Procedures Pertaining to Hypothermia and Hyperthermia
Procedures Pertaining to Hypothermia
With an increase in outdoor activities, changing weather patterns, and the growing epidemic of homelessness in our country, issues pertaining to hypothermia remain in the forefront. Hypothermia is not only a common diagnosis in rural areas but has also become more commonplace in urban centers across the nation secondary to inadequate housing or lack of preparation for cold weather changes.1 It is also important to note that numerous cases of accidental hypothermia (AH) are reported each year in areas typically considered warm weather locales such as Florida, Texas, California, and Alabama.2,3 Every year, many recreational and elite athletes participate in outdoor sporting events. The higher the environmental stress, the greater the potential for failure in performance and the development of hypothermia.4 The high-altitude expeditions on Mt. Everest in 1996, Mt. Denali in 2003, and Mt. Hood in 2006 are reminders that even well-protected, acclimatized individuals can succumb to cold-related fatalities. Optimal treatment of hypothermia remains controversial. It is a well-accepted practice to carry out resuscitation of these individuals for extended periods. The medical literature contains numerous anecdotal reports of profoundly hypothermic individuals who are successfully resuscitated and discharged neurologically intact.5–8 Despite these spectacular reports of survival, both morbidity and mortality from hypothermia are common. Between 1972 and 2002, 16,555 deaths in the United States were attributed to hypothermia, which equates to 689 deaths per year.9 Between 1999 and 2002 alone, 4607 death certificates in the United States had hypothermia-related diagnoses listed as the underlying cause of death.9 The actual number of patients seen in emergency departments (EDs) with hypothermia is unknown. Poverty, homelessness, alcoholism, and psychiatric illnesses are commonly associated conditions. This chapter critically reviews approaches and procedures appropriate to the management of several categories of hypothermic patients. The recommendations combine treatment efficacy with safety. Before describing procedures and making recommendations, essential terms are defined and the pathophysiology of hypothermia is briefly reviewed.
Definitions
Accidental hypothermia has been defined as an unintentional decrease in core (vital organ) temperature to below 35°C (<95°F).5 Victims of hypothermia can be separated into the following categories: mild hypothermia, 35°C to 32°C (95.0°F to 90.0°F); moderate hypothermia, lower than 32°C to 30°C (<90.0°F to 86.0°F); and severe hypothermia, colder than 30°C (<86.0°F). Other factors that may be useful in separating groups of patients with AH include the presence of underlying illness,1,10–15 altered neurologic state on arrival at the ED, hypotension, and the need for prehospital cardiopulmonary resuscitation (CPR). A hypothermia outcome score has been developed that incorporates some of these factors and may permit comparison of outcomes in patient groups treated with different modalities.16
Risk factors for the development of AH include burn injuries, extremes of age, ethanol intoxication, dehydration, major psychiatric illness, trauma, use of intoxicants, significant blood loss, sleep deprivation, malnutrition, and concomitant medical illnesses.17,18 Risk factors for the development of hypothermia indoors include advanced age, coexisting medical conditions, being alone at the time of illness, being found on the floor, and abnormal perception or regulation of temperature.13 Unlike healthy exposed outdoor enthusiasts, such as skiers or mountaineers,19 hypothermia in urban populations is most often associated with conditions that impair either thermoregulation or the ability to seek shelter. In the majority of studies of urban hypothermia, death has been attributed to the severity of the underlying disease.1
Because signs and symptoms may be vague and nonspecific, mild to moderate hypothermia may easily be overlooked in the ED. A common error is failure to routinely obtain an accurate core temperature in all patients at risk. The diagnosis is frequently delayed because of false reliance on standard oral temperatures. Symptoms such as confusion in the elderly and combativeness in intoxicated patients might not initially be recognized as symptoms of hypothermia. Hypothermic patients frequently will not feel cold or shiver, particularly the elderly population, who have impaired thermoregulatory responses because of their advanced age.20–22 Paradoxical undressing, a cold-induced psychiatric dysfunction, has been described in confused patients in whom a sensation of heat develops at lowered body temperatures. It occurs as a result of constricted blood vessels near the surface of the body that suddenly dilate. In many cases these patients are mislabeled as psychotic, thereby leading to further delays in appropriate treatment.23
Measurement of Core Temperature
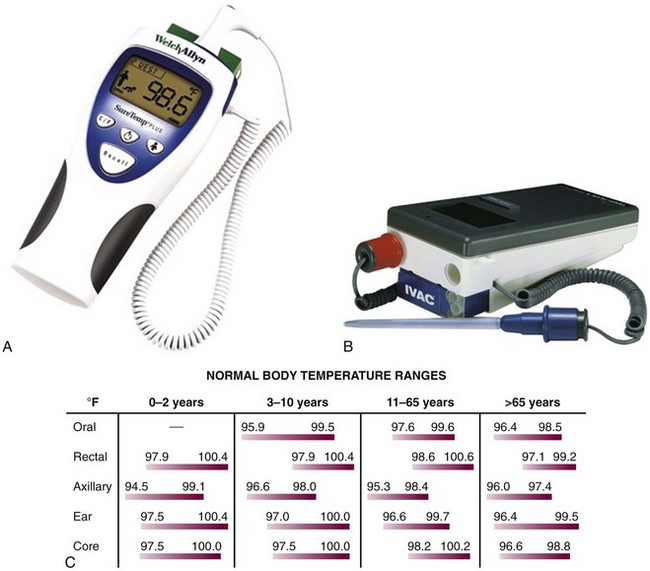
Because of the nonspecific nature of the symptoms of hypothermia, accurate assessment of temperature is a necessity when considering this diagnosis. It is of paramount importance not only for confirmation of the diagnosis but also for guidance in further diagnostic and therapeutic decisions. Any thermometer that does not record temperatures in the hypothermic range is inappropriate for evaluating significant hypothermia. Standard glass/mercury thermometers generally cannot record temperatures lower than 34°C (<93.2°F), although some models are available that record temperatures as low as 24°C (75.2°F) (Dynamed, Inc., Carlsbad, CA). An electronic probe with accompanying calibrated thermometer is recommended when monitoring this vital sign. Examples of thermometers with accompanying accuracy at various temperature ranges are shown in Figure 65-1A and B.
Core temperature is traditionally estimated with a rectal probe, but rectal temperature often lags behind core temperature because of large gradients within the body.22 Esophageal probes may be used, although they may be affected by warm humidified air therapy. Other possible sites for measurement of temperature include the tympanic membrane, nasopharyngeal tract, and urinary bladder.1,24,25 Fresh urine temperature can closely approximate core temperature.26 “Deep forehead” temperatures measured with a Coretemp thermometer (Teramo, Tokyo) have also demonstrated excellent accuracy and approximation of core temperatures.27 For continuous monitoring purposes, rectal or bladder probes are preferred. Infrared tympanic temperatures have demonstrated excellent correlation with core temperatures. However, studies show that although easier to use and faster, infrared tympanic temperatures can be inaccurate at extremes of temperature by underestimating higher temperatures and overestimating lower temperatures.28 When a rectal probe is used, it should be inserted at least 15 cm beyond the anal sphincter and its position verified frequently.6 One should remember that temperature gradients exist in the human body and therefore consistency of monitoring at one or more sites is mandatory. A chart and formula that convert centigrade to Fahrenheit temperatures will assist the clinician in assessing the severity of hypothermia (see Fig. 65-1C).
Pathophysiology
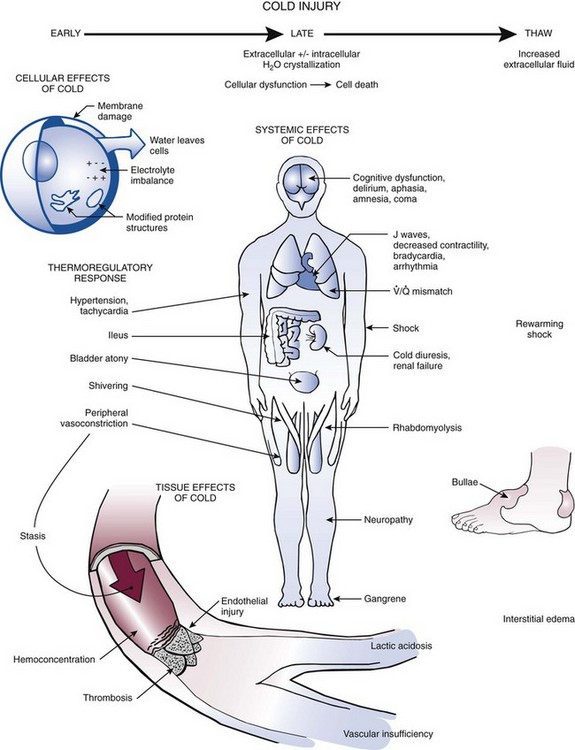
AH results from failure of the body’s thermoregulatory responses to generate enough heat to compensate for heat losses. These thermoregulatory responses include shivering, tachycardia, tachypnea, increased gluconeogenesis, peripheral vasoconstriction, and shunting of blood to central organs (Fig. 65-2).29,30 As core temperature drops despite these compensatory mechanisms, the patient becomes poikilothermic and cools to ambient temperature.
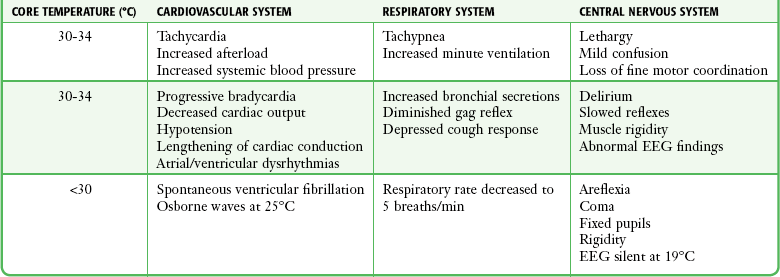
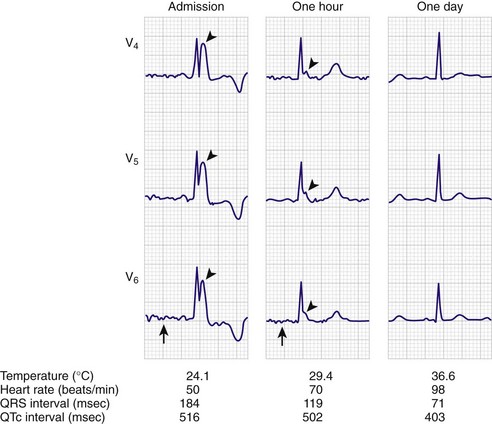
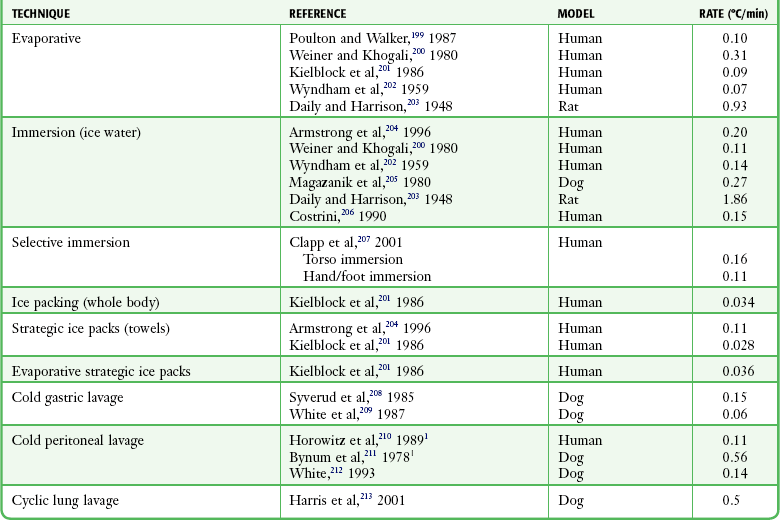
Four methods of heat loss affect the body: radiation, conduction, convection, and evaporation. Radiation involves transfer of heat from a warmer body to a cooler environment and accounts for approximately 60% of heat loss in a normothermic individual. Conduction refers to loss of heat from direct contact with a cooler surface. These losses are most profound with immersion hypothermia. Convection occurs when cool air currents pass by the body and accounts for 15% of heat loss, especially with a wind chill factor. Evaporation refers to significant loss of heat through sweating and insensible water loss.21,30 With hypothermia, the enzymatic rate of metabolism decreases twofold to threefold with each 10°C (18°F) drop, and cerebral blood flow decreases 6% to 7% per 1°C (1.8°F) drop. Signs and symptoms of hypothermia vary according to the core temperature. The overall functioning of all organ systems is impaired by the cold.31 The greatest effects are seen in the cardiovascular, neurologic, and respiratory systems (Table 65-1). As core body temperature drops below 33°C (<91.4°F), the patient becomes confused and ataxic.32 The initiation of involuntary motor activity (shivering) prevents the reduction in core temperature.33 Shivering thermogenesis in skeletal muscle operates on acute cold stress. In a malnourished patient, the mechanism may be rendered ineffective secondary to reduced muscle mass.34 Shivering stops at about 32°C (89.6°F), and shivering artifact on an electrocardiogram has been associated with increased survival of individuals with severe hypothermia.35 Atrial fibrillation occurs frequently as the temperature continues to drop and the patient loses consciousness. A J wave on the electrocardiogram often appears before ventricular fibrillation (Fig. 65-3).36,37 Though classically considered pathognomonic for hypothermia, the J or “Osborne” wave has no prognostic or predictive value in cases of hypothermia. Studies have found that Osborne waves are present in 36% of AH survivors and in 38% of nonsurvivors.35,38 Ventricular fibrillation may occur below 29°C (<84.2°F) and becomes common as the core temperature drops to 25°C (77°F).39 The electroencephalogram flattens at 19°C to 20°C (65.2°F to 68°F).40 Asystole commonly develops at 18°C (64.4°F) but has been seen at higher temperatures. Initial core temperature does not necessarily correlate with patient outcome.42 The lowest recorded temperature in a survivor of AH is 9.0°C (43.7°F).30
Initial Evaluation and Stabilization of Hypothermic Patients
Treatment of hypothermia can be divided into prehospital care and ED management.
Prehospital Care
In the prehospital setting, focus primarily on removing the patient from the current environment to prevent further decreases in core temperature. Studies have shown that oral temperatures are sufficiently accurate for field use41; however, infrared tympanic thermometers may not be reliable in the prehospital setting.42 Handle these patients with special care and anticipate the presence of an irritable myocardium because aggressive measures can inadvertently trigger cardiac dysrhythmias. Hypovolemia and a large temperature gradient often exist between the periphery and the core in a hypothermic patient.6 Avoid aggressive field management and prolonged transport times.43,44 After removing the patient’s wet clothing, wrap the patient in dry blankets or sleeping bags. “Field rewarming” is a misnomer because adding significant heat to a hypothermic patient in the field is extremely difficult. Studies have shown that for mild hypothermia, resistive heating (e.g., warming blankets) can be used safely in the prehospital setting. Resistive heating augments thermal comfort, increases core temperature by approximately 0.8°C/hr (33.4°F/hr), and reduces patient pain and anxiety during transport.45 In one study, resistive heating more than doubled the rewarming rate when compared with passive insulation and did not produce an afterdrop.46 With longer transport times, use active rewarming methods limited to heated inhalation and truncal heat application. Place insulated hot water bottles near the patient’s axilla or groin. The Res-Q-Air device (CF Electronics, Inc., Commack, NY) is lightweight and portable and delivers heated humidified air or oxygen at temperatures ranging from 42°C to 44°C (107.6°F to 111.2°F) and down to ambient conditions of −20°C (−4°F). In more remote settings, another option is to use a modified forced-air warming system in the field. The Portable Rigid Forced-Air Cover is heated with a Bair Hugger heater/blower (Augustine Medical, Inc., Eden Prairie, MN). It covers the patient’s trunk and thighs and can adapt to various transport vehicle power sources.6
Immobilize patients with potential traumatic injuries to the spine or extremities before transport. Pay continuous attention to airway maintenance. Initiate fluid resuscitation with intravenous (IV) crystalloid, preferably 5% dextrose in normal saline (D5NS). Alternatively, give warmed oral glucose-containing drinks to a patient who is awake and alert. Most hypothermic patients are dehydrated because fluid intake is reduced and cold causes diuresis. Avoid using lactated Ringer’s solution because it can theoretically decrease the metabolism of lactate by cold-induced hepatic dysfunction. If possible, use warmed IV fluids because they are generally well tolerated.47,48 If available, use a flameless heater, which is currently being used by military medical units and provides an easy and expedient means of warming fluids in the prehospital setting.49
Intubate unresponsive patients, but recognize that there is no universal agreement on when to intubate a hypothermic patient who has detectable vital signs. Pulse oximetry is not usually helpful because vasoconstriction limits blood flow to the periphery and readings may be inaccurate or not possible. Some authors suggest that pulseless victims with core temperatures below 32°C (<89.6°F) should be transported with continuous CPR.47 Other authors believe that it is unnecessary to perform CPR on a patient who has any perfusing cardiac rhythm because it may precipitate ventricular fibrillation.50 There is no universally accepted standard for intubation or CPR in hypothermic patients with detectable vital signs.51
Transport cold, stiff, cyanotic patients with fixed and dilated pupils because the treatment dictum for prehospital personnel remains, “No one is dead until warm and dead.” A succinct summary of the prehospital care of a hypothermic patient is rescue, examine, insulate, and transport.6
ED Management
Treatment priorities in the ED setting are to prevent further decreases in core body temperature; establish a steady, safe rewarming rate; maintain stability of the cardiopulmonary system; and provide sufficient physiologic support. Adjust the rate of rewarming and the techniques used according to the degree of hypothermia and the severity of the patient’s clinical condition (Table 65-2). Anticipate and prevent complications.
TABLE 65-2
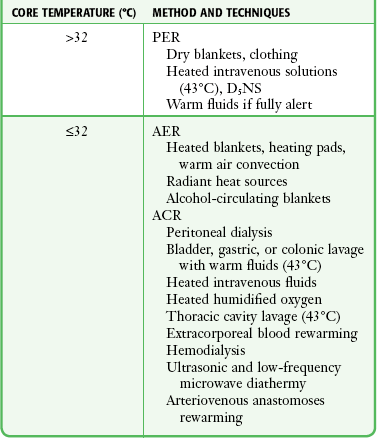
ACR, active core rewarming; AER, active external rewarming; PER, passive external rewarming.
With a mild to moderate reduction in core temperature, the level of mentation correlates with the severity of the AH, associated illnesses, or both. Noteworthy exceptions are alcoholics and diabetics, who can be in a coma at higher core temperatures because of concomitant hypoglycemia. Perform bedside glucose measurements on patients when they arrive in the ED. A high correlation exists between alcohol consumption and the development of hypothermia, especially in colder climates.1 A review of 68 cases of hypothermic deaths in Jefferson County, Alabama, found that a significant number of cases involved middle-aged men who had consumed alcohol.3 In the 22 cases of AH reviewed by Fitzgerald,52 all but 2 patients were alcoholics. The serum glucose level was less than 50 mg/dL in 41% (nine patients). This study noted glycosuria in two patients, even when low serum glucose values were evident, and described a renal tubular glycosuria in patients with AH. Such glycosuria may worsen or cause hypoglycemia. Glycosuria in AH is no guarantee of an adequate serum glucose concentration. This supports the routine use of supplemental IV glucose unless a normal serum glucose value can be quickly ensured. Consider administering IV thiamine (100 mg) and a trial dose of 0.4 to 2 mg of IV naloxone (Narcan) in a comatose patient. Although failure to rewarm spontaneously has been noted in victims with hypothyroidism and other endocrine deficiencies, reserve the use of thyroid hormones and corticosteroids for patients with suspected thyroid and adrenal insufficiency, respectively.
The thermoregulatory vasoconstriction caused by hypothermia significantly decreases subcutaneous oxygen tension.21 Good correlation exists between the incidence of wound infection and subcutaneous oxygen tension. As core temperatures decrease from 41°C to 26°C (105.8°F to 78.8°F), neutrophil function is significantly impaired.21 In animal models, hypothermia appears to decrease leukocyte sequestration within the brain parenchyma, thus offering some resistance to meningitis.53 Although antibiotics are not routinely indicated for victims of uncomplicated mild hypothermia, some authors advocate the routine empirical initiation of broad-spectrum antibiotic therapy on admission of severely hypothermic patients. In this setting, detection and treatment of the underlying cause, such as infection, may be more critical than treatment of the hypothermia.1
Management Guidelines
Hypothermia affects virtually every organ system because of generalized slowing of the body (see Fig. 65-2). Management goals depend on the severity of the hypothermia, but in all cases the primary goal is to increase core temperature and prevent further loss. In a patient with mild hypothermia, a conservative approach to rewarming is generally advocated. Overly aggressive methods may be more harmful to the patient by causing worsening hypotension, a paradoxical decrease in core temperature, and cardiac dysrhythmias. Other complications may include bleeding and infection of surgical incisions.21 The optimal rewarming rate remains unclear and varies with each case. Standard rewarming rates are a 0.5°C/hr to 2.0°C/hr (0.9°F/hr to 3.6°F/hr) rise in temperature in an otherwise stable patient (Table 65-3). Carefully consider and individualize invasive therapy to the severity of the hypothermia and the condition of the patient. Avoid overtreating and overusing invasive techniques in an otherwise stable hypothermic patient. In patients with severe underlying problems such as hypoglycemia, hyperglycemia, sepsis, adrenal crisis, drug overdose, or hypothyroidism, treat these conditions appropriately in addition to treating the hypothermia. Long-term outcome may depend more on treatment of the underlying illness than on treating the hypothermia.1,55
TABLE 65-3
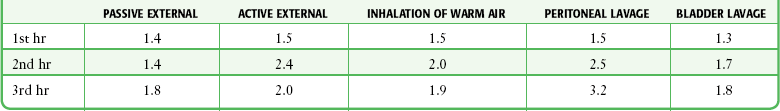
Note: Thoracic lavage had a median rewarming rate of 2.95°C/hr (see Plaisier54).
From Danzl D, Pozos RS. Multicenter hypothermia study. Ann Emerg Med. 1987;16:1042.
Passive External Rewarming
The cornerstone of the effectiveness of passive external rewarming relies on the body’s ability to restore normal body temperature through its own mechanisms for heat production. Stop further heat loss with insulation and manipulation of the environment. Give warm fluids containing glucose to patients who are fully alert. For patients with mild AH, remove wet clothing and then provide passive external rewarming with blankets. The technique is simple, but the patient must be capable of generating enough body heat for this method to be successful. Give warmed IV fluids to counteract the cold-induced diuresis. Internal heat generation is required for rewarming, and this effect will be relatively slow. In an otherwise stable patient, aggressive intervention with drugs and invasive monitoring might be more harmful than beneficial. Patients who cannot shiver, those who are hypotensive, or those who are intoxicated or malnourished may not have this capability. Survival rates with passive external rewarming have ranged from 55% to 100%.56–59
Active External Rewarming
Indications: Although there is some suggestion that active external rewarming of profoundly hypothermic patients by immersion may be associated with an increase in mortality over other treatments,16,60 more recent studies suggest that this technique is highly effective for mild hypothermia.32,61 Use it selectively and limit it to the trunk. Other forms of active external rewarming are increasingly being used in the ED as adjunctive care of moderately hypothermic, otherwise healthy individuals. Vasoconstriction limits the ability to increase core temperature with techniques that primarily warm the skin.62
Active external rewarming is most beneficial when the heat supplied by the external source is greater than the loss of rewarming heat incurred by the cessation of shivering. In more remote wilderness settings where more aggressive warming techniques are precluded because of the lack of equipment or personnel, active external rewarming with body-to-body contact may be the only option. The rewarming contribution of body-to-body contact appears to be limited, however.63
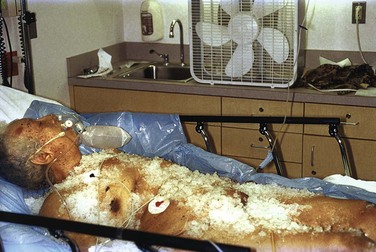
Equipment: Traditionally, immersion therapy has used a heated (40°C to 42°C [104.0°F to 107.6°F]) water tank of the type present in most burn units. Generally, immerse a hypothermic patient entirely except for the extremities and head, but immersion of the extremities may hasten rewarming.64,65 A major drawback is the inability to closely monitor patients undergoing immersion. Alternatively, use a warm water–filled heat exchange blanket (e.g., Blanketrol, Cincinnati Sub-Zero Products, Cincinnati, OH) for conduction warming. Intraoperative studies have demonstrated excellent results.66 A forced warm air convection system (Bair Hugger, Augustine Medical, Eden Prairie, MN; Snuggle Warm Convective Warming System, Sims Level 1, Inc., Rockland, MA) has been used for postsurgical rewarming.66,67 This approach has also been used successfully for ED-based AH therapy. Rewarming by warm air convection permits continued monitoring in the ED and is better tolerated than immersion because of the less rapid development of vasodilation in peripheral tissues.
Technique: Because profound fluid shifts can occur with conduction warming, give the patient supplemental IV fluid warmed to 40°C (104.0°F; Hotline Fluid Warmer, Sims Level 1, Inc., Rockland, MA) at a rate sufficient to generate a urinary output of 0.5 to 1.0 mL/kg/hr. Give an initial fluid bolus of 500 mL of D5NS. Note that blood pressure is not an accurate means of gauging fluid resuscitation since serious hypothermia is always accompanied by “physiologic” hypotension. Because patients requiring mechanical ventilation have rarely been subjected to tank immersion, it cannot be recommended for hypothermic patients who require intubation. Rewarming rates ranging from 0.9°C to 8.8°C (1.6°F to 15.8°F) per hour have been reported with immersion therapy.6,68
A heat exchange blanket allows the patient to receive other treatments that may be difficult or impossible to carry out in a tub, such as defibrillation, CPR, or more invasive warming techniques. Place the heating blanket and overlying cloth sheet underneath the patient. Set the blanket temperature to 40°C to 42°C (104.0°F to 107.6°F), and initiate the measures described in the section “Passive Rewarming Techniques.” Forced-air rewarming (convection) uses a blanket cradle to create an environment through which heated air is blown. Access to the patient is quite good with this system because the overlying blankets can be raised temporarily to evaluate the patient or perform procedures. Experience with mild immersion-induced hypothermia in volunteers suggests that the forced-air technique warms at a rate comparable to that of vigorous shivering, but with less metabolic stress and less afterdrop.69
Arteriovenous Anastomoses Rewarming: Arteriovenous anastomoses rewarming (AVR) involves immersion of the distal end of the extremities (hands, forearms, feet, and lower part of the legs). Advantages include rapid rewarming rates. A study of AVR immersion at temperatures of 45°C and 42°C (113.0°F and 107.6°F) in healthy volunteers demonstrated rewarming rates of 9.9°C/hr (±3.2°C/hr) for the former and 6.1°C/hr (±1.2°C/hr) (43.0°F ± 34.2°F/hr) for the latter.70 There was also a decrease in postcooling afterdrop. AVR is well tolerated by patients because of the rapid rise in core temperature and the shortened period of shivering.64,71
Complications: There is concern that surface warming with accompanying vasodilation may produce relative hypovolemia in a hypothermic patient. Other complications described with the active external rewarming method include core temperature afterdrop and rewarming acidosis. In core temperature afterdrop, colder peripheral blood is transported to the warmer core organs, thereby further reducing core temperature. In rewarming acidosis, colder blood and lactic acid return to the core organs and worsen the acidosis. To limit these complications in patients with moderate hypothermia, some authors advocate using active external warming only after active internal techniques have been initiated.71
Active Core Rewarming
There is evidence that active core rewarming may decrease mortality from severe hypothermic exposure when compared with other techniques. In the face of circulatory failure, often the best chance of survival is treatment with extracorporeal circulation (ECC) and warming of the blood.72 Several methods have been described, including the use of warm humidified air through an endotracheal tube or mask, peritoneal lavage, gastric or bladder lavage with warm fluid, thoracic tube lavage, cardiopulmonary bypass, AVR, peripheral vascular extracorporeal warming, hemodialysis, and thoracotomy with mediastinal lavage. These techniques transfer heat actively to the body core and achieve varying rewarming rates. The specific techniques and some of the advantages and disadvantages for each procedure follow.
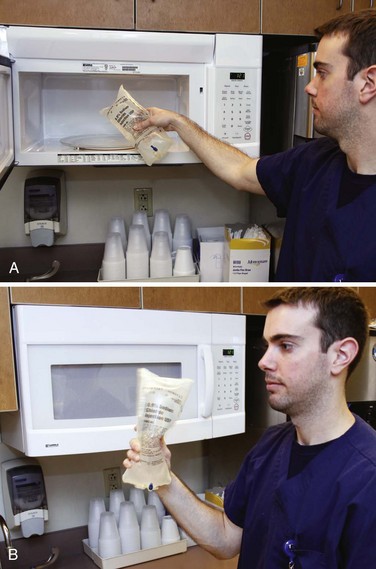
Emergency Warming of Saline in a Microwave: Under ideal circumstances, keep saline in a standard warming device. When large amounts of saline are required for such procedures as peritoneal lavage, warm 1-L saline bags rapidly in a standard microwave oven (Fig. 65-4).73 Although devices vary, a 650-W microwave oven has been demonstrated to warm 1 L of room-temperature non–dextrose-containing saline from 21.1°C to 38.3°C (70°F to 101°F) in 120 seconds on the high setting. At midcycle (i.e., after 60 seconds), interrupt the heating with agitation, and repeat the agitation at the end of the cycle before infusion.
Inhalation of Heated Humidified Oxygen or Air: The use of warm humidified oxygen to treat hypothermia has been well established. Average rates of rewarming of 1°C/hr (33.8°F/hr) via mask and 1.5°C/hr to 2.0°C/hr (34.7°F/hr to 35.6°F/hr) via endotracheal tube with heated aerosol at 40°C (104.0°F) can be obtained.6,32 Faster rewarming rates may be accomplished with a maximum safe aerosol temperature of 45°C (113°F). Core rewarming with this technique occurs through the following mechanisms. The warmed alveolar blood returns to the heart and warms the myocardium. The heated, humidified air delivered to the alveoli also warms contiguous structures in the mediastinum by conduction. Warming the inhaled air or oxygen eliminates a major source of heat loss.
Indications and Contraindications.: The use of heated humidified air or oxygen is a simple technique that should be used routinely in all patients with hypothermia, regardless of severity. If the correct equipment is available, it can be used in the field and in the hospital.44,45 One must address the risk for burns during the inhalation of warm air in the field environment.74 Mouth-to-tube ventilation in an intubated hypothermic prehospital patient has the theoretical advantage of providing warm humidified air without special equipment. A ventilating rescuer can inhale oxygen before expiring into the patient’s endotracheal tube to provide air with increased oxygen content. There are no contraindications to or reported complications from the use of warm humidified air for hypothermia, and there is no afterdrop.75
Technique.: Use a heated cascade nebulizer with a mask for patients with spontaneous respirations. Use a volume ventilator for intubated patients. Monitor the inspired air to maintain a temperature of approximately 45°C (≈113.0°F).76 Temperatures higher than 50°C (122°F) may burn the mucosa, and temperatures lower than 45°C (<113°F) do not deliver the maximum heat. Humidify the air or oxygen and note that the heater module may need modification because many units have feedback mechanisms that shut off at a given temperature. It may be difficult to deliver oxygen at the recommended temperature because of equipment limitations. In many cases the air temperature is only 30°C (86°F).
Summary.: Inhalation of warm humidified air or oxygen results in gradual rewarming of the core and should be the mainstay of all rewarming therapy. Studies have suggested that the rewarming rate of inhalation therapy is inferior to that of peritoneal lavage, thoracic lavage, and bath rewarming.6 Inhalation therapy can be combined with any and all other methods of rewarming and is relatively noninvasive and inexpensive. This therapy should be considered as the initial treatment of choice for hypothermic patients.
Peritoneal Dialysis (Lavage): Peritoneal dialysis (lavage) is an attractive treatment of severe hypothermia because it is available in most hospitals and does not require any unusual equipment or training. Rewarming rates of 2°C to 3°C (3.6°F to 5.4°F) per hour, depending on the dialysis rate, can be achieved without sophisticated equipment that may delay therapy or require transfer of the patient to a tertiary care facility.77 This technique can also be used to help correct electrolyte imbalances.
Rewarming by peritoneal dialysis was first used successfully in a patient in ventricular fibrillation with a temperature of 21°C (69.8°F).78 Since that time, there have been reports of successful rewarming with peritoneal lavage in stable, severely hypothermic patients and unstable hypothermic patients in cardiac arrest.79,80 Peritoneal lavage works via transfer of heat from lavage fluid to the peritoneal cavity. The peritoneal great vessels and abdominal organs provide a large surface area for exchange of heat. The use of warmed peritoneal lavage fluid is an effective approach to rewarming.81 There have been reports in the literature of success with rapid high-volume peritoneal lavage in pediatric patients. The technique involves the use of an infraumbilical “mini-laparotomy” incision followed by placement of a large silicone peritoneal dialysis catheter. The catheter is connected to a rapid infusion device with delivery of 1 L of warmed normal saline every 90 seconds.81
Indications and Contraindications.: Peritoneal dialysis is appropriate therapy in a severely hypothermic patient. In practice, it is often omitted if other measures appear to be successful. There are no universally established criteria for performing peritoneal lavage in hypothermic patients who have detectable vital signs. Though theoretically less effective than other techniques that directly warm the thorax in the setting of cardiac arrest, it has been used successfully in that situation. It is theoretically useful in hypothermic patients who have overdosed with a dialyzable toxin. Other less invasive methods, such as gastric or bladder lavage or warm nebulized air or oxygen inhalation, may be preferred in stable patients with temperatures higher than 26°C to 28°C (>78.7°F to 82.4°F). Peritoneal dialysis should not be performed on patients with previous abdominal surgery. It should be used with extreme caution in patients with a coagulopathy.
Equipment.: We recommend using the Seldinger technique with a commercially available disposable kit (e.g., Arrow Peritoneal Lavage Kit, product no. AK-09000, Arrow International, Inc., Reading, PA) because of the ease of performance and minimal morbidity associated with this procedure.
Technique.: In a noncritical patient, obtain a coagulation profile before the procedure, but in life-threatening situations, initiate the procedure immediately before laboratory studies. Place the patient in the supine position with a Foley catheter and nasogastric tube in place. After infiltrating with lidocaine, make an infraumbilical stab incision with a No. 11 scalpel blade, and place an 18-gauge needle into the peritoneal cavity directed toward the pelvis at a 45-degree angle. Insert a standard flexible J wire through the needle, and then remove the needle. Pass the 8-Fr dialysis catheter over the wire with a twisting motion, and then remove the wire.
Lavage rates of 4 to 12 L/hr can be achieved with two catheters. Warm the fluid with a standard blood warmer to 40°C to 45°C (104.0°F to 113.0°F). Use a standard 1.5% dextrose dialysate solution. Add potassium (4 mmol/L) if the patient becomes hypokalemic. Saline has also been used successfully. The rate should be at least 6 L/hr and preferably 10 L/hr.80
Complications.: The Seldinger method has a complication rate of less than 1%.82 A “mini-lap” performed via direct dissection may also be used but might have a higher complication rate.83 Further discussion of potential complications is provided in Chapter 43.
Summary.: Peritoneal dialysis is a useful method because it entails readily available fluid and can be done with a self-contained disposable kit.83 If a hospital also treats trauma victims, the lavage kit can be the same as that used for evaluation of abdominal trauma. If this technique is combined with warm nebulized inhalation, warming rates of 4°C/hr (7.2°F/hr) can be achieved.84 Peritoneal lavage rewarms the liver and restores its synthetic and metabolic properties.85
Gastrointestinal and Bladder Rewarming: Gastric or bladder irrigation offers some of the same advantages as peritoneal dialysis without invading the peritoneal cavity. Heat is delivered to structures in close proximity to the core. In the Multicenter Hypothermia Study, gastric/bladder/colon lavage had a first-hour rewarming rate of 1.0°C to 1.5°C/hr (33.8°F/hr to 34.7°F/hr) and a second-hour rewarming rate of 1.5°C/hr to 2.0°C/hr (34.7°F/hr to 35.6°F/hr) for severe hypothermia.83,86 In a multifactorial analysis of the Multicenter Hypothermia Study there was a trend toward improved survival in patients treated in this manner.16
Although the amount of heat delivered with gastric lavage appears to be less than that delivered with peritoneal dialysis, it is somewhat easier to use and less invasive. When combined with other methods, gastric or bladder lavage provides significant warming.83,84 Serum electrolyte levels should be monitored if large volumes of tap water are used because dilutional electrolyte disturbances may occur. Children and geriatric patients might be more susceptible to electrolyte changes with tap water irrigation.87
Indications and Contraindications.: Warmed gastric or bladder lavage may be used as adjunctive therapy for moderate or severe hypothermia. It can be combined with other warming techniques when rapid rewarming is needed. Patients who are obtunded and lack protective airway reflexes should undergo endotracheal intubation before gastric lavage to prevent aspiration of gastric contents. Refer to the appropriate chapters concerning nasogastric tube placement (see Chapter 40), gastric lavage (see Chapter 42), and urethral catheterization for specific contraindications to these procedures.
Equipment.: Use a large-diameter 32- to 40-Fr lavage tube with normal saline solution warmed to 40°C to 45°C (104.0°F to 113.0°F) in a microwave or blood warmer with verification of temperature before use. Although smaller tubes are easily passed nasally, use oral placement of the large lavage tubes. A modified Sengstaken tube with gastric and esophageal balloons may also be used.
Technique.: Instill 200- to 300-mL aliquots of fluid into the stomach before removal by gravity drainage. For bladder irrigation the optimal volume is not known, but avoid distention of the bladder (100- to 200-mL aliquots should be sufficient). The amount of time that the irrigant should be left in place before removal is not known, but use of rapid exchanges with a dwell time of 1 to 2 minutes is suggested.
Complications.: Complications of lavage include trauma to the nasal turbinates, gastric and esophageal perforation, dilutional hyponatremia, inadvertent placement of the tube in the lungs, and pulmonary aspiration, all of which can be minimized by careful, proper technique. Fluid overload and electrolyte disturbances when using tap water are potential complications in pediatric and geriatric patients.
Summary.: Gastrointestinal and bladder lavage with heated fluids is easily performed with equipment and solutions available in any hospital. The stomach, colon, and bladder are poor sites for body cavity lavage as a result of the small surface area for heat exchange.85 Because of its ease and availability, it can be started early in the resuscitation and be combined with any other rewarming method to significantly add heat,69 although its specific effect on morbidity and mortality is not known.
Thoracic Cavity Lavage: Thoracic cavity lavage can be performed either by closed means, through chest tubes placed in one hemithorax,88,89 or in open fashion, after resuscitative thoracotomy.90 The former approach offers the advantages of being less invasive and is an effective form of treatment in hospitals not equipped for cardiopulmonary bypass.89 Furthermore, closed-chest CPR can be continued while this technique is used. The open thorax approach offers the theoretical advantage of direct warming of the heart and the option of open-chest cardiac massage. Rapid warming rates of 6°C to 7°C (42.8°F to 44.6°F) in 20 minutes have been described.88,89 Pleural irrigation results in cardiac rewarming and might be the method of choice, particularly in patients with an arrhythmia.85
Indications and Contraindications.: Thoracic cavity lavage should be considered for patients requiring rapid core rewarming in the setting of cardiac arrest or inadequate perfusion (e.g., shock, during CPR) when cardiac bypass is not available. Open thoracic lavage should be considered in patients who will receive open-chest massage or thoracotomy for other reasons (e.g., hypothermic arrest with penetrating trauma). Thoracic lavage is not necessary for patients with mild or moderate hypothermia who can be rewarmed by other less invasive methods. Avoid the technique in patients with a coagulopathy unless required as a lifesaving measure.
Closed Thoracic Lavage.: An alternative that is more practical in the ED is pleural rewarming by repeatedly using warmed saline placed intermittently and then withdrawn through a chest tube. Place two large-bore thoracostomy tubes (e.g., 36 to 38 Fr in 70-kg adults) in one hemithorax. Infuse one chest tube with 3-L bags of heated normal saline (40°C to 41°C [104°F to 105.8°F]) via a high-flow fluid infuser (e.g., Level-1 Fluid Warmer, Technologies, Inc., Marshfield, MA). Collect the effluent with an autotransfusion thoracostomy drainage set (e.g., Pleur-evac, Deknatel A-5000-ATS, Fall River, MA). Empty the removable reservoir as needed. Alternatively, use a single–chest tube system with a Y-connector arrangement similar to that used for gastric lavage. Place aliquots of 200 to 300 mL with a 2-minute dwell time followed by suction drainage (at 20 cm H2O).
Open Thoracic Lavage.: Perform a left thoracotomy and pour saline warmed to 40°C to 41°C (104.0°F to 105.8°F) continuously into the thoracic cavity to bathe the heart while an assistant suctions the excess fluid from the lateral edge of the thoracotomy. Alternatively, add fluid to the thorax and mediastinum intermittently and suction after several minutes. Follow this with more warmed saline and repeat. This technique also allows direct monitoring of myocardial temperature. Perform direct cardiac massage until adequate spontaneous perfusion occurs. Perform direct cardiac defibrillation in a patient warmed to 30°C (86°F) with persistent ventricular fibrillation. When defibrillation is successful, continue direct myocardial warming until the patient’s temperature approaches 35°C (95°F). If defibrillation is unsuccessful at a core temperature of 30°C (86°F), continue warming while oxygenation, perfusion, and other physiologic parameters are optimized before further attempts at defibrillation.
Summary.: Thoracic lavage is an effective form of active core rewarming that is usually reserved for hypothermic arrest patients.54,89,90 Thoracic lavage may be considered when vital signs are inadequate or unstable enough to severely limit perfusion. Precise indications have not been clarified beyond patients in cardiac arrest.
Cardiac Bypass: The use of cardiac bypass or an extracorporeal shunt through either the femoral artery–femoral vein or the aortocaval procedure can result in rapid rewarming but requires surgical expertise, the availability of appropriate equipment, and technical support.5,50,91 This procedure has not been compared with other rewarming methods in a controlled fashion, and few centers have this modality available in a time frame that would affect survival rates. Its main advantages appear to be the rapid rate of warming that it produces and optimal patient oxygenation and perfusion. Femoral flow rates of 2 to 3 L/min with the warmer set at 38°C to 40°C (100.4°F to 104°F) will raise the core temperature 1°C to 2°C (33.8°F to 35.6°F) every 3 to 5 minutes.6 Drawbacks include potential delays in assembling the appropriate team and equipment, delays because of the time necessary to complete the operation, complications from the operation, the expense of the procedure and bypass equipment, and the potential for infection. Its use in extreme situations that may include cardiac arrest should be based on individual characteristics of the patient, clinician team, and hospital resources. If readily available, it should be strongly considered in hypothermic patients with asystole or ventricular fibrillation.41 If oxygenation is not a consideration, venovenous rewarming with an extracorporeal venovenous rewarmer can achieve rapid rewarming rates (2°C/hr to 3°C/hr [3.6°F/hr to 5.4°F/hr]), although they are slower than rates with cardiopulmonary bypass.71 Such a device is relatively easy to use, involves readily available technology, and probably does not require heparin. This equipment needs to be assembled before patients with hypothermia arrive.24 In severely hypothermic patients, extracorporeal rewarming using venovenous hemofiltration has also been reported to be successful.70,71 When compared with adults, children, especially smaller ones, require special consideration with regard to IV cannulation because drainage can be inadequate with femoral-femoral cannulation. In smaller hypothermic children, some sources recommend a more aggressive emergency median sternotomy for cardiopulmonary bypass.92
Cardiopulmonary bypass is indicated in the following situations: (1) cardiac arrest or hemodynamic instability with a temperature lower than 32°C (<89.6°F), (2) no response to less invasive techniques, (3) completely frozen extremities, or (4) rhabdomyolysis with severe hyperkalemia.2 A 47% long-term survival rate was obtained in a Swiss study of 32 young, otherwise healthy individuals, including mountain climbers, hikers, and victims of suicide attempts. Cardiopulmonary bypass is unlikely to confer similar benefit in older, poorly conditioned populations with underlying chronic diseases.93
Hemodialysis: Hemodialysis was first described for the management of AH in 1965.94 It is a rapid and efficient modality for rapid internal rewarming of patients with moderate to severe AH, but it is uncommonly used in clinical practice. One study reported that 26 patients with AH combined with circulatory arrest or severe circulatory failure were rewarmed to normothermia with the use of ECC.95 Core rewarming by hemodialysis has been achieved after placement of a dialysis catheter or with the use of an existing shunt. Some of the potential advantages and drawbacks of cardiac bypass also apply to this procedure, although slower warming rates have been reported. A range from 0.6°C/hr (33.1°F/hr) to rates as high as 4.5°C/hr (40.1°F/hr) have been achieved with fluid warmed to 40°C (104.0°F).87 For patients who have ingested a dialyzable toxin (such as barbiturates and toxic alcohols), hemodialysis can be used to both remove the toxin and rewarm the blood.94 In such cases its use may be appropriate.
Experimental Techniques: Ultrasonic, radiowave, and low-frequency microwave diathermy rewarming appears to be a rapid, safe, noninvasive technique that has shown promise in animal studies.67,96 Frequencies of 13.6 to 40.7 MHz are typically used. In a volunteer study the technique seemed to be less effective than immersion therapy and equivalent to passive rewarming techniques.96,97 Total liquid ventilation with warmed oxygenated perfluorocarbon is currently being studied in animals as a method of rapidly rewarming the core. Benefits include shorter rewarming times than with warm humidified oxygen (1.98 ± 0.5 hours versus 8.61 ± 1.6 hours; P < 0.0001), no afterdrop phenomenon, and no increase in lactate dehydrogenase and aspartate transaminase.75
Very hot IV fluids (65°C [149°F]) have been used in animals with little vascular damage or hemolysis. Trials in humans undergoing burn débridement have been very successful in preventing hypothermia during operative procedures. Saline heated to 60°C (140°F) with modified fluid warmers was infused through central venous access. There was no evidence of intravascular hemolysis or coagulopathy after the infusions.98 The role of hot IV fluids in the management of AH is currently undefined.
Special Situations
Cardiac arrest secondary to AH requires immediate treatment for the best chance of a successful outcome. Rapid rewarming and restoration of cardiac rhythm are essential for patients in cardiopulmonary arrest and can best be achieved with a combination of passive and multiple active core rewarming techniques. Because of numerous cases of survival from hypothermic cardiac arrest with prolonged external cardiac compression,5,41,99 thoracotomy is not mandatory. Thoracotomy does offer some theoretical advantages, however, such as increased cardiac output with open-chest massage,90 direct observation of cardiac activity, and direct warming of cardiac tissue with thoracic cavity lavage of warm fluid. Cardiopulmonary bypass is an effective technique for rapid rewarming. Blunt trauma and head trauma victims were previously not ideal candidates for cardiac bypass because of the anticoagulation requirement, but some authors have advocated this technique with heparin-bonded tubing even in the setting of known traumatic injury.5 A review of outcomes after hypothermic cardiac arrest from one institution found that the average time from thoracotomy to the development of a perfusing rhythm was 38 minutes (range, 10 to 90 minutes).5 The optimal rate of cardiac compressions in hypothermic patients is not known. Because of decreased oxygen consumption by vital organs, the rate required in hypothermic cardiac arrest is less than that recommended for normothermic cardiac arrest. Cardiac compressions should be initiated at half the normal rate in profoundly hypothermic patients. Guidelines developed by the American Heart Association and the Wilderness Medical Society recommend that CPR be initiated in patients with AH unless any of the following conditions exist: a “do-not-resuscitate” status is documented and verified, obvious lethal injuries are present, chest wall depression is impossible, no signs of life are present, or rescuers are endangered by delays in evacuation and altered triage conditions.6
The duration of CPR depends on the time required to raise the core temperature to a level at which defibrillation should be successful (i.e., >30°C [>86°F]). Previously, it was recommended that patients not receive a set of three countershocks until a core temperature above 30°C (>86°F) could be attained. There have been reports of successful defibrillation in patients with profound hypothermia with core temperatures of 25.6°C (78.1°F).86 The decision to terminate resuscitative efforts remains a clinical one, but there are certain poor prognostic factors. Certainly, survival is unlikely in patients who persist in asystole or go from ventricular fibrillation to asystole as they are warmed past 32°C (>89.6°F). Prognostic markers for patients with severe hypothermia and cardiac arrest have been proposed as contraindications to ED thoracotomy and cardiac bypass by some authors.5 Such markers include potassium levels elevated to above 10 mmol/L (mEq/L) and pH levels below 6.5. Nonetheless, there are reports of survival in patients with higher potassium levels and a pH as low as 6.29.92 The decision to continue resuscitative efforts should not be based solely on specific laboratory values or the initial core temperature.
Isolated reports of survival of hypothermic patients with prolonged CPR make extended efforts to resuscitate such patients reasonable. Children may be the best candidates for heroic measures.50 Under ideal conditions, hypothermic cardiac arrest patients may reasonably be admitted to an intensive care unit for a 4- to 5-hour trial of rewarming with CPR in progress. Manual CPR should be replaced by mechanical methods if the equipment is available. The oxygen-powered “thumper” has been successful during prolonged hypothermic resuscitation. Absence of responsiveness to treatment, in conjunction with a highly elevated potassium level, is an indication for termination of resuscitative efforts.
Airway Management
Maintain a secure functioning airway for hypothermic patients, just as in any critically ill patient. With mild hypothermia, deliver heated, humidified oxygen by face mask. Recognize that a hypothermic patient can be combative and uncooperative and may require arm restraints if a mask is used. Intubate patients with decreased sensorium who cannot reliably maintain their airway or hypothermic patients who may be hypoxic. Endotracheal intubation may be performed safely without the added risk of ventricular dysrhythmias.15 The technique for endotracheal intubation depends on the specific circumstances and the expertise of the operator. Once an endotracheal tube has been placed and secured, use it to provide warm humidified oxygen. There is no evidence that tracheal intubation is detrimental in severely hypothermic patients, and it should be considered if indicated for ventilation, oxygenation, or airway protection.
Acid-Base Disturbances
Acid-base disturbances are variable and can lead to metabolic acidosis from carbon dioxide retention and to lactic acidosis or metabolic alkalosis from decreased carbon dioxide production or hyperventilation. Interpretation of arterial blood gases in a hypothermic patient has been the cause of some confusion. Previously, it was suggested that all blood gases be corrected for temperature with correlation factors. With a decrease in temperature of 1°C (33.8°F), pH rises 0.015, carbon dioxide pressure (Pco2) drops by 4.4%, and oxygen pressure (Po2) drops 7.2% relative to values that would be obtained with blood analyzed under normal conditions. Despite the conversion guide, optimal or normal values in hypothermia have not been well documented.32 Other recent literature supports the use of uncorrected arterial blood gas values to guide therapy with bicarbonate or hyperventilation.30 This approach appears appropriate to support optimal enzymatic function. Gradual correction of acid-base imbalance will allow increased efficiency of the bicarbonate buffering system as the body warms. Arterial pH did not correlate with patient death in the Multicenter Hypothermia Study and should not be used as a prognostic guide to resuscitation.84
Coagulopathies
Abnormal clotting occurs frequently in hypothermic patients, probably because cold inhibits the enzymatic coagulation cascade.100–102 Hypothermia-induced coagulopathy does not result from excessive clot lysis, but rather from impaired clot formation.16,21 Platelet function is also impaired during hypothermia because production of thromboxane B2 is inhibited. Hypothermia-induced platelet aggregation with or without neutrophil involvement has been associated with neurologic dysfunction in patients undergoing surgical procedures.91 Hypercoagulability with a risk for thromboembolism may also occur, but the importance of cold-induced coagulopathy mainly involves patients with coincidental trauma. Such victims often have bleeding that is difficult to control. Replace appropriate clotting factors and use warm blood to limit further blood loss and worsening of the hypothermia.
Trauma and Hypothermia
Mortality is increased in trauma patients with temperatures below 32°C (<89.6°F). It is not clear whether this increased mortality is actually a result of the hypothermia or whether the hypothermia is merely an indicator of severe injury and response to a massive transfusion of cold fluid.21,103 Patients with severe trauma are prone to hypothermia because their injuries often expose them to environmental heat loss. Concurrent alcohol intoxication may add to the heat loss as a result of its vasodilatory effects on cutaneous vasculature and the prolonged cold exposure secondary to altered mental status. Victims of severe injury also lose heat because of exposure during resuscitation and rapid administration of cold fluids.
It is unknown to what degree correcting the hypothermia improves outcome. Nevertheless, devices to rapidly infuse warm fluids such as the Level 1 fluid warmer (Level 1 Technologies, Rockland, MA) and the Thermostat 900 (Arrow International, Reading, PA) are frequently used to warm large-volume fluid transfusions. Use of these devices seems reasonable to prevent the hypothermia associated with massive transfusions (see Chapter 28). Their use for hypothermia not associated with severe trauma is limited by the relatively low fluid requirements of patients with environmental exposure. Another Thermostat device (Aquarius Medical Corp., Phoenix, AZ) is used to accelerate recovery from hypothermia by mechanically distending blood vessels in the hand, thereby increasing transfer of exogenous heat to the body core. One article found that this particular rewarming device was not very effective in accelerating rewarming in hypothermic surgical patients after general anethesia.104
Pharmacotherapy and Monitoring
Hypothermia alters the pharmacodynamics of various drugs. It markedly alters drug kinetics, but not enough is known about this phenomenon to define specific therapeutic guidelines. Administer drugs with caution to hypothermic patients (Table 65-4). Because of the negative effects of hypothermia on both hepatic and renal metabolism, toxic levels of medications can accumulate rapidly after repeated use.105 Avoid certain drugs, such as digitalis. Sinus bradycardia and most atrial arrhythmias do not require pharmacologic treatment because the majority resolve with rewarming. Transient ventricular dysrhythmias also do not require treatment. Bretylium is the preferred agent for patients requiring medication for ventricular dysrhythmias, but lidocaine, magnesium, propranolol, and amiodarone have also been used.30 For severe acidosis (pH <7.1), IV sodium bicarbonate can be used with extreme caution. Vasopressors should be used with care, perhaps in much smaller doses than usual, because of the arrhythmogenic potential and the delayed metabolism of these agents. A review of intensive care unit admission of hypothermic patients found that treatment with vasoactive drugs was an independent risk factor for mortality, but this phenomenon remains poorly understood.41 In animal studies, use of epinephrine impaired myocardial efficiency in cases of moderate hypothermia.106 There was no advantage to repeated doses of epinephrine or high-dose epinephrine in hypothermic cardiac arrest animal models.107 The use of inamrinone, formerly known as amrinone, has been investigated in cases of deliberate mild hypothermia. Initial results indicate that amrinone accelerates the cooling rate of the core temperature, thereby potentially limiting its usefulness in management of AH.108
TABLE 65-4
Commonly Used Medications for Hypothermia
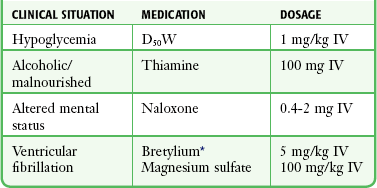
D50W, 50% dextrose in water; IV, intravenously.
*The role of more available antidysrhythmics such as amiodarone in patients with hypothermia remains to be determined.
Administer IV fluids slowly to prevent fluid overload potentiated by the decreased cardiac output. Fluids should be started early because intravascular volume is depleted in most hypothermic patients. D5NS has been advocated as the ideal initial resuscitation fluid.61,63,68 Avoid potassium until electrolytes are measured and normal renal function is confirmed. Check serum levels of creatine phosphokinase in hypothermic patients, which may indicate rhabdomyolysis. If elevated, carefully monitor renal function. Replace fluids aggressively because this may help prevent the development of renal failure. In severely hypothermic patients, consider placing a Swan-Ganz catheter and closely monitor urinary output to assist in fluid management. The risk of precipitating ventricular fibrillation should be weighed against the potential benefits of the Swan-Ganz catheter.
Frostbite
Hypothermic patients frequently suffer other forms of cold-related injuries in addition to their systemic hypothermia. The mildest form of frostbite is termed frostnip, a condition that involves only the skin and spares subcutaneous tissue. The skin is blanched and numb, but the injury is immediately reversible with no permanent sequelae if the area is quickly rewarmed. Rewarm rapidly in a water bath at 40°C to 42°C (104.0°F to 107.6°F). Frostnip occurs most frequently on the distal ends of the extremities, the nose, and the ears. Nonfreezing temperatures also produce trench foot, an intermediate step in the progression to true frostbite. Trench foot is the result of prolonged immersion in cold water. Rewarm patients and apply dry dressings.109,110
In frostbite, the body parts most susceptible are those farthest away from the body’s core: the hands, feet, earlobes, and nose. Exposure of the fingers to severe cold leads to cold-induced vasodilation.111,112 Apical structures rich in arteriovenous anastomoses can shunt blood flow away from tissues. Freezing of the corneas has been reported to occur in individuals who keep their eyes open in high–wind chill situations without protective goggles (e.g., snowmobilers and skiers).97
The pathophysiology of frostbite includes three pathways of tissue freezing: (1) through the extracellular formation of ice crystals, (2) hypoxia as a result of cold-induced local vasoconstriction, and (3) release of inflammatory mediators. These pathways often occur simultaneously and intensify the tissue damage. At the early stages of frostbite the “hunting reaction” is observed whereby the body alternates between periods of vasoconstriction and periods of vasodilation. As the temperature continues to decrease, the reaction stops and vasoconstriction persists.109,113 Cold also increases blood viscosity, promotes vasospasm, and precipitates the formation of microthrombi. Release of the inflammatory mediators prostaglandin F2 and thromboxane A2 causes further vasoconstriction leading to cell death. Release of these mediators peaks during rewarming, and cycles of recurrent freezing and rewarming only increase their tissue levels. Rewarming must be avoided until refreezing can be prevented.
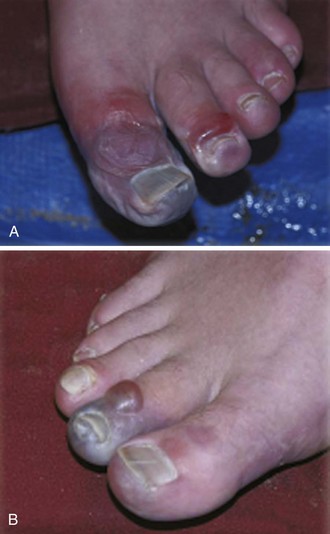
The clinical signs and symptoms of frostbite vary according to the degree of injury. Though useful clinically, the degree classification does not predict the extent of further tissue damage.32,92,100 The appearance of the affected extremity depends on the extent of the frostbite. With superficial frostbite, the affected extremity appears pale, waxy, and numb. The limb has poor capillary refill and is very painful on rewarming. With deeper frostbite, the affected extremity is hard, solid, and blanched. Hemorrhagic blisters may be present (Fig. 65-5). Initially, there is no pain or feeling in the frostbitten extremity. After rewarming, severe edema and blistering develop in the affected area, and victims eventually exhibit dry gangrene, mummification, and ultimately tissue sloughing.
Favorable prognostic signs for frostbite include intact sensation, normal color, warm tissues, early appearance of clear blisters, and edema. Early intervention is critical in terms of the ultimate outcome. Delay in seeking medical care for more than 24 hours is associated with an 85% likelihood that surgical intervention will be required. Patients seen within the first 24 hours require surgery less than 30% of the time.2,110 The predictive value of the initial physical examination is limited, but the presence of nonblanching cyanosis, hemorrhagic blisters, and impaired sensation appears to indicates a poor prognosis.2
Based on early bone scans and retrospective studies, researchers from France proposed a new classification for predicting frostbite outcomes on day 0.114 Four degrees of severity are defined. With first degree, there is complete recovery. Second degree often leads to soft tissue amputation. With third degree, bone amputation is needed, and with fourth degree, systemic effects occur.114
Rapid rewarming is the treatment of choice for frostbite.109 The aim is to limit the length of time that the tissue remains in the frozen state. The most practical way to rewarm an extremity is to totally immerse the area in warm water at 40°C to 42°C (104.0°F to 107.6°°F) for 15 to 30 minutes. Carefully protect the affected area to ensure that the tissue is not additionally injured by contact with the sides or rim of the container. After thawing, meticulously protect the area from injury. Elevate the extremity and place cotton or gauze between the toes or fingers to limit maceration. At some point, necrotic tissue should be débrided, most often after the ED encounter has allowed identification of viable tissue; however, the ideal timing and best method or intervention have not been elucidated. A conservative approach is advocated. One method is to débride white or clear blisters. Leave hemorrhagic or dark blisters intact because disruption may theoretically cause damage to the vascular supply and viable tissue.
Use topical aloe vera, a thromboxane inhibitor, and administer systemic antiprostaglandins such as ibuprofen. The use of semiocclusive dressings has shown promising results in the management of deep frostbite injuries of the fingertips.115 Provide tetanus prophylaxis. Adjuvant therapies involving the use of heparin or low-molecular-weight heparin, warfarin, vasodilators, corticosteroids, or immediate surgical sympathectomy have failed to improve outcomes.
The ideal intervention to ameliorate or limit tissue injury has not been proved, and it is uncertain if any protocol will prove effective. Mixed success has been achieved with the use of hyperbaric oxygen and thrombolytics.116 In a small study of frostbite victims, Twomey and coworkers suggested the following treatment algorithm for severe frostbite117: (1) rapid rewarming; (2) assessment of the patient’s clinical appearance; (3) early-phase 99mTc scintiscan to assess the distal circulation; (4) administration of tissue plasminogen activator (t-PA), 0.15 mg/kg by IV bolus, followed by 0.15 mg/kg/hr to a maximum dose of 100 mg over a 4- to 6-hour period, for patients with digits or limbs showing no flow and an absence of contraindications; (5) therapeutic heparin for 3 to 5 days; (6) administration of warfarin to an international normalized ratio two times control for 4 weeks; (7) pain management as needed; (8) ibuprofen, 400 to 600 mg orally four times daily; (9) light dressings with topical antimicrobials; and (10) no ambulation on frostbitten feet.117 Bruen and colleagues,118 in a small retrospective study, reported that administration of t-PA within 24 hours of frostbite injury improved tissue perfusion and reduced amputations. The protocol included t-PA administered at an initial rate of 0.5 to 1.0 mg/hr into the extremity via a femoral or brachial arterial catheter sheath. Heparin was also administered at 500 U/hr into the intraarterial catheter.118 Thrombolytic therapy for frostbite is encouraging, but the exact parameters for its use are still being investigated.
Agents that can inhibit the formation of free radicals are also promising. Such agents include superoxide dismutase, prostaglandin E1 analogues, and drugs containing antiplatelet activity such as pentoxifylline.109,113 The use of antibiotics is controversial, although some authors advocate agents effective against Staphylococcus and Streptococcus (e.g., cephalosporins, penicillins). Avoid débridement of tissue in the ED. Give analgesics (IV opioids) as needed.
Cold Water Immersion and Submersion
One of the leading causes of hypothermia remains cold water immersion or submersion.119 In a retrospective review of AH cases in a 3-year period, submersion hypothermia accounted for the greatest number of cases.120 Unlike cases of AH caused by cold exposure, risk factors are harder to identify because of the high mortality from drowning.100 Studies have shown that at cold water temperatures (8°C [46.4°F]), core cooling occurs at slower rates in persons with increased body mass and subcutaneous fat and at faster rates with increased voluntary activity (e.g., treading water). Risk factors for submersion hypothermia include impaired performance and the initial cardiorespiratory response to immersion. A study in healthy volunteers found that swimming efficiency and length of stroke decreased whereas the rate of stroke and swim angle increased as the water temperature dropped.121
The body’s response to cold water immersion (head out) has previously been described as occurring in three phases.67 The initial phase involves the “cold-shock response,” which typically occurs within the first 4 to 6 minutes. Signs include peripheral vasoconstriction, gasp reflex, hyperventilation, and tachycardia. At this stage there is a higher incidence of sudden death resulting from hypocapnia, inability to hold one’s breath, and increased cardiac output.67 After the initial cold-shock response, the body undergoes profound cooling of the peripheral tissues. The peripheral cooling tends to be the greatest in the hands, which leads to incoordination and difficulty grasping.67 With prolonged immersion in cold water, heat is lost from the body quicker than it is produced, with the individual quickly progressing to hypothermia.122–124
In cases of cold water submersion, researchers have found that rapid cooling is protective against neurologic impairment and increases the chance of survival.125 There are numerous reports in the literature of survival in children after cold water submersion but very few reports in adults. There are also reports of survival after up to 65 minutes of cold water submersion.126 Survival was reported in an elderly male after 22 minutes of submersion.127 Children tend to have a better prognosis because of the presence of the mammalian dive reflex and a greater body surface area–to-mass ratio, which allows more rapid cooling. A recent case was reported of a 2-year-old boy who suffered from severe hypothermia after falling into ice water.7 On discovery, cardiac arrest and asystole were present and the first measured temperature was 23.8°C (74.8°F). The patient was rewarmed by ECC with cardiopulmonary bypass and was discharged 9 days later without any sequelae. Orlowski identified five poor prognostic factors for near-drowning in pediatric patients128: (1) maximum submersion time longer than 5 minutes, (2) comatose on arrival at the ED, (3) arterial blood gas pH less than 7.10, (4) age younger than 3 years, and (5) resuscitation not attempted for at least 10 minutes after rescue. Adults tend to have higher mortality rates because of the following: (1) lack of the mammalian dive reflex and (2) slower rates of cooling secondary to lower body surface area–to-mass ratios than in children. Recent reports of hypothermia and drowning in commercial fishing deaths in Alaska noted a strong protective association with the use of personal floatation devices, particularly immersion suits, in surviving cold water–related events in adults.129
Various mechanisms of brain and body cooling during submersion hypothermia have been described, including the mammalian dive reflex, cold-induced changes in release of neurotransmitters, and water ventilation.130 The mammalian dive reflex prevents or delays aspiration or ventilation until the body has cooled to a point at which protection against hypothermia occurs. Much attention has focused on the theory of water ventilation as a key component of accelerated brain cooling. Animal studies comparing immersed (head out) and submersed dogs found that cooling rates were faster in submersed dogs than in immersed dogs. The submersed dogs cooled by convective heat exchange in the lungs, whereas the immersed dogs cooled by surface conduction only. Laboratory data obtained after the submersion indicated that there was indeed ventilation exchange in the water.126 The body also undergoes a relative bradycardia as another protective measure. Bradycardia is inversely proportional to the water temperature, with heart rates reaching 18 beats/min in water at 10°C (50°F).130 Many authors advocate therapies aimed at symptoms resulting from near-drowning rather than severe hypothermia because in fatal cases of submersion, death occurs too rapidly for hypothermia to be a significant contributor. Complications of near-drowning include pneumonia, lung edema, hemorrhagic pancreatitis, and skin edema.100
Conclusion
Mortality rates from AH are decreasing, and this is linked to increased recognition and advanced therapy. Caution should be used when extrapolating published data obtained in adults to children.56 With the exception of severe hypothermia, the prognosis correlates mostly with the presence or absence of underlying disease states. Studies have shown that the prognosis is excellent in patients in whom no hypoxic event precedes the hypothermia and no serious underlying disease states exist. Previously healthy individuals usually have full recovery with mortality rates lower than 5%, but patients with coexisting medical illnesses reportedly have mortality rates higher than 50%.55
Because death is related more to underlying illnesses than to hypothermia, some recent sources do not believe that invasive rewarming modalities are useful for poikilothermic patients with severe underlying disease.1
Procedures Pertaining to Hyperthermia
As a result of global climate change, it is projected that worldwide there will be a significant increase in the number and intensity of heat waves with resultant deaths from hyperthermia and heat-related illness.131 Temperature extremes and variability will remain important determinants of overall health, especially in the vulnerable populations of the elderly, children, and those with chronic illness. The mortality associated with heatstroke accounts for more than 200 deaths per year in the United States.132,133 In the United States from 1999 to 2003, a total of 3442 deaths were attributed to extreme heat exposure.134 During the heat wave of 2003, France reported 15,000 excess deaths as a result of the heat wave.135 The morbidity associated with heat-related illness is on the rise. Nationally, an estimated 54,983 patients were evaluated in U.S. EDs for exertional heat-related illness from 1997 to 2006.136 This represents a 133% increase over the 10-year period.136 Lack of heat acclimatization during extreme environmental conditions is responsible for the increasing percentage of heat-related illness, particularly in younger populations and agricultural workers.137
Other important causes of hyperthermia include malignant hyperthermia (MH) and neuroleptic malignant syndrome (NMS). Both MH and NMS are largely iatrogenic and are mostly triggered by modern pharmacologic therapy.138 There is evidence that MH involves a dose-dependent response, but the minimum dose is unknown.138 The incidence of hyperthermic conditions induced by psychostimulant drugs of abuse, such as morphine and amphetamine derivatives, continues to increase.139
Normal Thermoregulation
Body temperature typically follows a diurnal pattern, with an increase from about 36°C (96.8°F) in the early morning to 37.5°C (99.5°F) in the late afternoon, and is normally tightly regulated by an effective thermoregulatory system.140 Heat is produced as a by-product of metabolic processes and when ambient temperature exceeds body temperature. Body temperature increases when the rate of heat production exceeds the rate of heat dissipation. The brain’s thermal center is located in the preoptic nucleus of the anterior hypothalamus. In response to rising core temperature, this thermal center activates efferent fibers of the autonomic nervous system to produce vasodilation and increase the rate of sweating. Vasodilation dissipates heat by convection, and sweat dissipates heat by evaporation.
Hyperthermia occurs when the thermoregulatory mechanisms are overwhelmed by excessive metabolic production of heat, excessive environmental heat, or impaired heat dissipation. Different age-related thermoregulation strategies are used when dealing with heat stress. Children have a greater surface area–to-mass ratio and a lower sweating rate and rely more on “dry” heat exchange to dissipate heat. On the contrary, adults use evaporative heat loss as the primary heat dissipation technique. With primary aging, the reflex cutaneous vasoconstriction and vasodilation capabilities are impaired, thereby allowing increased susceptibility to complications from heat-related exposure.141 Fever occurs when the hypothalamic set-point is increased by the action of circulating pyrogenic cytokines, which cause peripheral mechanisms to conserve and generate heat until the body temperature rises to the elevated set-point. Hyperthermia and fever cannot be differentiated clinically on the basis of the magnitude of temperature or on the pattern of its changes.142,143
Types of Hyperthermia
Heat cramps and heat exhaustion are induced by a hot environment.144 The body’s heat dissipation mechanisms are generally able to keep up with heat production and absorption in these disorders. Symptoms are largely due to the mechanisms used by the body to dissipate heat, and body temperatures remain at or near normal. Rapid cooling techniques are not required, and supportive care and hydration in a cool environment are usually adequate therapy.
Heat cramps are intensely painful but generally benign involuntary skeletal muscle spasms. The pain most often occurs in the calf, hamstring, or quadriceps muscles but may also involve the arms and back. The cramps may be severe and prolonged but only rarely lead to rhabdomyolysis. Heat cramps occur after strenuous exercise or heavy labor in a hot environment. Heat cramps were previously thought to be the result of dehydration associated with significant loss of sodium chloride, but some clinical observations have proved that heat cramps can occur at rest or during exercise under any environmental conditions.145 Rest in a cool environment plus vigorous oral fluid replacement with isotonic solutions is usually adequate therapy, but in some cases IV saline is required.145 The benefits of oral rehydration over IV hydration directly relate to oropharyngeal stimulation, which influences the release of antidiuretic hormone (arginine vasopressin), cutaneous vasodilation, thirst sensation, and mean arterial pressure.146 A common mistake is to rely on thirst to indicate dehydration. The pain of severe cramping may be resistant to narcotics in the absence of adequate fluid replacement.
Heat exhaustion, commonly referred to as heat syncope, is a poorly defined syndrome with nonspecific symptoms that occur after heat exposure.147 Many have suggested replacing the current terminology of heat exhaustion with the term exercise-associated collapse.148 Malaise, flulike symptoms, orthostasis, dehydration, nausea, headache, and collapse may all occur. Previously it was believed that heat exhaustion is the result of dehydration-induced heat retention that is not severe enough to cause heatstroke.149 There is modern evidence that postural hypotension developing after exercise is the result of exercise-induced changes in blood pressure regulation. These changes involve recalibration of the arterial baroreflex to lower pressures after exercise, impaired sympathetic vascular regulation, and H1 and H2 receptor–mediated vasodilation.149 When compared with the more severe heat disorder of heatstroke, mental status is normal and body temperature is normal or mildly elevated with heat exhaustion. There does not appear to be any thermoregulatory failure in persons with heat exhaustion. Rehydration, rest, and supportive care in a cool environment are adequate therapy for heat exhaustion.144,145,148 Some authors advocate cooling and placing the patient either in the supine position with the legs elevated or seated with the head between the knees to decrease skin blood flow and increase venous blood flow to the heart.149 Recovery is usually evident within a few minutes to hours. Occasionally, heat exhaustion is accompanied by heat cramps, thus presenting a confusing scenario if the diagnosis is not suspected. Rapid cooling techniques, IV hydration, and advanced therapies are not usually required, but patients should be observed for progression to heatstroke because heat exhaustion and heatstroke are a continuum of one disease process.146,149
Heatstroke
When the body’s normal heat dissipation mechanisms are overwhelmed, core temperature elevation and heatstroke develop rapidly. Heatstroke is a state of thermoregulatory failure.133 Previously, the morbidity and mortality associated with heatstroke were attributed to the magnitude of the hyperthermic response.133 Recent literature has described a more complex interaction between cytokines, coagulation, and the systemic inflammatory response syndrome (SIRS), with endotoxin and cytokines being implicated as key mediators of heat-induced SIRS.150 Two forms of heatstroke are described in the literature. Classic (nonexertional) heatstroke usually occurs during summer heat waves. The poor, urban elderly, infants, homeless, and persons with impaired mobility are at greatest risk.151,152 Dehydration, lack of air-conditioning, obesity, neurologic disorders, hyperthyroidism, cardiovascular disease, impaired mentation, and medications that interfere with heat dissipation (e.g., phenothiazines, diuretics, and anticholinergics) predispose this population to heatstroke.151,152 Exertional heatstroke, a consequence of strenuous physical activity, usually afflicts a younger segment of the population. Highly motivated, poorly acclimatized, or unconditioned athletes and overweight military recruits are common victims, as are individuals who perform heavy physical labor in hot, humid conditions (Fig. 65-6).153–155 With exertional heatstroke, the risk appears to be greatest in individuals performing high-intensity exercise for relatively short durations.155 A retrospective review of long-distance cyclists participating in the California AIDS (acquired immunodeficiency disease) Ride found that as the number of chronic medical illnesses increased, so did the risk for development of an exertional heat-related illness. Human immunodeficiency virus seropositivity alone was not associated with an increased risk for exertional heat-related illness.155
Figure 65-6 Risk for heat exhaustion or heatstroke during intense work in the heat (adjusted to the American College of Sports Medicine position stand: prevention of thermal injuries during distance running2). (Adapted from Epstein Y, Moran D. Environmental aspects of travel medicine. In: Keystone JS, Kozarsky P, Freedman DO, et al, eds. Travel Medicine, ed 3. Philadelphia: Saunders; 2013.)
The degree of hyperthermia necessary to produce heatstroke in humans is unknown. In tissue culture cells, thermal injury is observed with temperatures in the range of 40°C to 45°C (104°F to 113°F). Studies of hyperthermia in patients undergoing cancer therapy have revealed that tissue sensitivity to heat is increased by relative hypoxia, ischemia, and acidosis.156
The key clinical findings in the diagnosis of heatstroke are (1) a history of heat stress or exposure, (2) a rectal temperature higher than 40°C (>104°F), and (3) central nervous system (CNS) dysfunction (altered mental status, disorientation, stupor, seizures, or coma). The cerebellum is very sensitive to heat, and ataxia may be an early clue. Although anhidrosis is described as a classic sign of heatstroke, investigations have demonstrates that cessation of sweating may be a late finding. Failure to consider the diagnosis of heatstroke in a diaphoretic patient with changes in mental status could prove disastrous.157
The sequelae of heatstroke are caused by thermal damage to multiple organ systems.158 Whole-body hyperthermia decreases pulmonary capillary wedge pressure and cerebral vascular conductance and causes an inotropic shift in the Frank-Starling curve.159 After a hyperthermic event, tissue injury continues.157,158 Delirium, seizures, and coma can result from the direct effects of heat on the CNS. Autopsies show profound brain edema after hyperthermic insults. Researchers suggest that in cases of instant death, brain edema from the increased permeability of the blood-brain barrier causes raised intracranial pressure and papilledema, followed by vascular infarction and brain herniation.160 Cardiovascular collapse results from dehydration, maximal cutaneous vasodilation, and direct heat-induced myocardial depression. Coagulopathies and liver dysfunction (elevated levels of bilirubin and transaminases) occur as a consequence of thermal breakdown, consumption of serum proteins, and direct heat damage to hepatic cells. Children often demonstrate diarrhea. Reduced intestinal blood flow causes barrier dysfunction and endotoxemia.161,162 Development of an acute abdomen, bloody diarrhea, dilated loops of bowel on radiographic studies, and unexplained shock should raise suspicion for colonic ischemia and pending colonic perforation.163 Renal failure can result from myoglobinuria (related to rhabdomyolysis) and acute tubular necrosis.163 Metabolic acidosis is the primary acid-base alteration observed in patients with heatstroke, with the prevalence increasing with the degree of hyperthermia.164
Treatment of these sequelae of acute heatstroke does not differ from that of other heat-related disorders, with the sole exception that rapid cooling is necessary to prevent further damage and reverse the heat stress. The more rapidly that rectal temperature is reduced to 38°C (100.4°F), the better the prognosis.151 The human body tolerates hyperthermia poorly. Unlike patients with hypothermia, in whom slow, gentle rewarming and supportive care often result in a favorable outcome, victims of severe heatstroke must be treated aggressively with measures designed to rapidly lower core temperature. Studies investigating precooling techniques to avoid heatstroke have been relatively unsuccessful in attenuating increases in core body temperature, and such techniques are not recommended.165 In contrast, whole-body precooling increases overall exercise endurance.166
MH
MH is a pharmacogenetic disease attributable to a medication that triggers a life-threatening, hypermetabolic syndrome.167 It results from a rare inherited autosomal dominant abnormality in the skeletal muscle membrane and has an incidence of 1 in 50,000 in adults.139 In response to certain stresses or drugs (Box 65-1), patients with this disorder sustain a potentially lethal hypermetabolic reaction with massive efflux of calcium from the skeletal muscle sarcoplasmic reticulum. This results in contraction of the sarcomeres, skeletal muscle rigidity, increased skeletal muscle metabolism, elevated serum creatine kinase levels, heat production, and finally, systemic hyperthermia.168,169 Hyperthermia is a late development and occurs after rigidity has been present for some time and the body’s normal heat dissipation mechanisms are overwhelmed. The earliest signs of MH are increased carbon dioxide production, muscle rigidity, and tachycardia.170 Cardiac output and cutaneous blood flow also increase to maximize the heat loss. Diagnosis of MH is based on the clinical triad of (1) exposure to an agent or stress known to trigger the condition, (2) skeletal muscle rigidity, and (3) hyperthermia.
MH is usually encountered in the operating room while patients are undergoing general anesthesia, particularly with halogenated inhalational agents and depolarizing muscle relaxants. Heat production in anesthetized patients can be profound with as much as a fivefold increase in oxygen consumption.169 Cases of MH may be encountered anywhere that general anesthetics or neuromuscular blocking agents are used.169,170 A massive increase in creatine kinase is a strong indicator of an MH reaction.167
As with heatstroke, treatment of MH requires rapid cooling and supportive care for the sequelae described previously. Unlike heatstroke, MH requires specific pharmacologic therapy to stop excessive heat production by skeletal muscle. Dantrolene sodium induces muscle relaxation in patients with MH by blocking release of calcium from the muscle cell sarcoplasmic reticulum.171 In all cases of MH, the inciting stimulus (see Table 65-4) should be discontinued immediately and dantrolene therapy administered. A dantrolene bolus of 2.5 mg/kg should started and repeated at 5-min intervals until normalization of the hypermetabolic state is achieved and all MH symptoms disappear.171 Procainamide has been used successfully when dantrolene is unavailable.171
It has been suggested that dantrolene administration speeds cooling of heatstroke victims by reducing skeletal muscle heat production.171 A randomized, controlled trial of the use of dantrolene for heatstroke found no difference between the treatment and placebo groups in terms of cooling time, complications, or length of stay.172 In 2005 a metaanalysis concluded that there was no role for the use of dantrolene in the management of heatstroke.173 Currently, dantrolene administration is best reserved for patients with clinical muscle rigidity or suspected MH. Routine use of this drug in heatstroke patients is not recommended.173 New and promising treatments of MH are being investigated. Researchers have discovered mutations in the gene coding for the ryanodine receptor calcium release channel (RyRI) in families with MH, which may be the functional basis for MH. Some studies have examined the effects of MH mutations on the sensitivity of the RyRI to drugs and endogenous channel effectors, including Ca2+ and calmodulin.174
NMS
First described in the late 1960s, NMS is characterized by hyperthermia, muscle rigidity, altered level of consciousness, and autonomic instability.175 Mortality from NMS is estimated to be 20% in patients in whom the condition develops.175,176 This idiosyncratic disorder follows the therapeutic use of neuroleptic drugs, including phenothiazines, butyrophenones, thioxanthenes, lithium, and tricyclic antidepressants. The reaction is triggered by blockade of dopaminergic receptors and results in skeletal muscle spasticity, which generates excessive heat and impairs hypothalamic thermoregulation and heat dissipation.176 Muscle rigidity, described as “lead pipe” rigidity in its most severe form,176 can be manifested as oculogyric crisis, dyskinesia, akinesia, dysphagia, dysarthria, or opisthotonos. NMS occurs in 0.2% of patients who take neuroleptic agents either chronically or acutely. Haloperidol and depot fluphenazine appear to be the most commonly offending agents.175 Temperatures can exceed 42°C (107.6°F). The initial agitation often progresses to stupor and coma. Catatonia and mutism may also be present. Autonomic instability is manifested as tachycardia, labile blood pressure, sweating, and incontinence. Ventilations are impaired by the chest wall rigidity.
This syndrome is more likely to occur at the initiation of or after an increase in neuroleptic dosage. Researchers suggest that NMS typically occurs over a period of several days (average in patients taking neuroleptic agents). It may also occur if the use of antiparkinsonian drugs is suddenly discontinued.176–179 NMS resembles MH but usually lasts longer (5 to 10 days) after use of the inciting drug is discontinued. The syndrome may be misinterpreted as worsening of an underlying psychiatric disorder, drug intoxication (e.g., cocaine and amphetamines), a severe dystonic reaction, tetanus, or a variety of CNS infections. In addition to agents with increased dopaminergic blocking activity, other risk factors for NMS include dehydration, previous history of dystonia, catatonia, agitation, and iron deficiency.175 The mortality rate is high, and respiratory failure, renal failure, cardiovascular collapse, or thromboembolic disease usually causes death.180
Treatment of severe NMS (i.e., hypotension, hyperthermia, marked rigidity) closely follows that of MH, except that therapy must be maintained for several days until the symptoms resolve. Therapy for NMS involves discontinuation of the triggering agent; rapid cooling; benzodiazepines; a combination of a central dopamine agonist, bromocriptine, or levodopa; dantrolene; and supportive treatment of the ensuing organ failure.179 Although the effects are not immediate, pharmaceutical therapy is directed at overriding the dopaminergic blockade caused by the offending neuroleptic agent or the dopamine depletion resulting from the cessation of antiparkinsonian medications. There are reports of successful treatment of NMS with subcutaneous apomorphine monotherapy or high-dose lorazepam and diazepam.180,181 As with MH, dantrolene (2.5 mg/kg) can be given to treat NMS-induced muscle rigidity.17 The beneficial response stems not only from effects at the sarcoplasmic reticulum of skeletal muscle but also from central dopamine metabolism of calcium in the CNS. Bromocriptine, a central dopamine agonist, is reported to be efficacious in treating NMS at doses of 2.5 to 10 mg three times a day.181 Although both these agents have been noted to reduce the duration of hyperthermia, there have been mixed results with the use of bromocriptine and dantrolene.178,181
A more recently described disorder often confused with NMS is serotonin syndrome.182 This syndrome involves the newer antidepressants fluoxetine, paroxetine, citalopram, fluvoxamine, venlafaxine, and sertraline,182 which are selective serotonin reuptake inhibitors. These drugs can adversely react with other stronger serotonin receptor agents such as monoamine oxidase inhibitors and nonselective serotonin reuptake inhibitors (clomipramine and tricyclic antidepressants) to induce a clinical picture similar to that of NMS, only milder. Serotonin syndrome classically occurs when two or more drugs that interfere with serotonin metabolism act synergistically on the 5-HT1A receptor and lead to overstimulation.178,182 Drugs that act at any of the other serotonin receptors are not likely to produce the syndrome.182 The range of symptoms varies from mild gastrointestinal upset, insomnia, and agitation to more severe symptoms that include muscle spasms, seizures, ataxia, rhabdomyolysis, and autonomic instability. Treatment is primarily supportive in milder cases and consists of prompt recognition and withdrawal of the offending agent. Most cases resolve spontaneously within 24 hours. For the most severe cases, aggressive intensive care unit management is warranted to prevent renal failure and death. The drug cyproheptadine (Periactin) has shown promise in managing the agitation often seen in severe cases.178,182 Cyproheptadine is an antihistamine with antiserotonergic properties. There has been limited success with benzodiazepines and β-blockers in these patients to treat agitation.182 In cases in which serotonin syndrome and NMS cannot be differentiated, benzodiazepines represent the safest therapeutic option.178 Further study of the newer antipsychotic drugs, such as ziprasidone, a powerful 5-HT1A receptor blocker, may delineate other possible benefits.182
Hyperthermia and Psychostimulant Overdose
As mentioned previously, the recognized incidence of hyperthermia induced by sympathomimetic psychostimulant drugs of abuse is on the rise. The offending agents most commonly described are cocaine, phencyclidine, amphetamine, and amphetamine derivatives such as 3,4-methylenedioxy-N-methamphetamine (MDMA) (“ecstasy”) and 3,4-methylenedioxy-N-ethylamphetamine (MDEA) (“Eve”).183,184 A number of studies have looked specifically at the club drug MDMA and its impairment of heat dissipation.183,184 Animal studies in rats suggest that MDMA-induced hyperthermia results not from MDMA-induced release of 5-HT but from increased release of dopamine acting at D1 receptors, thus suggesting a future role for the use of dopamine antagonists in clinical treatment.185
Hyperthermia is a common feature of these potentially severe to lethal poisonings with sympathomimetic psychostimulant drugs and may be the primary cause of fatality or MDMA-induced neurotoxicity.184 Because of the nonlinear pharmacokinetics of MDMA and γ-hydroxybutyrate, it is difficult to estimate a dose-response relationship.184 Some have applied a pathophysiologic model of exertional heatstroke or NMS to profound cocaine intoxication.185 In addition to profound hyperthermia (>42°C [>107.6°F]), acute rhabdomyolysis, disseminated intravascular coagulation, psychiatric and cognitive dysfunction, renal failure, coma, seizures, and death have been described in these patients.186–189 As demonstrated by Roberts and associates,188 even a patient with a core temperature of 45.5°C (114°F) because of acute cocaine intoxication may survive with aggressive cooling methods. Treatment requires prompt recognition, maintenance of adequate hydration, rapid cooling, and the aggressive use of sedatives or paralyzing agents (or both) to control agitation. Importantly, the longer that psychostimulant-overdosed patients remain hyperthermic, the higher their morbidity and mortality rates. Agitation and seizures must be chemically controlled because they lead to continued generation of heat and muscle injury. Therefore, liberal doses of benzodiazepines are recommended.188 Some have advocated the use of bromocriptine and dantrolene as for MH and NMS, but their efficacy in the setting of drug-associated hyperthermia remains controversial.189,190
Hemorrhagic Shock and Encephalopathy Syndrome
The condition of hemorrhagic shock and encephalopathy syndrome in children (mainly infants, but some older children also) resembles heatstroke in adults. The full-blown syndrome includes hyperthermia, coagulopathy, encephalopathy, and renal and hepatic dysfunction.191 Although there may be an association with concurrent viral illness, the condition generally follows an elevation in temperature, which may be triggered by “bundling” of a child with a low-grade fever. Therapy is largely supportive and includes volume replacement with rapid cooling of the hyperthermic child while sources of bacterial infection are sought and treated.
Cooling Techniques
Heatstroke mortality is proportional to the magnitude and duration of thermal stress measured in degree-minutes.192 Delay in cooling may be the single most important factor leading to death or residual disability in those who survive.144 In addition, advanced age and underlying disease states are significant contributing factors.144,145,151,152
Many exertional heatstroke victims are volume-depleted and may exhibit hypotension. Initial stabilization with cooled (room-temperature) IV fluids and correction of electrolyte abnormalities are valuable in hypotensive patients. Traditional sources recommend a rate of 1200 mL over the first 4 hours.193 Others advise a 2-L bolus over the first hour, with an additional 1 L/hr for the following 3 hours.194 Seraj and coworkers challenged this more aggressive recommendation.195 In their study of pilgrims who suffered heatstroke, 65% had normal or above normal central venous pressure (CVP) on arrival. These authors found that an average of 1 L of saline was sufficient to normalize CVP during the cooling period in their patients, who had a mean age of 55 years (range, 31 to 80 years). In older patients, fluid resuscitation should be monitored carefully to avoid pulmonary edema. Regarding antipyretics, there is no indication for either salicylates or acetaminophen in the setting of heatstroke because their efficacy depends on a normally functioning hypothalamus. Overzealous use of acetaminophen could potentiate hepatic damage, and salicylates may promote bleeding tendencies.196 A study comparing acetaminophen and physical cooling methods found that in patients treated with antipyretics only, mean body temperature increased by 0.2°C (32.4°F) on average.197
Given that rapid cooling is accepted as the cornerstone of effective heatstroke therapy, the clinician must choose which cooling technique to use. Studies in animal models are based on the assumption that the fastest cooling technique is the best. In clinical patient care, other factors also influence the choice of technique. Patient access, monitoring, safety, ease of use, availability, and speed are all considerations.198 A technique that may not be the most rapid but allows easy patient access and is readily available may be preferable to more cumbersome (albeit more rapid once established) cooling techniques in some clinical settings.
The cooling rates achieved in various human and animal studies of heatstroke are summarized in Table 65-5.199–213 The advantages and disadvantages of various cooling techniques are outlined in Table 65-6.
TABLE 65-6
Advantages and Disadvantages of Various Cooling Techniques
| TECHNIQUE | ADVANTAGES | DISADVANTAGES |
| Evaporative | Simple, readily available Noninvasive Easy monitoring and patient access Relatively fast |
Constant moistening of skin required |
| Immersion | Noninvasive Relatively fast Low mortality rates |
Cumbersome Patient access and monitoring difficult Shivering Poorly tolerated by conscious patients |
| Ice packing | Noninvasive Readily available |
Shivering Poorly tolerated by conscious patients |
| Strategic ice packs | Noninvasive Readily available Can be combined with other techniques |
Relatively slower cooling Shivering Poorly tolerated by conscious patients |
| Cold gastric lavage | Can be combined with other techniques | Relatively slower cooling Invasive Requires airway control Human experience limited |
| Cold peritoneal lavage | Rapid cooling | Invasive Human experience limited |
In addition to the cooling procedures outlined, it is imperative that the clinician institute the judicious use of sedation, muscle paralysis, or both to control agitation, suppress shivering, reduce energy expenditure, and make the patient receptive to sometimes unpleasant therapies.214 In general, IV benzodiazepines are the easiest and safest first-line drugs used for sedation.
Indications for Rapid Cooling
Rapid cooling should be instituted as soon as the diagnosis of heatstroke (rectal temperature >40°C [104°F], altered mental status, history of heat stress or exposure) is made. Rapid cooling is also indicated for the treatment of MH and NMS but should be instituted concurrently with discontinuation of the triggering agent or drug and administration of dantrolene. Because studies show that the degree of organ damage correlates with the degree and duration of temperature elevation above 40°C (>104°F), a reasonable clinical goal is to reduce the temperature to below 40°C (<104°F) within 30 minutes to an hour after the start of therapy.145,214
Evaporative Cooling
Evaporating water is thermodynamically a much more effective cooling medium than melting ice, given an appropriate water-vapor gradient. Evaporating 1 g of water requires 540 kcal. Melting 1 g of ice requires only 80 kcal. In theory, evaporative cooling should be approximately seven times more efficient than ice packing. In practice, evaporative cooling is more efficient. In separate human studies, Wyndham and colleagues and Weiner and Khogali found that evaporative cooling rates were substantially greater than cooling rates with water immersion at 14.4°C (57.9°F).200,202 Studies in primate models demonstrated faster cooling rates with evaporative cooling as an adjunct to ice bag placement.215 Methods using convection and evaporation were more effective than those involving conduction for the treatment of hyperthermia. In clinical practice, ice water immersion or ice packing causes heat loss by conduction and heat consumption by the phase change of melting ice. In healthy volunteers, evaporative cooling techniques (e.g., facial fanning) were associated with decreased thermal sensation and improved thermal comfort.216
Despite the continued enthusiasm of some clinicians for ice water immersion, evaporative cooling has been an effective noninvasive cooling technique in human studies.214,217 To maximize evaporative cooling rates, several factors must be optimized. Airflow rates must be high and therefore large fans are required. The air must be warm but not humid because evaporation is decreased at lower temperatures. The entire body surface must be exposed to airflow and continuously moistened with water. Ideally, the patient is suspended in a mesh sling to expose the back to airflow and moisture. Finally, the temperature of the water used to moisten the skin must be tepid (15°C [59°F]). Warm forced air is essential for effective evaporation. It maintains good peripheral perfusion and prevents shivering by warming the skin.217 If the water is ice cold, evaporation will be slowed. Conversely, if it is hot, conductive heat gain may occur. Studies conducted in heat-stressed laying hens demonstrated superior cooling rates with ventral cooling regimes over dorsal cooling.218
Weiner and Khogali constructed a sophisticated “body cooling unit” (BCU) to maximize evaporative cooling.200 Patients in the BCU are suspended in a mesh net. High airflow rates (30 m/min) at a temperature of 45°C (113°F) are maintained both anterior and posterior to the mesh net. Atomized water at 15°C (59°F) is continuously sprayed on all body surfaces. For EDs without access to a BCU,219 temporary units can be set up with shower sprays and fans, provided that the ambient temperature in the ED is relatively cool. An alternative less expensive, portable device developed at King Saud University involves covering the patient with a gauze sheet soaked in water at 20°C (68°F) while two fans direct room air over the patient.220 Cooling rates obtained with this device (0.087°C/min [32.2°F/min]) were nearly double the cooling rates achieved with the original BCU developed by Weiner and Khogali.200
Procedure: For evaporative cooling, undress the patient completely. Position a fan at the foot of the bed or stretcher, as close to the patient as possible. Then sponge or mist the patient’s skin with tepid water (15°C [59°F]). Spray water continuously over the skin to create a warm microclimate around the skin and to promote water evaporation.221 A single care provider can continue the technique and monitor the patient once cooling has been initiated. It is important to keep as much of the body surface area as moist as possible and exposed to airflow. Do not cover with sheets or clothing because this will impede skin evaporation and cooling. Studies of evaporative cooling in heatstroke patients show cooling rates of 0.046°C/min to 0.34°C/min (32.1°F/min to 32.6°F/min).217,221
Complications: Complications of evaporative cooling are rare and more often a result of the underlying disorder than the cooling technique. Wet skin may interfere with electrocardiographic monitoring, but this can usually be avoided by placing electrodes on the patient’s back. Shivering occurs infrequently with this technique when compared with other cooling methods since the water is relatively lukewarm.222 Because rectal temperature lags behind core (esophageal) temperature, evaporative cooling should be discontinued when rectal temperature reaches 39°C (102.2°F). In cases of mild hyperthermia, tympanic temperature also accurately reflects core temperature and can be useful in this setting.222 Continued cooling beyond this temperature may lead to subsequent “overshoot hypothermia” as a result of a continued drop in core temperature after active evaporative cooling is discontinued. Shivering indicates that core temperature has decreased to 37°C (89.6°F) or below.222
Immersion Cooling
It would seem obvious that the fastest way to cool a heatstroke patient would be immersion in ice water. In a case series of exertional heatstroke patients, iced water immersion cooled patients to lower than 39°C (<102.2°F) within 19.2 minutes.214 A recent study found that cold water immersion for 9 minutes in a 2.0°C circulated water bath until a rectal temperature of 38.6°C was achieved avoided any risk associated with overcooling.223 Some contemporary sources recommend ice water immersion as the cooling technique of choice for heatstroke.214,224 Plattner and associates demonstrated cooling rates with ice water immersion that were six times faster than rates seen with forced air or circulating water.225
Costrini reported no fatalities in 252 consecutive young marine recruits with exertional heatstroke who were treated by ice water immersion within 20 minutes of diagnosis.206 He regarded ice water immersion as superior to other conventional methods described in the literature in reducing mortality rates.
In clinical trials, cold water immersion remains one of the fastest noninvasive cooling techniques available (see Table 65-5). Cold water immersion takes advantage of the high-conductance property of water, which is 25 times that of air.224 When an adequate evaporative cooling system is not available, immersion may be the cooling technique of choice. Several factors are important in maximizing the rate of immersion cooling. Conductive heat loss depends on cutaneous blood flow to maintain a heat gradient from skin to water. Theoretically, contact with ice water causes skin and subcutaneous vasoconstriction, which blocks heat exchange and turns these structures into insulators.226 Intense cutaneous vasoconstriction will impede conductive heat loss. Mekjavic and coworkers reported that motion sickness actually potentiates core cooling during immersion by attenuating the vasoconstrictor response to skin and core cooling, thereby augmenting heat loss and the magnitude of the decrease in deep body temperature.227 Careful monitoring is required because this may predispose patients to hypothermia.
Researchers have suggested that ice water immersion may be superior to cold water immersion because of the establishment of a steeper thermal gradient between the skin and the environment.226 A study comparing the cooling capacity of ice water immersion (5.2°C [41.4°F]), tepid water immersion (14°C [57.2°F]), and passive cooling in experienced distance runners with body temperatures of 39.3°C to 39.6°C (102.7°F to 103.3°F) found comparable cooling rates with ice water and cold water immersion. Both techniques were superior to passive cooling techniques.226 The optimal water temperature for cooling human heatstroke patients has not been defined. Aggressive skin cooling may stimulate shivering and peripheral vasoconstriction, thus hindering cooling efficacy. Investigators suggest the inclusion of skin massage as a crucial component of immersion cooling techniques.228
Procedure: For immersion cooling, undress the patient completely and transfer the patient to a tub of water with a depth sufficient to cover the torso and extremities. Various water containers may be used. A regular bathtub can be used. Most clinical reports describe tubs that can be moved to the emergency treatment area when needed. A child’s plastic wading pool and a decontamination tub or stretcher with waterproof sides and drainage capability are examples of the latter approach. Support the patient’s head out of the tub at all times. When tubs are unavailable, place patients on water-impermeable sheets and in a sling apparatus while ice and water are poured into the sling. Securely attach temperature and electrocardiogram leads to the patient if monitoring is to be continued during immersion. Remove the patient from the bath when rectal temperature reaches 39°C (102.2°F) because core temperature will continue to drop for a short period even after the patient is removed. If available, use an electronic temperature monitor with a long flexible rectal probe for continuous monitoring of temperature during immersion. Studies show cooling rates with ice water immersion (1°C–5°C [33.8°F–41.0°F]) in heatstroke patients of 0.15°C/min to 0.23°C/min (32.3°F/min to 32.4°F/min).206,224,228
Complications: The common complications of immersion cooling are shivering, cutaneous vasoconstriction, discomfort, and loss of monitoring capability. Shivering generates considerable heat through muscle metabolism. Cutaneous vasoconstriction impedes conductive heat loss. If significant shivering does occur, it can be reduced with benzodiazepines. A recent study found that high-dose IV diazepam facilitates core cooling during cold saline infusion in healthy volunteers. Subjects were randomized to receive high-dose (20 mg) or low-dose (10 mg) diazepam or placebo during cold saline infusion. Administration of high-dose diazepam decreased the shivering threshold without compromising respiratory or cardiovascular status.229 The use of phenothiazines such as chlorpromazine has been advocated for shivering in the past. They are currently discouraged because administration of these agents may impair heat loss through anticholinergic effects on sweat glands, contribute to hypotension via α-adrenergic blockade, lower the seizure threshold, and cause dystonic reactions. In addition, phenothiazines possess central dopamine-blocking effects, which may exacerbate symptoms of NMS.175 Benzodiazepines are also valuable if the patient is hyperthermic secondary to sympathomimetic agents such as cocaine. Patient monitoring is a problem under water. Electrodes can be used on the nonimmersed upper part of the shoulders, but electrocardiographic artifact often becomes a major problem during vigorous shivering. Immersion cooling is not recommended for patients with unstable cardiac rhythms or those at risk for the development of these rhythms. A significant change in cardiac rhythm might go undetected during the labor-intensive process of immersion cooling.
Whole-Body Ice Packing
Packing a heatstroke victim in ice may enhance conductive heat loss without the attendant logistic problems caused by water immersion (Figs. 65-7 and 65-8). Constant attendance, as required for skin moistening with evaporative cooling and as described for immersion cooling, may not be necessary with ice packing. Kielblock and associates demonstrated in a human study of mild, exercise-induced hyperthermia that whole-body ice packing cooled just as fast as evaporative cooling did (see Table 65-5).201
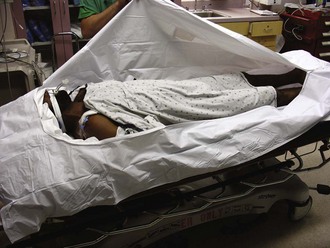
Figure 65-8 A body bag or plastic sheets may keep water from flooding the floor when packing the patient in ice.
Procedure: For whole-body ice packing, undress the patient completely and then cover the extremities and torso with crushed ice. A fan blown over the patient may increase cooling. As with any cooling technique, monitor the patient’s temperature constantly with an electric thermometer and a long, flexible rectal probe. A large supply of crushed ice will be needed whenever this technique is used. Logistically, ice packing may be problematic. Whole-body ice packing can usually be performed on an ED stretcher without additional equipment. Ideally, the patient is placed in a container that facilitates contact of ice with the skin and prevents water from dripping onto the floor. A body bag makes an ideal device. Iced cooling may also be accomplished by placing the patient in a child’s lightweight plastic pool, available in toy stores. Lacking this equipment, plastic cloths or trash bags may be placed under the patient with the edges curled up to form a slinglike apparatus. As with immersion cooling, electrocardiographic monitoring can potentially be difficult because of shivering artifact and displacement of electrodes. If the patient is alert and cannot tolerate the ice packing, use IV sedation or restraint. Treat excessive shivering with benzodiazepines if needed. Once rectal temperature reaches 39°C (102.2°F), remove the ice and dry the patient off. Studies show cooling rates of 0.34°C/min (32.6°F/min) in heatstroke patients with whole-body ice packing.201,224
Strategic Ice Packs
Noakes suggested that strategic placement of ice packs over areas of the body where large blood vessels run close to the skin may be an effective cooling technique.230 Cooling in these areas occurs despite cutaneous vasoconstriction because of direct conductive heat loss from blood within the vessel and across the vessel wall, subcutaneous tissue, and skin to ice. The most common areas used for strategic ice packing are the anterior aspect of the neck (carotid and jugular vessels), the axilla (axillary artery and vein), and the groin (femoral vessels). There have been numerous reports of successful cooling using ice packs as primary or adjunctive therapy (see Table 65-5).230,231 In addition, application of ice packs, though easier to perform than immersion or total-body ice packing, limits the conductive cooling offered by the latter two procedures. A study in pigtail monkeys demonstrated that a combination of strategic ice packs with evaporative cooling results in faster cooling than either technique alone, although the relative increase achieved by adding ice packs to evaporative cooling was small.232
In unconscious patients or in awake patients who can tolerate ice packs without excessive shivering, this technique could be added to evaporative cooling. Kielblock and associates found that the combination of strategic ice packs and evaporative cooling yielded higher cooling rates than did either method individually (0.036°C/min versus 0.027°C/min and 0.034°C/min).201 The clinical value of strategic ice packs alone or in combination with other techniques remains to be determined. Anecdotally, during the Chicago heat wave of 1995, the majority of heatstroke patients who went to EDs survived after being effectively cooled with the evaporation method accompanied by strategic placement of ice packs.233
Procedure: Place large plastic bags filled with crushed ice or an ice and water mixture in both axillae and over both femoral triangles. If the neck is used, place the packs laterally so that they do not compress the trachea or apply excessive weight over the carotid arteries. Do not pack the neck if the patient has carotid bruits or a history of cerebrovascular disease. Some sources advocate rubbing the body surface briskly with plastic bags containing ice after the body has been wet down with water. This is effective, provided that it is combined with evaporation therapy. Studies show ranges of cooling rates of 0.028°C/min to 0.087°C/min (32.0°F/min to 32.2°F/min) in heatstroke patients with strategic ice packs.234
External versus Core Cooling
All the external cooling techniques described previously are noninvasive and involve heat loss by evaporation or conduction across the skin as the primary cooling mechanism. With each of these techniques, central temperature will continue to drop even after the technique is discontinued and the skin is dried. This is due to a delay in establishment of an equilibrium between the cold skin and the core. The amount of “core afterdrop” can exceed 2°C (>35.6°F).217,219,234 For this reason, cooling is discontinued when the core temperature reaches 39°C (102.2°F).
Because the sites of significant cell damage with heatstroke are centrally located (e.g., liver, kidney, heart), central cooling techniques are theoretically preferable to external techniques. Core cooling techniques studied in both animal and human models include iced gastric lavage, intravascular cooling, bladder lavage, and peritoneal lavage.208–210,212,235,236 Central venous cooling is effective in rapidly decreasing core temperatures.235 Studies conducted in healthy volunteers have demonstrated that reductions in core temperature vary according to the temperature of the infused fluid. Subjects receiving 30-minute infusions of fluid at 4°C (39.2°F) experienced decreases in core temperature of 2.5°C ± 0.4°C (36.5°F ± 32.7°F). Subjects receiving 30-minute infusions of fluid at 20°C (68°F) experienced decreases of 1.4°C (±0.2°C [34.5°F ± 32.4°F]).235 Clinical trials investigating this method showed that cooling via the respiratory tract had no significant impact on temperature changes when used exclusively but did demonstrate effectiveness as an adjunctive measure to other external cooling techniques.224 Cool air (10°C [50°F]) was administered via a hood or mask. Cooling via the respiratory tract has been studied in animals but not investigated clinically.237–239 Central cooling techniques are necessarily more invasive than external techniques and have the potential for more significant complications.
Cold Gastric Lavage
The stomach lies in close proximity to the liver, great vessels, kidneys, and heart. The gastric mucosa is not subject to the intense vasoconstriction observed on exposure of the skin to ice water.208 For these reasons, lavage of the stomach might be expected to be an effective central cooling method. Studies of cold gastric lavage in a canine model produced cooling rates five times greater than in controls exposed to ambient air at room temperature (0.15°C/min versus 0.03°C/min).209 Human heatstroke victims have been cooled successfully with gastric lavage, but only in combination with external techniques.210 Cold gastric lavage seems to be best suited for use in patients with severe hyperthermia who are cooled at a slow rate with external techniques alone. The presence of an endotracheal tube and passage of a large-bore gastric tube make rapid lavage without aspiration possible. This technique should be reserved for patients whose airway is protected by endotracheal intubation and who do not have a contraindication to gastric tube placement.
Procedure: For cold gastric lavage, instill 10 mL/kg of iced tap water into the stomach as rapidly as possible (usually over a 30- to 60-second period). After a 30- to 60-second dwell time, remove the water by suction or gravity.213 Cooling will theoretically be faster if a high temperature gradient is maintained in the stomach. A faster lavage rate can be maintained if suction is used to withdraw the instilled fluid. A large container of ice-temperature water maintained 1 to 1.5 m above the patient’s body will facilitate the instillation of fluid. Connect this container directly to the lavage tubing and ideally allow passage of water but not ice, which may occlude the tube. Because large volumes of water are needed, it is helpful if additional ice can be added to the container without interrupting the lavage. A large syringe can be used as an alternative to gravity instillation, but this is usually slower.
Complications: A major potential complication of cold gastric lavage is pulmonary aspiration. Use of a cuffed endotracheal tube minimizes the incidence of this complication. Because of the large volume of water used and the frequent depression of airway reflexes seen with severe heatstroke, this technique should rarely be used in a patient who is not endotracheally intubated.
If tap water is used, water intoxication, hyponatremia, and other electrolyte disturbances are potential complications, particularly in pediatric or geriatric patients. Water is absorbed from the stomach and, with large-volume lavage, may pass the pylorus into the small intestine. In canine studies, large-volume gastric lavage with tap water did not cause electrolyte abnormalities.208 The actual incidence of these potential complications in human heatstroke has not been determined. Use of normal saline instead of tap water would eliminate this potential problem.
Theoretically, passage of cold water through the esophagus, located directly behind the heart, has the potential to induce cardiac dysrhythmias. Dysrhythmias have not been observed in canine studies or in case reports of human heatstroke victims cooled with this technique.212,236
Cold Peritoneal Lavage
The surface area and blood flow of the peritoneum greatly exceed those of the stomach. Peritoneal lavage is expected to exchange heat much faster than possible with gastric lavage. Peritoneal lavage achieves some of the fastest cooling rates ever reported in large animal or human studies (see Table 65-5). A case report of cooling via cold peritoneal lavage for hyperthermia after the ingestion of ecstasy demonstrated rapid cooling.236 As with gastric lavage, this central cooling technique offers the advantage of directly cooling the core organs that are most susceptible to thermal damage. Unlike gastric lavage, endotracheal intubation is not required. Peritoneal lavage is used extensively to treat hyperthermia under various conditions and typically decreases core temperatures 5°C/hr to 10°C/hr (41°F/hr to 50°F/hr).210,236
Peritoneal lavage is the most rapid central cooling technique. It can theoretically be combined with other techniques to speed cooling of heatstroke patients with refractory hyperthermia. As the most invasive cooling technique, it requires time, proper equipment, and surgical expertise to institute. Its use is probably best suited to situations in which heatstroke patients are not responding to external cooling and adequate equipment and personnel are readily available.217
Procedure: To institute cooling by peritoneal lavage, immerse 2 to 8 L of sterile saline in an ice water bath to cool while the catheter is being placed. Place a standard peritoneal lavage catheter (as for diagnostic use in trauma patients) via any of the techniques described in Chapter 43. Standard contraindications apply. Use of a larger peritoneal dialysis catheter may speed instillation and withdrawal of fluid. Actual lavage volumes and rates have not been established, however. One approach is to instill and withdraw 500 to 1000 mL every 10 minutes until adequate cooling is achieved. Rectal temperature may be falsely low during lavage because of the presence of cold water around the rectum at the level of the rectal temperature probe. It may be preferable to monitor temperature in the tympanic membrane or esophagus when using this technique. Stop the lavage when core temperature reaches 39°C (102.2°F) to avoid excessive core temperature afterdrop.
Other Cooling Techniques
“Rewarming” techniques are used to minimize ongoing heat loss via the respiratory tract in hypothermic patients.72 Although high-frequency jet ventilation (HFJV) achieves core cooling in critically ill patients,237 efforts to use the respiratory tract to cool heatstroke victims have been unsuccessful. In a canine model of heatstroke, HFJV was shown to be a relatively ineffective cooling technique.238 Heat loss by convection (air transfer) is relatively inefficient when compared with the conductive heat loss mechanism used by other cooling techniques. The use of dry, hot air to maximize evaporative heat loss from the lungs might cause respiratory complications.231
In human trials, ice water lavage of the bladder (300 mL of iced Ringer’s solution every 10 minutes) provided only minimal cooling at rates of 0.8°C/hr (±0.3°C/hr [33.4°F/hr ± 32.5°F/hr]).201 Iced water lavage of the rectum would theoretically provide faster cooling rates secondary to the increased surface area and better perfusion, but it has not been investigated in human trials.
Hemodialysis or partial cardiopulmonary bypass could theoretically be used to cool heatstroke patients. Before the availability of dantrolene in 1979, partial cardiopulmonary bypass was one of the treatments of MH.173 Drawbacks include the need for technical expertise and preparation time for the procedure. A recent case report described successful treatment of a heatstroke patient with multiple-organ failure refractory to conventional cooling techniques with cold hemodialysis initially at 30°C (86°F) and later at 35°C (95°F), followed by continuous hemodiafiltration with cold dialysate (35°C [95°F]) at a high flow rate of 18,000 mL/hr. Within 3 hours of starting this particular technique, the patient’s body temperature was below 38°C (<100.4°F).239
Cyclic lung lavage with cold perfluorochemicals is currently under investigation in animal models. Benefits include rapid cooling rates of 0.5°C/min (32.9°F/min) and minimally invasive nature in already mechanically ventilated subjects.213,240
Intravascular cooling catheters have demonstrated efficiency as cooling devices. They circulate temperature-controlled sterile saline placed in the bladder or inferior vena cava. Although these devices have not been used in heatstroke patients, studies have found the cooling catheters to be very effective for neurologic conditions in both human and animal models.241,242 Another promising cooling technique involves the use of a hypothermic retrograde jugular vein flush (HRJVF) for heatstroke. HRJVF has been studied only in animal models thus far. This technique involves the infusion of 4°C (39.2°F) isotonic sodium chloride solution through the external jugular vein (1.7 mL/100 g of body weight over a 5-minute period). Use of HRJVF was found to increase survival rates during heatstroke by attenuating cerebral oxidative stress, tissue ischemia or injury, systemic inflammation, and activated coagulation.243
Pharmacologic agents have demonstrated merit as adjunctive agents in the management of hyperthermia. There are anecdotal reports of enhanced reduction in temperature with IV ketorolac. Cienki and colleagues demonstrated enhanced decreases in temperature with the administration of ketorolac, 30 mg intravenously.244 All patients received standard hyperthermia treatment (e.g., ice packs, iced lavage, circulating air). Patients were randomized to receive ketorolac versus saline. In the group receiving ketorolac, the average rectal temperature after 90 minutes was two times lower than in those receiving placebo saline (3.7°C versus 1.6°C [38.7°F versus 34.9°F]).
Conclusion
Rapid cooling is the key step in the emergency management of heatstroke patients. Survival rates approach 90% when elevated temperatures are lowered in timely fashion.157,228 Evaporative cooling appears to be the technique of choice. It combines the advantages of simplicity and noninvasiveness with the most rapid cooling rates achieved with any external technique. It is also logistically easier to institute, maintain, and monitor evaporative cooling measures than with any other cooling technique. If a patient is not cooling rapidly with evaporative cooling, other techniques can be added. Strategic ice packs can be used. If the patient is endotracheally intubated, gastric lavage can be instituted. If facilities and personnel are available, peritoneal lavage cooling can be used as a rapid central cooling technique. If muscle rigidity is present or MH is suspected, dantrolene sodium should be administered. In addition, the clinician should have a heightened index of suspicion for NMS and toxicity from sympathomimetic drugs. Regardless of the cause, a reasonable clinical goal is to reduce rectal temperature to 40°C (104°F) or below within 30 minutes of instituting therapy.157,228
References
1. Jurkovich, GJ. Environmental cold-induced injury. Surg Clin North Am. 2007;87:247–267.
2. Delaney, K, Vassallo, S, Larkin, G, et al. Rewarming rates in urban patients with hypothermia: prediction of underlying infection. Acad Emerg Med. 2006;13:913.
3. Ulrich, AS, Rathlev, NK. Hypothermia and localized cold injuries. Emerg Med Clin North Am. 2004;22:281.
4. Windsor, JS, Firth, PG, Grocott, MP, et al. Mountain mortality: a review of deaths that occur during recreational activities in the mountains. Postgrad Med J. 2009;85:316–321.
5. Brunette, D, McVaney, K. Hypothermic cardiac arrest: an 11-year review of ED management and outcome. Am J Emerg Med. 2000;18:418.
6. Danzl, DF. Accidental hypothermia. In: Auerbach PS, ed. Wilderness Medicine. 5th ed. St. Louis: Mosby; 2007:125–159.
7. Maisch, S, Ntalakoura, K, Boettcher, H, et al. [Severe accidental hypothermia with cardiac arrest and extracorporeal rewarming: a case report of a 2-year-old child.]. Anaesthesist. 2007;2:25.
8. Moser, B, Voelckel, W, Gardetto, A, et al. One night in a snowbank: a case report of severe hypothermia and cardiac arrest. Resuscitation. 2005;6:365.
9. Murphy, T, Zumwalt, R, Fallico, F. Hypothermia-related deaths—United States 1999–2002 and 2005. MMWR Morb Mortal Wkly Rep. 2006;55(10):282–284.
10. Holowatz, LA, Kennedy, WL. Peripheral mechanisms of thermoregulatory control of skin blood flow in aged humans. J Appl Physiol. 2010;109:1538–1544.
11. Rawls, SM, Benamar, K. Effects of opioids, cannabinoids, and vanilloids on body temperature. Front Biosci (School Ed). 2011;3:822–845.
12. Gregory, RT. Accidental hypothermia, an Alaskan problem. Alaska Med. 2001;43:73.
13. Megarbane, B, Axler, O, Chary, I, et al. Hypothermia with indoor occurrence is associated with a worse outcome. Intensive Care Med. 2000;26:1843.
14. Thorsen, K, Ringdal, KG, Strand, K, et al. Clinical and cellular effects of hypothermia, acidosis and coagulopathy in major injury. Br J Surg. 2011;98:894–907.
15. Woods, S. Cold complications assessment and management of hypothermic patients. J Emerg Med Serv. 2001;26:68.
16. Danzl, DF. Accidental hypothermia. In: Rosen P, Barkin RM, Braen RG, et al, eds. Emergency Medicine: Concepts and Clinical Procedures. 4th ed. St. Louis: Mosby–Year Book; 1998:963.
17. Clifton, GL, Choi, SC, Miller, ER, et al. Intercenter variance in clinical trials of head trauma—experience of the National Acute Brain Injury Study: Hypothermia. J Neurosurg. 2001;95:751.
18. Macario, A, Dexler, F. What are the most important risk factors for a patient’s developing intraoperative hypothermia? Anesth Analg. 2002;94:215.
19. Gammons, M, Boynton, M, Russell, J, et al. On-mountain coverage of competitive skiing and snowboarding event. Curr Sports Med Rep. 2011;10:140–146.
20. Ranhoff, AH. Accidental hypothermia in the elderly. Int J Circumpolar Health. 2000;59:255.
21. Sessler, DI. Complications and treatment of mild hypothermia. Anesthesiology. 2001;95:531.
22. Kibayashi, K, Shojo, H. Accidental fatal hypothermia in elderly people with Alzheimer’s disease. Med Sci Law. 2003;43:127.
23. Michaud, K, Romain, N, Giroud, C, et al. Hypothermia and undressing associated with non-fatal bromazepam intoxication. Forensic Sci Int. 2001;124:112.
24. Akinyinka, OO, Omokhodion, SI, Olawuyi, JF, et al. Tympanic thermometry in Nigerian children. Ann Trop Paediatr. 2001;21:169.
25. Cronin, K, Wallis, M. Temperature taking in the ICU: which route is best? Aust Crit Care. 2000;13:59.
26. Lily, JK, Beland, JP, Zekan, S. Urinary bladder temperature monitoring: a new index of body core temperature. Crit Care Med. 1980;8:742.
27. Harioka, T, Matsukawa, T, Ozaki, M, et al. Deep-forehead temperature correlates well with blood temperature. Can J Anaesth. 2000;47:980.
28. Latman, NS, Hans, P, Nicholson, L, et al. Evaluation of clinical thermometers for accuracy and reliability. Biomed Instrum Technol. 2001;35:259.
29. Szekely, M, Petevari, E, Balasko, M. Thermoregulation, energy balance, regulatory peptides: recent developments. Front Biosci (School Ed). 2010;2:1009–1046.
30. Reamy, BV. Disturbances due to cold. In: Rakel RE, ed. Conn’s Current Therapy. 53rd ed. Philadelphia: Saunders; 2001:1171.
31. Castellani, JW, Sawk, MN, Degroot, DW, et al. Cold thermoregulatory responses following exertional fatigue. Front Biosci (School Ed). 2010;2:854–865.
32. Yoder, E. Disorders due to heat and cold. In: Goldman L, ed. Cecil Textbook of Medicine. 21st ed. Philadelphia: Saunders; 2001:513.
33. Swerey, MT, Sigg, DC, Tahvildari, S, et al. Shiver suppression using focal hand warming in unanesthetized normal subjects. Anesthesiology. 2001;95:1089.
34. Yokoyama, M, Noto, Y, Kida, H. Hypothermia with acute renal failure in a patient suffering from diabetic nephrology and malnutrition. Diabetes Metab. 2000;26:145.
35. Graham, CA, McNaughton, GW, Wyatt, JP. The electrocardiogram in hypothermia. Wilderness Environ Med. 2001;12:232.
36. Krantz, M, Lowery, CM. Giant Osborn waves in hypothermia. N Engl J Med. 2005;352:184.
37. Wong, FW. J wave and hypothermia. Dynamics. 2005;16:17.
38. Durakovic, Z, Misigoj-Durakovic, M, Corovic, N. Q-T + JT dispersion in the elderly with urban hypothermia. Int J Cardiol. 2001;80:221.
39. Aslam, AF, Aslam, AK, Vasavada, BC, et al. Hypothermia: evaluation, electrocardiographic manifestations, and management. Am J Med. 2006;119:297–301.
40. Alsafwah, S. ECG changes in hypothermia. Heart Lung. 2001;30:161.
41. Vassal, T, Benoit-Gonin, B, Carrat, F, et al. Severe accidental hypothermia treated in an ICU: prognosis and outcome. Chest. 2001;120:1998.
42. Rogers, IR, Obrien, DL, Wee, C, et al. Infrared emission tympanic thermometer cannot be relied upon in two wilderness settings. Wilderness Environ Med. 1997;10:201.
43. Giesbrecht, GG. Prehospital treatment of hypothermia. Wilderness Environ Med. 2001;12:24.
44. Streger, MR. Prehospital management of hypothermia. Emerg Med Serv. 2001;30:61.
45. Kober, A, Scheck, T, Fulesdi, B, et al. Effectiveness of resistive heating compared with passive warming in treating hypothermia associated with minor trauma: a randomized trial. Mayo Clin Proc. 2001;76:369.
46. Grieff, R, Rajek, A, Lacing, S, et al. Resistive heating is more effective than metallic-foil insulation in an experimental model of an accidental hypothermia: a randomized controlled trial. Ann Emerg Med. 2000;35:337.
47. Cassidy, ES, Adkins, CR, Rayl, JH, et al. Evaluation of warmed intravenous fluids in the prehospital setting. Air Med J. 2001;20:25.
48. Holmer, I. Assessment of cold exposure. Int J Circumpolar Health. 2001;60:413.
49. Garcia, GD, Modesto, VL, Lee, KT. Avoiding hypothermia in trauma: use of the flameless heater pack, meal ready to eat, as a field expedient means of warming crystalloid fluid. Mil Med. 2000;165:903.
50. Dobson, JAR, Burgess, JJ. Resuscitation of severe hypothermia by extracorporeal rewarming in a child. J Trauma. 1996;40:483.
51. American Heart Association. Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Part 10:4: hypothermia. Circulation. 2005;112:IV136.
52. Fitzgerald, FT. Hypoglycemia and accidental hypothermia in an alcoholic population. West J Med. 1981;133:105.
53. Irazuzta, JE, Pretzloff, R, Rowin, M, et al. Hypothermia as an adjunctive treatment for severe bacterial meningitis. Brain Res. 2000;881:88.
54. Plaisier, BR. Thoracic lavage in accidental hypothermia with cardiac arrest—report of a case and review of the literature. Resuscitation. 2005;66:99–104.
55. Henderson, ST. Hypothermia. In: Dambro, ed. Griffith’s 5-Minute Clinical Consult. Philadelphia: Lippincott, Williams & Wilkins; 2001:554.
56. Bernardo, LM, Gardner, MJ, Lucke, J, et al. The effects of core and peripheral warming methods on temperature and physiologic variables in injured children. Pediatr Emerg Care. 2001;17:138.
57. Brugger, H, Durrer, B, Adler-Kastner, L, et al. Field management of avalanche victims. Resuscitation. 2001;51:7.
58. Boyd, J, Brugger, H, Shuster, M. Prognostic factors in avalanche resuscitation: a systemic review. Resuscitation. 2010;81:645–652.
59. Koschel, MJ. Rewarming a hypothermic patient. Am J Nurs. 2001;101:85.
60. Miller, JW, Danzl, DF, Thomas, DM. Urban accidental hypothermia: 135 cases. Ann Emerg Med. 1980;9:456.
61. Giesbrecht, GG. Emergency treatment of hypothermia. Emerg Med. 2001;13:9.
62. Ereth, MH, Lennon, RL, Sessler, DI. Limited heat transfer between thermal compartments during rewarming in vasoconstricted patients. Aviat Space Environ Med. 1992;63:1065.
63. Giesbrecht, GG, Sessler, DI, Mekjavic, LB, et al. Treatment of mild immersion hypothermia by direct body-to-body contact. J Appl Physiol. 1994;76:2373.
64. Vanggaard, L, Eyolfson, D, Xu, X, et al. Immersion of distal arms and legs in warm water (AVA rewarming) effectively rewarms mildly hypothermic humans. Aviat Space Environ Med. 1999;70:1081.
65. Daanen, HAM, VandeLinde, FJG. Comparison of four noninvasive rewarming methods for mild hypothermia. Aviat Space Environ Med. 1992;63:1070.
66. Perl, T, Brauer, A, Quintel, M. Prevention of perioperative hypothermia with forced air warming system and upper body blankets. Surg Technol Int. 2006;15:19–22.
67. Ciufo, D, Dice, S, Coles, C. Rewarming hypothermic postanesthesia patients: a comparison between a water coil warming blanket and a forced-air warming blanket. J Post Anesth Nurs. 1995;10:155.
68. Giesbrecht, GG. Cold, stress, near drowning, and accidental hypothermia: a review. Aviat Space Environ Med. 2000;71:733.
69. Brauer, A, Quintel, M. Forced-air warming: technology, physical background and practical aspects. Curr Opin Anaesthesiol. 2009;22:769–774.
70. Tiruvoipati, R, Baiasubramanian, SK, Khoshbin, E, et al. Successful use of venovenous extracorporeal membrane oxygenation in accidental hypothermic cardiac arrest. ASAIO J. 2005;51:474.
71. Spooner, K, Hassani, A. Extracorporeal rewarming in a severely hypothermic patient using venovenous hemafiltration in the accident and emergency department. J Accid Emerg Med. 2000;17:422.
72. Kjaergaard, B, Tolboll, P, Lyduch, S, et al. A mobile system for the treatment of accidental hypothermia with extracorporeal circulation. Perfusion. 2001;16:453.
73. Werwath, DL, Schwab, CW, Scholten, JR, et al. Microwave ovens. A safe method of rewarming crystalloids. Am Surg. 1984;50:656.
74. Streba, JA. Efficacy and safety of prehospital rewarming techniques to treat accidental hypothermia. Ann Emerg Med. 1991;20:896.
75. Tobias, SW, Matthew, CB, Dubose, DA, et al. Comparison of oxygenated perfluorocarbon and humidified oxygen for rewarming miniswine. Mil Med. 2001;166:853.
76. Shanks, CA, Saia, CA. Temperature monitoring of the humidifier during the treatment of hypothermia. Med J Aust. 1972;2:1351.
77. Papadimos, TJ, Fowler, E. Hypothermia and rapid cold, peritoneal crystalloid infusion. Anesthesia. 2001;56:805.
78. Lash, RF, Burdett, SA, Ozil, T. Accidental profound hypothermia and barbiturate intoxication: report of “rapid core” rewarming by peritoneal dialysis. JAMA. 1967;201:269.
79. Laboto, MA. Peritoneal dialysis in emergency and critical care medicine. Clin Tech Small Anim Pract. 2000;15:126.
80. Lonning, PE, Skulberg, A, Abyholm, F. Accidental hypothermia. Acta Anaesthesiol Scand. 1986;30:601.
81. Vella, J, Farrell, J, Leavey, S, et al. The rapid reversal of profound hypothermia using peritoneal dialysis. Ir J Med Sci. 1996;165:113.
82. Howdieshell, TR, Osler, TH, Demarest, GB. Open versus closed peritoneal large with particular attention to time, accuracy and cost. Am J Emerg Med. 1989;7:367.
83. Weinberg Hypothermia. Ann Emerg Med. 1993;22:370.
84. Danzl, D, Pozos, RS. Multicenter hypothermia study. Ann Emerg Med. 1987;16:1042.
85. Petrone, P, Kuncir, EJ, Sensio, JA. Surgical management and strategies in the treatment of hypotherma and cold injury. Emerg Clin North Am. 2003;21:1165.
86. Thomas, R, Cahill, CJ. Successful defibrillation in profound hypothermia (core body temperature 25.6 degrees C). Resuscitation. 2000;47:317.
87. Peterson, LD. Electrolyte depletion following emergency stomach evacuation. Am J Hosp Pharm. 1979;35:1366.
88. Walters, DT. Closed thoracic cavity lavage for hypothermia with cardiac arrest. Ann Emerg Med. 1991;20:439.
89. Winegard, C. Successful treatment of severe hypothermia and prolonged cardiac arrest with closed thoracic cavity lavage. J Emerg Med. 1997;15:629.
90. Brunette, DD, Biros, M, Mlinek, EJ, et al. Internal cardiac massage and mediastinal irrigation in hypothermic cardiac arrest. Am J Emerg Med. 1992;10:32.
91. Gilbert, M, Busund, R, Skagseth, A, et al. Resuscitation from accidental hypothermia of 13.7°C with circulatory arrest. Lancet. 2000;355:375.
92. Wollenek, G, Honarawar, N, Golej, J, et al. Cold water and cardiac arrest in treatment of severe hypothermia and cardiopulmonary bypass. Resuscitation. 2002;53:255.
93. Walpoth, BH, Walpoth-Aslan, BN, Mattle, HP, et al. Outcome of survivors of accidental deep hypothermia and circulatory arrest treated with extracorporeal blood warming. N Engl J Med. 1997;337:1500.
94. Owda, A, Osama, S. Hemodialysis in the management of hypothermia. Am J Kidney Dis. 2001;38(2):E8.
95. Farstad, M, Anderson, KS, Koller, ME, et al. Rewarming from accidental hypothermia by extracorporeal circulation. A respective study. Eur J Cardiothorac Surg. 2001;20:58.
96. Kaufman, JW, Hamilton, R, Kejneka, KY, et al. Comparative effectiveness of hypothermia rewarming techniques: radiofrequency energy vs warm water. Resuscitation. 1995;29:203.
97. Long, WB, 3rd., Edlich, RF, Winters, KL, et al. Cold injuries. J Long Term Eff Med Implants. 2005;15:67.
98. Gore, DC, Beatson, J. Infusion of hot crystalloid during operative burn wound débridement. J Trauma. 1997;42:1112.
99. Papenhausen, M, Burke, L, Antony, A, et al. Severe hypothermia with cardiac arrest: complete neurologic recovery in a 4-year-old child. J Pediatr Surg. 2001;36:1590.
100. Hirvonen, J. Some aspects on death in the cold and concomitant frostbites. Int J Circumpolar Health. 2000;59:131.
101. Clift, J, Munro-Davies, L. Best evidence topic report. Is defibrillation effective in accidental severe hypothermia in adults? Emerg Med J. 2007;24:50–51.
102. Harman, KR, Herndon, TM. Cold-water immersion in a 22-year-old service member. Mil Med. 2006;171:459.
103. Eddy, VA, Morris, JA, Cullinane, DC. Hypothermia, coagulopathy, and acidosis. Surg Clin North Am. 2000;30:845.
104. Smith, CE, Parnard, A, Pinchak, AC, et al. The failure of negative pressure rewarming (Thermostat) to accelerate recovery from mild hypothermia in postoperative surgical patients. Anesth Analg. 1999;89:1541.
105. Trotorici, MA, Kochanek, PM, Poloyac, SM. Effects of hypothermia on drug disposition, metabolism, metabolism and response: a focus of hypothermia-mediated alterations on the cytochrome P450 enzyme system. Crit Care Med. 2007;35:2196–2204.
106. Schiffman, H, Gleiss, J, Von Hirscheydt, A, et al. Effects of epinephrine on the myocardial performance and haemodynamics of the isolated rat heart during moderate hypothermia—importance of calcium homeostasis. Resuscitation. 2001;50:309.
107. Kornberger, E, Lindner, KH, Mayr, VD, et al. Effects of epinephrine in a pig model of hypothermic cardiac arrest and closed-chest cardiopulmonary resuscitation combined with active rewarming. Resuscitation. 2001;50:301.
108. Inoue, S, Kawaguchi, M, Sakamoto, T, et al. Amrinone can accelerate the cooling rate of core temperature during deliberate mild hypothermia for neurosurgical procedures. Br J Anaesth. 2001;86:663.
109. McCaulay, RL, Killyon, GW, Smith, DJ, et al. Frostbite. In: Auerbach PS, ed. Wilderness Medicine. 5th ed. St. Louis: Mosby; 2007:195–210.
110. Biem, J, Koechcke, N, Classen, D, et al. Out in the cold: management of hypothermia and frostbite. CMAJ. 2003;168:305.
111. Golant, A, Nord, RM, Paksima, N, et al. Cold exposure injuries to the extremities. J Am Acad Orthop Surg. 2008;16:704–715.
112. Grieve, AW, Davis, P, Dhillon, S, et al. A clinical review of the management of frostbite. J R Army Corps. 2011;157:73–78.
113. Washington School of Medicine. Cold-induced illness. In Manual of Medical Therapeutics, 30th ed, St. Louis: Washington University School of Medicine; 2001:541.
114. Cauchy, E, Chetaille, E, Marchand, V, et al. Retrospective study of 70 cases of severe frostbite lesions: a proposed new classification scheme. Wilderness Environ Med. 2001;12:248.
115. Prommersberger, KJ, van Schoonhoven, J, Lanz, U. Treatment of 3rd degree fingertip frostbite in a mountain climber with semi-occlusive dressings. Handchir Mikrochir Plast Chir. 2001;33:95.
116. Von Heimburg, D, Noah, EM, Sieckmann, VP, et al. Hyperbaric oxygen treatment in deep frostbite of both hands in a boy. Burns. 2001;27:404.
117. Twomey, JA, Peltier, GI, Zera, RT. An open-label study to evaluate the safety and efficacy of tissue plasminogen activator in treatment of severe frostbite. J Trauma. 2005;59:1350.
118. Bruen, KJ, Ballard, JR, Morris, SE, et al. Reduction of the incidence of amputation in frostbite injury with thrombolytic therapy. Arch Surg. 2007;142:546.
119. Steinman, AM, Giebrecht, GG. Immersion in cold water. In: Auerbach PS, ed. Wilderness Medicine. 4th ed. St. Louis: Mosby; 2001:197.
120. Farstad, M, Andersen, KS, Koller, MC, et al. Rewarming from accidental hypothermia by extracorporeal circulation. A retrospective study. Eur J Cardiothorac Surg. 2001;20:58.
121. Tipton, M, Eglin, C, Gennser, M, et al. Immersion deaths and deterioration in swimming performance in cold water. Lancet. 1999;354:626.
122. Noakes, TD. Exercise and the cold. Ergonomics. 2000;43:1461.
123. Pitoni, S, Sinclair, HL, Andrews, PJ. Aspects of thermoregulation physiology. Curr Opin Crit Care. 2011;17:115–121.
124. Grigore, AM, Murray, CF, Ramakrishna, H, et al. A core review of temperature regimens and neuroprotection during cardiopulmonary bypass: does rewarming rate matter? Anesth Analg. 2009;109:1741–1751.
125. Kupchik, NL. Development and implementation of a therapeutic hypothermia protocol. Crit Care Med. 2009;37(7 Suppl):S279–S284.
126. Bolte, RG, Black, PG, Bowers, RS, et al. The use of extracorporeal rewarming in a child submerged for 66 minutes. JAMA. 1988;260:377.
127. Chochinov, A, Baydock, B, Bristow, G, et al. Recovery of a 62-year-old man from prolonged cold water submersion. Ann Emerg Med. 1998;31:127.
128. Orlowski, JP. Prognostic factors in pediatric cases of drowning and near-drowning. JACEP. 1979;8:176.
129. Hudson, D, Conway, G. The role of hypothermia and drowning in commercial fishing deaths in Alaska. Int J Circumpolar Health. 2004;63(suppl 2):1990–2002.
130. Giesbrecht, GG, Bristow, GK. A second postcoding afterdrop: more evidence for a convective mechanism. J Appl Physiol. 1992;73:1253.
131. O’Neill, MS, Carter, R, Kish, JK, et al. Preventing heat-related morbidity and mortality: new approaches in a changing climate. Maturitas. 2009;64:98.
132. O’Neill, MS, Ebi, KL. Temperature extremes and health: impacts of climate variability and change in the United States. J Occup Environ Med. 2009;51:13.
133. Leon, LR, Helwig, BG. Heat stroke: role of the systemic inflammatory response. J Appl Physiol. 2010;109:1980.
134. Centers for Disease Control and Prevention (CDC). Heat-related deaths—United States, 1999–2003. MMWR Morb Mortal Wkly Rep. 2006;55(29):796–798.
135. Canoui-Poitrine, F, Cadot, E, Spira, A, et al. Excess deaths during the August 2003 heat wave in Paris, France. Rev Epidemiol Sante Publique. 2006;54:127.
136. Nelson, NG, Collins, CL, Comstock, RD, et al. Exertional heat-related injuries treated in emergency departments in the US 1997-2006. Am J Prev Med. 2011;40:54.
137. Jackson, LL, Rosenberg, HR. Preventing heat-related illness among agricultural workers. J Agromedicine. 2010;15:200.
138. Hopkins, PM. Malignant hyperthermia: pharmacology of triggering. Br J Anaesth. 2011;107:48.
139. Sharma, HS, Sjoquist, PO, Ali, SF. Drugs of abuse-induced hyperthermia, blood-brain barrier dysfunction and neurotoxicity: newer protective effects of a new antioxidant compound. H-290/51. Curr Pharm Des. 2007;13:1903.
140. Sessler, DI. Thermoregulatory defense mechanisms. Crit Care Med. 2009;37:S203.
141. Holowatz, LA, Kenney, WL. Peripheral mechanisms of thermoregulatory control of skin blood flow in aged humans. J Appl Physiol. 2010;109:1588.
142. Falk, B, Dotan, R. Temperature regulation & elite young athletes. Med Sport Sci. 2011;56:126.
143. Dalal, S, Zhukovsky, DS. Pathophysiology and management of fever. J Support Oncol. 2006;4:9.
144. Becker, JA, Stewart, LK. Heat-related illness. Am Fam Physician. 2011;83:1325.
145. American College of Sports MedicineArmstrong, LE, Casa, DJ, et al. American College of Sports Medicine position stand. Exertional heat illness during training and competition. Med Sci Sports Exerc. 2007;39:556.
146. Van Rosendal, SP, Osborne, MA, Fassett, RG, et al. Intravenous versus oral rehydration in athletes. Sports Med. 2010;40:327.
147. Noakes, TD. A modern classification of the exercise-related heat illnesses. J Sci Med Sport. 2008;11:33.
148. Asplund, CA, O’Connor, FG, Noakes, TD. Exercise-associated collapse: an evidence-based review and primer for clinicians. Br J Sports Med. 2011;45:1157.
149. Zeller, L, Novack, V, Barski, L, et al. Exertional heatstroke: clinical characteristics, diagnostic and therapeutic considerations. Eur J Intern Med. 2011;23:296.
150. Leon, LR, Helwig, BG. Role of endotoxin and cytokines in the systemic inflammatory response to heat injury. Front Biosci (School Ed). 2010;2:916.
151. Jardine, DS. Heat Illness and heat stroke. Pediatric Rev. 2007;28:249.
152. Cleary, M. Predisposing risk factors on susceptibility to exertional heat illness: clinical decision-making considerations. J Sport Rehabil. 2007;16:204.
153. Howe, AS, Boden, BP. Heat-related illness in athletes. Am J Sports Med. 2007;35:1384.
154. Bedno, SA, Li, Y, Han, W, et al. Exertional heat illness among overweight US army recruits in basic training. Aviat Space Environ Med. 2010;81:107.
155. Krueger-Kalinski, MA, Schriger, DL, Friedman, L, et al. Identification of risk factors for exertional heat-related illnesses in long-distance cyclists: experience from the California AIDS ride. Wilderness Environ Med. 2001;12:81.
156. Horsman, MR. Tissue physiology and the response to heat. Int J Hyperthermia. 2006;22:197.
157. Becker, JA, Stewart, LK. Heat-related Illness. Am Fam Physician. 2011;83:1325.
158. Epstein, Y, Roberts, WO. The pathophysiology of heat stroke: an integrative view of the final common pathway. Scand J Med Sci Sports. 2011;21:742–748.
159. Wilson, TB, Brothers, RM, Tollund, C. Effect of thermal stress on Frank-Starling relations in humans. J Physiol. 2009;587:3383.
160. Sharma, HS. Hyperthermia-induced brain oedema: current status and future perspectives. Indian J Med Res. 2006;123:629.
161. Eshel, GM, Safar, P, Stezoski, W. The role of the gut in the pathogenesis of death due to hyperthermia. Am J Forensic Med Pathol. 2001;22:100.
162. Lee, CW, Perng, CL, Huang, YS. Multiple organ failure caused by non-exertional heat stroke after bathing in a hot spring. J Chin Med Assoc. 2010;73:212.
163. Tsai, MK, Chen, IH, Wang, CC, et al. Colon perforation as a critical complication of exertional heat stroke. Intern Med. 2010;49:2473.
164. Bouchama, A, DeVol, EB. Acid-base alterations in heatstroke. Intensive Care Med. 2001;27:680.
165. Bolster, D, Tappe, S, Short, K, et al. Effects of cooling on thermoregulation during subsequent exercise. Med Sci Sports Exerc. 1999;31:251.
166. Booth, J, Marino, F, Ward, J. Improved running performance in hot humid conditions following whole body precooling. Med Sci Sports Exerc. 1997;29:943.
167. Metterllein, T, Zink, W, Krank, E, et al. Cardiopulmonary bypass in malignant hyperthermia susceptible patients: a systemic review of published cases. J Thorac Cardiovasc Surg. 2011;141:1488.
168. Schneider, C, Pedrosa, F, Gil, F, et al. Intolerance to neuroleptics and susceptibility for malignant hyperthermia in a patient with proximal myotonic myopathy (PROMM) and schizophrenia. Neuromuscul Disord. 2002;12:31.
169. Nelson, TE. Heat production during anesthetic-induced malignant hyperthermia. Biosci Rep. 2001;21:169.
170. Igardi, T, Christensen, UC, Jacobsen, J, et al. How do anaesthesiologists treat malignant hyperthermia in a full-scale anaesthesia simulator? Acta Anaesthesiol Scand. 2001;45:1032.
171. Krause, T, Gerbershagen, MU, Fiege, M, et al. Dantrolene—a review of its pharmacology, therapeutic use, and new developments. Anaesthesia. 2004;59:364.
172. Bouchama, A, Cafege, A, Derol, EB, et al. Ineffectiveness of dantrolene sodium in the treatment of heatstroke. Crit Care Med. 1992;20:1192.
173. Hadad, E, Cohen-Sivan, Y, Heled, Y, et al. Clinical review: treatment of heat stroke: should dantrolene be considered? Crit Care. 2005;9:86.
174. Louis, CF, Balog, EM, Fruen, BR. Malignant hyperthermia: an inherited disorder of skeletal muscle at regulation. Biosci Rep. 2001;21:155.
175. Gillman, PK. Neuroleptic malignant syndrome: mechanisms, interactions, and causality. Mov Disord. 2010;25:1780.
176. Ong, KC, Chew, EL, Ong, YY. Neuroleptic malignant syndrome without neuroleptics. Singapore Med J. 2001;42:85.
177. Bhanushali, MJ, Tuite, PJ. The evaluation and management of patients with neuroleptic malignant syndrome. Neurol Clin North Am. 2004;22:389.
178. Nisljmak, K, Shloda, K, Iwamura, T. Neuroleptic malignant syndrome & serotonin syndrome. Prog Brain Res. 2007;162:81.
179. Susman, VL. Clinical management of neuroleptic malignant syndrome. Psychiatr Q. 2001;72:325.
180. Tsai, MC, Huang, TL. Severe neuroleptic malignant syndrome: successful treatment with high dose lorazepam & diazepam: a case report. Chang Gung Med J. 2010;33:576.
181. Hadad, E, Weinbroum, AA, Ben-Abraham, R. Drug-induced hyperthermia and muscle rigidity: a practical approach. Eur J Emerg Med. 2003;10:149.
182. Birmes, P, Coppin, D, Schmitt, L, et al. Serotonin syndrome: a brief review. CMAJ. 2003;168:1439.
183. Sarkar, S, Schmued, L. Neurotoxicity of ecstasy (MDMA): an overview. Curr Pharm Biotechnol. 2010;11:460.
184. Hall, AP, Hendry, JA. Acute toxic effects of “ecstasy” (MDMA) and related compounds: overview of pathophysiology and clinical management. Br J Anaesth. 2006;96:678.
185. Armstrong, LE, Hubbard, RW. Application of a model of exertional heat stroke pathophysiology to cocaine intoxication. Am J Emerg Med. 1990;8:178.
186. Daras, M, Kakkouras, L, Tuchman, AJ, et al. Rhabdomyolysis and hypothermia after cocaine abuse: a variant of the neuroleptic malignant syndrome. Acta Neurol Scand. 1995;92:161.
187. de la Torre, R, Farre, M, Roset, PN. Human pharmacology of MDMA: pharmacokinetics, metabolism, and disposition. Ther Drug Monit. 2004;26:137.
188. Roberts, JR, Quattrocchi, E, Howland, MA. Severe hyperthermia secondary to intravenous drug abuse. Am J Emerg Med. 1984;2:373.
189. Vasallo, SU. Pharmacologic effects on thermoregulation: mechanisms of drug-related heatstroke. Clin Toxicol. 1989;27:199.
190. Duffy, MR, Ferguson, C. Role of dantrolene in treatment of heat stroke associated with Ecstasy ingestion. Br J Anaesth. 2007;98:148.
191. Little, D, Wilkins, B. Hemorrhagic shock and encephalopathy syndrome. An unusual cause of sudden death in children. Am J Forensic Med Pathol. 1997;18:79.
192. Gaffin, SL, Gardner, JW, Flinn, S. Cooling methods for heatstroke victims. Ann Intern Med. 2000;132:678.
193. Stachenfeld, NS. American College of Sports Medicine position stand. Exercise and fluid replacement. Med Sci Sports Exerc. 2007;39:377.
194. Shapiro, Y, Seidman, DS. Field and clinical observations of exertional heat stroke patients. Med Sci Sports Exerc. 1990;22:6.
195. Seraj, MA, Channa, AB, Al Harthi, SS, et al. Are heat stroke patients fluid depleted? Importance of monitoring central venous pressure as a simple guideline for fluid therapy. Resuscitation. 1991;21:33.
196. Plaisance, KI. Toxicities of drugs used in the management of fever. Clin Infect Dis. 2000;31:S224.
197. Henker, R, Rogers, S, Kramer, DJ, et al. Comparison of fever treatments in the critically ill: a pilot study. Am J Crit Care. 2001;10:276.
198. Bouchama, A, Dehbi, M, Chaves-Carbello, E. Cooling and hemodynamic management in heatstroke: practical recommendation. Crit Care. 2007;11:R54.
199. Poulton, TJ, Walker, RA. Helicopter cooling of heat stroke victims. Aviat Space Environ Med. 1987;58:358.
200. Weiner, JS, Khogali, M. A physiological body-cooling unit for treatment of heat stroke. Lancet. 1980;1:507.
201. Kielblock, AJ, Van Rensburg, JP, Franz, RM. Body cooling as a method for reducing hyperthermia. S Afr Med J. 1986;69:378.
202. Wyndham, CH, Strydom, NB, Cookett, M, et al. Methods of cooling subjects with hyperpyrexia. J Appl Physiol. 1959;14:771.
203. Daily, WM, Harrison, TR. A study of the mechanism and treatment of experimental heat pyrexia. Am J Med Sci. 1948;215:42.
204. Armstrong, LE, Crago, AE, Adams, R, et al. Whole body cooling of hyperthermic runners: comparison of two field therapies. Am J Emerg Med. 1996;14:355.
205. Magazanik, A, Epstein, Y, Udassin, R, et al. Tap water, an efficient method for cooling heatstroke victims—a model in dogs. Aviat Environ Med. 1980;51:864.
206. Costrini, AM. Emergency treatment of exertional heatstroke and comparison of whole body cooling techniques. Med Sci Sports Exerc. 1990;22:15.
207. Clapp, AJ, Bishop, PA, Muir, I, et al. Rapid cooling techniques in joggers experiencing heat strain. J Sci Med Sport. 2001;4:160.
208. Syverud, SA, Barker, WJ, Amsterdam, JT, et al. Iced gastric lavage for treatment of heatstroke: efficacy in a canine model. Ann Emerg Med. 1985;14:424.
209. White, JD, Riccobene, E, Nucci, R, et al. Evaporation versus iced gastric lavage treatment of heatstroke: comparative efficacy in a canine model. Crit Care Med. 1987;15:748.
210. Horowitz, BZ. The golden hour in heatstroke: use of iced peritoneal lavage. Am J Emerg Med. 1989;7:616.
211. Bynum, GD, Pandolf, KB, Schuette, WH, et al. Induced hyperthermia in sedated humans and the concept of critical thermal maximum. Am J Physiol. 1978;235:625.
212. White, JD. Evaporation versus iced peritoneal lavage treatment of heatstroke: comparative efficacy in a canine model. Am J Emerg Med. 1993;11:1.
213. Harris, SB, Darwin, MG, Russell, SR, et al. Rapid (0.5 degrees C/min) minimally invasive induction of hypothermia using cold perfluorochemical lung lavage in dogs. Resuscitation. 2001;50:189.
214. Smith, JE. Cooling methods used in the treatment of exertional heat illness. Br J Sports Med. 2005;39:503.
215. Eshel, GM, Safar, P, Stezoski, W. Evaporative cooling as an adjunct to ice bag use after resuscitation from heat-induced arrest in a primate model. Pediatr Res. 1990;27:264.
216. Kato, M, Sugenoya, J, Matsumoto, T, et al. The effects of facial fanning on thermal comfort sensation during hyperthermia. Pflugers Arch. 2001;443:175.
217. Hadad, E, Rav-Acha, M, Heled, Y, et al. Heat stroke: a review of cooling methods. Sports Med. 2004;34:501.
218. Wolfenson, D, Bachrach, D, Mamam, M, et al. Evaporative cooling of ventral regions of the skin in heat stressed laying hens. Poult Sci. 2001;80:958.
219. Hee-Nee, P, Rupano, M, Lee, VJ. Treatment of exertional heat injuries with portable body cooling unit in a mass endurance event. Am J Emerg Med. 2010;28:246.
220. Desruelle, AV, Candad, V. Thermoregulatory effects of three different types of head cooling in humans during a mild hyperthermia. Eur J Appl Physiol. 2000;81:33.
221. DeWitte, J, Sessler, D. Perioperative shivering: physiology and pharmacology. Anesthesiology. 2002;96:467.
222. Sithinamsuwan, P, Plyavechviratana, K, Kitthaweesin, T, et al. Exertional heatstroke: early recognition and outcome with aggressive combined cooling—a 12 year experience. Mil Med. 2009;174:496.
223. Gagnon, D, Lemire, BB, Casa, DJ. Cold water immersion and the treatment of hyperthermia: using 38.6 C as a safe rectal temperature cooling unit. J Athl Train. 2010;45:439.
224. Duffield, R. Cooling interventions for the protection and recovery of exercise performance from exercise-induced heat stress. Med Sport Sci. 2008;53:89.
225. Plattner, O, Kurz, A, Sessler, DI. Efficacy of cooling methods in malignant hyperthermia crisis. Anesthesiology. 1997;87:487.
226. Proulx, CI, Ducharme, MB, Kenny, GP. Effect of water temperature on cooling efficiency during hyperthermia in humans. J Appl Physiol. 2003;94:1317.
227. Mekjavic, IB, Tipton, MJ, Gennser, M, et al. Motion sickness potentiates core cooling during immersion in humans. J Physiol. 2001;535:619.
228. Pease, S, Bouadma, L, Kermarrec, N, et al. Early organ dysfunction course, cooling time and outcome in classic heatstroke. Intensive Care Med. 2009;35:1454.
229. Hostler, D, Northington, WE, Callaway, CW. High-dose diazepam facilitates core cooling during cold saline infusion in healthy volunteers. Appl Physiol Nutr Metab. 2009;34:582.
230. Noakes, TD. Heatstroke during the 1981 national cross-country running championships. S Afr Med J. 1982;61:145.
231. McDermott, BP, Casa, DJ, O’Conner, FG, et al. Cold-water dousing with ice massage to treat exertional heat stroke: a case series. Aviat Space Environ Med. 2009;80:720.
232. Eshel, GM, Safar, P, Stezoski, W. Evaporative cooling as an adjunct to ice bag use after resuscitation from heat-induced arrest in a primate model. Pediatr Res. 1990;27(3):264–267.
233. Dematte, J, O’Mara, K, Buescher, J, et al. Near-fatal heatstroke during the 1995 heat wave in Chicago. Ann Intern Med. 1998;129:173.
234. Lucas, RA, Ainslie, PN, Fan, JL. Skin cooling aids cerebrovascular function more effectively under severe than moderate heat stress. Eur J Appl Physiol. 2010;106:101.
235. Rajek, A, Greif, R, Sessler, DI, et al. Core cooling by central venous infusion of ice-cold (4 degree C and 20 degrees C) fluid: isolation of core and peripheral thermal compartments. Anesthesiology. 2000;93:629.
236. Ferrie, R, Loveland, R. Bilateral gluteal compartment syndrome after “ecstasy” hyperpyrexia. J R Soc Med. 2000;93:260.
237. Kessler, M, Klein, R, McMlellan, L, et al. Effects of conventional and high frequency jet ventilation on lung parenchyma. Crit Care Med. 1982;10:514.
238. Smith, RB, Cutaia, F, Hoff, BH, et al. Long term transtracheal high-frequency ventilation in dogs. Crit Care Med. 1981;9:311.
239. Wakino, S, Hori, S, Mimura, T, et al. Heat stroke with multiple organ failure treated with cold hemodialysis and cold continuous hemodiafiltration: a case report. Ther Apher Dial. 2005;9:423.
240. Kelly, KP, Stenson, BJ, Drummond, GB. Randomized comparison of partial liquid ventilation, nebulized perfluorocarbon, porcine surfactant, artificial surfactant, and combined treatments on oxygenation, lung mechanics, and survival in rabbits after saline lung lavage. Intensive Care Med. 2000;26:1523.
241. Mack, WJ, Huang, J, Winfree, C, et al. Ultrarapid, convection-enhanced intravascular hypothermia: a feasibility study in nonhuman primate stroke. Stroke. 2003;34:1994.
242. Schmutzhard, E, Engelhardt, K, Beer, R, et al. Safety and efficacy of a novel intravascular cooling device to control body temperature in neurologic intensive care patients: a prospective pilot study. Crit Care Med. 2002;30:2481.
243. Hsu, SF, Niu, KC, Lin, CL, et al. Brain cooling causes attenuation of cerebral oxidative stress, systemic inflammation, activated coagulation, and tissue ischemia/injury during heatstroke. Shock. 2006;26:210.
244. Cienki, JJ, Sevald, J, Frisch, M, et al. An evaluation of ketorolac in hyperthermia. Ann Emerg Med. 2000;36:S6.