Principles of sterilization
Susannah E. Walsh and Jean-Yves Maillard
Chapter contents
Key points
• A number of dosage forms, medical products and devices need to be free of microorganisms.
• High-level disinfection is used for the ‘sterilization’ of certain medical devices.
Introduction
Previous chapters in this Part of the book have described the types and properties of microorganisms (Chapter 13) and the action of heat and chemical agents upon them (Chapter 15). This chapter will build on those fundamentals and describe the principles underlying the different methodologies available to achieve sterility. These will be described both for pharmaceutical preparations and also for medical products and devices. This chapter will also describe the criteria used to measure sterility. The practicalities associated with the processes of sterilization are described in Chapter 17.
By definition, a sterile preparation is described as the absolute absence of viable microbial contaminants. In practice, this definition is not achievable as a preparation cannot be guaranteed to be sterile. This remark is discussed further in Chapter 17.
Certain pharmaceutical preparations, medical devices and items for which usage involves contact with broken skin, mucosal surfaces or internal organs, injection to the blood stream and other sterile parts of the body, are required to be sterile. These are frequently referred to in pharmacopoeias as sterile products or sterile dosage forms. Microbiological materials, such as soiled dressings and other contaminated items, also need to be sterilized before disposal or reuse.
Sterilization is the process by which a product is rendered sterile, i.e. the destruction or removal of microorganisms. The majority of the processes recommended by pharmacopoeias (i.e. steam under pressure, dry heat, gaseous and ionizing radiation) are terminal sterilization processes for which the preparation is sterilized in its final container or packaging. For other multiple component preparations that cannot be sterilized with such methodologies, filtration sterilization can be used. Finally, high-level disinfection is used for the ‘sterilization’ of medical devices.
Need for sterility
As mentioned in the introduction, certain pharmaceutical preparations, medical products and devices are required to be sterile (further information is given in Chapter 17, Table 17.1). Briefly, these include:
• ophthalmic preparations – eye drops, lotions and ointments and some contact lens solutions
• implants
• surgical ligatures and sutures (absorbable and non-absorbable)
Failure to achieve sterility can result in serious consequences. In the best case scenario, surviving microorganisms induce spoilage of the product (i.e. chemical and physical degradation) that might be identified before the preparation is used. The product (or batch of product) is then removed from use and destroyed. In the worst case scenario, where microbial survival cannot be identified through deleterious effects to the product, infection (sometimes fatal) might result from the use of the contaminated preparation. There have been many reports in the literature of such incidents over the years. For example, in the Devonport incident (in 1971–1972) the death of five patients (from acute endotoxic shock) was traced to dextrose 5% infusion bottles sterilized with a faulty autoclave. In 1996, in Romaira (Brazil), 35 newborn infants died of sepsis attributed to locally produced intravenous solutions (Editorial 1998). In 2005, a contaminated heparin intravenous flush was responsible for infecting several patients in different states in the USA (Editorial 2005). In these cases, inappropriate quality control procedures were implicated. These incidents emphasize that not only must an appropriate sterilization regimen be used but appropriate monitoring and control must be performed. This requires an understanding of the principles of sterilizing processes and their control and validation.
Sterilization parameters
The inactivation kinetics of a pure culture of microorganisms exposed to a physical or chemical sterilization process is generally described by an exponential relationship between the number of organisms surviving and the extent of treatment (ISO 2000), although variations from this are likely (Chapter 15 gives more details). Survivor curves have been used to generate inactivation data for specific sterilization processes using specific biological indicators (see Chapter 17). These data are important for calculating a number of sterilization parameters which help to establish a sterilizing regimen adapted to a specific preparation or product.
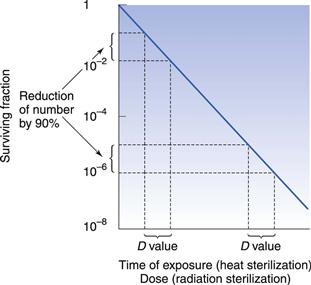
D value and Z value
One of the important concepts in sterilization is the D value (Fig. 16.1). This parameter is calculated as the time taken to achieve a one log (90%) reduction in the number of microorganisms. Another important concept is the Z value, which calculates the increase in temperature for steam (under pressure) or dry heat, or dose for radiation sterilization, required to produce a one log (90%) reduction in D value for a particular microorganism. This parameter is used to compare the heat (or dose) resistance of different biological indicators following alterations in temperature or radiation. Chapter 15 provides more information on both these parameters.
Inactivation factor and most probable effective dose
The inactivation factor is the total reduction in the number of viable microorganisms brought about by a defined sterilization process. This parameter can be calculated from the D value but only if the destruction curve follows the linear logarithmic model. To overcome problems caused by variations from this model, a most probable effective dose value can be used. This is the dose needed to achieve n decimal reductions in the number of microorganisms.
F value
The F value is a measure of total lethality of a heat sterilization process for a given microorganism and is used to compare the lethality of different heat sterilization processes. A reference value (Fo) of Geobacillus stearothermophilus (formerly Bacillus stearothermophilus) spores at 121 °C is often used with a Z value of 10 °C. The total Fo of a process includes the heating up and cooling down phases of the sterilization cycle.
For dry heat sterilization the F value concept has some limited application. The FH value is used and corresponds to the lethality of a dry heat process in terms of the equivalent number of minutes exposure at 170 °C. A Z value of 20 °C is used for the calculation.
Principles of sterilization processes
Five main types of sterilization processes are usually recommended for pharmaceutical products (British Pharmacopoeia 2012). Among these, steam sterilization (sometimes referred to as steam under pressure sterilization or high-temperature steam sterilization) still represents the gold standard. Novel sterilization processes are being developed and have already been applied in the food industry. These are mentioned in the ‘New Technologies’ section later in this chapter.
Heat sterilization
Heat has been employed as a purifying agent since early historical times, and is now used worldwide in sterilization. Boiling is not a form of sterilization as higher temperatures are needed to ensure the destruction of all microorganisms.
Microorganisms vary in their response to heat. Species of bacterial spores are thought to be some of the most heat-resistant forms of life and can survive temperatures above 100 °C. Non-sporulating bacteria are destroyed at lower temperatures (50–60 °C) and vegetative forms of yeasts and moulds have a similar response. Cysts of amoeba (e.g. Acanthamoeba polyphaga) are less sensitive than their vegetative cells which are inactivated at 55–60 °C. It is generally thought that viruses are less resistant than bacterial spores (McDonnell 2007). The agents responsible for spongiform encephalopathies, the prions, are worth mentioning due to their infectious nature and high resistance to heat (current thermal sterilization procedures are not effective in inactivating prions; see Chapter 15).
Despite the widespread use of heat sterilization, the exact mechanisms and target sites involved are still uncertain. It is likely that several mechanisms and targets are implicated and those proposed include damage to the outer membrane (Gram-negative bacteria), cytoplasmic membrane, RNA breakdown and coagulation, damage to DNA and denaturation of proteins, probably as a result of an oxidation process. For hydrated cells (steam sterilization), it is likely that the chemical lethal reactions occur more rapidly with the presence of water. The denaturation and coagulation of key enzymes and structural proteins probably result from a hydrolytic reaction.
The thermal death of bacterial cells and spores is usually thought to have a first-order reaction kinetic. Although some controversy over this does exist, the use of an exponential inactivation model for the kinetics of spores is unlikely to be underestimating the heat required (Joslyn 2001; McDonnell 2007). One way to express the order of death as a first-order reaction is:
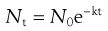 (16.1)
(16.1)
This relationship is discussed further in Chapter 15.
Heat sterilization processes usually occurs in three phases:
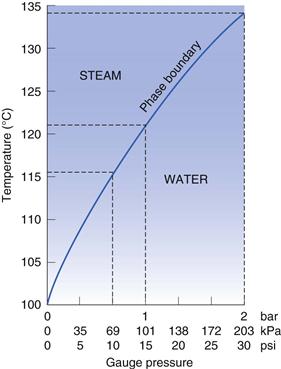
Principles of steam sterilization
Steam sterilization relies on a combination of steam, temperature and pressure. Steam is used to deliver heat to the product to be sterilized. There are different types of steam but only steam at the phase boundary has the appropriate characteristics for maximum effectiveness (Fig. 16. 2). Steam at the phase boundary between itself and its condensate has the same temperature as the boiling water that produced it but holds much more latent heat. This latent heat is available for transfer (without a decrease in temperature) when it condenses on to a cooler surface. This rapid transfer of latent heat is responsible for the rapid rise in temperature (to the sterilization temperature) of any items it touches (Chapter 15 provides more information). The condensed water also aids the process by hydrating microorganisms and making them more sensitive. The condensation of steam contracts it to a very small volume that creates a pressure decrease into which more steam is drawn to re-establish the pressure. This aids the penetration of steam into porous items such as dressings. Wet saturated steam is less effective than dry saturated steam as not as much condensation is produced and the latent heat available is less (Fig. 16.2). It can also saturate loads with free water and interfere with steam penetration. Superheated steam is another potential problem that must be limited (Chapter 15). Although it is hotter than dry saturated steam, superheated steam is less efficient at releasing its heat to cooler objects, as it is only as efficient as hot air at the same temperature.
Principles of dry heat sterilization
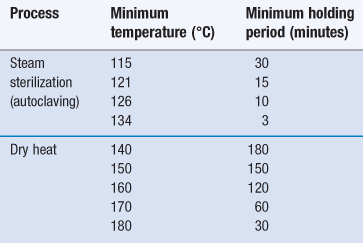
Sterilization using dry heat is less efficient than moist heat but is the preferred method for items that are thermostable but moisture sensitive or impermeable to steam (Sharp 2000). This includes some metal devices, glassware, oils/oily injections and some powders (see Chapter 17, Table 17.1). In addition, temperatures greater than 220 °C are used for depyrogenation of glassware (in this case the process needs to demonstrate a three log reduction in heat-resistant endotoxin). The main advantage of dry heat over steam sterilization is its ability to penetrate items and kill microorganisms via oxidation. The process is generally slower and holding times for dry heat are much longer than those needed for steam sterilization (Table 16.1).
Combination treatments
The amount of heat required for sterilization can be reduced by using a combination treatment of heat plus a reduced pH (below 4.5) or low water potential (Aw). Although some spores may survive the initial treatment, they then germinate and die, reducing the numbers of organisms present as time passes (a process called autosterilization). This technique has been used in the food industry with meat-based canned foods to avoid high-temperature sterilization. In addition, the combination of heat and hydrostatic pressure is thought to be synergistic. This is further discussed in the ‘Ultrahigh pressure’ section below.
In the pharmaceutical industry, another type of combination treatment involves low-temperature steam formaldehyde (LTSF). The steam is below atmospheric pressure (70–80 °C) and formaldehyde gas provides the sporicidal effect. Formaldehyde has been used to sterilize medical devices but care must be taken with its use as it is acutely toxic to humans and is also mutagenic and possibly carcinogenic (Sharp 2000). Some caution should be attached to the sterility assurance level (see Chapter 17) of the process, as it has been suggested that revival of spores after LTSF treatment is possible (McDonnell 2007). Finally, the processing of thermolabile medical equipment such as endoscopes is conducted in automated washer disinfectors (Fig. 16.3). These make use of high-level disinfectants and slightly raised temperature. This is further discussed in the ‘High-level disinfection’ section below.
Alternative means for heat delivery and control
Systems for the delivery of heat via direct application of flame, heating using alternating electric currents and microwave energy have been developed and adopted in some parts of the world. The use of infrared radiation allows a rapid elevation in temperature. Advances in packaging have improved heat penetration and made it more uniform. These include flexible pouches, polypropylene rigid containers, thermoformed containers, tin-free steel and foil-plastic combinations.
Gaseous sterilization
When sterilization by heat is not possible, one alternative is to use a sterilizing gas. Not many gases are used in the pharmaceutical industry for sterilization and it is important to note that some of these gases are also used for disinfection (as either a liquid or a gas) under different conditions. Pharmacopoeias usually recommend the use of ethylene oxide for gaseous sterilization of pharmaceutical preparations, although other chemicals are available, such as formaldehyde, hydrogen peroxide, chlorine dioxide, peracetic acid and ozone. The chemical biocides are generally separated, according to their mode of action, into alkylating and oxidizing agents.
Alkylating gases
Some alkylating gases can permeate through many polymeric materials and are therefore not limited to just surface applications.
Ethylene oxide is widely used in pharmaceutical manufacturing but less so in hospitals. It occurs in gaseous form at room temperature (boiling point 10.7 °C) and penetrates narrow spaces well. Ethylene oxide has been shown to possess bactericidal, fungicidal, virucidal, sporicidal and protozocidal properties (Dusseau et al 2004).
The use of (LTSF) sterilization has been mentioned already. Formaldehyde is a surface sterilant only and cannot be used to sterilize occluded areas. Penetration into porous materials can be inhibited by the formation of polymers that crosslink, preventing further sterilant access (Chapter 15 provides more information).
Oxidizing gases
Oxidizing gases are relatively unstable and their decomposition can lead to microenvironments within the load that are not exposed to the full concentration of the agent.
Hydrogen peroxide gas has been shown to be effective against spores at a range of temperatures. Its action is greatest when used at near-saturation levels on clean dry surfaces and it does not leave a toxic residue (McDonnell 2007). The vapour is usually obtained via evaporation of a heated stock solution. Water condensation on surfaces that are being sterilized can reduce local concentrations of hydrogen peroxide and hence its activity; decomposition by catalytic activity and absorption by cellulosic materials (e.g. paper) can also reduce its effectiveness. The combination of hydrogen peroxide with cold plasma, cupric or ferric ions, ozone or UV has been shown to enhance activity (Dusseau et al 2004).
Ozone has been demonstrated to be an effective sterilant, but the complex control of humidity required and corrosion problems have limited its applications and it is not routinely used. Ozone as a disinfectant for water currently shows more promise and commercial systems are available for several applications.
Chlorine dioxide is a broad-spectrum biocide with a sporicidal activity and it is mainly used for the high-level disinfection of medical devices. Its applications are limited due to its effect on materials such as uncoated aluminium foil, uncoated copper, polycarbonate and polyurethane (McDonnell 2007).
Peracetic acid exists as a liquid at room temperature and is used for high-level disinfection (discussed below). Vaporized peracetic acid can be used for the sterilization of surfaces and devices, although a long contact time is required. Peracetic acid can cause corrosion of certain metals and rubbers and has low penetrating power.
Vaporized oxidizing agents are often used in combination or with plasma. Formulations also contain excipients that reduce their negative impact, such as smell and corrosiveness.
Radiation sterilization
There are two main types of radiation: electromagnetic and particulate.
Of these, only γ-rays and high-speed electron beams are used for sterilization of pharmaceutical products, since other forms of radiation have not been shown to be effective as sterilants and/or are not suitable (McDonnell 2007). Both γ– and β-rays are forms of ionizing radiation (Chapter 15 provides more information on mode of action and resistance). One advantage of irradiation is that it does not cause a significant rise in temperature and hence has been called ‘cold’ sterilization. The main target site for radiation is DNA but damage to other vital components such as RNA, enzymes and cell membranes is also involved. Single-strand or double-strand DNA breaks will inhibit DNA synthesis or cause errors in protein synthesis. Damage to the sugars and bases may also occur (McDonnell 2007).
Ionizing radiation can be used to sterilize items that cannot be sterilized with heat (see Chapter 17, Table 17.1). This is not a process that is normally carried out in a standard pharmaceutical manufacturing facility; instead specialized plants are used (Sharp 2000).
D values are used in radiation sterilization (see Fig. 16.1) and can be calculated from the formula:
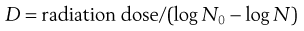 (16.2)
(16.2)
where N0 and N represent a one log difference in numbers.
As with heat sterilization, the dose–response curve to radiation can vary (shoulders and tailing off can occur) but in general the exponential model holds. The initial lag that causes a shoulder is thought to result from multiple targets being hit before death or from DNA repair taking place (see Chapter 17).
Despite having been shown to have activity against bacterial spores, viruses and vegetative cells, UV radiation is not used for sterilization of pharmaceutical products because of its low penetrative power and absorption by glass and plastics. It is used for disinfection of surfaces, including isolators and safety cabinets, and can be used as part of the treatment for drinking water (Lambert 2004).
Filtration sterilization
Thermolabile solutions can be sterilized by filtration through filters that remove bacteria. Filtration sterilization is the complete removal of microorganisms within a specific size range from liquids or gases. It is a non-terminal sterilization process, and strict aseptic techniques need to be observed. Because of their small size, viruses are not removed by sterilization filtration and therefore, where possible, terminal sterilization processes should be preferred. Items that might be filter sterilized include heat-sensitive injections and ophthalmic solutions, biological products and air and other gases for supply to aseptic areas (Denyer & Hodges 2004).
There are two main types of filtration mechanisms:
Generally, only membrane filters are thought to be suitable for the removal of microorganisms. The types used for pharmaceutical applications are usually made from cellulose esters or other polymers and are highly uniform with regular spaces or holes. When a liquid passes through the filter, all particles and microorganisms larger than the holes are retained. Membrane filters have the advantage of removing particles and microorganisms efficiently while retaining very little of the product in their holder or housing. However, they can become blocked quickly if the liquid being sterilized contains a lot of particles and smaller particles can become trapped in the holes, causing a pressure differential (Levy 2001). Because of this, prefilters are often used.
High-level disinfection
The term ‘high-level’ disinfection is often employed with chemical biocides that have demonstrated sporicidal activity. They are sometimes described as ‘sterilants’ or ‘chemosterilants’. High-level disinfectants are usually considered to be highly reactive against macromolecules and are generally divided into two main groups of highly reactive biocides: alkylating and oxidizing agents (Table 16.2). Among the former, the aldehydes, notably formaldehyde (gas), glutaraldehyde and ortho-phthalaldehyde (OPA) are the most important. Oxidizing agents are composed of a wider family, such as peroxygen compounds (e.g. peracetic acid and hydrogen peroxide) chlorine dioxide and superoxidized water (McDonnell 2007). Peracetic acid has also been used for the cold sterilization of some pharmaceutical preparations such as emulsions, hydrogels, ointments and powders.
Table 16.2
Sterilization and high-level disinfection using chemical biocides
| Chemical agents | Usage |
| Hydrogen peroxide | Gas plasma sterilization (endoscopes) |
| Peracetic acid | Endoscopes, pharmaceutical preparations |
| Chlorine dioxide | Gas-phase chlorine dioxide sterilization (medical equipment) |
| Glutaraldehyde Ortho-phthalaldehyde Formaldehyde |
High-level disinfection (endoscopes) High-level disinfection (endoscopes) Gaseous sterilization (LTSF)* |
| Ethylene oxide | Liquid and gaseous sterilization |
Oxidizing agents have been shown to react extensively with macromolecules such as amino acids, peptides and proteins, lipids, notably through a reaction with sulphydryl (-SH) groups, sulphur bonds (S-S), fatty acids double-bonds (Finnegan et al 2010), however the mechanisms by which they bring lethality to the bacterial cell has not been solved, although there is evidence that the primary target site for oxidising agents used at a high (in use) concentration is the bacterial nucleic acid. Oxidising agents also lead to the formation of peroxyl radicals (fatty acid; lipids), hydroxyl radicals and other reactive species, although the role of these species in the lethality of these agents at a high concentration is subject to debate.
Alkylating agents have been shown to react with macromolecules through a reaction with amino, carboxyl, thiol, hydroxyl, imino groups and amide substituents. Their interactions with proteins and enzymes and their cross-linking ability are thought to be responsible for their mechanisms of bactericidal action. The lipophilicity of OPA is thought to be responsible for a better penetration of the aldehyde through the cell wall and for an increase in activity compared with glutaraldehyde (Fraud et al 2003).
On occasion, the use of chemical biocides has been combined with a physical process, such as temperature (e.g. LTSF as discussed earlier in this chapter). For the disinfection of medical devices such as endoscopes, high-level disinfectants are often used in automated washer disinfectors, which are designed for specific devices and ensure automation of the process, although a pre-cleaning stage is often necessary before disinfection takes place.
New technologies
Although only five sterilization procedures are usually recommended in pharmacopoeias, there has been an interest in developing alternative methodologies to palliate the disadvantages of existing ones (see Chapter 17). Most of the progress has been made in the food area. These technologies include ultrahigh pressure, high-intensity light pulse, ultrasonication and gas plasma, the last being the most promising for the sterilization of medical devices and products. The principles of these new sterilization processes, although they are not used for pharmaceutical preparations, are worth mentioning briefly.
Ultrahigh pressure
The principle of using high pressure is that vegetative microorganisms are inactivated at pressures above 100 MPa and bacterial spores at pressures above 1200 MPa. The use of high pressure processing for food preservation has been combined with the chemical effect of the preservative system, such as a low pH in certain foodstuff (e.g. jams, fruit juices, etc.). The low pH ensures the prevention of outgrowth of bacterial spores. The advantage of this process is that the quality and taste of products tend not to be affected by such a system. Indeed, high pressure tends to preferably denature macromolecules rather than low molecular weight flavour and odour compounds (Yordanov & Angelova 2010). The microbial cell offers multiple pressure-sensitive target sites (e.g. enzymes, membranes, genomic material, etc.), although bacterial spores are more resilient to high pressures. The combination of high pressure with elevated temperature has been shown to be synergistic, although the process varies depending upon the combination and the type of spores.
The use of high pressure for the sterilization of pharmaceutical products has been considered and might offer an appropriate alternative to existing sterilization processes. High pressure is an innocuous process that can be performed at a relatively low temperature. Another advantage of using high pressure is the rapid control of thermal processes. Indeed, the application of pressure raises the temperature but conversely the temperature decreases as the pressure is reduced. Hence, rapid temperature changes following a rapid change in pressure can help in controlling a thermal process and reduce heat-induced damage to a product (Heinz & Knorr 2001). However, detailed studies with pharmaceutical preparations and detailed process validation are needed for high pressure to be used for the sterilization of sterile dosage forms (van Doorne 2008).
High-intensity light pulse
The application of intense light, such as a high-intensity laser, has been known to inactivate microorganisms. This principle already has applications in the food industry and also in the medical area, notably in dentistry. Such processes have been shown to inactivate vegetative microorganisms and bacterial spores. In the food industry, broad-spectrum light with pulse durations from 10−6 to 10−1 seconds and with energy densities ranging from 0.1 to 50 J/cm2 is used. In dentistry, high-intensity light pulse (i.e. laser) has been combined with the use of antimicrobial dyes (e.g. toluidine blue) to achieve a better inactivation of microorganisms in the treatment of root canal, and such a combination is also referred to as antimicrobial photodynamic treatment (Soukos & Goodson 2011).
For pharmaceutical preparations, a potential application is the terminal sterilization of clear solutions such as water, saline, dextrose and ophthalmic products. The type of container is of prime importance since it must not hinder the transmission of light.
Ultrasonication
The use of sonication to inactivate microorganisms was first reported more than 30 years ago. The principle is based on cavitation through the material exposed, resulting in the formation and collapse of small bubbles. The ensuing shock waves associated with high temperatures and pressures can be sufficiently intense to disrupt the microbial cell; however, spores are highly resistant. A synergistic effect has been reported by combining ultrasound and heat to a certain extent (O’Donnell et al 2010). Interestingly, such a combination has been reported to reduce the heat resistance of microorganisms.
Gas plasma
Gas plasma is generated with the application of a strong magnetic field to a gaseous phase compound (e.g. hydrogen peroxide). This process creates a mixture of charged nuclei, free electrons and other reactive species such as free radicals that can then damage cellular components (e.g. membrane, nucleic acid), a mechanism of action similar to that of oxidizing agents. Plasma is generally considered as the ‘fourth’ state of matter. This dry sterilization process is effective against vegetative microorganisms and also bacterial spores and has the advantage of being non-thermal (Moreau et al 2008). The use of gas plasma offers an appropriate alternative to traditional sterilization processes and finds many applications for the sterilization of medical devices, but also in drug delivery with the treatment of biomaterials (Cheruthazhekatt et al 2010; Fig. 16.4).
Summary
Sterilization is an essential process for the manufacture of sterile dosage forms, and reprocessing medical devices and products. There are several processes that can be used to achieve appropriate sterilization for a given preparation or product/device. Each of these processes presents advantages and disadvantages, although steam sterilization remains the reference standard. The advances in non-thermal sterilization and the coming new technologies, although mainly applied to the food industry to date, offer potentially valuable alternatives. The demonstration of sporicidal activity of a new technology, as well as its control and reproducibility, remains essential.
Common to all these processes is the need for the user to understand the technology, its activity and limitations, to follow the appropriate guidelines but also, importantly, to ensure the validation of the process. Failure to provide the appropriate documentation and to control a sterilization process adequately might result in failure of the process, with potentially fatal consequences.
It is important to note at this point that although terminal sterilization ensures the destruction of possible microbial contaminants, it needs to be operated alongside good manufacturing practice. Therefore, suitable measures must be taken to ensure the microbiological quality of pharmaceutical preparations during manufacture but also during packaging, storage and distribution. These important aspects are discussed in more depth in Chapter 17.
References
1. British Pharmacopoeia. Appendix XVIII Methods of sterilization (methods of preparation of sterile products). London: Pharmaceutical Press; 2012.
2. Cheruthazhekatt S, Cernak M, Slavicek P, Havel J. Gas plasmas and plasma modified materials in medicine. Journal of Applied Biomedicine. 2010;8:55–66.
3. Denyer SP, Hodges NA. Sterilization: filtration sterilization. In: Fraise AP, Lambert PA, Maillard J-Y, eds. Principles and Practice of Disinfection, Preservation and Sterilization. 4th edn Oxford: Blackwell Science; 2004.
4. Dusseau J-Y, Duroselle P, Freney J. Sterilization: gaseous sterilization. In: Praise AP, Lambert PA, Maillard J-Y, eds. Principles and Practice of Disinfection, Preservation and Sterilization. 4th edn Oxford: Blackwell Science; 2004.
5. Editorial. Morbidity and Mortality Weekly Report. 1998;47:610–612.
6. Editorial. Morbidity and Mortality Weekly Report. 2005;54:269–272.
7. Finnegan M, Denyer SP, McDonnell G, Simons C, Maillard J-Y. Mode of action of hydrogen peroxide and other oxidizing agents: differences between liquid and gas forms. Journal of Antimicrobial Chemotherapy. 2010;65:2108–2115.
8. Fraud S, Hann AC, Maillard J-Y, Russell AD. Effects of ortho-phthalaldehyde, glutaraldehyde and chlorhexidine diacetate on Mycobacterium chelonae and M. abscessus strains with modified permeability. Journal of Antimicrobial Chemotherapy. 2003;51:575–584.
9. Heinz V, Knorr D. Effects of high pressure on spores. In: Hendrickx MEG, Knorr D, eds. Ultra High Pressure Treatments of Foods. New York: Kluwer Academic/Plenum Publishers; 2001.
10. International Standards Organization (ISO). Sterilization of health care products – general requirements for characterization of a sterilizing agent and the development, validation and routine control of a sterilization process for medical devices. ISO 14937 Geneva: ISO; 2000.
11. Joslyn LJ. Sterilization by heat. In: Block SS, ed. Disinfection, Sterilization, and Preservation. 5th edn Philadelphia: Lippincott Williams and Wilkins; 2001.
12. Lambert PA. Sterilization: radiation sterilization. In: Fraise AP, Lambert PA, Maillard J-Y, eds. Principles and Practice of Disinfection, Preservation and Sterilization. 4th edn Oxford: Blackwell Science; 2004.
13. Levy RV. Sterile filtration of liquids and gases. In: Block SS, ed. Disinfection, Sterilization, and Preservation. 5th edn Philadelphia: Lippincott Williams and Wilkins; 2001.
14. Moreau M, Orange N, Feuilloley MGJ. Non-thermal plasma technologies; New tools for bio-decontamination. Biotechnology Advances. 2008;26:610–617.
15. O’Donnell CP, Tiwari BK, Bourke P, Cullen PJ. Effect of ultrasonic processing on food enzymes of industrial importance. Trends in Food Science & Technology. 2010;21:358–367.
16. Sharp J. Sterile products: basic concepts and principles. In: Quality in the Manufacture of Medicines and Other Healthcare Products. London: Pharmaceutical Press; 2000.
17. Soukos NS, Goodson JM. Photodynamic therapy in the control of oral biofilms. Periodontology 2000. 2011;55:143–166.
18. Van Doorne H. High-pressure treatment, a potential antimicrobial treatment for pharmaceutical preparations? A survey. PDA Journal of Pharmaceutical Science and Technology. 2008;62:273–291.
19. Yordanov DG, Angelova GV. High pressure processing for food preserving. Biotechnology & Biotechnological Equipment. 2010;24:1940–1945.
Bibliography
1. Block SS, ed. Disinfection, Sterilization, and Preservation. 5th edn Philadelphia: Lippincott Williams and Wilkins; 2001.
2. British Society for Gastroenterology Working Party. Cleaning and disinfection of equipment for gastrointestinal endoscopy. Gut. 1998;42:585–593.
3. Fraise AP, Lambert PA, Maillard JY, eds. Principles and Practice of Disinfection, Preservation and Sterilization. 4th edn Oxford: Blackwell Science; 2004.
4. McDonnell G, ed. Antisepsis, Disinfection and Sterilization: Types, Action and Resistance. Washington, DC: ASM Press; 2007.