Procedural ultrasound for surgeons
(CONSULTANT-LEVEL EXAMINATION)
Liver
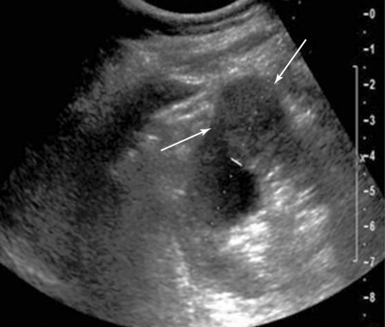
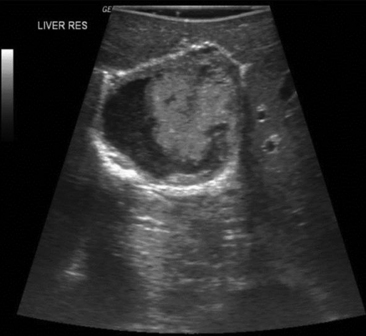
Primary hepatocellular carcinoma usually occurs in the setting of dense cirrhosis, which makes visualization and palpation difficult. Furthermore, many hepatomas are smaller than 5 cm and are not fully evident by exploration and palpation alone. IOUS aids with anatomic localization, definition of satellite lesions, and imaging of segmental/subsegmental vascular invasion of the portal or hepatic veins (Figure 44-1).
Figure 44-1 Intraoperative ultrasound (IOUS) image of hepatocellular carcinoma invading the right hepatic vein. An isoechoic mass protrudes into the lumen of the vein in a cirrhotic liver.
The same principles apply to hepatic metastases of all tissue types. IOUS has higher (>90%) sensitivity and specificity than preoperative computed tomography (CT), US, or surgical inspection in screening for these lesions, largely because IOUS detects tumors less than 1 cm.1 When performed as a routine screening procedure during colorectal resections, occult metastases are identified nearly 10% of the time. Likewise, in determining resectability of other intra-abdominal malignancies (pancreas, gallbladder, stomach), identification of occult metastases in the liver dramatically changes the surgical approach from curative to palliative intent. The results of many studies show that IOUS changes the clinical management in up to 50% of patients undergoing hepatic resection for malignancy. Even when intraoperative US does not change surgical management, it results in corrected staging and, consequently, alters postoperative treatment in 11% of patients.2,3
Bloed et al4 showed that despite preoperative triphasic CT, intraoperative US still provided additional useful information in 50% of their patients, which resulted in a change in the surgical procedure in 15%. In a study done at Royal Prince Alfred Hospital, 20 of 30 hepatic resections (67%) were changed or guided by IOUS. IOUS detected 26 more metastases (44%) in 10 of 19 patients (1-5 per patient). Two patients had preoperatively suspected metastases refuted by IOUS-guided biopsy. Eight of the 11 patients (73%) undergoing resection of primary carcinoma of the liver had the planned procedure changed or guided by IOUS.5
Gall bladder
IOUS has equivalent efficacy while providing cost and time savings when compared with cholangiography in biliary disease and has great utility in identifying common bile duct (CBD) pathology during open operations, especially because these procedures typically occur amidst inflammation, malignancy, or prior surgery. In cases of unresectable disease, biopsy can be guided by IOUS, and during reconstructive procedures, transhepatic biliary stent placement can be facilitated.
In a recent study, IOUS identified significant biliary abnormalities in 20 patients (40%) undergoing cholecystectomy, including a foreshortened cystic duct (<1 cm) in 7 patients (14%), CBD stones in 4 patients (8%), abnormal cystic duct anatomy in 4 patients (8%), and abnormal vascular anatomy in 8 patients (16%).6
Kidney
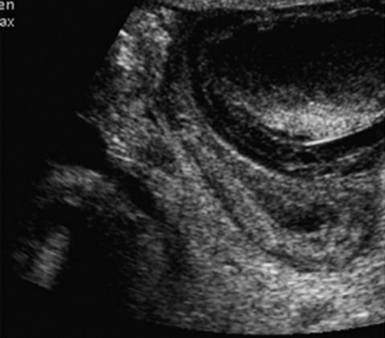
The relationship of renal tumor to structures deeper within the sinus may be more readily appreciated using IOUS. In a series of patients with hereditary renal carcinoma, IOUS identified tumors that were undetectable by the surgeon in 25% of cases, some as large as 4 cm, of which 50% were pathologically proven renal cell carcinoma (Figure 44 E-1).
Pancreas
IOUS techniques are particularly useful in the pancreas because of its retroperitoneal location. It augments the staging of pancreatic malignancies, especially neuroendocrine tumors. Perhaps one of the best-defined applications for IOUS is for localization of pancreatic islet cell tumors, both functional and nonfunctional. These tumors are characteristically small (<5 mm) and hypoechoic on US, but more important, preoperative and even intraoperative identification can be unyielding. Intrapancreatic insulinomas can be detected with IOUS between 85% and 100% of the time, and gastrinomas are identified with a 95% frequency, although these rates correlate directly with IOUS experience.7 In parenchymal-sparing enucleation procedures for tumors or cysts, IOUS helps reveal the relationship of the lesion to the main pancreatic duct in order to protect against the development of a significant pancreatic fistula.
IOUS may be used for pancreatic pseudocyst drainage procedures and can help determine whether multiple pseudocysts are connected, or not, and assess the adequacy of drainage (Figure 44-2). Complications of chronic pancreatitis, such as pseudocysts, dilatated and strictured pancreatic ducts, and portal/splenic venous hypertension, may require surgical intervention but can be difficult to understand anatomically by using visible and tactile exploration alone. IOUS is also excellent for determining the size and characteristics of an obstructed pancreatic duct, an important consideration in choosing a ductal drainage procedure over resection in some cases of chronic pancreatitis. Intraductal papillary projections can occasionally be seen by using IOUS in cases of intraductal papillary mucinous neoplasms, although this is usually unnecessary if the preoperative endoscopic and imaging evaluation was adequate.
Colon
With the increase in laparoscopic surgery for colorectal neoplasms, there is difficulty encountered in localizing neoplasms without the benefit of tactile feedback. The most commonly used localizing techniques are tattooing with India ink and intraoperative colonoscopy. Although, widely used, these methods are far from perfect. Injected dyes tend to migrate away from the lesion or fail to show it. Intraoperative colonoscopy is cumbersome, and the insufflated gas causes bowel distension that may compromise the operative domain in laparoscopic cases. IOUS has been described as a method to identify these lesions; however, excellent imaging requires the bowel to be filled with saline,8,9 and this technique has not been widely adopted.
Ultrasound-guided percutaneous surgical procedures
Suprapubic catheter insertion
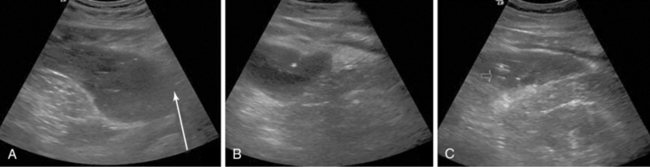
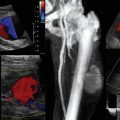
Ultrasound-guided suprapubic catheter (USSPC) insertion is the safest method, especially in patients with previous abdominal or pelvic surgery, because it reduces the incidence of bowel injury observed in blind SPC while avoiding general anesthesia. The goals of USSPC are to evaluate bladder filling, identify interposed bowel, and guide the needle to the appropriate site (Figure 44 E-2). In patients with sufficient bladder distension and intervening bowel excluded, the puncture site is usually 2 to 4 cm above the symphysis pubis. The position should be confirmed using US before and after balloon inflation to avoid accidental insertion of the catheter tip into the urethra or a diverticulum.
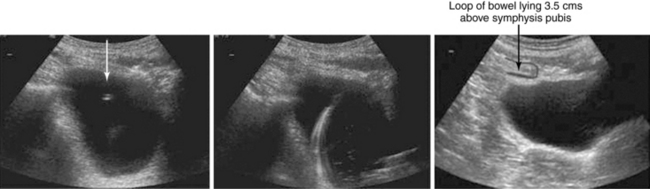
Figure 44 E-2 Images (from left to right) show needle puncture under ultrasound (US) guidance, guidewire confirmed on US, and loop of intervening bowel over bladder. (Reproduced with permission from Jacob P, Rai BP, Todd AW: Suprapubic catheter insertion using an ultrasound-guided technique and literature review. BJU Int 110:779-784, 2012.)
Although the literature on USSPC is sparse, the few studies that have been done show reduced complication rates, improved first-time success rates, and subsequent reduction in infections.10 As a result, recent British Association of Urological Surgeons guidelines recommend US guidance for SPC insertion whenever possible.11
Percutaneous cholecystostomy
Percutaneous cholecystostomy (PC) is used as a temporizing measure in critically ill patients with acute cholecystitis who are not candidates for immediate surgical gallbladder removal (Figure 44-3). Once the patient’s condition is stable enough for surgery, definite treatment is still cholecystectomy. One exception is acalculous cholecystitis, where percutaneous drainage may be the only treatment required.
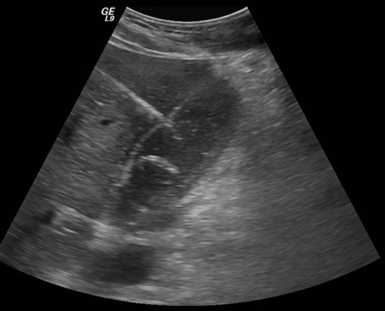
Figure 44-3 Image shows a percutaneous cholecystostomy catheter entering an inflamed gallbladder in a man with acute cholecystitis that complicates renal failure after a necrotizing soft tissue infection.
Surgical gallbladder removal is the best treatment for both acute calculous and acalculous cholecystitis because of its curative nature and low mortality rate of 0% to 0.8%.12,13 However, the mortality rate in elderly or critically ill patients with comorbid disease skyrockets to 14% to 30%14,15; therefore PC provides a safe alternative in the critically ill and elderly.16,17
Percutaneous abscess drainage
The success of USPAD is well documented and has become the standard of care in treating intraabdominal and pelvic abscesses (Figure 44 E-3).
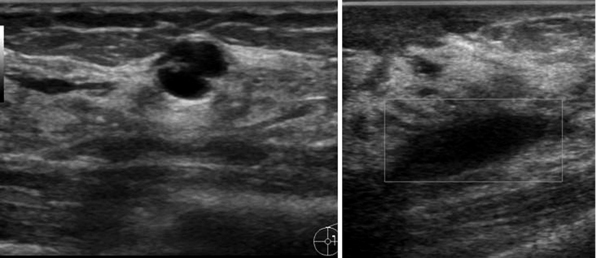
Breast cyst drainage
Breast cysts are a common problem affecting women of all ages. Although asymptomatic stable cysts do not require treatment, larger cysts may cause pain and benefit from aspiration (Figure 44 E-4). In addition, any cyst with a potential solid component should be evaluated to exclude malignancy. Fine-needle aspiration is done using ultrasound to ensure proper placement within the fluid-filled cavity. The procedure is used to both diagnose and treat the cyst. If the aspirated fluid is clear and the mass resolves, malignancy is unlikely. If the fluid is bloody or if there is a residual mass after aspiration, referral for core-needle or excisional biopsy is indicated.
Postoperative seroma drainage
A seroma is a collection of clear serous fluid that can develop after surgery. Seromas are differentiated from hematomas by a lack of red blood cells and from abscesses by a lack of pus. Seromas commonly develop after mastectomies, facial plastic surgeries, lymph node removal, abdominal surgeries, and, in some cases, brachytherapy. Most seromas will resolve without intervention, but persistent seromas may require percutaneous drainage with US guidance. The technique is similar to cyst aspiration as discussed earlier.
Pearls and highlights
• IOUS facilitates identification of occult hepatic lesions and small neuroendocrine pancreatic tumors and determines resectability of larger pancreatic and liver cancers.
• US provides the safest method of suprapubic urinary catheter insertion.
• US-guided cholecystostomy is a safe and effective alternative to surgical gallbladder removal in the critically ill.
• US guidance is standard of care in seroma and breast cyst aspiration.
References
1. Callery, MP, Strasberg, SM, Doherty, GM, et al, Staging laparoscopy with laparoscopic ultrasonography: optimizing resectability in hepatobiliary and pancreatic malignancy. J Am Coll Sur. 1997; 185:33–39.
2. Paul, MA, Mulder, LS, Cuesta, MA, et al. Impact of intraoperative ultrasonography on treatment strategy for colorectal cancer. Br J Surg. 1994; 81:1660–1663.
3. Boutkan, H, Luth, W, Meyer, S, Cuesta, M, et al. The impact of intraoperative ultrasonography of the liver on the surgical strategy of patients with gastrointestinal malignancies and hepatic metastases. Eur J Surg Oncol. 1992; 18:342–346.
4. Bloed, W, van Leeuwen, MS, BorelRinkes, IH. Role of intraoperative ultrasound of the liver with improved preoperative hepatic imaging. Eur J Surg. 2000; 166:691–695.
5. Solomon, MJ, Stephen, MS, Gallinger, S, White, GH. Does intraoperative hepatic ultrasonography change surgical decision making during liver resection. Am J Surg. 1994; 168:307–310.
6. Pfluke, JM, Bowers, SP, Jr., Laparoscopic intraoperative biliary ultrasonography: findings during laparoscopic cholecystectomy for acute disease. J Laparoendosc Adv Surg Tech . 2011; 21:505–509.
7. John, TG, Wright, A, Allan, PL, et al. Laparoscopy with laparoscopic ultrasonography in the TNM staging of pancreatic carcinoma. World J Surg. 1999; 23:870–881.
8. Luck, A, Thomas, M, Roediger, WEW, Hewett, PJ. Localization of the nonpalpable colonic lesion with intraoperative ultrasound. Surg Endosc. 1999; 13:526–527.
9. Greif, F, Belenky, A, Aranovich, D, et al, Intraoperative ultrasonography: a tool for localizing small colonic polyps. Int J Colorectal Di. 2005; 20:502–506.
10. Ahluwalia, RS, Johal, N, Kouriefs, C, et al. The surgical risk of suprapubic catheter insertion and long-term sequelae. Ann R Coll Surg Engl. 2006; 88:210–213.
11. Harrison, SC, Lawrence, WT, Morley, R, et al. British Association of Urological Surgeons’ suprapubic catheter practice guidelines. BJU Int. 2011; 107:77–85.
12. Gilliland, TM, Traverso, LW. Modern standards for comparison of cholecystectomy with alternative treatments for symptomatic cholelithiasis with emphasis on long-term relief of symptoms. Surg Gynecol Obstet. 1990; 170:39–44.
13. Pickleman, J, Gonzalez, RP. The improving results of cholecystectomy. Arch Surg. 1986; 121:930–934.
14. Houghton, PW, Jenkinson, LR, Donaldson, LA, Cholecystectomy in the elderly: a prospective study. Br J Surg. 1985;72:220–222.
15. Frazee, RC, Nagorney, DM, Mucha, P, Jr. Acute acalculous cholecystitis. Mayo Clin Proc. 1989; 64:163–167.
16. Radder, RW. Ultrasonically guided percutaneous catheter drainage for gallbladder empyema. Diagn Imaging. 1980; 49:330–333.
17. Bakkaloglu, H, Yanar, H, Guloglu, R, et al. Ultrasound guided percutaneous cholecystostomy in high-risk patients for surgical intervention. World J Gastroenterol. 2006; 12:7179–7182.