Chapter 4 Principles of Surgical Positioning
• Surgical positioning is a critical step of every operation that needs to be planned and executed carefully in order to avoid any significant potential complications.
• It is crucial to avoid traction on the patient’s extremities and to ensure proper padding of all bony prominences, as these steps will prevent the occurrence of easily avoidable complications.
• Venous air embolism (VAE) can occur in any surgery where the wound is above the heart but is seen more frequently with the sitting position. Less favored these days, this position still offers great advantages but requires adequate preoperative investigation and intraoperative monitoring to decrease the risks for the patient.
• Surgeons must play a leading role in ensuring the safety and comfort of the patient, for themselves, and for every other personnel member in the operating room.
General Principles of Patient Positioning
A thorough preoperative assessment by the anesthesia and nursing staff is necessary, as is a general understanding of the indications, advantages, disadvantages, and potential complications that may arise from commonly utilized neurosurgical patient positions. The final position of the patient should be conveyed to the entire team as early as possible by the surgeon so that appropriate equipment is readily available and to ensure optimal selection and placement of the endotracheal tube, intravenous and arterial lines, noninvasive equipment, and monitors.1–4
Typically, patient positioning occurs subsequent to the induction of general anesthesia, intubation, acquisition of vascular access, and bladder catheterization. Neurophysiological monitoring leads are placed at various stages throughout the perioperative period. Unlike other surgical subspecialties, the planar rotation of the operative table varies depending on the planned surgical approach. The table can be neutral, turned 90 degrees, or often positioned at 180 degrees to allow adequate space and optimal placement of equipment required during complex neurosurgical procedures. This equipment may include the operating microscope, image guidance equipment, C-arm, endoscope tower, headlight sources, and occasionally intraoperative magnetic resonance imaging (MRI) or computed tomography (CT).1
During table rotation and patient positioning, it is often necessary to disconnect the ventilator and monitors temporarily. All members of the team should pay special attention to the duration of this period in order to avoid hypoxic insult to the patient. Eye protection with lubrication and tape provides a barrier that prevents corneal abrasion and introduction of caustic material into the eyes during positioning, intubation, and patient preparation and draping.2
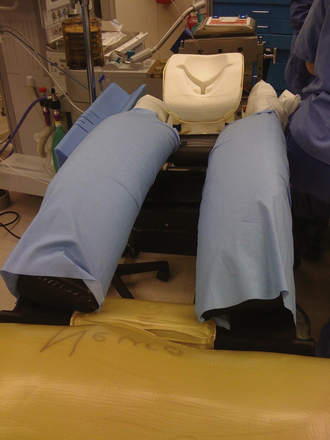
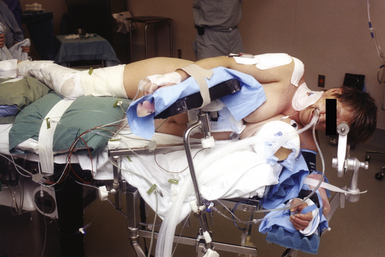
Principles of padding, taping, and positioning of the patient’s extremities are based on the Summary of Task Force Consensus on the Prevention of Perioperative Peripheral Neuropathies Relevant to Positioning for Neurosurgery (Box 4.1).5 In general, maintaining the patient’s arms and legs in an anatomically neutral and relaxed position with soft protective barriers over areas with associated bony prominences helps prevent neurovascular compression, muscle damage, and cutaneous pressure injuries. A combination of gel pads, foam cushions, pillows, and padded arm rests are used for these purposes. For thoracoabdominal and pelvic protection and positioning, large gel rolls or specially designed frames and operative tables provide padding while simultaneously allowing relaxation of this region, aiding in both ventilation and venous return (Fig. 4.1). Such devices include but are not limited to the Wilson frame, Relton-Hall frame, Andrews frame, and Jackson table and frame.3,4,5
Upper Extremity Positioning
 Arm abduction should be limited to 90 degrees in supine patients; patients who are positioned prone may comfortably tolerate arm abduction greater than 90 degrees.
Arm abduction should be limited to 90 degrees in supine patients; patients who are positioned prone may comfortably tolerate arm abduction greater than 90 degrees.
 Arms should be positioned to decrease pressure on the postcondylar groove of the humerus (ulnar groove). When arms are tucked at the side, a neutral forearm position is recommended. When arms are abducted on armboards, either supination or a neutral forearm position is acceptable.
Arms should be positioned to decrease pressure on the postcondylar groove of the humerus (ulnar groove). When arms are tucked at the side, a neutral forearm position is recommended. When arms are abducted on armboards, either supination or a neutral forearm position is acceptable.
 Prolonged pressure on the radial nerve in the spiral groove of the humerus should be avoided.
Prolonged pressure on the radial nerve in the spiral groove of the humerus should be avoided.
 Extension of the elbow beyond a comfortable range may stretch the median nerve.
Extension of the elbow beyond a comfortable range may stretch the median nerve.
Protective Padding
 Padded armboards may decrease the risk of upper extremity neuropathy. The use of chest rolls in laterally positioned patients may decrease the risk of upper extremity neuropathies.
Padded armboards may decrease the risk of upper extremity neuropathy. The use of chest rolls in laterally positioned patients may decrease the risk of upper extremity neuropathies.
 Padding at the elbow and at the fibular head may decrease the risk of upper and lower extremity neuropathies, respectively.
Padding at the elbow and at the fibular head may decrease the risk of upper and lower extremity neuropathies, respectively.
Documentation
 Charting specific positioning actions during the care of patients may result in improvements of care by (1) helping practitioners focus attention on relevant aspects of patient positioning; and (2) providing information that continuous improvement processes can use to lead to refinements in patient care.
Charting specific positioning actions during the care of patients may result in improvements of care by (1) helping practitioners focus attention on relevant aspects of patient positioning; and (2) providing information that continuous improvement processes can use to lead to refinements in patient care.
From American Society of Anesthesiologists Task Force on the Prevention of Perioperative Peripheral Neuropathies: Practice Advisory for the Prevention of Perioperative Peripheral Neuropathies. Anesthesiology 2000;92:1168-1182.
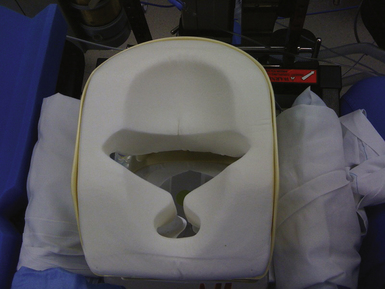
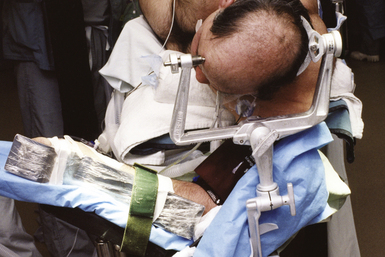
Cranial surgeries and occasionally posterior cervical surgeries require rigid skull fixation. Historically the use of stereotactic navigation universally required skull fixation; however, the development of electromagnetic systems has allowed the head to be mobile during a stereotactic procedure, obviating the need for pins. If rigid fixation is not required, the head may be placed on a gel or foam doughnut (Fig. 4.2) or in a padded horseshoe. The Mayfield frame (Fig. 4.3) is a three-pin device that is typically used for rigid skull fixation.1,2 Both radiolucent and metal versions exist depending on the need for intraoperative x-ray. In cases in which spinal distraction and reduction may be necessary, Gardner-Wells tongs or a halo ring with attached weights are used. For prone positioning the head can be maintained in the Mayfield frame or placed on a foam pillow that is usually precut with openings for the eyes, nose, and endotracheal tube (Fig. 4.4).1 Lateral positioning requires the use of specially positioned arm rests and usually an axillary roll to prevent brachial plexus compression.5 More detailed requirements for the various positions are discussed in the respective sections later in this chapter.
In positions other than prone, placement of the Mayfield frame is carried out on the operative table prior to patient positioning. In the prone position, the pins are usually inserted on the hospital bed prior to transferring the patient to the operative table. In adults pins are placed under 60 pounds per square inch (psi) of pressure.1 Inserting and tightening the pins has a profoundly stimulating effect on the patient that usually leads to an increase in heart rate and blood pressure. This hemodynamic change may incur potential complications, for example, in patients with unsecured aneurysms or intracerebral hemorrhage. The timing of pin insertion should be clearly communicated between the surgeon and anesthesiologist so that the depth of anesthesia can be increased with additional bolus administration of an agent such as propofol (Diprivan). The patient’s vital signs should be closely monitored during this time. This assumes that appropriate invasive and noninvasive forms of monitoring are in place and functioning prior to this step. Additionally, antibiotic ointment should be applied to each of the pins prior to their percutaneous insertion as a bacterial barrier and to avert air embolism, particularly in the sitting position.2,6
Head and neck configuration is perhaps the most important aspect of neurosurgical patient positioning. Final orientation of the head and neck is based on the planned surgical approach and exposure. There are several basic cranial approaches that determine head and neck positioning. The head can be safely rotated approximately 45 degrees in the supine position in healthy individuals. Access to the skull beyond 45 degrees requires manipulation and rotation of the patient’s body into any of the specific positions described later in the chapter.2 Various degrees of anteroposterior and lateral flexion and extension of the neck provide additional modification to the surgical trajectory.1
Avoidance of neurovascular complications during head and neck positioning requires vigilance on the part of the surgeon and the anesthesiologist. Hyperflexion beyond approximately 2 to 3 fingerbreadths between the mandibular protuberance and the manubrium is considered the upper limit of safe neck flexion. Hyperflexion of the neck in both the anteroposterior and lateral planes may lead to a series of complications. These complications include decreased cranial venous return and lymphatic outflow leading to facial swelling, macroglossia, and raised intracranial pressure, compression of the vertebral arteries leading to ischemia, and increased airway pressures affecting ventilation and oxygenation. Awareness of the distance between the patient’s chin and the edge of the operative table is also important. In the prone position if the patient shifts downward on the table, the chin can press against the edge, leading to skin necrosis.1,2,7
Supine Position
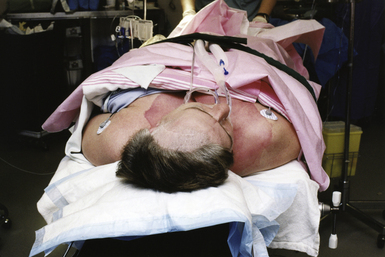
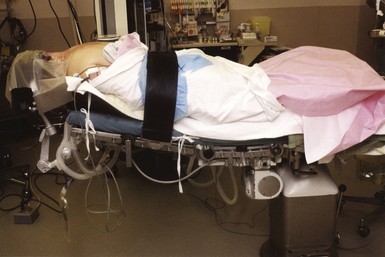
The supine position (Fig. 4.5) is perhaps the most commonly used patient position in neurosurgery and across all surgical specialties. Because it is a familiar position, it is arguably the safest with the fewest number of associated complications. As an additional advantage, no special equipment is required.
In the supine position, the patient’s head can be free on a padded doughnut or horseshoe, rigidly fixed in the Mayfield clamp, or in traction with Gardner-Wells tongs or a halo ring. The elbows, wrists, and heels are appropriately padded with gel or foam cushions. The knees are maintained in a slightly flexed position over a pillow. The arms are generally maintained at the patient’s side on padded arm rests.3 The head can safely be turned 45 degrees, as previously mentioned. Additional rotation can be achieved by placing a roll or bolster under the shoulder ipsilateral to the surgical side. If pins are not used and the head is turned, the ear and contralateral scalp should be protected with a gel doughnut or foam pad to avoid compressive injury to the pinna and to prevent pressure alopecia.2 If a shoulder roll is used, the contralateral or dependent arm is often placed in a slightly abducted position on an arm rest. The ipsilateral arm is either placed in a flexed position across the abdomen or maintained at the side on an arm rest, depending on the degree of patient rotation and whether access to the abdomen is desired (e.g., for ventriculoperitoneal shunt or if abdominal fat graft is desired).1 Arms should not be abducted more than 90 degrees at the shoulder and supination of the forearm is recommended in order to minimize ulnar nerve injury.5
In addition to patient position, bed configuration plays an important role in the supine patient. In anterior spinal procedures and endarterectomies, the bed is maintained in the horizontal position. In cranial procedures in which both venous drainage from the brain and venous return from the legs are desired, the lawn chair position is preferable. Maximal venous drainage from the head is achieved with either the Fowler or reverse Trendelenburg position, both of which help to minimize venous bleeding and to reduce cerebral swelling.1,2
Prone Position
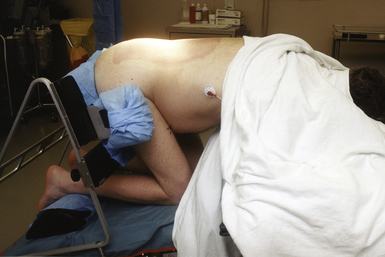
The prone position refers to three primary patient configurations: straight prone, Concorde, and kneeling (Figs. 4.6 to 4.8). In general, the prone position is used for access to the suboccipital region and the posterior spine. For prone procedures the patient is placed under general anesthesia and intubated on the hospital bed in the supine state. Vascular access is also obtained and a bladder catheterization occurs prior to placing the patient into the final position on the operative table.3
Extreme caution should be used when rotating the patient from the hospital bed to the prone position on the operative table, particularly in cases of presumed or known spinal instability. The surgeon, not the anesthesiologist, should be responsible for control of the cervical spine and skull during this maneuver. Gripping the Mayfield clamp during this step does not provide optimal craniocervical stabilization. Instead, the Mayfield clamp should be locked in place, the surgeon’s receiving hand should be placed directly onto the patient’s face with the endotracheal tube stabilized between two fingers on the same hand, and the surgeon’s other hand should be placed on the occiput. This configuration offers maximal airway protection and control of the patient’s cervical spine and skull simultaneously. All members of the team should monitor intravenous/arterial lines, catheters, and tubing during this transition. Many of the circuits are intentionally disconnected prior to this maneuver.1,2,7
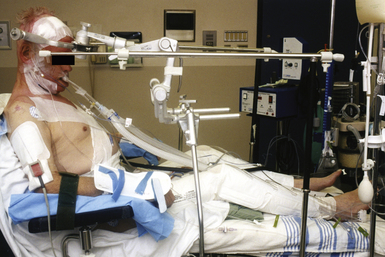
The use of large gel pads for the chest or special padded frames or tables allows for appropriate cushioning while preventing excessive thoracoabdominal compression. Female breasts and nipples should be positioned medially and male genitalia should hang freely.3 The patient’s knees are padded and usually flexed. The wrists and elbows are also appropriately padded.5 The neck is maintained in either a neutral or flexed position, the degree of which is determined by the surgeon and the indication for surgery. The head may or may not be fixed and can be tilted up to 30 degrees to one side and rotated up to 45 degrees, also depending on the precise location of the pathology being treated and surgeon preference.2
Posterior cervical surgeries may be performed with the patient in a three-point headholder, halo ring, or traction tongs, or with the face on a padded pillow with cutouts for the endotracheal tube and eyes. Special consideration should be given to corneal lubrication, lid taping, and eye positioning so as to avoid ocular pressure that may lead to blindness.2,8 The neck is often maintained in a neutral position, particularly if fusion is desired. For thoracolumbar surgeries, the patient is often placed on a Jackson table or Wilson frame, and the head is placed on a padded pillow with cutouts as mentioned earlier.2 For spinal procedures in which intraoperative x-rays are planned, it is important to consider the placement of the patient’s arms. For thoracolumbar procedures the arms are maintained in abduction at approximately 90 degrees at the shoulder and elbow (“airplaned”) on arm rests. For cervical and cervicothoracic junction procedures, the arms are often padded and wrapped at the patient’s side. To enhance radiographic visualization of the cervicothoracic junction, traction applied toward the feet by using either tape on the shoulders or soft wrist restraints anchored to the foot of the bed is often necessary. However, excessive traction should be avoided because it can lead to neuromuscular and vascular injuries, cutaneous burns, and very rarely joint dislocation.3,5
For cranial approaches, especially the occipital transtentorial and supracerebellar infratentorial approaches, the Concorde position is advocated. In this position, the skull is fixed in pins, the patient’s neck and knees are flexed, the arms are maintained in the neutral position at the side with thumbs pointing downward, and the thoracolumbar region is extended so that the head is elevated slightly above the level of the heart. The Concorde position is commonly used for suboccipital approaches and has a relative benefit over the sitting position (used for similar surgical approaches) in that it is associated with a significantly lower incidence of venous air embolism.1,9
Finally, the kneeling prone position is rarely used. In this position, the patient is placed on an operative table that approximates the outline of the letter Z. Historically patients were placed in the kneeling position to minimize the amount of intraoperative blood loss. Although this was demonstrated clinically and experimentally, the disadvantages of the kneeling position are well documented and include increased potential for neurovascular and muscular pressure injuries,5 hypotension from pooling of blood in the dependent lower extremities, a concomitant increase in the incidence of venous thrombosis and pulmonary embolism, and muscle necrosis and rhabdomyolysis that can lead to renal failure. For these reasons, placing the patient in the kneeling configuration is rarely justified.2
Lateral Position
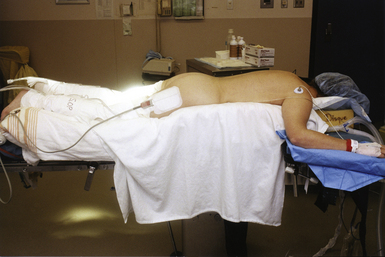
The lateral position (Figs. 4.9 to 4.11) allows the best access to the temporal lobe, the lateral skull base, and the lateral suboccipital area. From a spine perspective, the lateral position is utilized for transthoracic and retroperitoneal approaches to the thoracolumbar spine as well as posterior approaches to the lumbar spine for unilateral decompressive procedures. Specific uses of the lateral position also include lumboperitoneal and syringoperitoneal shunts, intrathecal baclofen pumps, pain pumps, and dorsal sympathectomies. From a cranial standpoint, the lateral position can be approximated from supine position by using a shoulder bolster. However, the true lateral position usually requires that the patient’s hips be perpendicular to the floor.1
This position is achieved by first placing the patient supine on the operative table. A bean bag is often used to secure the patient in the lateral position and is placed on the table prior to the patient.3 The patient is then rotated laterally, and an axillary roll the diameter of the upper arm is inserted approximately 4 cm below the dependent armpit to avoid injury to the long thoracic nerve and the C5-C6 roots.5 Finally the bean bag is compressed against the patient’s torso and deflated. For cranial procedures the skull is pinned, secured to the table, and the dependent arm is then placed in a hanging position on a padded arm rest. This arm rest is placed between the Mayfield clamp and the edge of the operative table. The shoulder is slightly abducted with the elbow minimally flexed, and the entire extremity is positioned outstretched in front of the patient. A modification in the lateral position, the park bench position (see Fig. 4.11), has the nondependent arm taped in a slightly flexed position at the elbow over the patient’s side, the neck flexed toward the floor, and the head rotated contralaterally in order to allow access to the posterior fossa.1,2
For spinal procedures, a similar configuration is achieved with the head resting on a padded doughnut and the table flexed at the level of the lumbar spine or with the kidney rest elevated. This maneuver theoretically opens the interlaminar space on the nondependent side of the vertebral column (i.e., the side of the pathology).1
In all lateral configurations, the lower extremities are positioned with a pillow between the legs and the dependent knee flexed to avoid compressive injury to the peroneal nerve over the fibular head. Care should be taken not to hyperflex the knee as this position is often associated with excess hip flexion that can lead to undue traction on the lateral femoral cutaneous nerve.5
Three-Quarter Prone Position
The three-quarter prone position (Fig. 4.12) resembles the lateral position in many ways. Indications for this position include approaches to the parieto-occipital region, the posterior fossa, and the pineal region. Although it is not the most popular position for spinal surgeries, reports of its use have been documented for thoracic extracavitary approaches. Compared to the sitting position, the three-quarter prone position leads to lower risks of air embolism.9 It also allows less retraction of the parietal and occipital lobes during a parafalcine approach performed on the hemisphere positioned inferiorly.1 Another important advantage of this position is that, in comparison to the sitting position, it provides more comfort to the surgeon and decreases fatigue imposed on the arms and shoulders.10
The patient is placed on the table supine and undergoes general anesthesia, intubation, and placement of invasive and noninvasive monitors. Although not an obligatory step, the skull is generally fixed in pins at this stage. The patient’s body is then rotated approximately 130 degrees while keeping the head and torso centered on the table. As previously stated the surgeon should be in charge of mobilizing and positioning the head. Careful avoidance of excessive neck movement is important because the patient is under the effects of general anesthesia and muscle relaxant. The dependent arm is then positioned either next to the body or, as in the lateral position, dependent on a padded arm rest at the head of the table. In order to decrease the risk of injury to the brachial plexus and the rib cage, an axillary roll is inserted in the same manner as described for the lateral position. The superior nondependent arm is then flexed and placed on a soft barrier over the chest. Gentle traction toward the feet can be applied on the superior shoulder. This traction can be achieved by applying tape from the lateral aspect of the shoulder to the foot of the bed. However, care should be taken to avoid traction or direct compression of the brachial plexus. The lower extremities are positioned with the dependent leg straight, underneath, and the superior leg slightly flexed to allow for greater stability and to decrease the risk of stretch neuropathy. Both legs are separated by pillows. The head can finally be secured to the table in the desired position with varying degrees of rotation and flexion in the direction of the floor to allow for maximal exposure of the surgical site. The importance of making sure that all bony prominences are padded and that there is no stretch on any extremity cannot be emphasized enough because these complications are easily avoidable.1–35
As in any other position, because the surgical site is above the heart, there is an increased risk of air embolism, but this risk is significantly less than that for the sitting position. If the Mayfield frame is not used and the patient’s head is placed on a horseshoe or doughnut, potential ocular complications such as blindness can occur. The risk of injury to the brachial plexus or other peripheral nerves is decreased by appropriately padding the extremities and avoiding excessive traction on the arms as delineated earlier.2,5,8
Sitting Position
Traditionally, the sitting position (Fig. 4.13) has been the preferred position for posterior fossa and posterior cervical spine surgeries. Over time this position has fallen out of favor because of a higher risk of complications associated with it such as quadriplegia, pneumocephalus, and venous air embolism (VAE). However, the sitting position can still offer significant advantages, and many surgeons continue to use this position for posterior fossa disease, pineal region lesions, and posterior cervical spine approaches. Today the sitting position and its variations are used most commonly during deep-brain stimulation (DBS) procedures. In certain scenarios the benefits of this position outweigh the associated risks, and it is preferred by many surgeons. Specifically, the position offers excellent anatomical exposure, lowers intracranial pressure and venous pressure, allows drainage of cerebrospinal fluid and blood to gravity, reduces the need for cerebellar retraction during the supracerebellar infratentorial approach, and provides direct access to the face for control of the airway and for cranial nerve monitoring. Careful preoperative planning, perioperative monitoring, as well as close collaboration with an experienced anesthesia team are required in order to minimize and avoid complications.1,2
Relative contraindications should be determined and evaluated before a patient is deemed a suitable candidate for the sitting position. There are several key considerations that are unique to this position and should be assessed during the preoperative period. Although the effects of gravity may have advantages on the technical aspects of surgery, they contribute to the majority of the serious complications that arise in this configuration. These effects are compounded by general anesthesia and muscle relaxation. Hypotension is one such risk that requires careful consideration. Relative contraindications to the sitting position in the context of hypotension include a patient history of orthostasis, cardiac atherosclerotic disease, and long-standing antihypertensive therapy. Slow verticalization during table positioning and the use of vasopressors can minimize the likelihood of hypotension. The use of trunk and lower extremity compressive bandages, elastic leg stockings, and sequential compression devices decreases the extent of venous pooling. However, these measures may be inadequate in obese patients.2
Cases of cervical spinal cord injury resulting in quadriparesis or quadriplegia are well documented in the literature. Although this complication occurs more frequently in patients with a history of cervical stenosis, this devastating outcome can occur in healthy patients. In the latter case, the injury is thought to be secondary to hemodynamic changes induced in the spinal cord vasculature during neck flexion. The most commonly reported level of injury is C5. In order to reduce the risk of postoperative position-induced myelopathy, a simple preoperative evaluation can be performed by asking the patient to simulate the surgical position and to hold the head flexed for at least 5 minutes. If the patient complains of any neck pain or other neurological symptom, additional preoperative investigation should be performed or another surgical position should be considered. Unfortunately this simple bedside test cannot completely eliminate the risk of neurological complications, but by allowing early identification of high-risk patients, it may reduce the occurrence of complications. Additionally, the use of intraoperative monitoring can be helpful in detecting and preventing spinal cord injury in this setting.2,7,11–14
Finally, well-documented cases of fatal VAE occurring in the sitting position have been reported. Although VAE is most commonly discussed in the context of the sitting position, any surgical position that maintains the patient’s wound above the heart carries a theoretical and actual risk of this complication. However, in the sitting position this distance is greatest and is therefore seen more commonly. In the context of VAE, an absolute contraindication arises in patients with a patent foramen ovale (PFO) or other type of right-to-left shunt. In these patients paradoxical air emboli can cross into the systemic arterial circulation with devastating consequences. This topic has been a popular subject in the literature.6,8,9,15–22 A recent review published by Fathi and associates concluded that, when considering the sitting position, routine preoperative investigation with transesophageal echocardiography or contrast-enhanced transcranial Doppler to rule out PFO is necessary because its incidence in the general population is as high as 20% to 25%.20 If the sitting position appears to be the only suitable position, patients with a PFO can undergo a low-risk percutaneous procedure to achieve closure of the foramen and reduce the risk of paradoxical air embolism.23
Upon completion of the preoperative assessment, the patient is brought to the operating room, positioned supine on the operative table, placed under general anesthesia, and intubated. Standard noninvasive cardiopulmonary monitoring is used with or without the use of additional invasive monitors, depending on surgeon preference. An arterial line and a central venous catheter are almost universally advocated in this position. Central access to the venous system allows the patient’s volume status to be continuously monitored while also providing a means to treat VAE should it occur. Although it remains controversial, the central line can be used to aspirate air directly from the superior vena cava and the right atrium. To achieve proper monitoring for VAE, Mirski and colleagues suggested using different combinations of end-tidal nitrogen and carbon dioxide monitoring as well as esophageal stethoscope, precordial Doppler, transcranial Doppler, or transesophageal echocardiography.9 According to an editorial published by Leonard and Cunningham, at least three of these monitoring modalities should be used.24 Unfortunately, intraoperative interpretation of transesophageal echocardiography and transcranial Doppler by an anesthesiologist require expertise that is not widely available at every center. The use of precordial Doppler offers excellent sensitivity, allowing detection of air embolus of about 0.05 mL/kg, which is well below the estimated lethal 3 to 5 mL/kg volume. Because hypotension can have deadly consequences, continuous knowledge of the patient’s fluid status is also crucial. Although the use of a Swan-Ganz catheter is not advised for every patient, it may be indicated in certain high-risk patients with tenuous cardiovascular and cardiopulmonary conditions.9,16,19,20,22 Intermittent compression devices or compressive elastic stockings and bandages are typically applied as a way to reduce venous pooling in the lower extremities and the lower part of the trunk. Using a G-suit or military antishock trouser, although proved to increase right atrial pressure while the patient is in the sitting position, is not advocated because the potential complications outweigh the benefits. These complications include lower extremity compartment syndrome, abdominal organ hypoperfusion, and lower vital capacity.2,9,19,20,22
Once the various forms of invasive and noninvasive monitors are in place, the Mayfield head clamp is applied, and the operative table is flexed in the middle. The head and the thighs are slowly elevated and pillows are placed underneath the knees. The foot of the table is slightly lowered, thereby flexing the knees and minimizing pressure on the sciatic nerve. The entire table is then tilted backward and the head slowly elevated to the sitting position, which varies from 45 to 90 degrees. At this stage careful observation of the blood pressure is necessary. The next step involves flexing the head in a manner that avoids compression of the cervical spinal cord and allows proper venous drainage. A distance of 2 to 3 fingerbreadths between the chin and the sternum is generally acceptable. The head is then fixed to the table using the Mayfield clamp, which is secured anteriorly to the foot of the bed. The table and its remote control are then locked to avoid any alteration in the general position and prevent serious injury to the cervical spine. The table can still be tilted during the surgery to accommodate the surgical team’s needs as long as the general position remains the same. After the desired position is achieved, the arms are cautiously placed either on arm boards or directly on the patient. Standard measures should be undertaken to prevent traction on the brachial plexus and avoid pressure on neurovascular structures and bony prominences.1–35
Because the most common complication cited by surgeons as a reason to avoid the sitting position is the risk of VAE, additional consideration should be given to this topic. Only a 5-cm gravitational gradient between the wound and the heart is necessary for air to enter the venous system. Although VAE is most commonly discussed in the context of the sitting position, this short distance is also present in many of the more standard positions described here. In fact, a retrospective analysis published by Black and associates compared posterior fossa surgeries in the sitting position with surgeries performed in the horizontal position. The occurrence of VAE was 45% in the first group versus 12% in the latter. However, when the patient is sitting, the incidence of VAE can range from as low as 7% to as high as 60%, depending on the means used for its detection. Clinically significant VAE still remains rare with reported morbidity rates ranging from 0.5% to 2% in different published series. Other factors that contribute to the development of VAE include traversing noncompressible large venous structures such as dural sinuses or intraosseous emissary veins.6,12,16
Important preoperative and perioperative steps discussed earlier must be taken to prevent the occurrence of VAE. Both anesthesia and the surgical teams play important roles in recognizing and treating the condition at the onset of systemic symptoms. VAE can affect the cardiovascular, pulmonary, and neurological systems. For a patient under general anesthesia, these symptoms may range from early cardiac arrhythmia and electrocardiogram changes to complete vascular collapse secondary to right-sided heart failure. The respiratory alterations can include bronchoconstriction, a decrease in oxygen saturation and end-tidal CO2, and an increase end-tidal N2 and systemic CO2. Neurological symptoms associated with VAE occur from hypoperfusion secondary to vascular collapse or direct occlusion of the cerebral vasculature by paradoxical emboli. Postoperative neurological consequences vary from simple mental status alteration to focal neurological deficit to coma. The presence and degree of such symptoms will depend on two main factors: the volume and rate of air accumulation. In humans, the acute lethal volume is estimated to be approximately 200 to 300 mL.11,14,25 The rate of accumulation can be significant as demonstrated by Flanagan and co-workers, who proved that a 5-cm gravity gradient applied to a 14-gauge needle could lead to entrapment of air as quickly as 100 mL/second.26 At this rate rapid actions need to be taken to prevent dramatic consequences from occurring. The first step involves actively irrigating the wound and covering with sponges. The operating table can be tilted backward to reduce the gravity gradient. Prompt identification of the origin of the air embolus is necessary. Waxing of all exposed bony surfaces and repairing or coagulating open veins and dural sinuses are obligatory actions to prevent further entry of air into the circulation. While the surgical team is attempting to identify and eliminate the source, the anesthesiology team must maintain hemodynamic stability. Strategies include increasing the inspiratory oxygen, discontinuing nitrous oxide, and using vasopressors as necessary to keep the blood pressure within a normal range.9,11,14,25 If a central line is in place, an attempt at aspirating the entrapped air should be made and has been successful according to many published reports independent of the volume of air removed. However, there is no indication to proceed with emergent line placement if one was not previously placed. In the case of refractory vascular collapse, chest compression can be performed, facilitating mobilization and dispersion of the air embolus. Hyperbaric oxygen therapy has also been used postoperatively as long as the patient remains hemodynamically stable. These patients should be monitored closely in the intensive care unit for the delayed development of pulmonary edema.15,20,26
Although less frequently mentioned, other potential complications have been reported with the sitting position. According to a recent study published by Sloan, postoperative supratentorial pneumocephalus related to decreased intracranial pressure was seen in more than 40% of the patients. Simple pneumocephalus occurs more often when the patient has a ventriculostomy catheter and if the surgery is prolonged. Simple pneumocephalus tends to resolve spontaneously over the course of 2 to 3 postoperative days. More severe complications include tension pneumocephalus as well as supratentorial hematoma, both of which have been reported after surgery in the sitting position. Very little can be done to prevent these adverse events, but fortunately they rarely occur.27
Surgical Ergonomics
The importance of patient positioning has been thoroughly described in previous sections. However, the ergonomics of the operating room and its impact on the surgical team cannot be underestimated. Neurosurgical operations can be long and strenuous both mentally and physically. They often require a high degree of concentration, repetitive movements of the upper extremities over long periods of time, and very fine motor control. The presence of various types of equipment such as the operating microscope, C-arm, intraoperative CT or MRI, image guidance systems, and two- and three-dimensional displays all leads to crowded operating rooms and a more restricted corridor to access the surgical field (Fig. 4.14). It is known from laparoscopic experience that the strategic placement of devices such as these has been shown to reduce fatigue and improve concentration. Although this issue has not been specifically studied in the neurosurgical literature, many would argue that these principles are universal across surgical specialties. Simple maneuvers such as providing rests for the arms and hands, keeping the elbows flexed at 90 degrees, sitting for prolonged operations that require fine repetitive movements, avoiding excessive neck flexion for the surgeon, maintaining the operating scope perpendicular to the surgical trajectory, altering the bed position to allow additional anatomical visualization, and placing displays and navigation equipment in the surgeon’s line of site will all aid in reducing discomfort, fatigue, and injury. Surgical ergonomics encompasses all aspects of patient, surgeon, and equipment placement and position. It impacts each member of the operating room team, but its effective implementation is ultimately the responsibility of the operating surgeon.10
Mirski M.A., Lele A.V., Fitzsimmons L., Toung T.J. Diagnosis and treatment of vascular air embolism. Anesthesiology. 2007;106(1):164-177.
Practice advisory for the prevention of perioperative peripheral neuropathies: a report by the American Society of Anesthesiologists Task Force on Prevention of Perioperative Peripheral Neuropathies. Anesthesiology. 2000;92(4):1168-1182.
Rozet I., Vavilala M.S. Risks and benefits of patient positioning during neurosurgical care. Anesthesiol Clin. 2007;25(3):631-653.
St. Arnaud D., Paquin M.J. Safe positioning for neurosurgical patients. AORN J. 2008;87(6):1156-1168. quiz 1169–1172
Winn H.R., editor, Youmans Neurological Surgery. 5th ed. Philadelphia: WB Saunders; 2004:4. vols., 38 pp plates
Please go to expertconsult.com to view the complete list of references.
1. Winn H.R., editor, Youmans Neurological Surgery. 5th ed. Philadelphia: WB Saunders; 2004:4. vols., 38 pp plates
2. Rozet I., Vavilala M.S. Risks and benefits of patient positioning during neurosurgical care. Anesthesiol Clin. 2007;25(3):631-653.
3. St. Arnaud D., Paquin M.J. Safe positioning for neurosurgical patients. AORN J. 2008;87(6):1156-1168. quiz 1169–1172
4. Butler V.M., Dean L.S., Little J.R. Positioning the neurosurgical patient in the operating room: “a team effort.”. J Neurosurg Nurs. 1984;16(2):89-95.
5. Practice advisory for the prevention of perioperative peripheral neuropathies: a report by the American Society of Anesthesiologists Task Force on Prevention of Perioperative Peripheral Neuropathies. Anesthesiology. 2000;92(4):1168-1182.
6. Prabhakar H., Ali Z., Bhagat H. Venous air embolism arising after removal of Mayfield skull clamp. J Neurosurg Anesthesiol. 2008;20(2):158-159.
7. Morandi X., Riffaud L., Amlashi S.F., Brassier G. Extensive spinal cord infarction after posterior fossa surgery in the sitting position: case report. Neurosurgery. 2004;54(6):1512-1515. discussion 1515–1516
8. Practice advisory for perioperative visual loss associated with spine surgery: a report by the American Society of Anesthesiologists Task Force on Perioperative Blindness. Anesthesiology. 2006;104(6):1319-1328.
9. Mirski M.A., Lele A.V., Fitzsimmons L., Toung T.J. Diagnosis and treatment of vascular air embolism. Anesthesiology. 2007;106(1):164-177.
10. Albayrak A., van Veelen M.A., Prins J.F., et al. A newly designed ergonomic body support for surgeons. Surg Endosc. 2007;21(10):1835-1840.
11. Matjasko J., Petrozza P., Cohen M., Steinberg P. Anesthesia and surgery in the seated position: analysis of 554 cases. Neurosurgery. 1985;17(5):695-702.
12. Black S., Ockert D.B., Oliver W.C.Jr., Cucchiara R.F. Outcome following posterior fossa craniectomy in patients in the sitting or horizontal positions. Anesthesiology. 1988;69(1):49-56.
13. Harrison E.A., Mackersie A., McEwan A., Facer E. The sitting position for neurosurgery in children: a review of 16 years’ experience. Br J Anaesth. 2002;88(1):12-17.
14. Liutkus D., Gouraud J.P., Blanloeil Y. The sitting position in neurosurgical anaesthesia: a survey of French practice. Ann Fr Anesth Reanim. 2003;22(4):296-300.
15. Losasso T.J., Muzzi D.A., Black S., Cucchiara R.F. The “risk” of nitrous oxide in neurosurgical patients operated upon in the sitting position: a prospective, randomized study. J Neurosurg Anesthesiol. 1989;1(2):131-132.
16. Black S., Muzzi D.A., Nishimura R.A., Cucchiara R.F. Preoperative and intraoperative echocardiography to detect right-to-left shunt in patients undergoing neurosurgical procedures in the sitting position. Anesthesiology. 1990;72(3):436-438.
17. Duke D.A., Lynch J.J., Harner S.G., et al. Venous air embolism in sitting and supine patients undergoing vestibular schwannoma resection. Neurosurgery. 1998;42(6):1282-1286. discussion 1286–1287
18. Bithal P.K., Pandia M.P., Dash H.H., et al. Comparative incidence of venous air embolism and associated hypotension in adults and children operated for neurosurgery in the sitting position. Eur J Anaesthesiol. 2004;21(7):517-522.
19. Kwapisz M.M., Deinsberger W., Müller M., et al. Transesophageal echocardiography as a guide for patient positioning before neurosurgical procedures in semi-sitting position. J Neurosurg Anesthesiol. 2004;16(4):277-281.
20. Fathi A.R., Eshtehardi P., Meier B. Patent foramen ovale and neurosurgery in sitting position: a systematic review. Br J Anaesth. 2009;102(5):588-596.
21. Hooper A.K., Okun M.S., Foote K.D., et al. Venous air embolism in deep brain stimulation. Stereotact Funct Neurosurg. 2009;87(1):25-30.
22. Jadik S., Wissing H., Friedrich K., et al. A standardized protocol for the prevention of clinically relevant venous air embolism during neurosurgical interventions in the semisitting position. Neurosurgery. 2009;64(3):533-538. discussion 538–539
23. Webb S.T., Klein A.A., Calvert P.A., et al. Preoperative percutaneous patent foramen ovale closure before neurosurgery in the sitting position. Br J Anaesth. 2009;103(2):305. author reply 306
24. Leonard I.E., Cunningham A.J. The sitting position in neurosurgery—not yet obsolete!. Br J Anaesth. 2002;88(1):1-3.
25. Leslie K., Hui R., Kaye A.H. Venous air embolism and the sitting position: a case series. J Clin Neurosci. 2006;13(4):419-422.
26. Flanagan J.P., Gradisar I.A., Gross R.J., Kelly T.R. Air embolus: a lethal complication of subclavian venipuncture. N Engl J Med. 1969;281:488-489.
27. Sloan T. The incidence, volume, absorption, and timing of supratentorial pneumocephalus during posterior fossa neurosurgery conducted in the sitting position. J Neurosurg Anesthesiol. 2010;22(1):59-66.