Procedural Sedation and Analgesia
Perspective
The performance of painful diagnostic and therapeutic procedures is common in emergency care. Many of these are associated with significant anxiety, especially in children.1–5 Procedural sedation and analgesia (PSA) has therefore become a fundamental and required skill for emergency physicians and an integral part of the core training of emergency medicine residents.4–8
PSA improves the quality of patient care and satisfaction through relief of pain and anxiety and by facilitating the timeliness and success of therapeutic or diagnostic procedures.7,9–14 These include fracture or joint reduction, incision and drainage of abscesses, cardioversion, tube thoracostomy, lumbar puncture, complex wound repair, and imaging studies in young or uncooperative patients.
Many of the agents used for PSA have the potential to cause significant respiratory, cardiovascular, or central nervous system (CNS) depression.15–25 The Joint Commission (TJC), the Centers for Medicare and Medicaid Services (CMS), the American College of Emergency Physicians (ACEP), and the American Society of Anesthesiologists (ASA) have produced expert consensus or evidence-based documents concerning its use26–28 (Box 4-1). Although significant controversy continues with respect to credentialing and oversight of PSA outside the operating room, the advent of these guidelines has led to PSA becoming a safe, common, and practical emergency department (ED) procedure.9,29 It has been further improved by the development of shorter-acting, more effective drugs and the use of noninvasive monitoring devices.
Terminology
Anxiolysis is a state of decreased apprehension concerning a particular situation in which the patient’s level of awareness does not change.
Analgesia refers to the relief of pain without the intentional alteration of mental status, such as occurs in sedation. An altered mental state may be a secondary effect of the medications administered for this purpose.
Dissociation is a trancelike cataleptic state induced by an agent such as ketamine and characterized by a profound analgesia and amnesia. Protective reflexes, spontaneous respirations, and cardiopulmonary stability are retained.
Sedation is a controlled reduction of environmental awareness.
Procedural sedation and analgesia is a technique of administering a sedative or dissociative agent, usually along with an analgesic, to induce a state that allows the patient to tolerate unpleasant procedures while maintaining adequate spontaneous cardiorespiratory function. It is intended to result in a depressed level of consciousness that allows the patient to maintain oxygenation and airway control independently and continuously. The drugs, doses, and techniques used are not likely to produce a loss of the protective airway reflexes.28
Prior terminology defined three levels of sedation: conscious sedation, deep sedation, and general anesthesia. The term conscious sedation was often misinterpreted, confusing, and imprecise. It was coined in 1985 to describe lightly sedated dental patients. It was then further incorporated into pediatric sedation guidelines to distinguish a level of sedation from which the patient is easily arousable from the more advanced techniques of deep sedation, in which patients are difficult to arouse, and general anesthesia, in which patients are not arousable.30–32 Despite the focused intent of these definitions, practitioners quickly labeled all levels of procedural sedation taking place outside the operating room as “conscious sedation.”
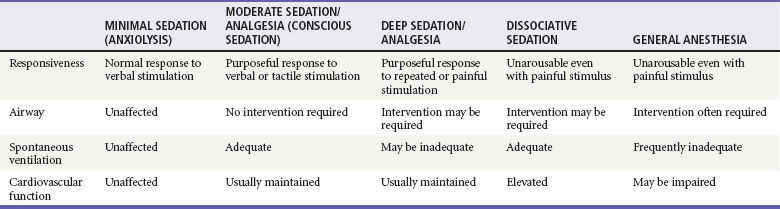
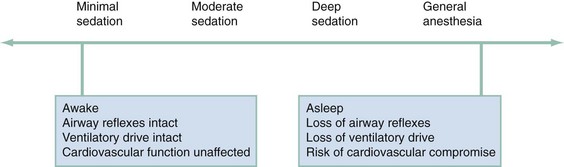
In 2001 TJC adopted the ASA definition of sedation and analgesia that was created to better describe the continuum of sedation and analgesia33 (Fig. 4-1). Although this truly is a continuum, the ASA divided PSA into four distinct subgroups: minimal sedation, moderate sedation, deep sedation, and general anesthesia. A fifth category, dissociative sedation, has since been added34 (Table 4-1). This new nomenclature is more intuitive, clear, and logical.
Minimal sedation (anxiolysis) is a drug-induced state during which patients respond normally to verbal commands. Although cognitive functions and coordination may be impaired, ventilatory and cardiovascular functions are unaffected.
Moderate sedation/analgesia (formerly called “conscious sedation”) refers to a drug-induced depression of consciousness during which patients respond purposefully to verbal commands, either alone or accompanied by light tactile stimulation. Reflex withdrawal from the painful stimulus is not considered a purposeful response. No interventions are required to maintain a patent airway, and spontaneous ventilation is adequate. Cardiovascular function is always maintained.
Dissociative sedation is a trancelike cataleptic state induced by the dissociative agent ketamine and characterized by profound analgesia and amnesia, while protective airway reflexes, spontaneous respirations, and cardiopulmonary stability are maintained.
Deep sedation/analgesia describes a drug-induced depression of consciousness during which patients cannot be easily aroused but respond purposefully after repeated or painful stimulation. The ability to independently maintain ventilatory function may be impaired. Patients may require assistance in maintaining a patent airway, and spontaneous ventilation may be inadequate. Cardiovascular function is usually maintained.
General anesthesia is a drug-induced loss of consciousness during which patients are not arousable even with painful stimulation. The ability to independently maintain ventilatory function is often impaired. Patients often require assistance in maintaining a patent airway, and positive-pressure ventilation may be required because of depressed spontaneous ventilation or drug-induced depression of neuromuscular function. Cardiovascular function may be impaired.
Approach to Procedural Sedation and Analgesia for Procedures
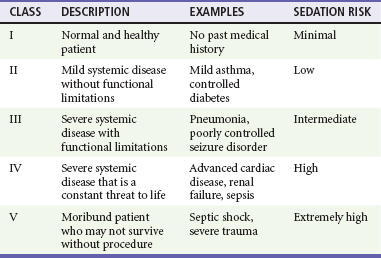
To date, no outcome-based studies have demonstrated clear benefit from extensive evaluation beyond vital signs, mental status, and airway and cardiopulmonary assessment before PSA.35,36 Despite this, consensus guidelines suggest that an increased risk of adverse events may exist in select subsets of patients. These include patients at the extremes of age, patients with difficult facial or neck anatomy or any other reason for potential intubation or bag-valve-mask ventilation difficulty, and patients with underlying significant disease states.15,16,25,36 A patient’s general physical status is conventionally categorized according to the ASA’s classification system37 (Table 4-2). Most practice guidelines require that a history and focused physical examination be performed and documented before PSA. There is no literature to support the need for routine diagnostic testing other than diagnostic testing driven by the patient’s current status, including comorbidities.
Preprocedural Fasting
The need for preprocedural fasting in PSA remains controversial.38–49 Currently the ASA recommends a period of 2 hours after ingestion of clear liquids, a period of 4 hours after ingestion of breast milk, and a period of 6 hours after ingestion of other liquids (infant formula, nonhuman milk) or solids before PSA, but there are no outcome studies to support these recommendations.50 These guidelines are based on expert consensus and extrapolated from data describing circumstances in which patients received sedation to the level of general anesthesia followed by the manipulation of the airway during intubation and extubation.20 PSA in the ED attempts to avoid both of these specific situations.
Many studies fail to support the notion that gastric emptying has any effect on the incidence of complications or outcome with PSA. There have been no published studies demonstrating an increased risk of aspiration after a liquid or solid meal and no studies showing a benefit of fasting before PSA. In one large study of nearly 5000 children and 18,000 adults, no clinically significant differences with airway complications, emesis, or other adverse effects were observed between various groups of patients classified by their preprocedural fasting status.4,40 During PSA, the combination of vomiting and the loss of the airway protective reflexes is an extremely rare occurrence. Furthermore, most episodes of vomiting and aspiration occur during airway manipulation, which is also very unlikely to occur during PSA.
Personnel
TJC and most institutional policies suggest that PSA providers should have adequate training to administer the agents effectively and safely, the skills to monitor the patient’s response to the medications given, and the expertise needed to manage all potential complications.51–56 This generally implies that PSA in the ED should be supervised by an emergency physician or other appropriately trained and credentialed physician. It is also recommended that a qualified support person (nurse, respiratory therapist) be present for the continuous monitoring of the patient. Such support persons should focus on the patient’s status and not take part in the procedure. They should also be able to recognize and respond to the complications of PSA. They may assist with minor, interruptible tasks; however, they should have no other responsibilities that would interfere with the level of monitoring and documentation appropriate for the planned level of sedation. They should be free to monitor the patient from the start of the procedure through the completion of the recovery phase.28
Supplies and Equipment
PSA may result in an allergic reaction, oversedation, respiratory depression, or, rarely, cardiopulmonary arrest. The incidence of these complications depends on patient selection, the drugs used, the rate and dosage of administration, and specific patient sensitivities. Consequently, appropriate equipment to monitor the patient’s condition at all times; to manage airway complications, allergic reactions, and drug overdoses; and to treat respiratory or cardiopulmonary arrest should be readily available. Supportive equipment includes oxygen, suction, patient-monitoring devices, basic and advanced airway management equipment, a monitor/defibrillator, advanced life-support medications, reversal or rescue agents, and vascular access equipment (Box 4-2).
The requirement for supplemental oxygen, and its benefits during PSA, have not been well studied and remain somewhat controversial.56–62 Supplemental oxygen may prevent hypoxemia in many patients; however, significant respiratory depression in these patients may not be detected because of their normal oxygen saturation. This may delay the recognition of respiratory compromise and hypercarbia when capnography is not used. On the other hand, transient hypercarbia is not harmful, and maintenance of adequate oxygen saturation is much more important. The use of capnography eliminates this issue, because ventilatory status is displayed continuously. We recommend the use of supplemental oxygen in administering PSA to a patient in the ED.
Monitoring
The most important aspect of monitoring during PSA is the visual observation and assessment of the patient. The patient’s ability to follow commands in response to varied levels of stimulation is useful in quantifying the level of consciousness. Furthermore, the patient’s ventilatory rate may be readily assessed by direct observation, although depth of respiration (tidal volume) is much harder to estimate clinically. Other components of monitoring, which should be documented, include respiratory rate, heart rate, blood pressure, oxygen saturation, and perhaps cardiac rhythm and capnometry.63–67 Pulse oximetry is a reliable and important monitoring modality, used in conjunction with close and continuous observation of the patient and the response to medications and procedures.
There is no evidence that cardiac monitoring during PSA is of any benefit, but it certainly is not harmful, is readily available, and is inexpensive to use.68–70 We recommend continuous electrocardiographic monitoring in older patients and in patients with a history of cardiovascular disease, hypertension, or dysrhythmia. In young healthy patients without underlying significant disease, this may be safely replaced by continuous pulse oximetry, which also displays the heart rate, but, in most circumstances, monitors capable of showing heart rate, blood pressure, and pulse oximetry will also easily facilitate cardiac rhythm monitoring.
Capnometry or capnography measures end-tidal carbon dioxide (CO2) partial pressure and has been shown to detect cases of inadequate ventilation earlier than clinical assessment or detection of hypoxemia by oximetry.60,62,71–80 Several studies have demonstrated this, but none have shown an effect on clinical outcome to date. In July 2011, the ASA updated its procedural sedation standards to include capnography during moderate or deep sedation to evaluate the adequacy of ventilation in addition to continual observation of qualitative clinical signs.81,82 Capnography should be used when deep sedation is planned, as respiratory depression is common in patients undergoing deep sedation. It is optional when only light sedation is planned, but even in such cases, it will help the observer recognize unintended oversedation with respiratory depression.
The Bispectral Index (BIS) is monitored via a noninvasive device attached to the patient’s forehead and derives a depth of sedation level via frontal lobe electroencephalographic measurements. It has been used in the operating room as an objective measure of sedation depth.83–85 Studies have shown that it may be beneficial in preventing oversedation in PSA and reducing the time to discharge.86 These investigations have also suggested that its use may better guide the depth of sedation endpoint than traditional sedation scales have, and it may have further benefit for PSA in children, as they frequently require deeper levels of sedation for prevention of movement.87–90 Early ED studies for its use in PSA to discriminate between mild-to-moderate and moderate-to-deep levels of sedation have not been reliable, nor has it been shown to be predictive of patients sedated to the point of general anesthesia from those with lesser degrees of sedation. BIS monitoring may have a beneficial role for emergency medicine use and PSA in the future but requires more investigation before its possible uses and benefits can be completely defined.
The highest risk of serious adverse events generally occurs within 5 to 20 minutes of receiving the last dose of intravenous medication and at the completion of procedures, when the patient remains sedated but is no longer receiving the painful stimulus. Similarly, patients undergoing prolonged procedures in which deeper sedation is desired to reduce motion (e.g., magnetic resonance imaging [MRI]) are also at an increased risk.16–19,23,36,91 Patients should continue to be monitored closely at these times, and this should continue until clinical recovery has occurred.
Discharge Criteria and Instructions
Before discharge, baseline cognitive and motor function should be achieved. The patient should be able to follow commands, speak clearly, and ambulate or sit unassisted (infants). Vital signs and respiratory status should be back to baseline and within normal limits. Residual pain should be addressed. Nausea should be minimal, and vomiting should be resolved. It is preferable that all patients, including adults, be sent home with a responsible adult, but if this is not possible, the patient remains in the ED until normal baseline has been achieved.4,92–96
Patients should be advised not to drive or participate in other dangerous activities for 12 to 24 hours. Despite the short clinical duration of most of the agents used, many people may exhibit subtle signs of cognitive deficits and mild drowsiness.94,97 It is therefore preferable that they remain in the company of a responsible adult at home for 4 to 8 hours. For children, light play at home should be the extent of activities, with no bicycle riding, swimming, or other complex motor activity until the next day. An antinauseant and progressive diet is helpful if nausea or vomiting is experienced. Standard discharge instructions should also be provided for the presenting complaint, and all patients should be instructed to immediately return if any confusion or respiratory symptoms arise.
Pharmacology
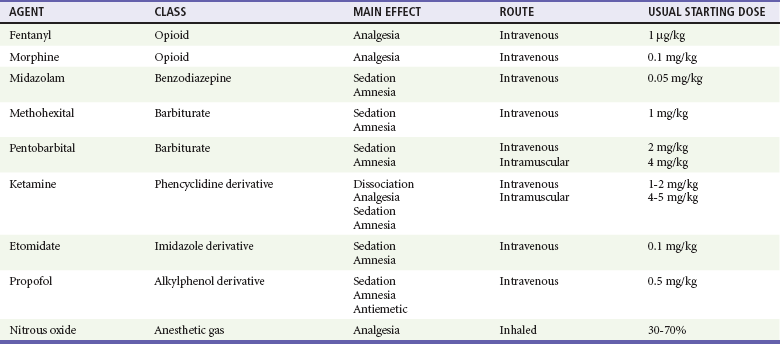
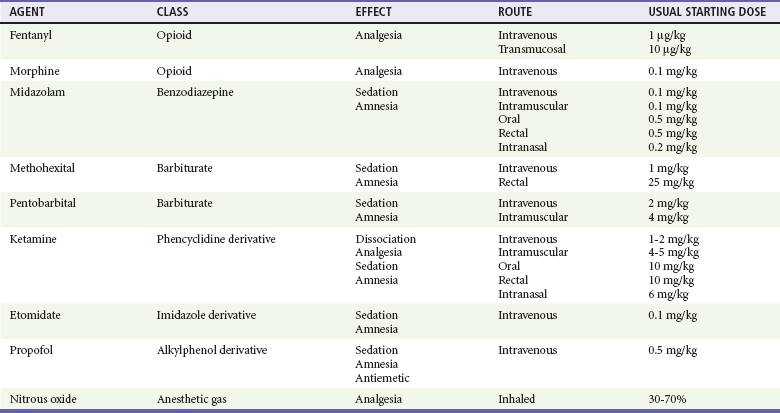
The specific agents for PSA and dosage recommendations for adult patients are provided in Table 4-3 and for pediatric patients in Table 4-4. Benefits and adverse effects are provided in Table 4-5. Individual agents are discussed in greater detail in the following sections.
Table 4-5
Procedural Sedation and Analgesia Agents—Benefits and Adverse Effects
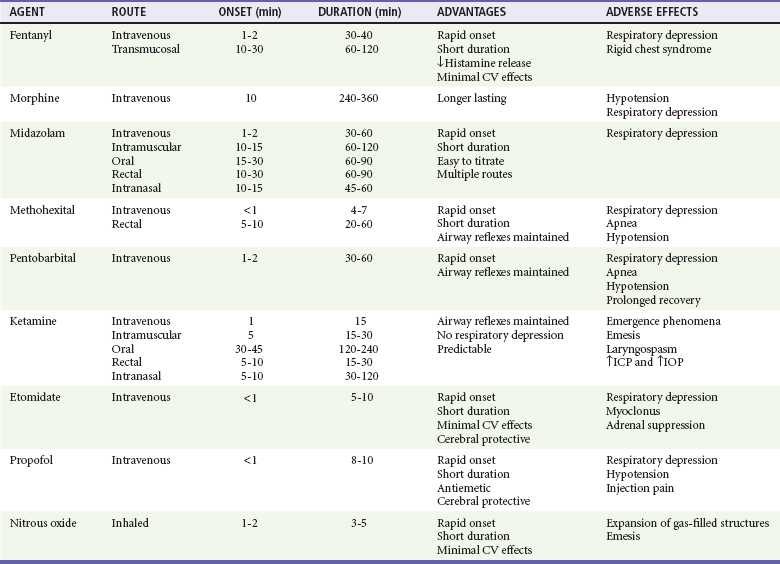
CV, cardiovascular; ICP, intracranial pressure; IOP, intraocular pressure.
Route of Administration
Route of administration should also be determined by the procedure and the specific patient. In most situations, intravenous titration to the desired level of sedation and analgesia provides the safest, most rapid, and most predictable results. Drugs given by the intramuscular, oral, transmucosal, intranasal, or rectal route generally have a slower onset of action, are difficult to titrate, have unpredictable results, and may lead to prolonged sedation. These routes are virtually never used for PSA in adults. In children, however, ketamine has been shown to provide consistent and predictable results when given intramuscularly (IM).98–100 Nitrous oxide has predictable behavior when used as a sole inhalational PSA agent in children but is also frequently used as an analgesic adjunct to a sedating agent.101–104 In pediatric patients, the benefits of intravenous drug administration may be outweighed by the difficulty and distress to the patient in obtaining intravenous access. In this situation, drugs given by the alternative routes may be preferred.
Drugs should be administered by titrated slow intravenous bolus to minimize hypotension or respiratory depression in many situations. It is important to allow adequate time between doses to achieve and assess peak effect before an additional dose is given. Lower initial doses should be chosen in sensitive patients or when drugs from multiple classes are being administered. One exception is ketamine. Unlike the other agents described, it possesses a threshold response rather than an additive dose-response continuum. Smaller doses of ketamine cause analgesia and disorientation. Dissociation occurs when a dosage threshold of 1 to 1.5 mg/kg IV in adult patients or 2 to 2.5 mg/kg in younger pediatric patients is reached. Higher doses do not enhance or deepen the sedation.105
Fentanyl
Fentanyl has many advantages as an analgesic agent for PSA, given its rapid onset of action, short duration of activity, lack of histamine release, and favorable cardiovascular profile.62,106–110 Fentanyl rapidly crosses the blood-brain barrier and produces analgesia in as little as 90 seconds. Serum levels rapidly decline from peak concentrations because of extensive tissue uptake followed by hepatic metabolism. It has a duration of action of 30 to 40 minutes and a serum half-life of approximately 90 minutes. These properties permit the administration of multiple small doses that can be easily titrated to the desired clinical effect. Because fentanyl readily creates a reservoir in the adipose tissue, accumulated large doses may result in a progressively increasing duration of effect. This does not generally occur in doses less than 10 µg/kg.111–113
Respiratory depression is more likely at higher doses, when the drug is given rapidly, or when it is combined with other CNS depressants such as benzodiazepines or alcohol. Other side effects may include vomiting and pruritus, although these are less common than with other opioids. Hypotension and bradycardia are rare but may occur with high doses. Chest wall rigidity and glottic spasm, which may make ventilation difficult, are unique complications seen with high doses (anesthetic) of fentanyl given rapidly (generally more than 7 µg/kg).114 Many of these adverse effects may be readily reversed by naloxone. The exception to this is chest wall rigidity, which may not reliably be antagonized and may necessitate neuromuscular blockade and intubation to enable adequate ventilation. This complication is extremely rarely reported with the doses of fentanyl used for PSA.114
In children, oral or transmucosal fentanyl has been used widely as a premedication for anesthesia and intravenous placement. It has also been used for PSA when intravenous access is not feasible. This is generally in the form of a fentanyl-impregnated, sweetened matrix in lozenge form on a holder—the “fentanyl lollipop.” Transmucosal delivery allows rapid onset of action by avoiding first-pass metabolism in the liver. It has been shown to decrease activity and relieve pain in 10 to 30 minutes, resulting in scores similar to those after comparable intravenous doses of fentanyl.110,115,116 Despite this fairly rapid onset, transmucosal delivery does not allow for easy titration. The general dose is 10 to 15 µg/kg. Larger doses have been shown to cause more nausea and vomiting without improving analgesia or activity scores. The combination of transmucosal fentanyl and transmucosal midazolam has not been shown to have additional benefit over either agent used singularly for laceration repair in the ED and has been shown to increase adverse events.117 Its use before other agents such as propofol has not been studied well. The use of transmucosal fentanyl for PSA has largely been limited by unacceptable levels of nausea and vomiting, which approach 20 to 40% of patients.118
Benzodiazepines
Benzodiazepines are potent amnestic, hypnotic, and anxiolytic medications. They also have anticonvulsant and purported muscle relaxant properties but do not have analgesic effects. Because of this, they are commonly coadministered with an analgesic agent such as fentanyl or morphine. They may be given IV, IM, orally (PO), intranasally (IN), or per rectum (PR) but are virtually always used IV for PSA in adults.119–121 Midazolam is the most commonly used agent because of its favorable pharmacokinetics.
Midazolam
Midazolam has many advantages for PSA, given its rapid onset of action and short duration of activity compared with other benzodiazepines. Its amnestic properties also appear to be superior to many of the others.122 The starting intravenous dose is 0.05 mg/kg. Children may need slightly higher doses. Onset of sedation is generally within 1 to 2 minutes, and the duration of action is 30 to 60 minutes. Alternatives to the intravenous route are often used in children, particularly when sedation alone, without analgesia, is desired as for performance of a radiologic study. With intramuscular administration the same doses may be used, but the onset of action is delayed and the duration of effect is increased. Oral doses are often used in children, with a recommended starting dose of 0.5 mg/kg. Sedation is generally achieved within 30 minutes. Rectal administration may also be useful with doses of 0.5 mg/kg. The response when the drug is given by this route may be less predictable, and the rectal route is generally not accepted by older children. Intranasal administration may be useful but is irritating and often difficult in older children as well. The starting dose for this route is 0.2 mg/kg and results in sedation in 10 to 15 minutes. Of note, 1% of children younger than 5 years may experience a paradoxical reaction and become excited and agitated when given midazolam. If necessary, the agitated state is reversible by flumazenil. Midazolam has been shown to be an extremely safe and effective agent for PSA, both alone and when used in combination with fentanyl.123–125
Methohexital
Depressed respiratory drive and apnea are more common than with other agents but are generally transient and mild. Most patients respond to supplemental oxygen and repositioning of the airway. Its use in the ED appears to be safe and effective, particularly for brief orthopedic joint reductions, cardioversions, and radiologic imaging procedures. Only a small percentage of patients require brief periods of assisted ventilation. In several studies, no ED patients required intubation or change in disposition after having received methohexital for PSA.126–129 Methohexital does have the potential to cause significant hypotension as well and should be used with caution in patients with hemodynamic instability or occult blood loss. It also may cause activation of the respiratory reflexes, inducing coughing, hiccups, and, rarely, laryngospasm. In contrast to the other barbiturates, methohexital may worsen or precipitate seizures, and it is prudent to avoid it in patients with a known seizure disorder.128,130,131 Propofol has largely supplanted methohexital for PSA because it has more reliable, consistent effects, is easily titrated, and can be administered as an infusion for longer procedures.
Ketamine
Ketamine is a well-studied, safe, and predictable agent for use in the pediatric population for PSA.124,128,132,133 It continues to gain popularity for use in adults as well.124,129–131 It is a derivative of the street drug phencyclidine and is classified as a dissociative agent. It causes disruption between the thalamoneocortical and limbic systems, preventing the higher centers from perceiving visual, auditory, or painful stimuli. Because of this, its use leads to profound analgesia, amnesia, and catalepsis. It does not produce unconsciousness, but rather a trancelike state. Patients often experience nystagmus, roving eye movements, and random movements of the extremities unrelated to painful stimuli. Parents observing procedures in which ketamine is used may be disturbed at seeing this and should be forewarned.
Ketamine has several advantages over other PSA agents.124,128,132–135 The most notable are its profound analgesic effect and the lack of significant respiratory depression. The protective airway reflexes, such as coughing, swallowing, and muscular tone of the tongue and pharynx, are well maintained or slightly enhanced. This is particularly useful in the ED setting where preprocedural fasting might not be assured. Its use further leads to blockade of catecholamine reuptake, and blood pressure is generally well supported. It also induces bronchial smooth muscle relaxation and is well tolerated in patients with reactive airway disease. It has a fast onset and offset and is predictable when given by the intravenous or intramuscular route. After administration, it is rapidly distributed and taken up by the cerebral tissues. The effects are maintained until the drug redistributes into the peripheral tissues and is metabolized by the liver. Because of this mechanism, repeat doses are well tolerated in longer procedures with predictable results but may lead to longer recovery times and increased incidence of emesis.
The most common side effect seen with ketamine is the emergence phenomenon.99,136–140 This occurs in approximately 15% of patients and is mild in almost all. They may wake up with unpleasant, vivid dreams or hallucinations or report nighttime awakenings as a result of unpleasant dreams for several days after the administration of ketamine. Less than 1 to 2% of patients have significant emergence agitation. This is more commonly seen in female patients, adolescents or adults, and in those with underlying psychiatric disorders. Its rare occurrence, especially in children, should not limit ketamine’s use when indicated. Some studies have suggested that preprocedural or concurrent administration of midazolam may mitigate this reaction, but others have disputed the utility of this strategy. The most recent clinical practice guideline update does not recommend this practice in children.53,106,141–144 We do not recommend the routine concomitant use of a benzodiazepine when administering ketamine sedation, unless it is judged to be of benefit for preprocedural anxiety. Benzodiazepines are useful for treating emergence phenomena, however, if such phenomena occur during recovery. Other side effects seen with ketamine use are transient apnea, laryngospasm, and emesis.145–151 These are also rare but more common with rapid intravenous administration or with larger doses. Doses given slowly, at a rate of 0.5 mg/kg/min, may limit much of this. Ketamine also stimulates tracheobronchial and salivary secretions. In young children and in any patient undergoing airway examination (e.g., fiberoptic laryngoscopy), pretreatment with glycopyrrolate, 0.01 mg/kg given 10 minutes before the ketamine, may be beneficial. Because airway reflexes are maintained, this generally is not a concern in other patients. Prophylactic pretreatment with anticholinergics is unnecessary, and we no longer recommend this practice.53,152–156 Postrecovery nausea and vomiting are also frequently seen but generally short lived and respond well to typical antiemetics such as ondansetron.149
Etomidate
Etomidate is a short-acting sedative-hypnotic agent that is structurally unrelated to the other PSA agents and has no analgesic properties. Its use leads to the very rapid onset of profound sedation and hypnosis by enhancing neurotransmission at γ-aminobutyric acid (GABA) receptors. Etomidate has been used for deep sedation because of its rapid onset, short duration of action, and, most important, minimal effects on respiratory and cardiovascular function.157–165
Adverse effects that may limit its usefulness include apnea, respiratory depression, myoclonus, nausea, vomiting, and adrenal suppression.163,164,166–170 These side effects are more common with rapid intravenous administration, when higher doses are used, and in older patients. Although respiratory depression is rare and generally transient, few patients may require brief periods of assisted ventilation. Myoclonus is also typically described as mild and brief but at times may interfere with the procedure. Vomiting is unlikely with doses administered in the ED. Etomidate suppresses adrenal function by inhibiting 11-β-hydroxylase activity. This is generally not clinically relevant when a single sedating dose is used (see Chapter 1).171–178
Propofol
Propofol is another ultra-short-acting sedative-hypnotic agent that is structurally unrelated to the other PSA drugs and has no analgesic properties. It has an extremely rapid onset, a short duration of action, and predictable efficacy for inducing deep sedation.10,125,164,179–192 Sedation quickly clears completely, permitting superior titration and earlier recovery and discharge. Propofol also possesses potent antiemetic properties and decreases intracranial pressure. Because of its properties, propofol is a highly desirable and widely used agent for deep sedation in the ED. It does not provide analgesia, however, and should be preceded by an opioid for painful procedures. As with all sedative agents, the addition of an opioid may increase the risk of deeper than anticipated sedation, respiratory depression, apnea, and hypotension, and smaller starting doses are recommended.
Adverse effects include dose-dependent respiratory depression, apnea, hypotension, and pain on injection. Other agents, such as opioids, may intensify these effects. In most cases apnea is transient, and patients will not experience significant falls in oxyhemoglobin levels before recovering their respiratory function. This underscores the values of supplemental oxygen administration during sedation. If the oxygen saturation falls into the low 90s, the patient may require a brief period of assisted ventilation. Propofol commonly results in a transient drop in systolic and diastolic blood pressure, which rarely necessitates fluid administration and is well tolerated by healthy patients. The hypotensive effect is exaggerated in the elderly and in patients with hypovolemia, and the initial dose of propofol should be reduced to 0.25 to 0.5 mg/kg in such patients. Pain associated with propofol injection occurs in the majority of patients and can be reduced with small infusions of intravenous lidocaine under tourniquet before its administration. Despite these concerns, propofol has been shown to be reliable and safe when used with proper monitoring in the ED setting. In a series of more than 25,000 pediatric patients in whom propofol was administered by emergency physicians, serious adverse effects were rare and there were no adverse outcomes.180
Ketamine Plus Propofol (“Ketofol”)
Ketamine is commonly combined with propofol for PSA, especially in children. The two completely different agents are felt to have synergistic effects that balance each other’s deficits while decreasing the overall dose of propofol. Ketamine provides the analgesic effects that propofol lacks, and the respiratory depression and hypotension caused by propofol are balanced by the cardiorespiratory stimulating effects of ketamine. Furthermore, vomiting and recovery hallucinations from ketamine are mitigated by the antiemetic and hypnotic effects of propofol.106,141,142,144,193–197
In several recent studies, ketofol was not shown to be clinically superior to either agent used alone with regard to respiratory depression or airway complications.106,141,142,144 There was statistically significant benefit in provider satisfaction, sedation quality, and decreased emesis and clinically insignificant improvement in recovery time. Although the use of this combination is extremely popular, it cannot be currently recommended as superior to propofol, propofol plus fentanyl, or ketamine with or without ondansetron.
Reversal and Rescue Agents
Careful titration of PSA drugs to the desired level of sedation is the goal. At times, unanticipated deeper levels of sedation may be reached, and respiratory depression or apnea may be experienced. Airway repositioning, supplemental oxygen, and bag-valve-mask ventilation may be required. If these periods are prolonged, partial or complete reversal of agents such as opioids or benzodiazepines may be necessary. Elective reversal of PSA after the completion of the procedure is not recommended.198,199
Drug Selection and Administration
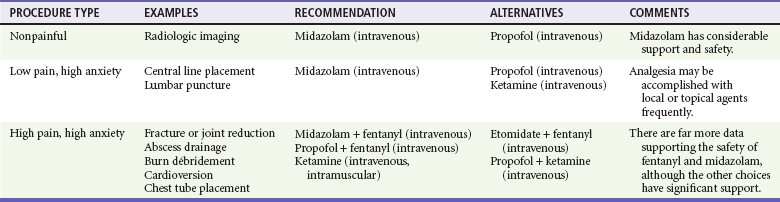
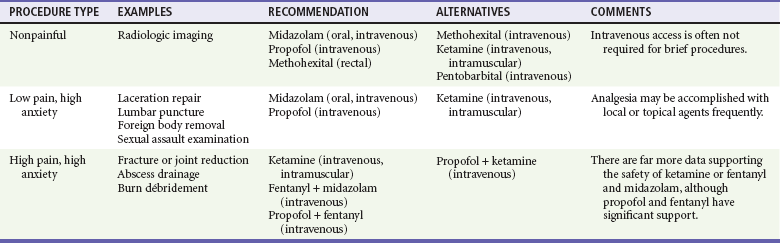
When choosing a strategy for PSA, it is important to consider the type of procedure being performed (painful or not), the length of the procedure, specific procedural requirements (anxiolysis vs. immobility), and whether sedation may need to be prolonged. The need for intravenous access generally is an issue only in small children. Planned adjuncts, such as topical, local, or regional anesthesia, are also considered. Patient factors, including age, medications, alcohol and drug use, and comorbid conditions, are considered when selecting both the agent and the initial dose. Procedures necessitating sedation may be broadly divided into three categories: nonpainful procedures requiring immobilization (e.g., computed tomography [CT], MRI); low-pain, high-anxiety procedures (e.g., laceration repair, lumbar puncture); and highly painful, high-anxiety procedures (e.g., fracture or joint reduction, tube thoracostomy, abscess drainage, cardioversion). These are summarized in Table 4-6 for adult patients and Table 4-7 for pediatric patients.
Special Considerations for Pediatric Populations
The advent of ultrafast spiral CT scanners has nearly eliminated the need for sedation for this procedure. For prolonged CT studies or MRI, deep sedation is generally required in young children to ensure immobility. The need and desire for intravenous access in children should also be considered when planning PSA. Agents such as midazolam may be very effective in infants, but they do not reliably ensure immobility in young children except at high doses.200–203 Methohexital and pentobarbital have demonstrated efficacy for sedating children for these longer imaging procedures.202,204–211 Ketamine has been the most extensively studied and has been shown to be highly effective with a large margin of safety.212–214 Many of these drugs have been largely supplanted by propofol as studies continue to show its efficacy and safety in pediatric PSA.205,206,212,215–219
Chloral hydrate, a pure sedative-hypnotic agent, has been historically popular for sedating children for a wide variety of procedures. It offers no advantage over currently available agents.209,210,220,221 It has a poor safety record, delayed onset of 45 minutes, prolonged recovery time of hours, and residual sedation of nearly a day. Its use should be discontinued. Likewise, the combination of meperidine (Demerol), promethazine (Phenergan), and chlorpromazine (Thorazine), also known as DPT, is not recommended. It has an unacceptable rate of sedation failure, prolonged sedation times, high risk of respiratory depression, and frequent dystonic reactions.222
References
1. Atkinson, P, Chesters, A, Heinz, P. Pain management and sedation for children in the emergency department. BMJ. 2009;339:b4234.
2. Cordell, WH, et al. The high prevalence of pain in emergency medical care. Am J Emerg Med. 2002;20:165–169.
3. Ratnapalan, S, Mason, KP, Mace, SE. Pediatric pain management and sedation. Int J Pediatr. 2010;2010:454731.
4. Mace, SE, et al. Clinical policy: Critical issues in the sedation of pediatric patients in the emergency department. Ann Emerg Med. 2008;51:378–399.
5. Mace, SE, et al. Clinical policy: Evidence-based approach to pharmacologic agents used in pediatric sedation and analgesia in the emergency department. Ann Emerg Med. 2004;44:342–377.
6. Breakey, VR, Pirie, J, Goldman, RD. Pediatric and emergency medicine residents’ attitudes and practices for analgesia and sedation during lumbar puncture in pediatric patients. Pediatrics. 2007;119:e631–e636.
7. Godwin, SA, et al. Clinical policy: procedural sedation and analgesia in the emergency department. Ann Emerg Med. 2005;45:177–196.
8. Perina, DG, et al. The 2007 Model of the Clinical Practice of Emergency Medicine: The 2009 update 2009 EM Model Review Task Force. Ann Emerg Med. 2011;57:e1–e15.
9. O’Connor, RE, et al. Procedural sedation and analgesia in the emergency department: recommendations for physician credentialing, privileging, and practice. Ann Emerg Med. 2011;58:365–370.
10. Zed, PJ, Abu-Laban, RB, Chan, WW, Harrison, DW. Efficacy, safety and patient satisfaction of propofol for procedural sedation and analgesia in the emergency department: A prospective study. CJEM. 2007;9:421–427.
11. Miner, JR, Krauss, B. Procedural sedation and analgesia research: State of the art. Acad Emerg Med. 2007;14:170–178.
12. Shavit, I, Keidan, I, Augarten, A. The practice of pediatric procedural sedation and analgesia in the emergency department. Eur J Emerg Med. 2006;13:270–275.
13. Krauss, B, Green, SM. Procedural sedation and analgesia in children. Lancet. 2006;367:766–780.
14. Bahn, EL, Holt, KR. Procedural sedation and analgesia: A review and new concepts. Emerg Med Clin North Am. 2005;23:503–517.
15. Caperell, K, Pitetti, R. Is higher ASA class associated with an increased incidence of adverse events during procedural sedation in a pediatric emergency department? Pediatr Emerg Care. 2009;25:661–664.
16. Melendez, E, Bachur, R. Serious adverse events during procedural sedation with ketamine. Pediatr Emerg Care. 2009;25:325–328.
17. Cravero, JP, et al. The incidence and nature of adverse events during pediatric sedation/anesthesia with propofol for procedures outside the operating room: A report from the Pediatric Sedation Research Consortium. Anesth Analg. 2009;108:795–804.
18. Green, SM, et al. Predictors of airway and respiratory adverse events with ketamine sedation in the emergency department: An individual-patient data meta-analysis of 8,282 children. Ann Emerg Med. 2009;54:158–168.
19. Strayer, RJ, Nelson, LS. Adverse events associated with ketamine for procedural sedation in adults. Am J Emerg Med. 2008;26:985–1028.
20. Hollman, GA, Schultz, MM, Eickhoff, JC, Christenson, DK. Propofol-fentanyl versus propofol alone for lumbar puncture sedation in children with acute hematologic malignancies: Propofol dosing and adverse events. Pediatr Crit Care Med. 2008;9:616–622.
21. Babl, FE, Oakley, E, Seaman, C, Barnett, P, Sharwood, LN. High-concentration nitrous oxide for procedural sedation in children: Adverse events and depth of sedation. Pediatrics. 2008;121:e528–e532.
22. Bell, A, Treston, G, McNabb, C, Monypenny, K, Cardwell, R. Profiling adverse respiratory events and vomiting when using propofol for emergency department procedural sedation. Emerg Med Australas. 2007;19:405–410.
23. Cravero, JP, et al. Incidence and nature of adverse events during pediatric sedation/anesthesia for procedures outside the operating room: Report from the Pediatric Sedation Research Consortium. Pediatrics. 2006;118:1087–1096.
24. Heistein, LC, et al. Chloral hydrate sedation for pediatric echocardiography: Physiologic responses, adverse events, and risk factors. Pediatrics. 2006;117:e434–e441.
25. Roback, MG, Wathen, JE, Bajaj, L, Bothner, JP. Adverse events associated with procedural sedation and analgesia in a pediatric emergency department: A comparison of common parenteral drugs. Acad Emerg Med. 2005;12:508–513.
26. Administration of propofol. Clin Privil White Pap. 2010:1–20.
27. Moderate sedation. Clin Privil White Pap. 2010:1–20.
28. Pitetti, R, et al. Effect on hospital-wide sedation practices after implementation of the 2001 JCAHO procedural sedation and analgesia guidelines. Arch Pediatr Adolesc Med. 2006;160:211–216.
29. Sedation in the emergency department. Ann Emerg Med. 2011;57:469.
30. Ratnapalan, S, Schneeweiss, S. Guidelines to practice: The process of planning and implementing a pediatric sedation program. Pediatr Emerg Care. 2007;23:262–266.
31. Practice guidelines for sedation and analgesia by non-anesthesiologists. Anesthesiology. 2002;96:1004–1017.
32. Poe, SS, et al. Ensuring safety of patients receiving sedation for procedures: Evaluation of clinical practice guidelines. Jt Comm J Qual Improv. 2001;27:28–41.
33. Epstein, BS. The American Society of Anesthesiologist’s efforts in developing guidelines for sedation and analgesia for nonanesthesiologists: The 40th Rovenstine Lecture. Anesthesiology. 2003;98:1261–1268.
34. Green, SM, Mason, KP. Reformulation of the sedation continuum. JAMA. 2010;303:876–877.
35. Misra, S, Mahajan, PV, Chen, X, Kannikeswaran, N. Safety of procedural sedation and analgesia in children less than 2 years of age in a pediatric emergency department. Int J Emerg Med. 2008;1:173–177.
36. Bhatt, M, et al. Consensus-based recommendations for standardizing terminology and reporting adverse events for emergency department procedural sedation and analgesia in children. Ann Emerg Med. 2009;53:426–435.
37. Fitz-Henry, J. The ASA classification and peri-operative risk. Ann R Coll Surg Engl. 2011;93:185–187.
38. Schmitz, A, et al. Fasting times and gastric contents volume in children undergoing deep propofol sedation—an assessment using magnetic resonance imaging. Paediatr Anaesth. 2011;21:685–690.
39. Bhatt, M, Currie, GR, Auld, MC, Johnson, DW. Current practice and tolerance for risk in performing procedural sedation and analgesia on children who have not met fasting guidelines: A Canadian survey using a stated preference discrete choice experiment. Acad Emerg Med. 2010;17:1207–1215.
40. Molina, JA, Lobo, CA, Goh, HK, Seow, E, Heng, BH. Review of studies and guidelines on fasting and procedural sedation at the emergency department. Int J Evid Based Healthc. 2010;8:75–78.
41. Thorpe, RJ, Benger, J. Pre-procedural fasting in emergency sedation. Emerg Med J. 2010;27:254–261.
42. Taylor, D. Pre-procedural fasting for sedation: Do we need to do it? Emerg Med J. 2010;27:253.
43. McGlone, R, Carley, S. Towards evidence based emergency medicine: Best BETs from the Manchester Royal Infirmary. BET 3: Does the time of fasting affect complication rates during ketamine sedation? Emerg Med J. 2008;25:367–369.
44. Paris, PM, Yealy, DM. A procedural sedation and analgesia fasting consensus advisory: One small step for emergency medicine, one giant challenge remaining. Ann Emerg Med. 2007;49:465–467.
45. Green, SM, Roback, MG, Miner, JR, Burton, JH, Krauss, B. Fasting and emergency department procedural sedation and analgesia: A consensus-based clinical practice advisory. Ann Emerg Med. 2007;49:454–461.
46. Roback, MG, Bajaj, L, Wathen, JE, Bothner, J. Preprocedural fasting and adverse events in procedural sedation and analgesia in a pediatric emergency department: Are they related? Ann Emerg Med. 2004;44:454–459.
47. Treston, G. Prolonged pre-procedure fasting time is unnecessary when using titrated intravenous ketamine for paediatric procedural sedation. Emerg Med Australas. 2004;16:145–150.
48. Green, SM. Fasting is a consideration—not a necessity—for emergency department procedural sedation and analgesia. Ann Emerg Med. 2003;42:647–650.
49. Agrawal, D, Manzi, SF, Gupta, R, Krauss, B. Preprocedural fasting state and adverse events in children undergoing procedural sedation and analgesia in a pediatric emergency department. Ann Emerg Med. 2003;42:636–646.
50. Practice guidelines for preoperative fasting and the use of pharmacologic agents to reduce the risk of pulmonary aspiration: Application to healthy patients undergoing elective procedures: An updated report by the American Society of Anesthesiologists Committee on Standards and Practice Parameters. Anesthesiology. 2011;114:495–511.
51. Smally, AJ, Nowicki, TA, Simelton, BH. Procedural sedation and analgesia in the emergency department. Curr Opin Crit Care. 2011;17:317–322.
52. Couloures, KG, Beach, M, Cravero, JP, Monroe, KK, Hertzog, JH. Impact of provider specialty on pediatric procedural sedation complication rates. Pediatrics. 2011;127:e1154–e1160.
53. Green, SM, Roback, MG, Kennedy, RM, Krauss, B. Clinical practice guideline for emergency department ketamine dissociative sedation: 2011 update. Ann Emerg Med. 2011;57:449–461.
54. Metzner, J, Domino, KB. Risks of anesthesia or sedation outside the operating room: The role of the anesthesia care provider. Curr Opin Anaesthesiol. 2010;23:523–531.
55. Chambers, WA, Patey, RE. Sedation—is delegation appropriate? Anaesthesia. 2010;65:439–442.
56. Hemelaar, AE, Lemson, J. Patients under sedation should always be monitored by well-trained personnel and should be given supplemental oxygen. Pediatrics. 2008;122:1415–1416.
57. Deitch, K, Chudnofsky, CR, Dominici, P, Latta, D, Salamanca, Y. The utility of high-flow oxygen during emergency department procedural sedation and analgesia with propofol: A randomized, controlled trial. Ann Emerg Med. 2011;58:360–364.
58. Rozario, L, Sloper, D, Sheridan, MJ. Supplemental oxygen during moderate sedation and the occurrence of clinically significant desaturation during endoscopic procedures. Gastroenterol Nurs. 2008;31:281–285.
59. Keidan, I, et al. Supplemental oxygen compromises the use of pulse oximetry for detection of apnea and hypoventilation during sedation in simulated pediatric patients. Pediatrics. 2008;122:293–298.
60. Deitch, K, Chudnofsky, CR, Dominici, P. The utility of supplemental oxygen during emergency department procedural sedation with propofol: A randomized, controlled trial. Ann Emerg Med. 2008;52:1–8.
61. Green, SM, Krauss, B. Supplemental oxygen during propofol sedation: Yes or no? Ann Emerg Med. 2008;52:9–10.
62. Deitch, K, Chudnofsky, CR, Dominici, P. The utility of supplemental oxygen during emergency department procedural sedation and analgesia with midazolam and fentanyl: A randomized, controlled trial. Ann Emerg Med. 2007;49:1–8.
63. Eichhorn, V, Henzler, D, Murphy, MF. Standardizing care and monitoring for anesthesia or procedural sedation delivered outside the operating room. Curr Opin Anaesthesiol. 2010;23:494–499.
64. Lamas, A, Lopez-Herce, J. Monitoring sedation in the critically ill child. Anaesthesia. 2010;65:516–524.
65. Wildsmith, JA. Monitoring during sedation—the setting is all. Anaesthesia. 2008;63:1144–1145.
66. Fanning, RM. Monitoring during sedation given by non-anaesthetic doctors. Anaesthesia. 2008;63:370–374.
67. Cote, CJ, Wilson, S. Guidelines for monitoring and management of pediatric patients during and after sedation for diagnostic and therapeutic procedures: An update. Paediatr Anaesth. 2008;18:9–10.
68. Win, NN, et al. Haemodynamic changes and heart rate variability during midazolam-propofol co-induction. Anaesthesia. 2007;62:561–568.
69. Win, NN, Fukayama, H, Kohase, H, Umino, M. The different effects of intravenous propofol and midazolam sedation on hemodynamic and heart rate variability. Anesth Analg. 2005;101:97–102.
70. Grime, ID, Robb, N. Conscious sedation. The role of monitoring. SAAD Dig. 1996;13:7–16.
71. Sivilotti, ML, Murray, HE, Messenger, DW. Does end-tidal CO2 monitoring during emergency department procedural sedation and analgesia with propofol decrease the incidence of hypoxic events? Ann Emerg Med. 2010;56:702–703.
72. Sivilotti, ML, Messenger, DW, van Vlymen, J, Dungey, PE, Murray, HE. A comparative evaluation of capnometry versus pulse oximetry during procedural sedation and analgesia on room air. CJEM. 2010;12:397–404.
73. Green, SM, Pershad, J. Should capnographic monitoring be standard practice during emergency department procedural sedation and analgesia? Pro and con. Ann Emerg Med. 2010;55:265–267.
74. Deitch, K, Miner, J, Chudnofsky, CR, Dominici, P, Latta, D. Does end tidal CO2 monitoring during emergency department procedural sedation and analgesia with propofol decrease the incidence of hypoxic events? A randomized, controlled trial. Ann Emerg Med. 2010;55:258–264.
75. Krauss, B, Hess, DR. Capnography for procedural sedation and analgesia in the emergency department. Ann Emerg Med. 2007;50:172–181.
76. Green, SM. Research advances in procedural sedation and analgesia. Ann Emerg Med. 2007;49:31–36.
77. Anderson, JL, Junkins, E, Pribble, C, Guenther, E. Capnography and depth of sedation during propofol sedation in children. Ann Emerg Med. 2007;49:9–13.
78. Lightdale, JR, et al. Microstream capnography improves patient monitoring during moderate sedation: A randomized, controlled trial. Pediatrics. 2006;117:e1170–e1178.
79. Burton, JH, Harrah, JD, Germann, CA, Dillon, DC. Does end-tidal carbon dioxide monitoring detect respiratory events prior to current sedation monitoring practices? Acad Emerg Med. 2006;13:500–504.
80. Hatlestad, D. Capnography in sedation and pain management. Emerg Med Serv. 2005;34:65–69.
81. Waugh, JB, Epps, CA, Khodneva, YA. Capnography enhances surveillance of respiratory events during procedural sedation: A meta-analysis. J Clin Anesth. 2011;23:189–196.
82. Verschuren, F, Kabayadondo, MG, Thys, F. Expired CO measurement in intubated or spontaneously breathing patients from the emergency department. J Vis Exp. 2011;47:2508.
83. Adesanya, AO, Rosero, E, Wyrick, C, Wall, MH, Joshi, GP. Assessing the predictive value of the bispectral index vs patient state index on clinical assessment of sedation in postoperative cardiac surgery patients. J Crit Care. 2009;24:322–328.
84. Apan, A, Doganci, N, Ergan, A, Büyükkoçak, U. Bispectral index-guided intraoperative sedation with dexmedetomidine and midazolam infusion in outpatient cataract surgery. Minerva Anestesiol. 2009;75:239–244.
85. Mayer, J, Boldt, J, Schellhaass, A, Hiller, B, Suttner, SW. Bispectral index-guided general anesthesia in combination with thoracic epidural analgesia reduces recovery time in fast-track colon surgery. Anesth Analg. 2007;104:1145–1149.
86. Camci, E, Koltka, K, Celenk, Y, Tugrul, M, Pembeci, K. Bispectral index-guided desflurane and propofol anesthesia in ambulatory arthroscopy: Comparison of recovery and discharge profiles. J Anesth. 2006;20:149–152.
87. Weaver, CS, Hauter, WH, Duncan, CE, Brizendine, EJ, Cordell, WH. An assessment of the association of bispectral index with 2 clinical sedation scales for monitoring depth of procedural sedation. Am J Emerg Med. 2007;25:918–924.
88. Malviya, S, et al. Effect of age and sedative agent on the accuracy of bispectral index in detecting depth of sedation in children. Pediatrics. 2007;120:e461–e470.
89. Sadhasivam, S, Ganesh, A, Robison, A, Kaye, R, Watcha, MF. Validation of the bispectral index monitor for measuring the depth of sedation in children. Anesth Analg. 2006;102:383–388.
90. Agrawal, D, Feldman, HA, Krauss, B, Waltzman, ML. Bispectral index monitoring quantifies depth of sedation during emergency department procedural sedation and analgesia in children. Ann Emerg Med. 2004;43:247–255.
91. Shavit, I, et al. Comparison of adverse events during procedural sedation between specially trained pediatric residents and pediatric emergency physicians in Israel. Acad Emerg Med. 2008;15:617–622.
92. Scherrer, PD. Safe and sound: Pediatric procedural sedation and analgesia. Minn Med. 2011;94:43–47.
93. Sury, M, Bullock, I, Rabar, S, Demott, K. Sedation for diagnostic and therapeutic procedures in children and young people: Summary of NICE guidance. BMJ. 2010;341:c6819.
94. Steurer, LM, Luhmann, J. Adverse effects of pediatric emergency sedation after discharge. Pediatr Nurs. 2007;33:403–407.
95. Cote, CJ. Discharge criteria for children sedated by nonanesthesiologists: Is “safe” really safe enough? Anesthesiology. 2004;100:207–209.
96. Newman, DH, Azer, MM, Pitetti, RD, Singh, S. When is a patient safe for discharge after procedural sedation? The timing of adverse effect events in 1367 pediatric procedural sedations. Ann Emerg Med. 2003;42:627–635.
97. Lepere, AJ, Slack-Smith, LM. Average recovery time from a standardized intravenous sedation protocol and standardized discharge criteria in the general dental practice setting. Anesth Prog. 2002;49:77–81.
98. Deasy, C, Babl, FE. Intravenous vs intramuscular ketamine for pediatric procedural sedation by emergency medicine specialists: A review. Paediatr Anaesth. 2010;20:787–796.
99. Treston, G, et al. What is the nature of the emergence phenomenon when using intravenous or intramuscular ketamine for paediatric procedural sedation? Emerg Med Australas. 2009;21:315–322.
100. Ramaswamy, P, Babl, FE, Deasy, C, Sharwood, LN. Pediatric procedural sedation with ketamine: Time to discharge after intramuscular versus intravenous administration. Acad Emerg Med. 2009;16:101–107.
101. Zier, JL, Tarrago, R, Liu, M, Level of sedation with nitrous oxide for pediatric medical procedures. Anesth Analg. 2010;110:1399–1405.
102. Denman, WT, et al. The PediSedate device, a novel approach to pediatric sedation that provides distraction and inhaled nitrous oxide: Clinical evaluation in a large case series. Paediatr Anaesth. 2007;17:162–166.
103. Burnweit, C, et al. Nitrous oxide analgesia for minor pediatric surgical procedures: An effective alternative to conscious sedation? J Pediatr Surg. 2004;39:495–499.
104. Luhmann, JD, Kennedy, RM, Jaffe, DM, McAllister, JD. Continuous-flow delivery of nitrous oxide and oxygen: A safe and cost-effective technique for inhalation analgesia and sedation of pediatric patients. Pediatr Emerg Care. 1999;15:388–392.
105. Dallimore, D, Herd, DW, Short, T, Anderson, BJ. Dosing ketamine for pediatric procedural sedation in the emergency department. Pediatr Emerg Care. 2008;24:529–533.
106. Nejati, A, Moharari, RS, Ashraf, H, Labaf, A, Golshani, K. Ketamine/propofol versus midazolam/fentanyl for procedural sedation and analgesia in the emergency department: A randomized, prospective, double-blind trial. Acad Emerg Med. 2011;18:800–806.
107. McQueen, A, Wright, RO, Kido, MM, Kaye, E, Krauss, B. Procedural sedation and analgesia outcomes in children after discharge from the emergency department: Ketamine versus fentanyl/midazolam. Ann Emerg Med. 2009;54:191–197.
108. Gorchynski, J, Wang, S, Anderson, C, Montano, J. Conscious sedation and emergency department length of stay: A comparison of propofol, ketamine, and fentanyl/versed. Cal J Emerg Med. 2006;7:4–7.
109. Godambe, SA, Elliot, V, Matheny, D, Pershad, J. Comparison of propofol/fentanyl versus ketamine/midazolam for brief orthopedic procedural sedation in a pediatric emergency department. Pediatrics. 2003;112(1 Pt 1):116–123.
110. Lind, GH, et al. Oral transmucosal fentanyl citrate for analgesia and sedation in the emergency department. Ann Emerg Med. 1991;20:1117–1120.
111. Shibutani, K, Inchiosa, MA, Jr., Sawada, K, Bairamian, M. Pharmacokinetic mass of fentanyl for postoperative analgesia in lean and obese patients. Br J Anaesth. 2005;95:377–383.
112. [Should the dosage of fentanyl be based on the body weight when the patient is obese? A proposal of the appropriate “pharmacokinetic body mass”]. Masui. 2004;53(Suppl):S145–S152.
113. Shibutani, K, Inchiosa, MA, Jr., Sawada, K, Bairamian, M. Accuracy of pharmacokinetic models for predicting plasma fentanyl concentrations in lean and obese surgical patients: Derivation of dosing weight (“pharmacokinetic mass”). Anesthesiology. 2004;101:603–613.
114. Fahnenstich, H, Steffan, J, Kau, N, Bartmann, P. Fentanyl-induced chest wall rigidity and laryngospasm in preterm and term infants. Crit Care Med. 2000;28:836–839.
115. Epstein, RH, et al. The safety and efficacy of oral transmucosal fentanyl citrate for preoperative sedation in young children. Anesth Analg. 1996;83:1200–1205.
116. Schutzman, SA, Liebelt, E, Wisk, M, Burg, J. Comparison of oral transmucosal fentanyl citrate and intramuscular meperidine, promethazine, and chlorpromazine for conscious sedation of children undergoing laceration repair. Ann Emerg Med. 1996;28:385–390.
117. Klein, EJ, et al. A randomized, clinical trial of oral midazolam plus placebo versus oral midazolam plus oral transmucosal fentanyl for sedation during laceration repair. Pediatrics. 2002;109:894–897.
118. Stanley, TH, et al. Oral transmucosal fentanyl citrate (lollipop) premedication in human volunteers. Anesth Analg. 1989;69:21–27.
119. Fitch, RW, Kuhn, JE. Intraarticular lidocaine versus intravenous procedural sedation with narcotics and benzodiazepines for reduction of the dislocated shoulder: A systematic review. Acad Emerg Med. 2008;15:703–708.
120. Everitt, IJ, Barnett, P. Comparison of two benzodiazepines used for sedation of children undergoing suturing of a laceration in an emergency department. Pediatr Emerg Care. 2002;18:72–74.
121. Gempeler, F, Park, GR. Sedation with benzodiazepines. Middle East J Anesthesiol. 1995;13:117–145.
122. Bulach, R, Myles, PS, Russnak, M. Double-blind randomized controlled trial to determine extent of amnesia with midazolam given immediately before general anaesthesia. Br J Anaesth. 2005;94:300–305.
123. Lamont, T, Matthew, L, Cousins, D, Green, J. Avoiding midazolam overdose: Summary of a safety report from the National Patient Safety Agency. BMJ. 2009;339:b4459.
124. Sim, TB, Seet, CM. To study the effectiveness and safety of ketamine and midazolam procedural sedation in the incision and drainage of abscesses in the adult emergency department. Eur J Emerg Med. 2008;15:169–172.
125. Hohl, CM, Sadatsafavi, M, Nosyk, B, Anis, AH. Safety and clinical effectiveness of midazolam versus propofol for procedural sedation in the emergency department: A systematic review. Acad Emerg Med. 2008;15:1–8.
126. Austin, T, Vilke, GM, Nyheim, E, Kelly, D, Chan, TC. Safety and effectiveness of methohexital for procedural sedation in the emergency department. J Emerg Med. 2003;24:315–318.
127. Lerman, B, Yoshida, D, Levitt, MA. A prospective evaluation of the safety and efficacy of methohexital in the emergency department. Am J Emerg Med. 1996;14:351–354.
128. Vardy, JM, et al. Audit of the safety and effectiveness of ketamine for procedural sedation in the emergency department. Emerg Med J. 2008;25:579–582.
129. Newton, A, Fitton, L. Intravenous ketamine for adult procedural sedation in the emergency department: A prospective cohort study. Emerg Med J. 2008;25:498–501.
130. Chudnofsky, CR, et al. A combination of midazolam and ketamine for procedural sedation and analgesia in adult emergency department patients. Acad Emerg Med. 2000;7:228–235.
131. Slonim, AD, Ognibene, FP. Sedation for pediatric procedures, using ketamine and midazolam, in a primarily adult intensive care unit: A retrospective evaluation. Crit Care Med. 1998;26:1900–1904.
132. Green, SM, et al. Intramuscular ketamine for pediatric sedation in the emergency department: Safety profile in 1,022 cases. Ann Emerg Med. 1998;31:688–697.
133. Dolansky, G, Shah, A, Mosdossy, G, Rieder, M. What is the evidence for the safety and efficacy of using ketamine in children? Paediatr Child Health. 2008;13:307–308.
134. Bourgoin, A, et al. Safety of sedation with ketamine in severe head injury patients: Comparison with sufentanil. Crit Care Med. 2003;31:711–717.
135. Green, SM, et al. Intravenous ketamine for pediatric sedation in the emergency department: Safety profile with 156 cases. Acad Emerg Med. 1998;5:971–976.
136. Grace, RF. The effect of variable-dose diazepam on dreaming and emergence phenomena in 400 cases of ketamine-fentanyl anaesthesia. Anaesthesia. 2003;58:904–910.
137. Kudoh, A, Katagai, H, Takazawa, T. Anesthesia with ketamine, propofol, and fentanyl decreases the frequency of postoperative psychosis emergence and confusion in schizophrenic patients. J Clin Anesth. 2002;14:107–110.
138. Carley, S, Martin, B. Towards evidence based emergency medicine: Best BETs from the Manchester Royal Infirmary. Midazolam and emergence phenomena in children undergoing ketamine sedation. Emerg Med J. 2001;18:273–274.
139. Green, SM, Li, J. Ketamine in adults: What emergency physicians need to know about patient selection and emergence reactions. Acad Emerg Med. 2000;7:278–281.
140. Kumar, A, Bajaj, A, Sarkar, P, Grover, VK. The effect of music on ketamine induced emergence phenomena. Anaesthesia. 1992;47:438–439.
141. Andolfatto, G, Willman, E. A prospective case series of single-syringe ketamine-propofol (ketofol) for emergency department procedural sedation and analgesia in adults. Acad Emerg Med. 2011;18:237–245.
142. David, H, Shipp, J. A randomized controlled trial of ketamine/propofol versus propofol alone for emergency department procedural sedation. Ann Emerg Med. 2011;57:435–441.
143. Sener, S, Eken, C, Schultz, CH, Serinken, M, Ozsarac, M. Ketamine with and without midazolam for emergency department sedation in adults: A randomized controlled trial. Ann Emerg Med. 2011;57:109–114.
144. Shah, A, et al. A blinded, randomized controlled trial to evaluate ketamine/propofol versus ketamine alone for procedural sedation in children. Ann Emerg Med. 2011;57:425–433.
145. Green, SM, Rothrock, SG. Transient apnea with intramuscular ketamine. Am J Emerg Med. 1997;15:440–441.
146. Green, SM, Roback, MG, Krauss, B. Laryngospasm during emergency department ketamine sedation: A case-control study. Pediatr Emerg Care. 2010;26:798–802.
147. Cohen, VG, Krauss, B. Recurrent episodes of intractable laryngospasm during dissociative sedation with intramuscular ketamine. Pediatr Emerg Care. 2006;22:247–249.
148. Thorp, AW, Brown, L, Green, SM. Ketamine-associated vomiting: Is it dose-related? Pediatr Emerg Care. 2009;25:15–18.
149. Langston, WT, Wathen, JE, Roback, MG, Bajaj, L. Effect of ondansetron on the incidence of vomiting associated with ketamine sedation in children: A double-blind, randomized, placebo-controlled trial. Ann Emerg Med. 2008;52:30–34.
150. Green, SM, et al. Predictors of emesis and recovery agitation with emergency department ketamine sedation: An individual-patient data meta-analysis of 8,282 children. Ann Emerg Med. 2009;54:171–180.
151. McKee, MR, Sharieff, GQ, Kanegaye, JT, Stebel, M. Oral analgesia before pediatric ketamine sedation is not associated with an increased risk of emesis and other adverse events. J Emerg Med. 2008;35:23–28.
152. Green, SM, Roback, MG, Krauss, B. Anticholinergics and ketamine sedation in children: A secondary analysis of atropine versus glycopyrrolate. Acad Emerg Med. 2010;17:157–162.
153. Adjunctive atropine is unnecessary during ketamine sedation in children. Emerg Med J. 2009;26:230.
154. Carley, S, Body, R. Towards evidence based emergency medicine: Best BETs from the Manchester Royal Infirmary. BET 2: Is atropine needed as an adjunct in paediatric ketamine administration? Emerg Med J. 2008;25:366–367.
155. Brown, L, et al. Adjunctive atropine is unnecessary during ketamine sedation in children. Acad Emerg Med. 2008;15:314–318.
156. Heinz, P, Geelhoed, GC, Wee, C, Pascoe, EM. Is atropine needed with ketamine sedation? A prospective, randomised, double blind study. Emerg Med J. 2006;23:206–209.
157. Levins, T. Etomidate in procedural sedation. Air Med J. 2011;30:45–48.
158. Cicero, M, Graneto, J. Etomidate for procedural sedation in the elderly: A retrospective comparison between age groups. Am J Emerg Med. 2011;29:1111–1116.
159. Lee-Jayaram, JJ, et al. Ketamine/midazolam versus etomidate/fentanyl: Procedural sedation for pediatric orthopedic reductions. Pediatr Emerg Care. 2010;26:408–412.
160. Kaufman, D. Etomidate versus ketamine for sedation in acutely ill patients. Lancet. 2009;374:1240–1241.
161. Mongardon, N, Singer, M. Etomidate versus ketamine for sedation in acutely ill patients. Lancet. 2009;374:1240.
162. Bordo, D, Chan, SB, Shin, P. Patient satisfaction and return to daily activities using etomidate procedural sedation for orthopedic injuries. West J Emerg Med. 2008;9:86–90.
163. Di Liddo, L, et al. Etomidate versus midazolam for procedural sedation in pediatric outpatients: A randomized controlled trial. Ann Emerg Med. 2006;48:433–440.
164. Miner, JR, Danahy, M, Moch, A, Biros, M. Randomized clinical trial of etomidate versus propofol for procedural sedation in the emergency department. Ann Emerg Med. 2007;49:15–22.
165. Hunt, GS, Spencer, MT, Hays, DP. Etomidate and midazolam for procedural sedation: Prospective, randomized trial. Am J Emerg Med. 2005;23:299–303.
166. Kosarek, L, et al. Increase in venous complications associated with etomidate use during a propofol shortage: An example of clinically important adverse effects related to drug substitution. Ochsner J. 2011;11:143–146.
167. Perrone, J, Band, RA, Mathew, R. Agitation complicating procedural sedation with etomidate. Am J Emerg Med. 2006;24:511–512.
168. Van Keulen, SG, Burton, JH. Myoclonus associated with etomidate for ED procedural sedation and analgesia. Am J Emerg Med. 2003;21:556–558.
169. Vinson, DR, Bradbury, DR. Etomidate for procedural sedation in emergency medicine. Ann Emerg Med. 2002;39:592–598.
170. Yealy, DM. Safe and effective … maybe: Etomidate in procedural sedation/analgesia. Acad Emerg Med. 2001;8:68–69.
171. Albert, SG, Ariyan, S, Rather, A. The effect of etomidate on adrenal function in critical illness: A systematic review. Intensive Care Med. 2011;37:901–910.
172. Majesko, A, Darby, JM. Etomidate and adrenal insufficiency: The controversy continues. Crit Care. 2010;14:338.
173. Hildreth, AN, et al. Adrenal suppression following a single dose of etomidate for rapid sequence induction: A prospective randomized study. J Trauma. 2008;65:573–579.
174. Mullins, ME, Theodoro, DL. Lack of evidence for adrenal insufficiency after single-dose etomidate. Arch Surg. 2008;143:808–809.
175. Vinclair, M, et al. Duration of adrenal inhibition following a single dose of etomidate in critically ill patients. Intensive Care Med. 2008;34:714–719.
176. Kamp, R, Kress, JP. Etomidate, sepsis, and adrenal function: Not as bad as we thought? Crit Care. 2007;11:145.
177. Lundy, JB, Slane, ML, Frizzi, JD. Acute adrenal insufficiency after a single dose of etomidate. J Intensive Care Med. 2007;22:111–117.
178. Zed, PJ, Mabasa, VH, Slavik, RS, Abu-Laban, RB. Etomidate for rapid sequence intubation in the emergency department: Is adrenal suppression a concern? CJEM. 2006;8:347–350.
179. McGrane, O, Hopkins, G, Nielson, A, Kang, C. Procedural sedation with propofol: a retrospective review of the experiences of an emergency medicine residency program 2005 to 2010. Am J Emerg Med. 2011.
180. Mallory, MD, et al. Emergency physician-administered propofol sedation: A report on 25,433 sedations from the pediatric sedation research consortium. Ann Emerg Med. 2011;57:462–468.
181. Rahman, NH, Hashim, A. The use of propofol for procedural sedation and analgesia in the emergency department: A comparison with midazolam. Emerg Med J. 2011;28:861–865.
182. Lamond, DW. Review article: Safety profile of propofol for paediatric procedural sedation in the emergency department. Emerg Med Australas. 2010;22:265–286.
183. Miner, JR, Gray, RO, Bahr, J, Patel, R, McGill, JW. Randomized clinical trial of propofol versus ketamine for procedural sedation in the emergency department. Acad Emerg Med. 2010;17:604–611.
184. Miner, JR, Gray, RO, Stephens, D, Biros, MH. Randomized clinical trial of propofol with and without alfentanil for deep procedural sedation in the emergency department. Acad Emerg Med. 2009;16:825–834.
185. Hohl, CM, Nosyk, B, Sadatsafavi, M, Anis, AH. A cost-effectiveness analysis of propofol versus midazolam for procedural sedation in the emergency department. Acad Emerg Med. 2008;15:32–39.
186. Weaver, CS, et al. Emergency department procedural sedation with propofol: Is it safe? J Emerg Med. 2007;33:355–361.
187. Dunn, T, Mossop, D, Newton, A, Gammon, A. Propofol for procedural sedation in the emergency department. Emerg Med J. 2007;24:459–461.
188. Miner, JR, Burton, JH. Clinical practice advisory: Emergency department procedural sedation with propofol. Ann Emerg Med. 2007;50:182–187.
189. Symington, L, Thakore, S. A review of the use of propofol for procedural sedation in the emergency department. Emerg Med J. 2006;23:89–93.
190. Pershad, J, Godambe, SA. Propofol for procedural sedation in the pediatric emergency department. J Emerg Med. 2004;27:11–14.
191. Bassett, KE, et al. Propofol for procedural sedation in children in the emergency department. Ann Emerg Med. 2003;42:773–782.
192. Jackson, R, Carley, S. Towards evidence based emergency medicine: Best BETs from the Manchester Royal Infirmary. Use of propofol for sedation in the emergency department. Emerg Med J. 2001;18:378–379.
193. Green, SM, Andolfatto, G, Krauss, B. Ketofol for procedural sedation? Pro and con. Ann Emerg Med. 2011;57:444–448.
194. Andolfatto, G, Willman, E. A prospective case series of pediatric procedural sedation and analgesia in the emergency department using single-syringe ketamine-propofol combination (ketofol). Acad Emerg Med. 2010;17:194–201.
195. Arora, S. Combining ketamine and propofol (“ketofol”) for emergency department procedural sedation and analgesia: A review. West J Emerg Med. 2008;9:20–23.
196. Willman, EV, Andolfatto, G. A prospective evaluation of “ketofol” (ketamine/propofol combination) for procedural sedation and analgesia in the emergency department. Ann Emerg Med. 2007;49:23–30.
197. Babl, FE, Belousoff, J, Deasy, C, Hopper, S, Theophilos, T. Paediatric procedural sedation based on nitrous oxide and ketamine: Sedation registry data from Australia. Emerg Med J. 2010;27:607–612.
198. George, MS, Sury, MR. Reversal of paradoxical excitement to diazepam sedation. Paediatr Anaesth. 2008;18:546–547.
199. Shannon, M, et al. Safety and efficacy of flumazenil in the reversal of benzodiazepine-induced conscious sedation. The Flumazenil Pediatric Study Group. J Pediatr. 1997;131:582–586.
200. Singh, R, Kumar, N, Vajifdar, H. Midazolam as a sole sedative for computed tomography imaging in pediatric patients. Paediatr Anaesth. 2009;19:899–904.
201. Tschirch, FT, et al. Multicenter trial: comparison of two different formulations and application systems of low-dose nasal midazolam for routine magnetic resonance imaging of claustrophobic patients. J Magn Reson Imaging. 2008;28:866–872.
202. Moro-Sutherland, DM, Algren, JT, Louis, PT, Kozinetz, CA, Shook, JE. Comparison of intravenous midazolam with pentobarbital for sedation for head computed tomography imaging. Acad Emerg Med. 2000;7:1370–1375.
203. Harcke, HT, Grissom, LE, Meister, MA. Sedation in pediatric imaging using intranasal midazolam. Pediatr Radiol. 1995;25:341–343.
204. Cote, CJ, Ferrari, LR, Liu, LM. Rectal methohexital for sedation of children during imaging procedures. AJR Am J Roentgenol. 1994;162:465–466.
205. Mallory, MD, Baxter, AL, Kost, SI. Propofol vs pentobarbital for sedation of children undergoing magnetic resonance imaging: Results from the Pediatric Sedation Research Consortium. Paediatr Anaesth. 2009;19:601–611.
206. Pershad, J, Wan, J, Anghelescu, DL. Comparison of propofol with pentobarbital/midazolam/fentanyl sedation for magnetic resonance imaging of the brain in children. Pediatrics. 2007;120:e629–e636.
207. Mason, KP, et al. Infant sedation for MR imaging and CT: Oral versus intravenous pentobarbital. Radiology. 2004;233:723–728.
208. Kienstra, AJ, et al. Etomidate versus pentobarbital for sedation of children for head and neck CT imaging. Pediatr Emerg Care. 2004;20:499–506.
209. Mason, KP, et al. Superiority of pentobarbital versus chloral hydrate for sedation in infants during imaging. Radiology. 2004;230:537–542.
210. Rooks, VJ, et al. Comparison of oral pentobarbital sodium (Nembutal) and oral chloral hydrate for sedation of infants during radiologic imaging: preliminary results. AJR Am J Roentgenol. 2003;180:1125–1128.
211. Mason, KP, et al. Sedatives used in pediatric imaging: Comparison of IV pentobarbital with IV pentobarbital with midazolam added. AJR Am J Roentgenol. 2001;177:427–430.
212. Eich, C, et al. Low-dose S-ketamine added to propofol anesthesia for magnetic resonance imaging in children is safe and ensures faster recovery—a prospective evaluation. Paediatr Anaesth. 2011;21:176–178.
213. Luscri, N, Tobias, JD. Monitored anesthesia care with a combination of ketamine and dexmedetomidine during magnetic resonance imaging in three children with trisomy 21 and obstructive sleep apnea. Paediatr Anaesth. 2006;16:782–786.
214. Tomatir, E, Atalay, H, Gurses, E, Erbay, H, Bozkurt, P. Effects of low dose ketamine before induction on propofol anesthesia for pediatric magnetic resonance imaging. Paediatr Anaesth. 2004;14:845–850.
215. Machata, AM, Kabon, B, Willschke, H, Prayer, D, Marhofer, P. Upper airway size and configuration during propofol-based sedation for magnetic resonance imaging: An analysis of 138 infants and children. Paediatr Anaesth. 2010;20:994–1000.
216. Heard, C, et al. A comparison of dexmedetomidine-midazolam with propofol for maintenance of anesthesia in children undergoing magnetic resonance imaging. Anesth Analg. 2008;107:1832–1839.
217. Machata, AM, Willschke, H, Kabon, B, Kettner, SC, Marhofer, P. Propofol-based sedation regimen for infants and children undergoing ambulatory magnetic resonance imaging. Br J Anaesth. 2008;101:239–243.
218. Koroglu, A, et al. A comparison of the sedative, hemodynamic, and respiratory effects of dexmedetomidine and propofol in children undergoing magnetic resonance imaging. Anesth Analg. 2006;103:63–67.
219. Hasan, RA, Shayevitz, JR, Patel, V. Deep sedation with propofol for children undergoing ambulatory magnetic resonance imaging of the brain: Experience from a pediatric intensive care unit. Pediatr Crit Care Med. 2003;4:454–458.
220. Allegaert, K, Naulaers, G. Procedural sedation of neonates with chloral hydrate: A sedation procedure does not end at the end of the acquisition of the images. Paediatr Anaesth. 2008;18:1270–1271.
221. D’Agostino, J, Terndrup, TE. Chloral hydrate versus midazolam for sedation of children for neuroimaging: A randomized clinical trial. Pediatr Emerg Care. 2000;16:1–4.
222. Parks, BR, Snodgrass, SR. Reappraisal of lytic cocktail/Demerol, Phenergan, and Thorazine (DPT) for the sedation of children. Pediatrics. 1996;97:779–780.