because they have a characteristic structure. Fig. 9.2 shows glandular hairs that are typical of the Lamiaceae and Asteraceae.
An example of a simple biophysical method is the swelling index (Eur. Ph. 2002, Chapter 2.8.4). This index is an indicator for the amount of polysaccharides present in a certain drug. It is defined as the volume (in ml) occupied by 1 g of a drug, including any adhering mucilage, after it has swollen in an aqueous liquid for 4 h. The drug is treated with 1.0 ml of ethanol (96%) and 25 ml water in a graduated cylinder, shaken every 10 min for 1 h and allowed to stand as specified. Some of the drugs are tested without pretreatment (e.g. fenugreek, ispaghula, linseed); others have to be powdered to a defined particle size prior to measuring the swelling index (e.g. marshmallow root). The required minimal swelling indices for a variety of botanical drugs are given in Table 9.4. If these values are not reached, it may be an indication that the botanical drug is contaminated with other drugs or that it is not of adequate quality (e.g. because it was not stored properly).
| Drug | Swelling indexa |
|---|---|
| Agar (from several genera of marine algae, especially Gelidium and Gracilaria) | > 10 |
| Cetraria (Lichen islandicus, from Cetraria islandica (L.) Ach. s.l.) | > 4.5 |
| Fenugreek (Foenugraeci semen, from Trigonella foenum-graecum L.) | > 6 |
| Ispaghula husk (Plantaginis ovatae testae, from Plantago ovata Forssk.) | > 40 (determined on 0.1 g of powder) |
| Ispaghula seed (Plantaginis ovatae semen) | > 9 |
| Linseed (Lini semen, whole drug from Linum usitatissimum L.) | > 4 |
| Linseed (Lini semen, powdered drug) | > 4.5 |
a Measured as a multiple of the original volume.
• HPLC (high-performance liquid chromatography), used especially in the quantification of compounds and fingerprinting of extracts
• GC (gas chromatography), used mostly for essential oils, and sometimes combined with mass spectrometry (MS)
• TLC (thin-layer chromatography), which is simple and cheap and provides a good analytical tool for establishing the identity of a drug and for detecting contaminants containing similar types of compounds. The method is widely used, but not detailed, and it is not possible to quantify substances fully using TLC
• NIR (near-infrared spectroscopy), used to assess the identity and quality of a botanical sample, and also used for crude drug material. This technique is gaining in popularity
DNA-barcoding, that is the identification of a specific DNA sequence of selected genes in a species, is currently being developed to clearly identify from which species a botanical drug is derived. This method allows for the authentication of a species, but does not provide information on the purity of a botanical drug (i.e. if other plant parts were included, too) nor does it provide information on the quality of the material (e.g. its level of active constituents).
For most widely used botanical drugs, the relevant protocols can be found in the respective pharmacopoeias (see Box 9.1) or in protocols provided by the manufacturer. In the future, we may see the use of DNA-fingerprinting techniques as a novel and very sensitive tool for analysing the quality of all sorts of botanical material, including medicinal drugs.
Box 9.1 Sample monograph from the European Pharmacopoeia
Devil’s claw root
Harpagophyti radix
Identification
A. It consists of thick, fan-shaped or rounded slices or of roughly crushed discs. The darker outer surface is traversed by tortuous longitudinal wrinkles. The paler cut surface shows a dark cambial zone and xylem bundles distinctly aligned in radial rows. The central cylinder shows fine concentric striations. Seen under a lens, the cut surface presents yellow to brownish-red granules.
B. Reduce to a powder (355). The powder is brownish-yellow. Examine under a microscope using chloral hydrate solution R. The powder shows the following diagnostic characters: fragments of cork layer consisting of yellowish-brown, thin-walled cells; fragments of cortical parenchyma consisting of large, thin-walled cells, sometimes containing reddish-brown granular inclusions and isolated yellow droplets; fragments of reticulately thickened vessels and tracheidal vessels with associated lignified parenchyma from the central cylinder; small needles and crystals of calcium oxalate are present in the parenchyma. The powder may show rectangular or polygonal pitted sclereids with dark reddish-brown contents. With a solution of phloroglucinol in hydrochloric acid, the parenchyma turns green.
C. Examine by thin-layer chromatography (2.2.27), using a suitable silica gel as the coating substance.
Reference solution. Dissolve 1 mg of harpagoside R in 1 ml of methanol R.
Tests
Starch. Examine the powdered drug (355) under a microscope using water R. Add iodine solution R1. No blue colour develops.
Foreign matter (2.8.2). It complies with the test for foreign matter.
Loss on drying (2.2.32). Not more than 12.0%, determined on 1.000 g of the powdered drug (355) by drying in an oven at 100–105°C.
Assay
Examine by liquid chromatography (2.2.29) using methyl cinnamate R as the internal standard.
– a stainless steel column 0.10 m long and 4 mm in internal diameter packed with octadecylsilyl silica gel for chromatography R (5 μm)
– as mobile phase at a flow rate of 1.5 ml/min a mixture of equal volumes of methanol R and water R
Calculate the percentage content of harpagoside from the expression:
m1 = mass of the drug, in grams
m2 = mass of methyl cinnamate R, in grams in the internal standard solution
F1 = area of the peak corresponding to harpagoside in the chromatogram obtained with the test solution
F2 = area of the peak corresponding to methyl cinnamate, in the chromatogram of the test solution.
(reprinted, with modifications, from European Pharmacopoeia 2002, p 1013–1014, with permission)
Drug: solvent ratio and drug extract ratio
Drug:extract ratio (DER): The ratio of a botanical drug to the amount of extract obtained, for example, 4 to 1 (4:1) – four units of a dried starting material (the botanical drug) yield one unit of extract (e.g. kg)
Drug:solvent ratio (DSR): The ratio of a botanical drug to the amount of solvent used in the extraction, for example, 1 to 8 (1:8) – eight units are used to extract one unit of a botanical drug. In general m/v (mass/volume) or m/m (mass/mass) are used as units and very often a range is given (e.g. 1 to 6–10). In addition, the solvent and the type of extraction must also be stated.
Standardization
• reproducible composition and generally higher quality of the product. Standardization may require that the quantity of unwanted material in the extract should not exceed a certain limit, while active ingredients will have to be above a minimum concentration
• provided that the product is registered, it thus becomes a medicine that should comply with the basic standards required for all drugs
• standardization allows comparison of the clinical effectiveness, pharmacological effects and side effects of a series of products (e.g. against placebo). If a product is not standardized to active compounds, the comparison with other products derived from the same botanical drug is more problematic, since the composition may vary greatly
• such products give patients greater (objective and subjective) security and thus increase the level of trust people have in herbal products
• ensuring the quality of products sold has always been, and continues to be, a key responsibility of the pharmacist.
Types of extract
• (Truly) standardized extracts (type A): extracts standardized to active constituents
• Quantified extracts (type B1): extracts standardized to constituents that contribute to the activity
• Other extracts (type B2): extracts standardized to lead compounds of unknown pharmacological relevance, which serve as quality markers.
This first classification heavily impacts on strategies for pharmaceutical quality control.
Standardized extracts
• digitalis leaf (Digitalis folium, foxglove)
• senna dry extracts: standardized to 5.5–8.0% hydroxyanthracene glycosides, calculated as sennoside B with reference to dried extract (Eur. Ph.)
• belladonna leaf dry extract (Belladonnae folium from Atropa belladonna L., deadly nightshade): standardized to 0.95–1.05% of alkaloids calculated as hyoscyamine (Eur. Ph.).
Examples of drugs and their quality control and standardization
Standardized extracts
Digitalis purpurea folium (foxglove leaves)
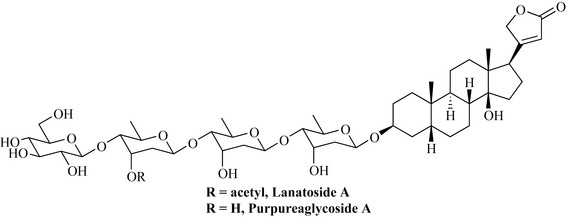
William Withering’s ‘An account of foxglove and some of its medical uses’ (1785) introduces foxglove as a remedy for dropsy and oedema, later additionally used for heart conditions, especially congestive heart failure. This is largely due to an inhibitory effect on Na+/K+-ATPase. Today, pure compounds (including semi-synthetic derivatives of digitalis glycosides, rather than standardized extracts) are used. The glycosides digoxin (the most widely used in the UK), digitoxin and lanatoside C all have monographs in the Eur. Ph. and are isolated industrially from the botanical drug using a multistep process. Therapeutically used digitalis glycosides are generally not chemically pure but may contain up to 5% (Eur. Ph.) or even 11% (USP) of other compounds. Digitalis lanata Ehrh. (Scrophulariaceae) is the species cultivated for obtaining the raw pharmaceutical product. Digitoxin is a degradation product of lanatoside A (from Digitalis lanata) [as well as of purpurea glycoside A (from D. purpurea L.)] (Fig. 9.3).
Quality control
Typical microscopical characteristics of the pulverized drug (see Fig. 9.4) include:
• 2–7-celled clothing trichomes (clothing hairs) with cells often collapsed and glandular hairs composed of a characteristic gland and a unicellular stem cell (pedicel)
• characteristic structure of polygonal epidermal cells and stomata
In a last step, pyruvate is metabolized by lactate dehydrogenase to yield lactate:
• RIA An antigen (digoxin, etc., covalently bound to a hapten) is used to induce antibody production in a rabbit. The quantity of digitoxin is determined by co-incubating the labelled antigen–hapten (defined amount) with the unlabelled antigen (unknown); if more unlabelled antigen is present, the amount of bound antigen is reduced. The analysis of trace amounts is possible, but such a highly specific method is generally only available in specialized laboratories.
• ELISA The antibodies are fixed to the surface of, for example, a microtitre plate. The sample with an unknown amount of antigen (digitoxin) is then added together with hapten-bound enzyme-labelled (e.g. peroxidase) antigen (also digitoxin). Both compete for the binding positions. After washing, the read-out is the rate of production of a coloured compound, which is quantified photometrically. The amount of digoxin in a sample is calculated from the amount of labelled antigen, which is highest when the least unbound antigen is present.
(Truly) standardized extracts
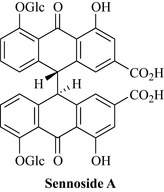
Sennae folium (senna leaf)
• Sennosides, including sennosides A and B (Fig. 9.5)
• Glucosides of rhein (e.g. rhein-8O-glucoside) and aloe-emodin.
Quality control
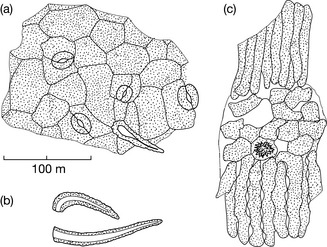
Some characteristic microscopic features of the drug include the unique non-lignified warty hairs up to 250 μm long, and small cluster crystals as well as prisms of calcium oxalate. Another characteristic are the stomata with two cells with the long axes parallel to the pore (diacytic stomata), the mid-rib bundle and larger veins of the leaves surrounded by a zone of lignified pericyclic fibres and a sheath of parenchymatous cells containing prisms of calcium oxalate (Fig. 9.6).
Quantified extracts
Ginkgo biloba leaves
• Flavonoids (0.5–1%): flavone and flavonol glycosides, acetylated flavonol glycosides, biflavonoids
Quality control
Microscopy is less useful in quality control here, so phytochemical methods are predominant.
• Flavonoids: ethyl acetate/formic acid/glacial acetic acid/water (100:11:11:26), detection with diphenylboryloxyethylamine.
• Biflavones: chloroform/acetone/formic acid (75:16.5:8.5), detection with diphenylboryloxyethylamine.
• Terpene lactones: toluene/acetone (70:30), detection with acetic acid reagent.
Other extracts
Passiflorae herba (passion flower)
Quality control
Microscopic analysis gives a very characteristic picture, including prominent pieces of the lower epidermis with cells around the stomata, which cannot be distinguished from the other cells of the epidermis (anomocytic), and cluster crystals below the surface. The material is also characterized by typical hairs (Fig. 9.7).
• Qualitative analysis: TLC analysis of a methanolic extract (Eur. Ph.) using rutin and hyperoside as reference; plate development with acetic acid/water/ethyl methyl ketone/ethyl acetate (10:10:30:50), detection at 365 nm (UV) after spraying with diphenylboryloxyethylamine
• Quantitative analysis: spectrophotometric or fingerprinting with HPLC.
European Pharmacopoeia. Council of Europe (Directorate for the Quality of Medicines), fourth ed. Strasbourg: European Pharmacopoeia; 2002.
European Pharmacopoeia. European Directorate for the Quality of Medicines and HealthCare (EDQM). France: Strasbourg; 2003.
Evans W.C. Trease and Evans’s pharmacognosy, sixteenth ed. London: WB Saunders; 2009.
Franz C. Züchtung und Anbau von Arnzeipflanzen. In: Rimpler H., editor. Biogene Arzneistoffe, 2 Aufl. Stuttgart: Wissenschaftliche Verlagsgesellschaft; 1999:1-19.
Goodman J., Walsh V. The story of taxol. In Nature and politics in the pursuit of an anti-cancer drug. Cambridge: Cambridge University Press; 2001.
Lange D. Conservation and sustainable use of Adonis vernalis, a medicinal plant in international trade. Plant species conservation monographs. Bonn: Federal Agency for Nature Conservation, 2000;vol. 1.
Eschrich W. Pulver-Atlas der Drogen der deutschsprachigen Arzneibücher. Stuttgart: Deutscher Apotheker Verlag; 1999.
Heinrich M., Pieroni A., Bremner P. Medicinal plants and phytomedicines. In: Nesbitt M., Prance G., editors. The cultural history of plants. New York: Routledge (Taylor and Francis); 2005:205-238.
Heinrich M., Modarai M., Kortenkamp A. Herbal extracts used for upper respiratory tract infections: Are there clinically relevant interactions with the Cytochrome P450 enzyme system? Planta Med.. 2008;74:657-660.
Hohmann B., Reher G., Stahl-Biskup E. Mikroskopische Drogenmonographien der deutschsprachigen Arzneibücher. Stuttgart: Wissenschaftliche Verlagsgesellschaft; 2001.
Mukherjee P.K., Mukherjee D., Venkatesh M., Rai S., Heinrich M. The sacred lotus (Nelumbo nucifera) – phytochemical and therapeutic profile. J. Pharm. Pharmacol.. 2009;61:407-422.
Rahfeld B. Mikroskopischer Farbatlas pflanzlicher Drogen. Spektrum Akademischer. Heidelberg: Verlag; 2009.
Rimpler H. Biogene Arzneistoffe. Stuttgart: Wissenschaftliche Verlagsgesellschaft; 1999.
Stewart K.M., Cole D. The commercial harvest of devil’s claw (Harpagophytum spp.) in southern Africa: The devil’s in the details. J. Ethnopharmacol.. 2005;100:225-236.