6 Principles of the surgical management of cancer
The biology of cancer
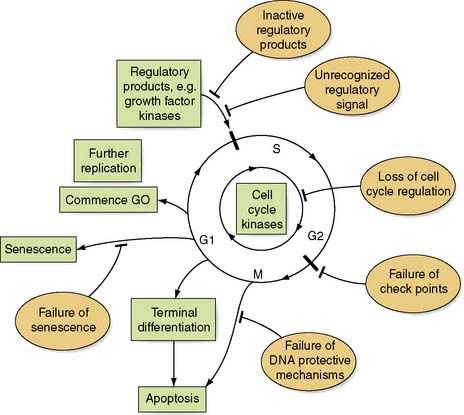
A neoplasm or new growth consists of a mass of transformed cells that does not respond in a normal way to growth regulatory systems. These transformed cells serve no useful function and proliferate in an atypical and uncontrolled way to form a benign or malignant neoplasm. In normal tissues, cell replication and death are equally balanced and under tight regulatory control. However, when a cancer arises, this is generally due to genomic abnormalities that either increase cell replication or inhibit cell death (Fig. 6.1). The mechanisms by which this abnormal growth activity is induced (carcinogenesis) are complex and can be influenced in many ways: for example, inherited genetic make-up, residential environment, exposure to ionizing radiation or carcinogens, viral infection, diet, lifestyle and hormonal imbalances. These cellular insults give rise to alterations in the genomic DNA (mutations) and it is these mutations that lead to cancer. Mutations can lead to disruption of the cell replication cycle at any point and lead to either activation or over-expression of oncogenes, or the inactivation of tumour suppressor genes, or a combination of the two (Table 6.1). Defining which genes have been mutated in the primary and metastatic cancers may ultimately help predict prognosis. For example the amplification and over expression of C-erbB-2 oncogene can give an indication of the aggressiveness of breast cancer. Scientists have now been able to sequence the entire human genome. This will allow identification of new genes and hence proteins involved in the formation of cancer that will eventually lead to a greater understanding of the development of cancer, and new treatments.
Table 6.1 Examples of gene mutations that can lead to cancer formation
| Gene | Point of action in cell cycle |
|---|---|
| p16, CDK4, Rb | Cell cycle check point |
| MSH2, MLH1 | DNA replication and repair |
| p53, fas | Apoptosis |
| E cadherin | Cellular adhesion |
| erb-A | Cellular differentiation |
| Ki-ras, erb-B | Regulatory kinases |
| TGF-β | Growth factors |
Summary Box 6.1 Factors leading to loss of cell cycle regulation
Growth of a cancer is due to loss of cell cycle regulation, which is dependent on:
The adenoma–carcinoma progression
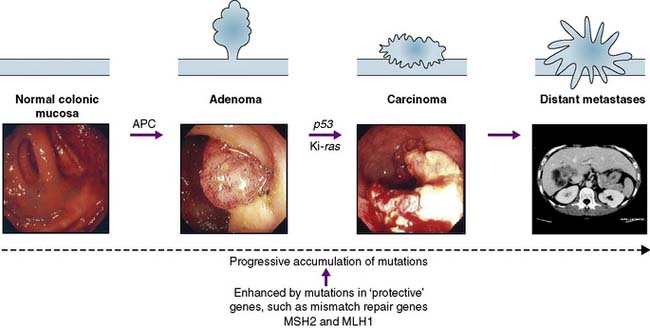
Neoplasms may be benign or malignant; the essential difference is the capacity to invade and metastasize. The cells of benign tumours do not invade surrounding tissues but remain as a local conglomerate. Malignant tumours are invasive and their cells can directly invade adjacent tissues or enter blood and lymphatic channels, to be deposited at remote sites. This malignant genotype develops as a result of the progressive acquisition of cancer mutations (by point mutation, chromosomal loss or translocation). This progressive accumulation of mutations may lead to the formation of cancer stem cells. In a similar manner to other stem cells these cancer stem cells are pleuripotent (i.e. have the ability to give rise to more than one cell type). It is proposed they produce cells that form the epithelial, structural, and vascular components needed for cancer formation. However the cells arising from a cancer stem cell lack the normal response to the normal cell cycle controls and are, therefore, tumour forming. Such cancer stem cells could explain why cancers can relapse or metastasize. The acquisition of the malignant phenotype can be recognized histologically as a tumour develops from a benign adenoma through to a dysplastic lesion, and finally into an invasive carcinoma (Fig. 6.2). The concept of tumour progression from a benign to malignant phenotype provides the rationale behind screening and early detection programmes; i.e. if benign or pre-invasive lesions are removed, this will prevent invasive disease.
Invasion and metastasis
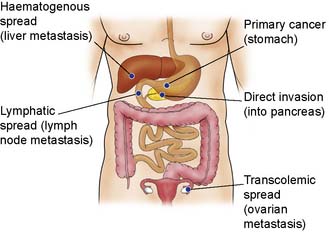
The process of invasion and metastasis is complex (Fig. 6.3) and is dependent on the biology of the tumour. For metastases to occur it would appear that further mutations need to occur in the cancer cells. These extra mutations can be called the metastatic signature. Some tumours metastasize earlier in their clinical course than others. This variation may depend on the tissue of origin of the primary tumour, but can also vary widely according to the phenotype of individual tumours. For example, cancer of the breast is thought to metastasize early, and micrometastases are often present but not detectable when the patient first presents. Some patients with apparently localized colorectal cancer are cured by radical surgery, but others receiving the same treatment deteriorate rapidly with metastatic disease.
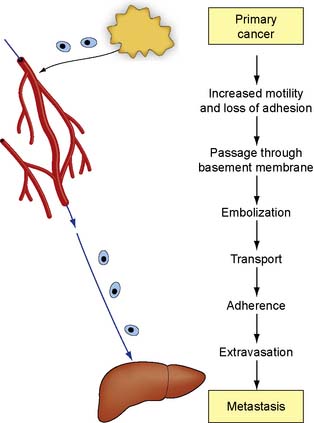
The mechanisms that control invasion and metastasis are obscure (Fig. 6.4). Local pressure effects from the expanding tumour and the increased motility of tumour cells may play a role in local invasion. Malignant cells secrete a number of factors that may determine their biological behaviour and promote growth at both primary and metastatic sites. The matrix metalloproteinases (MMPs) are endoproteinases with enzymatic activity directed against components of the extracellular matrix. Their action facilitates tumour cell invasion and metastasis by degrading extracellular collagens, laminins and proteoglycans. Other proteases, such as urokinase, plasminogen-activating factor and the cathepsins, are also involved in metastasis formation. Clumps of cancer cells can then embolize to distant tissues and form metastases. The location for the development of metastases could be a simple mechanical property with organs that have fine capillary beds, such as liver and lung, trapping circulating malignant cells which then develop into metastases. The survival of metastatic deposits depends on angiogenesis, which is mediated by an imbalance between positive and negative regulatory molecules released by the tumour cells and surrounding normal cells. Negative factors, such as angiostatin or endostatin, will inhibit new vessel formation. Positive factors, such as vascular endothelial growth factors or fibroblast growth factors, will enhance metastasis. Cancer cells also secrete prostaglandins, which can induce osteolysis and may promote the development of skeletal deposits.
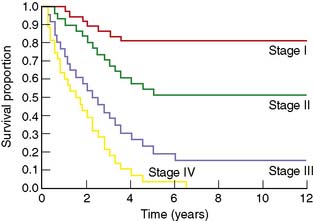
Natural history and estimate of cure
Measures used in survival analysis include, survival and hazard probabilities, Kaplan–Meier equations and graphs (Fig 6.5), Cox’s proportional hazard models, univariate and multivariate analysis. Survival is the probability that a subject survives from the starting point to the end point of the study period. Hazard is the probability that the subject has a specified event at one particular moment in time. ‘Cure’ rates of individual cancers are assessed by survival rates at various times after treatment. Conventionally, 5- and 10-year intervals are used. Cure rates vary according to the aggressiveness of the disease and the success of treatment. In some patients with cancer (e.g. stomach and lung), metastases grow rapidly and cause death within a few years of clinical presentation. In others (e.g. cancer of the breast and melanoma), many years may elapse before metastatic spread becomes evident and, even when metastases have occurred, life may be long. It is for this reason that 5-year survival rates cannot provide a satisfactory estimate of cure for all tumours.
The management of patients with cancer
Screening
If cancer can be detected before it causes symptoms, then it is generally smaller, has less chance of having metastasized and is therefore more amenable to cure. Detecting benign lesions with malignant potential, pre-invasive cancer, and invasive malignancy before it becomes symptomatic is called screening (Fig. 6.6). Screening is expensive and its effectiveness in relation to cost must be critically evaluated before routine use (EBM 6.1). Screening is most effective when targeted at specific risk groups and when the screening test has a high level of acceptability to the target population. For successful screening, the test used must be able to detect the cancer at a stage when earlier treatment will lead to fewer deaths from the cancer. In any given population, the likelihood of a cancer being present is generally low (< 1%); hence, the test must be sensitive in order to detect these relatively rare lesions. The test must also be specific (i.e. have a low false-positive rate); otherwise, individuals will undergo unnecessary investigation or even inappropriate treatment. Finally, the proposed treatment of a cancer patient detected by a screening programme must be effective. In the UK, cervical cytology is offered to women on a 3-yearly basis until the age of 60, and mammographic screening (Fig. 6.7) is offered to women between 50 and 64 years on a 3-yearly basis. Other tumour types that might be amenable to screening are listed with their relevant screening tests in Table 6.2.
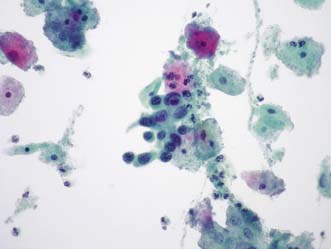
Fig. 6.6 Cervical cytology.
A group of severely dyskaryotic squamous cells in a ThinPrep liquid-based cytology preparation
(Courtesy of Dr A.R.W. Williams, Senior Lecturer/Honorary Consultant in Pathology, University of Edinburgh).
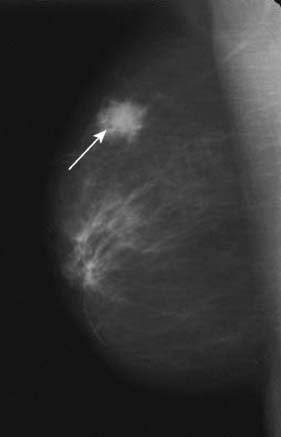
Fig. 6.7 Single mammogram showing malignancy (arrow) in peripheral breast tissue.
(Courtesy of Mr M. Barber, Consultant Breast Surgeon, Western General Hospital, Edinburgh).
Table 6.2 Examples of cancer types that are or could be the subject of screening programmes
| Cancer | Screening test |
|---|---|
| Breast | Mammography |
| Cervix | Smear cytology |
| Colon | Faecal occult blood test and flexible sigmoidoscopy |
| Colonoscopy | |
| Prostate | Prostate-specific antigen (PSA) |
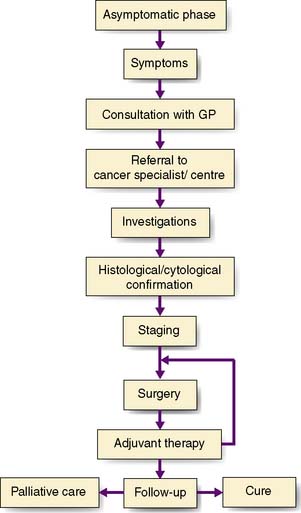
The cancer patient’s journey
The management of cancer frequently involves surgery, be it radical for cure or palliative to relieve distressing symptoms. Because of the complexity of modern cancer management, cancer services in a hospital are currently organized around a multidisciplinary team approach. The team commonly includes surgeons, oncologists (radiotherapy/chemotherapy), radiologists, pathologists and clinical nurse specialists (Table 6.3).
| Medical staff |
Good communication with the patient and between team members forms the basis of optimal patient care. There are several key stages in the management of the patient with cancer, which can be regarded as a journey from the onset of symptoms to definitive treatment and subsequent follow-up (Fig. 6.8). The exact sequence of events may differ from one patient to the next. For example, it may be necessary to remove the tumour to obtain full information on staging before an adequate treatment plan can be evolved. Patients usually begin their ‘cancer journey’ by deciding that a symptom or symptoms they have developed are serious enough to merit consultation with their GP. These symptoms may be a result of local or systemic effects of the cancer.
Investigations
Diagnostic investigations
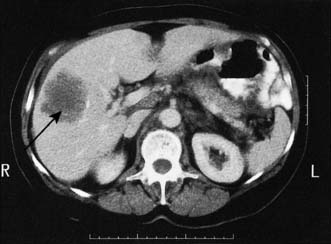
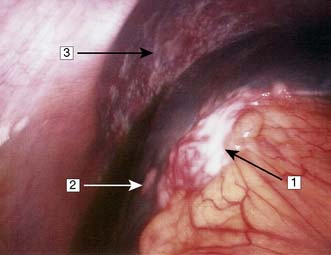
Initial investigations to make the diagnosis should proceed in a logical order, starting with simple blood tests (e.g. tumour markers) and progressing through more complex imaging investigations, with the ultimate aim of obtaining histological or cytological confirmation of the diagnosis (Table 6.4). Plain radiology may demonstrate a soft tissue tumour, e.g. tumours of the lung or bone, but for tumours of the stomach or intestine, contrast studies are necessary. For some deep-seated tumours, e.g. those of the pancreas or brain, other methods of imaging are needed. These include angiography, radioactive scintigraphy and ultrasonography (US), CT (Fig. 6.9), MRI and positron emission tomography (PET). Neoplastic disease can be confirmed cytologically, e.g. by the demonstration of malignant cells in secretions, in washings from hollow viscera, or in needle aspirates. Biopsies obtained at either upper or lower gastrointestinal endoscopy can provide material for histology, as can ultrasound or CT-guided Tru-cut needle biopsies. In some instances, it may be necessary to perform an examination under anaesthetic or diagnostic laparoscopy (Fig. 6.10) to obtain suitable diagnostic material. In general, a treatment plan for the management of a patient cannot be formulated until a histological or cytological diagnosis has been made. However, there are circumstances in which this is not possible (e.g. in certain patients with pancreatic cancer), and then clear radiological evidence may be used instead.
| Blood tests |
Staging investigations
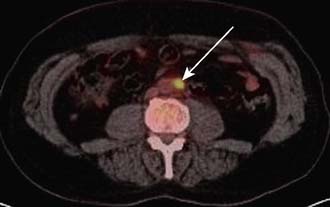
Staging investigations will depend on the site of the primary cancer and the relevant common sites of metastasis. Local invasion can be assessed – for example, in oesophageal cancer – by endoscopic ultrasound. CT or MRI scans can also be usefully employed to assess local invasion. Metastatic spread can be determined by a variety of investigations, e.g. bone scans, CT, PET scan and laparoscopy. When combined with CT, PET can be a particularly powerful way to detect metastatic disease (Fig. 6.11). Often, staging investigations will have been undertaken as part of the diagnostic process, e.g. CT scans.
Treatment
Malignant tumours
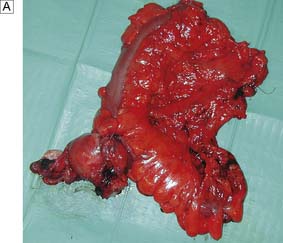
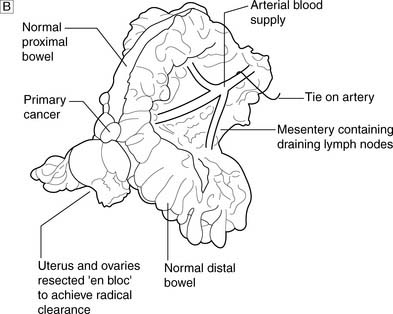
Complete radical excision, which is confirmed by histological examination with no evidence of lymph node metastasis, carries a high chance of surgical cure. A good example is the regional lymphadenectomy performed for colon cancer (Fig. 6.12). During any operation for cancer, care is taken to try to avoid the spillage of malignant cells which may cause cancer recurrence. In some sites (e.g. testis), it is usual to ligate the main vessels draining the area before the tumour is mobilized, so that further malignant cells are not shed into the circulation. Overall, a careful and meticulous approach to all aspects of the operation is vital. Attention to each detail improves the outcome of surgery.
There are data to suggest that surgery performed in specialist centres where surgeons are regularly performing radical operations produces better survival rates than surgery in non-specialist centres. Hence, surgeons are increasingly sub-specialized and concentrate on performing selected operations (EBM 6.2). One of the recent advances in surgical techniques is minimally invasive surgery. Using minimally invasive surgery the trauma related to surgery can be significantly reduced which can enhance the postoperative recovery. These techniques are being increasingly employed in the treatment of cancer. Early results suggested there might be an increase in metastases to the surgical wounds using these techniques, however, it is now recognised that minimally invasive surgical techniques can be used in the treatment of patients with cancer with no detriment to their outcome (EBM 6.3).
Follow-up
In most patients with tumours amenable to surgical treatment, it is important to check subsequently that there is no local recurrence of disease and that the patient is symptom-free (EBM 6.4). In general, patients are seen more frequently in the early months after surgery, as this is the period when recurrence is most likely; it is also the period when it is necessary to detect and treat non-cancer-related postoperative complications. The nature of the surgery will influence the follow-up strategy; patients undergoing palliative surgery will have different follow-up requirements from those undergoing curative surgery. However, it is often difficult to detect recurrence or metastasis in the asymptomatic postoperative patient, and some would question the value of routine sophisticated investigations in the detection of metastatic disease in such cases. Current evidence suggests that, in some cases, once the primary therapy has been undertaken, patients may be discharged back to their GP for follow-up with re-referral to the multidisciplinary team as necessary.