Chapter 488 Principles of Treatment
The best chance for cure of cancer is during the initial course of treatment; the cure rates for patients with recurrent disease are much lower than those for patients with primary disease. All patients with cancer should be referred to an appropriate specialized center as soon as possible when the diagnosis of cancer is suspected. All such centers in North America are identified on the Children’s Oncology Group website (www.childrensoncologygroup.org) and on the National Cancer Institute cancer trials website (www.clinicaltrials.gov). The remarkable increases in cure rates for childhood malignancies since the 1980s would not have occurred without the collective participation of patients and their physicians in clinical research programs at these centers. In the USA, the National Cancer Institute’s Clinical Trials Cooperative Groups Program has been associated with a >80% reduction in the incidence of mortality due to cancer among children <15 yr of age despite an overall increase in cancer incidence during this interval (Fig. 488-1). This remarkable achievement represents the effects of an effective multimodal, multi-institutional, multidisciplinary collaboration.
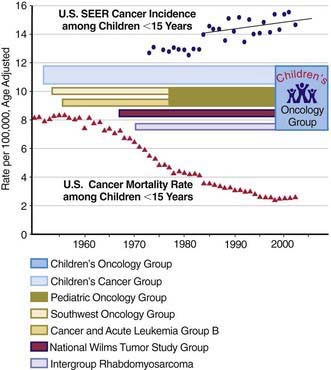
(Incidence and mortality rate data from Ries LAG, Eisner MP, Kosary CL, et al, editors: SEER Cancer Statistics Review, 1975-2002, Bethesda, MD, National Cancer Institute; http://seer.cancer.gov/csr/1975_2002/, based on November 2004 SEER (Surveillance Epidemiology and End Results) data submission, posted to the SEER website 2005. The mortality rate data are national rates, and the incidence data are derived from the SEER program, representing about 15% of the USA.)
The most current information on treatment of all types of childhood cancer is available in the PDQ (Physician Data Query) on the National Cancer Institute website (www.cancer.gov/cancertopics/pdq/pediatrictreatment).
A Multimodal, Multidisciplinary Approach
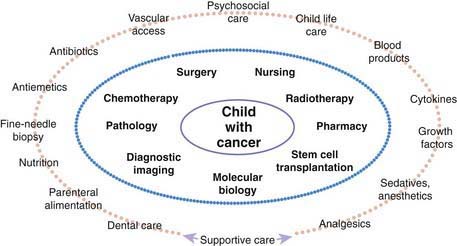
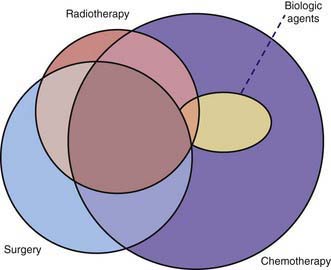
Many pediatric subspecialties are involved in the evaluation, treatment, and management of children with cancer, including provision of primary therapy and supportive care services (Fig. 488-2). More than 2 of the primary modalities are often used together, with chemotherapy being the most widely used, followed, in order of use, by surgery, radiation therapy, and biologic agent therapy (Fig. 488-3).
The leukemias that occur in childhood usually are managed with chemotherapy alone, with a small proportion of patients receiving cranial or craniospinal radiation therapy to prevent or treat overt central nervous system (CNS) leukemia. Children with non-Hodgkin lymphoma also are treated with chemotherapy alone, with the exception of radiation therapy for CNS involvement. Localized therapy with surgery or irradiation, or both, is an important component of treatment of most solid tumors, including Hodgkin lymphoma, but systemic multiagent chemotherapy usually is necessary because tumor dissemination generally is present even if it is undetectable. Chemotherapy alone usually is not adequate to eradicate gross residual tumors. Hence, it is not unusual for children with malignant tumors to require treatment with all three modalities (see Fig. 488-3). Unfortunately, most treatments that are effective in children with cancer have a narrow therapeutic index (a low ratio of efficacy to toxicity). The acute and chronic adverse effects of these treatments can be minimized but not entirely avoided.
Over the past 15 yr, biologic agent therapy has become an important modality in a few childhood cancers (see Fig. 488-3). This type of treatment generally refers to immunotherapy, biologic response modifiers, or endogenously occurring molecules that have therapeutic effects in supraphysiologic doses. Examples are retinoic acid therapy in acute promyelocytic leukemia, monoclonal antibody therapy for neuroblastoma and certain non-Hodgkin lymphomas, imatinib mesylate for chronic myelogenous and Philadelphia chromosome–positive leukemias, and radioactive metaiodobenzylguanidine therapy for neuroblastoma.
Development of selective, highly effective therapy for cancer in both children and adults had been hindered by a lack of understanding of the molecular mechanisms that underlie malignant transformation. De novo or acquired resistance to chemotherapy and radiation therapy remains an obstacle to cure. Ongoing discoveries of molecular and cellular mechanisms that explain the cancer process have led to increasingly specific antineoplastic therapies, generally referred to as molecularly targeted therapies. Their most prominent feature is a relative lack of normal tissue toxicity, such that the additional therapeutic benefit occurs with minimum additional toxicity. Many of the new biologic agent therapies, such as imatinib and rituximab, fall into this category (Table 488-1). Complementary and alternative remedies are increasingly being provided by parents to their children with cancer, with or without knowledge of the medical professionals entrusted with the child’s care (Chapter 59). Many of these have not been evaluated by rigorous testing and most are ineffective; some are toxic or interfere with the metabolism of other drugs. Although dramatic advances in the discipline have reduced the empiricism of therapy for cancer, much remains to be discovered.
Table 488-1 PROTEIN TYROSINE KINASE INHIBITORS AND MONOCLONAL ANTIBODIES
| AGENT | KINASE | MALIGNANCY |
|---|---|---|
| Imatinib | BCR-ABL | CML Philadelphia chromosome positive ALL |
| PDGFRα | Hypereosinophilic syndrome Systemic mastocytosis |
|
| PDGFRβ | CMML | |
| cKIT | Systemic mastocytosis Gastrointestinal stromal tumor |
|
| Dasatinib | BCR-ABL | CML Philadelphia chromosome-positive ALL |
| Nilotinib | BCR-ABL | CML Philadelphia chromosome-positive ALL |
| Gefitinib | EGFR | Non–small cell lung cancer |
| Erlotinib | EGFR | Non–small cell lung cancer |
| Trastuzumab | ERBB2/HER-2 | Breast cancer |
| Cetuximab | EGFR | Non–small cell lung cancer Squamous cell cancer of head/neck |
| Bevacizumab | VEGFR-1, -2 | Non–small cell lung cancer Breast cancer Renal cell carcinoma Colorectal cancer Glioblastoma |
ALL, acute lymphoblastic leukemia; CML, chronic myelogenous leukemia; CMML, chronic mono myelogenous leukemia.
Treatment
Chemotherapy
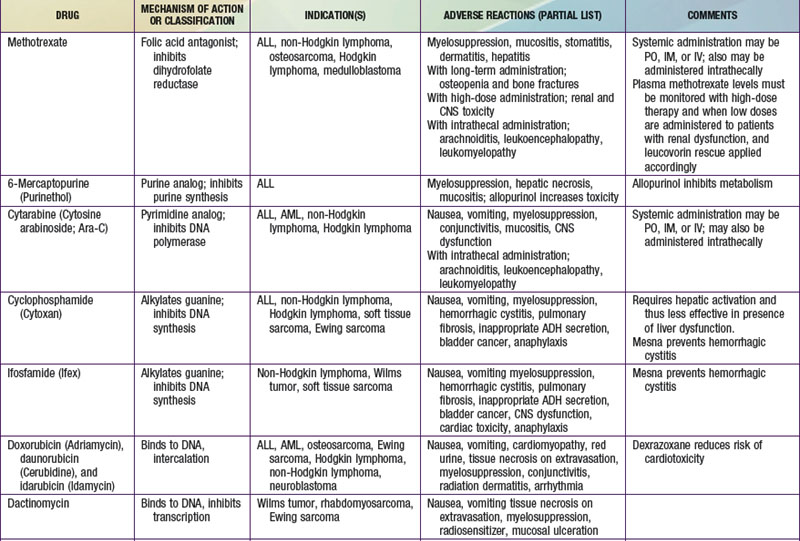
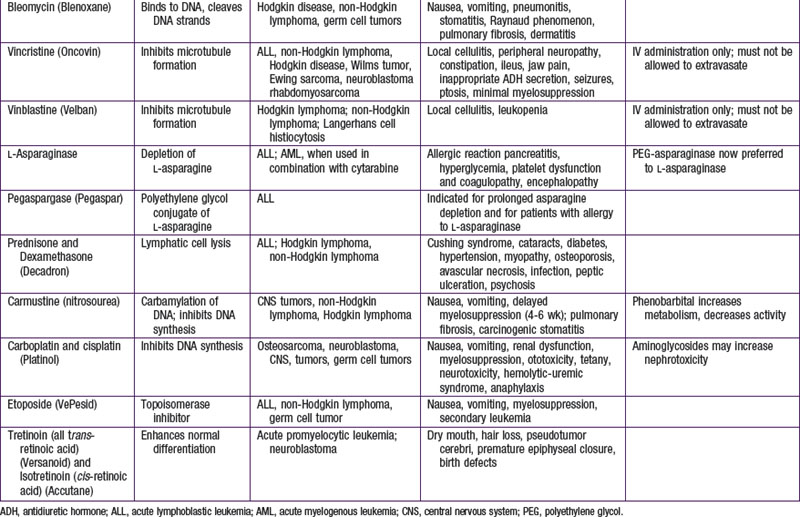
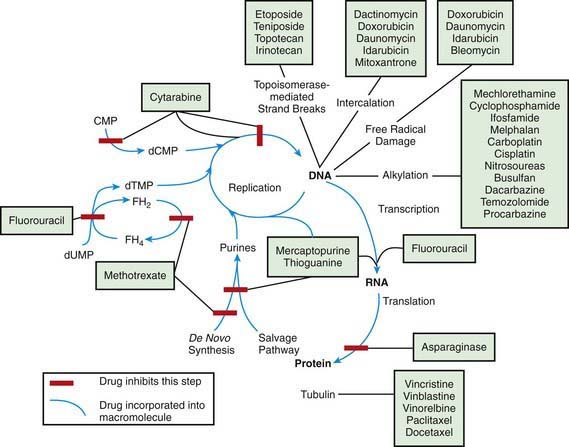
The most widely used modality in pediatric cancer therapy is chemotherapy (see Fig. 488-3). Therapy nearly always involves combinations of drugs, such as VAC (vincristine, dactinomycin [Actinomycin D], and cyclophosphamide) and CHOP (cyclophosphamide, doxorubicin [Adriamycin], vincristine [Oncovin], and prednisone). Historically, sequential single-drug therapy rarely resulted in complete responses, and partial responses usually were infrequent and transient and grew progressively shorter in duration with each drug used. Combination chemotherapy became the standard when combinations of drugs with different mechanisms of action and nonoverlapping toxicities (e.g., POMP [mercaptopurine, vincristine (Oncovin), methotrexate, and prednisone], VAMP [vincristine, doxorubicin (Adriamycin), methotrexate, and prednisone], and MOPP [nitrogen mustard, vincristine (Oncovin), prednisone, and procarbazine] were first demonstrated to be effective in childhood leukemia. Most of the cytotoxic drugs for childhood cancer are selected from several classes of agents, including alkylating agents, antimetabolites, antibiotics, hormones, plant alkaloids, and topoisomerase inhibitors (Table 488-2). The increased metabolic and cell cycle activity of malignant cells makes them more susceptible to the cytotoxic effects of these types of agents (Fig. 488-4).
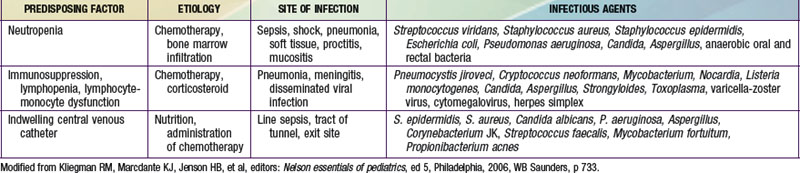
Because most antineoplastic agents are cell cycle dependent, their adverse effects usually are related to the proliferation kinetics of individual cell populations. Most susceptible are tissues or organs with high rates of cell turnover: bone marrow, oral and intestinal mucosa, epidermis, liver, and spermatogonia. The most common acute adverse effects are myelosuppression (with neutropenia and thrombocytopenia being the most problematic), immunosuppression, nausea and vomiting, hepatic dysfunction, upper and lower gastrointestinal mucositis, dermatitis, and alopecia. Fortunately, the tissues affected also recover relatively quickly, so that the acute adverse effects are nearly always reversible. Life-threatening effects of many chemotherapy agents include severe neutropenia with infection, fungemia or fungal pneumonia due to immunosuppression, and septicemia, not infrequently linked to indwelling intravascular devices (Table 488-3; Chapters 171 and 172). Cardiomyopathy caused by anthracyclines (e.g., doxorubicin and daunorubicin) and renal failure from platinum-containing agents also may be life threatening or disabling.
Surgery
With the exception of brainstem tumors and retinoblastoma, all solid tumors in children require a tissue diagnosis; therefore, biopsy of the suspected neoplasm is paramount. Staging with sentinel node biopsies has become the standard of care for several pediatric malignancies. Surgical expertise is essential for implantation of vascular access devices and removal and replacement of such devices when infection or thrombosis supervenes (Chapter 172).
Acute Toxic Effects and Supportive Care
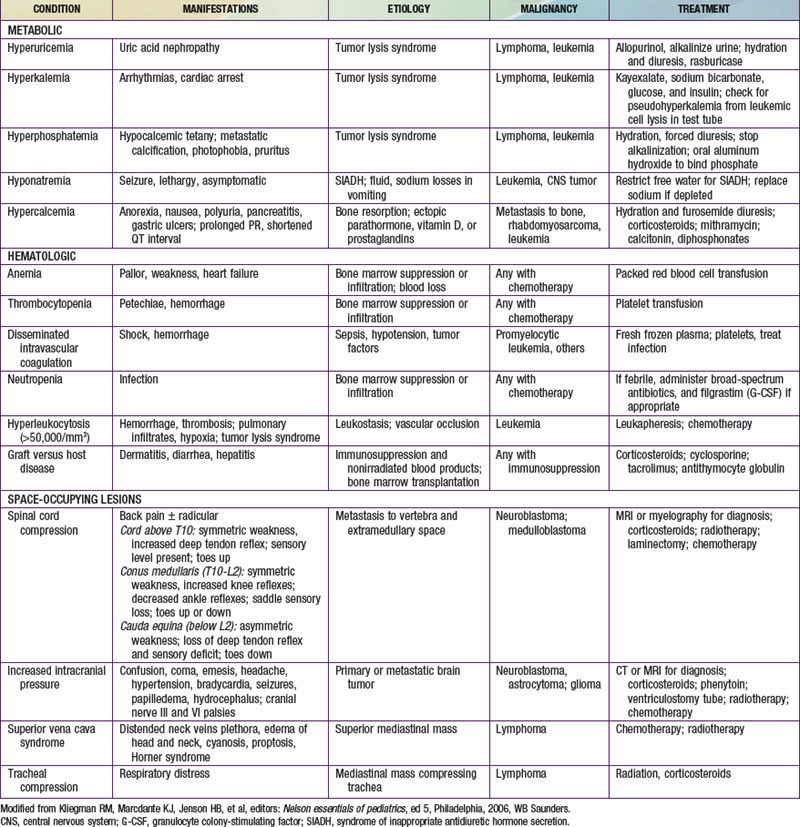
Adverse treatment effects that occur early in therapy can result in oncologic emergencies. These include metabolic disorders, bone marrow suppression, and compression by tumors on vital structures (Table 488-4). In tumor lysis syndrome (TLS), uric acid, phosphates, and potassium are released in the circulation in large quantities from death of tumor cells. Hyperuricemia can lead to impairment of renal function, which further exacerbates the metabolic abnormalities. TLS can occur before therapy in patients with large tumor burden (e.g., Burkitt lymphoma, lymphoblastic lymphoma, and high white blood cell count leukemia) but is usually seen within 12-48 hours of initiating chemotherapy. TLS is infrequently reported in other tumors (Hodgkin lymphoma, neuroblastoma, hepatoblastoma). Before therapy is initiated, the serum levels of uric acid, electrolytes, calcium, phosphorus, and creatinine should be measured and adequate hydration ensured. Allopurinol (a xanthine oxidase inhibitor) should be started to prevent further accumulation of uric acid. In patients with established TLS with high uric acid levels or those at high risk for TLS, rasburicase (an enzyme that degrades uric acid) should be given instead of allopurinol. Symptomatic hyperkalemia and hyperphosphatemia with subsequent hypocalcemia can develop in the setting of inadequate renal function.
Virtually all chemotherapy regimens can produce myelosuppression, as can malignancies that invade and replace bone marrow. Anemia can be corrected by transfusions of packed erythrocytes, and thrombocytopenia can be corrected by platelet infusions. Patients receiving immunosuppressive therapy should receive irradiated blood products to prevent graft versus host disease and leukoreduced blood products to prevent transfusion-associated reactions and infections. Neutropenia (neutrophil counts <500/mm3) poses a risk of life-threatening infection. Febrile neutroopenic patients should be hospitalized and treated with empiric broad-spectrum intravenous antimicrobial therapy pending the results of appropriate cultures of blood, urine, or any obvious sites of infection (Chapter 171). Treatment is continued until fever resolves and the neutrophil count rises. If fever persists for >3-5 days while the patient is receiving broad-spectrum antibiotics, the possibility of fungal infection must be considered. Fungal infections caused by Candida and Aspergillus are common in immunosuppressed patients. Opportunistic organisms such as Pneumocystis jiroveci can produce fatal pneumonia. Prophylactic treatment with trimethoprim-sulfamethoxazole is given when severe or prolonged immunosuppression is anticipated.
Adequate pain management is critical. The World Health Organization (WHO) guidelines are particularly useful in the management of pain associated with cancer and cancer therapy (Chapter 71).
Late Adverse Effects
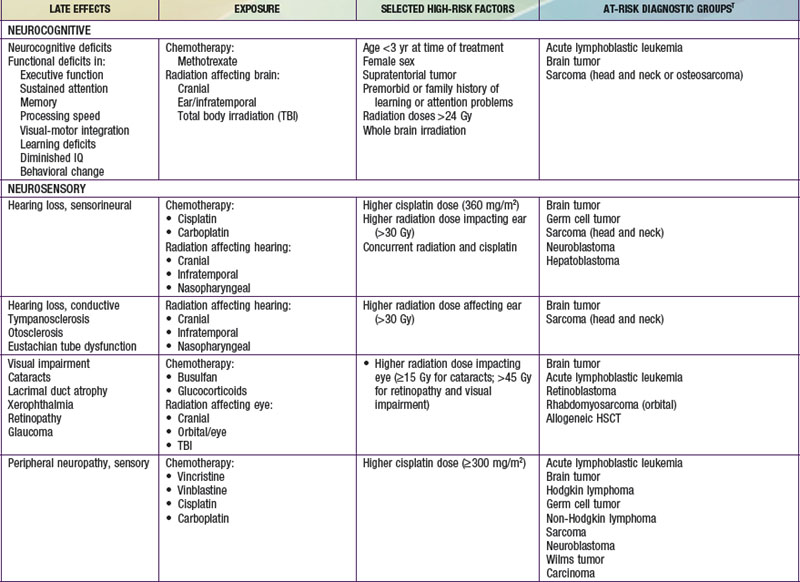
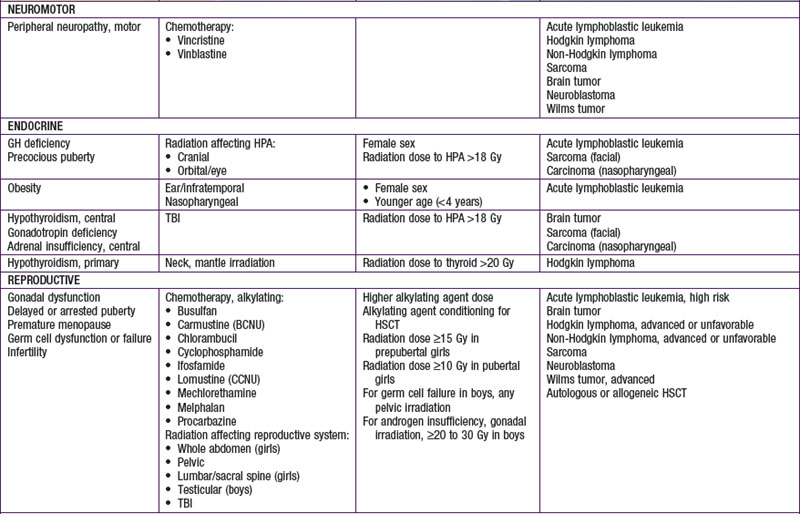
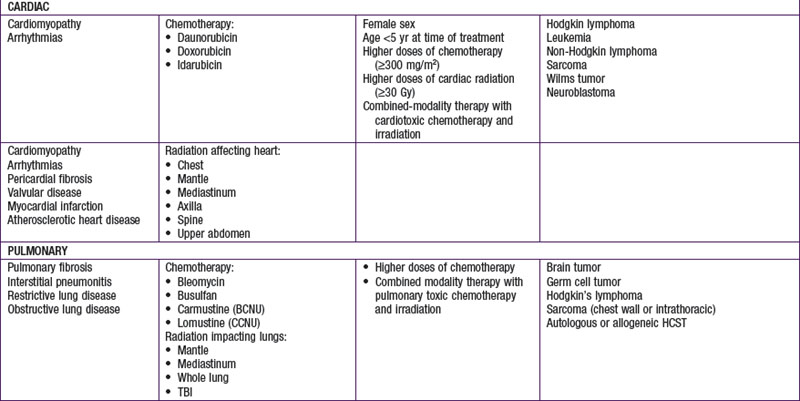
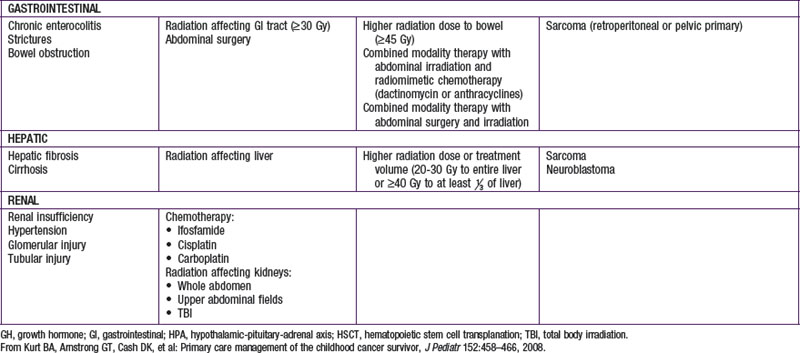
Injury to tissues with low repair potential often results in long-lasting or permanent deficit. These effects can be either from the tumor or its treatment. For example, a brain or spinal tumor can leave the child with a permanent paresis or autonomic dysfunction, anthracycline-induced cardiomyopathy usually produces refractory cardiac dysfunction, and the leukoencephalopathy caused by intrathecal methotrexate and by CNS radiation therapy often is only partially reversible. The potential types of late adverse effects depend on the child’s age at the time of treatment, the location(s) of the cancer, and the therapy administered. A good resource for the pediatrician, patient, and family who have to anticipate the possibilities is available at www.survivorshipguidelines.org.
Late adverse effects of therapy can cause substantial morbidity (Table 488-5). Successful surgical resection can result in loss of important functional structures. Irradiation can produce irreversible organ damage, with symptoms and functional limitations depending on the organ involved and the severity of the damage. Many problems related to radiation therapy do not become obvious until the patient is fully grown, such as asymmetry between irradiated and nonirradiated areas or extremities. Irradiation of fields that include endocrine organs can cause hypothyroidism, pituitary dysfunction, or infertility. In sufficient doses, cranial irradiation can produce neurologic dysfunction and spinal irradiation can produce growth retardation.
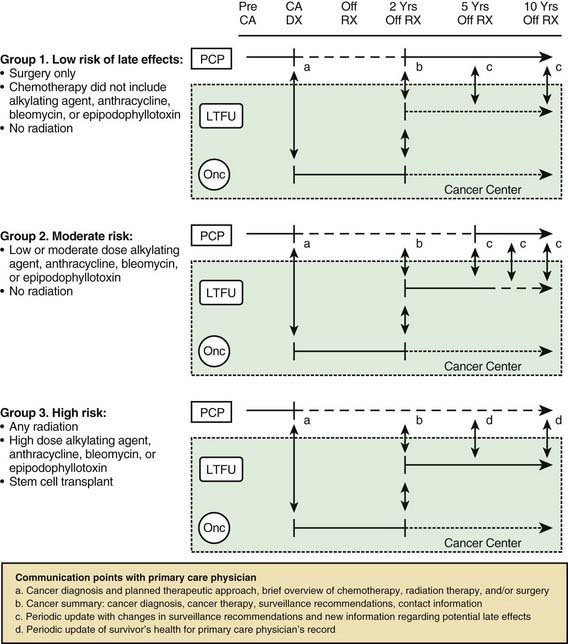
Perhaps the most serious late adverse effect is the occurrence of second cancers in patients successfully cured of a first malignancy. The risk appears to be cumulative, increasing by about 0.5% per year, resulting in approximately a 12% incidence at 25 yr after treatment. Patients who have been treated for childhood cancer should be examined annually, with particular attention to possible late adverse effects of therapy, including second malignancies (Fig. 488-5).
Palliative Care
At all stages of caring for children with cancer, principles of palliative care should be applied to relieve pain and suffering and to provide comfort (Chapter 40). Pain is a serious cause of suffering among patients with cancer. It may be the result of organ obstruction or compression or bone metastasis, or it may be neuropathic. Pain should be managed in a stepwise manner, as recommended by the WHO, in accordance with the principles of selecting the appropriate analgesic, prescribing the appropriate dosage, administering the drug by the appropriate route, and choosing an appropriate dosing schedule to prevent persistent pain and to relieve breakthrough pain (Chapter 71). In addition, the dosage should be titrated aggressively while attempts are made to prevent, anticipate, and manage side effects. Adjuvant drugs and sequential trials of analgesic drugs should be considered.
American Academy of Pediatrics Section on Hematology/Oncology Children’s Oncology Group. Long-term follow-up care for pediatric cancer survivors. Pediatrics. 2009;123:906-915.
Bradlyn AS. Health-related quality of life in pediatric oncology: current status and future challenges. J Pediatr Oncol Nurs. 2004;21:137-140.
Cardous-Ubbink MC, Heinen RC, Langeveld NE, et al. Long-term cause-specific mortality among five-year survivors of childhood cancer. Pediatr Blood Cancer. 2004;42:563-573.
Dickerman JD. The late effects of childhood cancer therapy. Pediatrics. 2007;119:554-568.
Dolmans MM, Marinescu C, Saussoy P, et al. Reimplantation of cryopreserved ovarian tissue from patients with acute lymphoblastic leukemia is potentially unsafe. Blood. 2010;116(16):2908-2914.
Eshelman D, Landier W, Sweeney T, et al. Facilitating care for childhood cancer survivors: integrating children’s oncology group long-term follow-up guidelines and health links in clinical practice. J Pediatr Oncol Nurs. 2004;21:271-280.
Fallon M, Hanks G, Cherny N. Principles of control of cancer pain. BMJ. 2006;332:1022-1024.
Fong SL, van den Heuvel-Eibrink MM, Eijkemans MJC, et al. Pregnancy outcome in female childhood cancer surviviors. Hum Reprod. 2010;25(5):1206-1212.
Giulino LB, Bussel JB, Neufeld EJ, et al. Treatment with rituximab in benign and malignant hematologic disorders in children. J Pediatr. 2007;150:338-344.
Green DM, Kawashima T, Stovall M, et al. Fertility of female survivors of childhood cancer: a report from the childhood cancer survivor study. J Clin Oncol. 2009;27:2677-2685.
Grunberg SM, Rolski J, Strausz J, et al. Efficacy and safety of casopitant mesylate, a neurokinin 1 (NK-1)-receptor antagonist, in prevention of chemotherapy-induced nausea and vomiting in patients receiving cisplatin-based highly emetogenic chemotherapy: a randomized, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:549-558.
Harris MB. Palliative care in children with cancer: which child and when? J Natl Cancer Inst Monogr. 2004;32:144-149.
Hoffer FA. Interventional radiology in pediatric oncology. Eur J Radiol. 2005;53:3-13.
Hudson MM, Mulrooney DA, Bowers DC, et al. High-risk populations identified in childhood cancer survivor study investigations: implications for risk-based surveillance. J Clin Oncol. 2009;27:2405-2414.
Jenney M, Levitt G. Survivors of childhood cancer. BMJ. 2010;340:3-4.
Joensuu A. Sunitinib for imatinib-resistant GIST. Lancet. 2006;368:1303-1304.
Juweid ME, Cheson BD. Positron-emission tomography and assessment of cancer therapy. N Engl J Med. 2006;354:496-507.
Kantarjian H, Shah NP, Hochhaus A, et al. Dasatinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2010;362:2260-2270.
Kelly KM. Complementary and alternative medical therapies for children with cancer. Eur J Cancer. 2004;40:2041-2046.
Koehn BH, Schoenberger SP. Tumor immunotherapy: making an immortal army. Nat Med. 2009;15:731-732.
Krause DS, Van Etten RA. Tyrosine kinases as targets for cancer therapy. N Engl J Med. 2005;353:172-187.
Kurt BA, Armstrong GT, Cash KC, et al. Primary care management of the childhood cancer survivor. J Pediatr. 2008;152:458-466.
Lobo RA. Potential options for preservation of fertility in women. N Engl J Med. 2005;353:64-73.
Mack JW, Grier HE. The day one talk. J Clin Oncol. 2004;22:563-566.
The Medical Letter. Palifermin (kepivance) for myelotoxic-therapy-related mucositis. Med Lett. 2005;47:36-37.
Mocellin S, Mandruzzato S, Bronte V, et al. Part 1: vaccines for solid tumours. Lancet Oncol. 2004;5:681-689.
Mocellin S, Semenzato G, Mandruzzato S, et al. Part II: vaccines for haematological malignancy disorders. Lancet Oncol. 2004;5:727-737.
Mulrooney DA, Yeazel MW, Kawashima T, et al. Cardiac outcomes in a cohort of adult survivors of childhood and adolescent cancer: retrospective analysis of the childhood cancer survivor study cohort. BMJ. 2009;339:b4606.
Nathan PC, Furlong W, Barr RD. Challenges to the measurement of health-related quality of life in children receiving cancer therapy. Pediatr Blood Cancer. 2004;43:215-223.
Neal DE, Donovan JL, Martin RM, Hamdy FC. Second primary cancers in survivors of childhood cancer. Lancet. 2009;374:1484-1485.
Oeffinger KC, Ford JS, Moskowitz CS, et al. Breast cancer surveillance practices among women previously treated with chest radiation for a childhood cancer. JAMA. 2009;301:404-414.
Oeffinger KC, Mertens AC, Sklar AC, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355:1572-1582.
Oeffinger KC, Nathan PC, Kremer LCM. Challenges after curative treatment for childhood cancer and long-term follow up of survivors. Pediatr Clin N Am. 2008;55:251-273.
Offit K, Sagi M, Hurley K. Preimplantation genetic diagnosis for cancer syndromes. JAMA. 2006;296:2727-2730.
Patenaude AF, Kupst MJ. Psychosocial functioning in pediatric cancer. J Pediatr Psychol. 2005;30:9-27.
Patiño-García A, Zalacaín M, Marrodàn L, et al. Methotrexate in pediatric osteosarcoma: response and toxicity in relation to genetic polymorphisms and dihydrofolate reductase and reduced folate carrier 1 expression. J Pediatr. 2009;154(5):688-693.
Pollock BH, Knudson AG. Preventing cancer in adulthood: advice for the pediatrician. In: Pizzo PA, Poplack DG, editors. Principles and practice of pediatric oncology. ed 5. Philadelphia: Lippincott Williams & Wilkins; 2006:1617-1628.
Prasad D, Schiff D. Malignant spinal-cord compression. Lancet Oncol. 2005;6:15-24.
Pui CH, Relling MV. Can the genotoxicity of chemotherapy be predicted? Lancet. 2004;364:917-918.
Reaman GH. Pediatric cancer research from past successes through collaboration to future transdisciplinary research. J Pediatr Oncol Nurs. 2004;21:123-127.
Reulen RC, Winter DL, Frobisher C, et al. Long-term cause-specific mortality among survivors of childhood cancer. JAMA. 2010;304(2):172-178.
Ross CJD, Katzov-Eckert H, Dubé MP, et al. Genetic variants in TPMT and COMT are associated with hearing loss in children receiving cisplatin chemotherapy. Nat Genet. 2009;41:1345-1349.
Ross L, Johansen C, Dalton SO, et al. Psychiatric hospitalization among survivors of cancer in childhood or adolescence. N Engl J Med. 2003;349:650-656.
Saglio G, Kim DW, Issaragrisil S, et al. Nilotinib versus imatinib for newly diagnosed chronic myeloid leukemia. N Engl J Med. 2010;362:2251-2259.
Soussain C, Ricard D, Fike JR, et al. CNS complications of radiotherapy and chemotherapy. Lancet. 2009;374:1639-1650.
Shamberger RC, Jaksic T, Ziegler MM. General principles of surgery. In: Pizzo PA, Poplack DG, editors. Principles and practice of pediatric oncology. ed 5. Philadelphia: Lippincott Williams & Wilkins; 2006:405-420.
Sharma R, Tobin P, Clarke SJ. Management of chemotherapy-induced nausea, vomiting, oral mucositis, and diarrhoea. Lancet Oncol. 2005;6:93-102.
Simard PF, Bolton RM, Tarbell NJ. Anti-inflammatory cream reduces skin damage induced by ionizing radiation. Oncologist. 2009;14:197-198.
Spielberger R, Stiff P, Bensinger W, et al. Palifermin for oral mucositis after intensive therapy for hematologic cancers. N Engl J Med. 2004;351:2590-2598.
Steward AF. Hypercalcemia associated with cancer. N Engl J Med. 2005;352:373-378.
Tarbell NJ, Yock T, Kooy H. General principles of radiation oncology. In: Pizzo PA, Poplack DG, editors. Principles and practice of pediatric oncology. ed 5. Philadelphia: Lippincott Williams & Wilkins; 2006:421-432.
Uren A, Toretsky JA. Pediatric malignancies provide unique cancer therapy targets. Curr Opin Pediatr. 2005;17:14-19.
van Casteren NJ, van der Linden GHM, Hakvoort-Cammel GAJ, et al. Effect of childhood cancer treatment on fertility markers in adult male long-term survivors. Pediatr Blood Cancer. 2009;52:108-112.
Viswanathan AN. Childhood cancer survivors: stillbirth and neonatal death. Lancet. 2010;376:570-572.
Weiner LM, Dhodapkar MV, Ferrone S. Monoclonal antibodies for cancer immunotherapy. Lancet. 2009;373:1033-1040.