Chapter 139 Principles of Scalp Surgery and Surgical Management of Major Defects of Scalp
Throughout history, advances in plastic surgery have been reflected in the management of scalp wounds. Hippocrates, Gallen, and Celsus described the treatment of a denuded skull by perforating the dry black sequestrum formed above bare cranium with an awl.1 In the 17th century, Augustin Belloste advocated perforating the outer table of the skull to permit granulation tissue formation and subsequent epithelialization.2 In 1871, Netolitzky described the technique of skin grafting granulating scalp wounds,3 and in 1908, Robinson described the use of skin graft on intact periosteum.4 In 1953, Kazanjian first described the use of superficial incisions through the galea to increase tissue availability.5 In 1967 and 1971, Orticochea published his four- and three-flaps technique, respectively, for coverage of large scalp defects.6,7 Miller, in 1976, replanted microsurgically for the first time an avulsed scalp.8 In 1978, Radovan was the first to report successful clinical applications of tissue expansion and to demonstrate prototypes of the expanders in clinical use today.9–11
Anatomy of the Scalp
The rich vascular supply of the scalp receives vascular contribution from the internal and external carotid arteries. The forehead and anterior scalp are mainly supplied by terminal branches of the ophthalmic artery, which is a branch of the internal carotid artery: the supratrochlear and supraorbital arteries. The supratrochlear vessels arise 1.6 to 2.3 cm from the midline, which is usually located at the medial border of the eyebrow.12 The supraorbital vessels arise through the supraorbital notch which is located 2.4 to 2.9 cm from the midline.13 The external carotid artery provides three branches to the scalp. The terminal branch of the external carotid artery, the superficial temporal artery, ascends anterior to the helix within the superficial temporal fascia and it supplies the temporal and parietal region. The posterior auricular artery ascends between the auricle and mastoid process and supplies blood to the scalp posterior to the auricle. The occipital artery, which supplies blood to the back of the scalp, pierces the fascia connecting the cranial attachment of the sternocleidomastoid and trapezius muscle, and ascends at a mean distance of 4.2 cm from the midline of the external occipital protuberance.14
Planning Scalp Incisions
The single most important means of preventing or minimizing wound healing complications of the scalp is the thoughtful planning of the initial scalp incision. Because the current treatment of malignant brain tumors commonly requires repeated craniotomies, scarring and a loss of tissue elasticity can be predictably anticipated. Soft tissue is frequently further compromised by external beam radiation, resulting in scalp wound breakdown, failure to heal, or skin necrosis. Therefore, in anticipation of such compromised wound healing, the initial operative incision should be placed so as to maximize blood flow to the healing incision, and to allow for alternate salvage maneuvers in the event of wound breakdown. Generally, linear scalp incisions that run parallel to the major arteries of the scalp are preferred over traditional U-shaped craniotomy flaps. Such linear incisions are well perfused, heal favorably, and offer broader surgical options in the event of wound breakdown.15 Ideally, the scalp incision lies adjacent to, rather than directly over, underlying anticipated cranial osteotomies, microplates, and screws, to avoid hardware exposure and infection of the craniotomy bone segments in the event of minor incisional dehiscence.
Wounds with Tissue Loss
The management of scalp wounds with soft-tissue loss is determined by the amount of soft tissue lost and the type of tissue exposed. Even relatively small scalp defects can present a reconstructive challenge. The inherent inelasticity of the galea aponeurotica contributes to a property known as “stretch-back,” the tendency for the scalp to contract back toward its original state.16 Stretch-back leads to increased tension and ischemia across the healing incision, with sequelae ranging from alopecia and widened scars, to nonhealing wounds and tissue necrosis. The convex curvature of the cranium also complicates scalp closures, requiring additional flap length to achieve the desired tissue advancement. Finally, although local scalp flaps provide the best cosmetic outcome, hair-bearing scalp is a limited resource, and one that can be further depleted by pre-existing scars across the axial blood supply.
Local Advancement (Galeal Scoring)
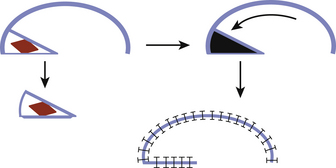
The surface area of the scalp adjacent to the defect can be enlarged considerably by galeal scoring to allow closure by advancement of the wound edge. Each cut, which is made at 1-cm intervals in a parallel or crosshatch fashion, allows the scalp to be stretched approximately 4 to 6 mm. Galeotomies should be oriented perpendicular to the desired direction of advancement, and must be done carefully so as to avoid compromising the vascular supply to the scalp with incisions that are unnecessarily deep. This scoring maneuver requires a complete division of the substantial galeal layer (Fig. 139-1). Tissue loss of more than 1 to 2 cm may require extension of the laceration to allow greater undermining and scoring. Defects that are 3 cm in diameter can be routinely closed with this technique.
Skin Grafts
Meshed grafts are those that are mechanically perforated in a grid pattern, which allows them to be expanded and to conform to irregular surfaces. The perforations also provide egress for wound drainage. The resultant improved graft bed contact optimizes conditions for graft take (Fig. 139-2). For scalp defects, meshed grafts should not be perforated and expanded more than 1.5 times their normal size unless donor skin is in short supply. A widely expanded graft is less desirable because larger open areas take longer to epithelialize and provide poorer protective coverage.
Another method involves making small drill holes 1 cm apart through the outer table down into the diploë space. Usually, granulation tissue arises from these holes and grows over the exposed calvarium to coalesce and form a suitable bed for skin grafting.
Recently, multiple studies showed that vacuum-assisted closure devices hasten soft-tissue contraction as well as granulation tissue formation in various trunk and extremity wounds.17 In the scalp, two small studies demonstrated a possible role for the vacuum-assisted closure device. Molnar et al. treated four patients with exposed skull by removing the outer table, immediate application of a split thickness skin graft to the diploë and treatment of the wound with a vacuum-assisted closure device for 3 to 4 days. The author reported a 100% graft take without complications.18 In a another study, Umesh et al. reported a successful case report where the author closed a 10 × 12 cm wound with exposed dura by using the vacuum-assisted closure device over the dura for a period of 3 weeks and then grafting the granulated wound bed with a split thickness skin graft.19
Flaps
The two basic principles of flap coverage follow:1 move available tissue with its intact circulation from an area of relative excess to the area of deficiency, and2 optimize vascularity of the flap. In the scalp, the lateral and posterior aspects are usually used as donor sites to avoid distortion of the forehead or frontal hairline. The flaps are designed to include axial vessels in their bases. Usually, the superficial temporal artery or the occipital artery provides the basic blood supply to these flaps. The flaps should be designed with respect to previous incisions, which may block vascular inflow to the flap. Many types of flaps are possible in the scalp. The most commonly used flaps include rotation flaps and bipedicle advancement flap.20 Orticochea flap and double-scalping flap are occasionally used for large defects. The flap’s design requires considerable expertise and extensive mobilization of the scalp tissues. Usually most of the scalp must be degloved in the subgaleal space, and the galea should be scored to allow primary closure of the secondary defect.
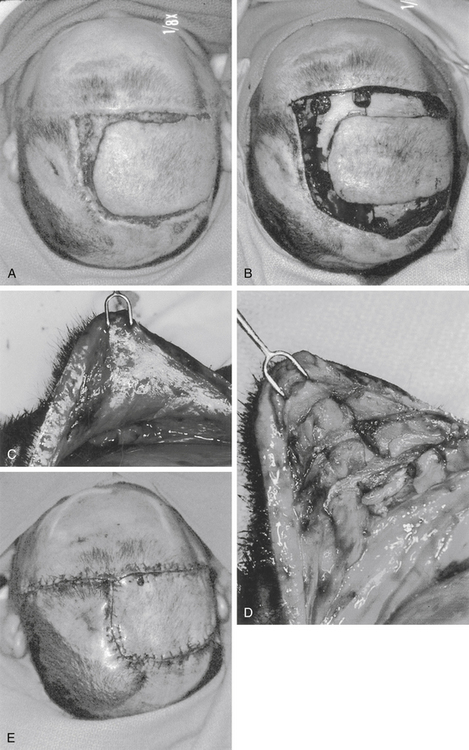
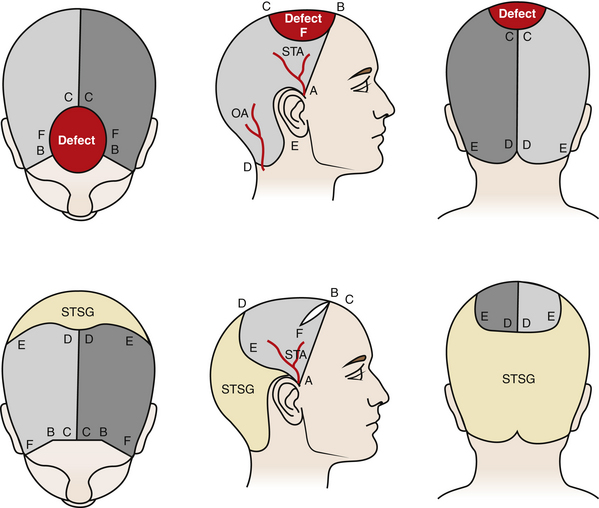
Rotation flaps use adjacent tissue rotated in an arc to close the defect. An isoceles triangle is designed around the defect, making the shortest side the base of the triangle. Next, a semicircular arc is outlined by creating a half circle that incorporates the triangular base at one end. The length of the flap’s arc are four to six times the size of the base, i.e., the diameter of the defect (Fig. 139-3). In the scalp, rotation flap are generally used to transfer an anterior hairline defect posterior. In general, a skin graft is needed to close the posterior donor site (Fig. 139-4).
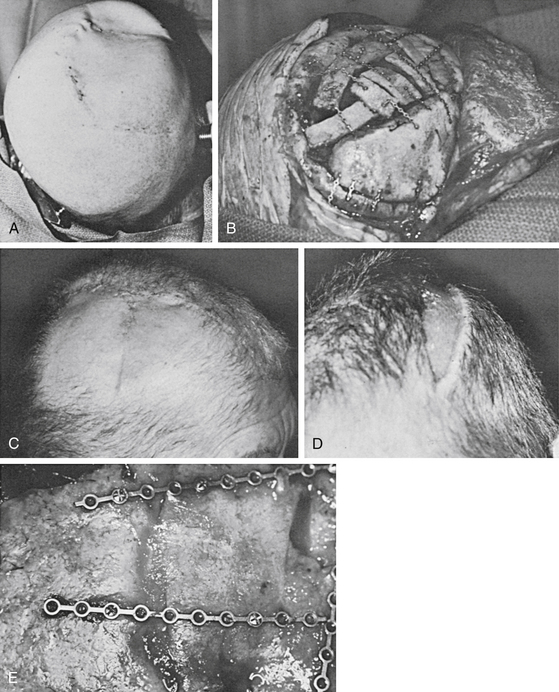
Advancement flaps slide adjacent tissue to close the defects. In the scalp, bipedicle flaps are the most commonly used advancement flaps. They are generally used to close large parietal defects. These flaps are designed to allow advancement into the adjacent defect in a vector that is perpendicular to the flap axis. They are created by making a parallel incision to the longitudinal axis of the wound. The flap length-to-width ratio of 4:1 may be achieved. The occipital and supraorbital arteries are generally incorporated in the design of the flap for parietal wounds. The flap, between the wound edge and the skin incision, is undermined in a subgaleal plane. The flap is then advanced to close the defect. At most, the bipedicle advancement flap could be advanced a distance equivalent to the width of the flap. In most cases, back-grafting of the donor site is required (Figs. 139-5 and 139-6; see case report 1).
Orticochea initially described his flap in 1967 and then modified in 1971. The Orticochea flap is valuable for large anterior or occipital defects, i.e., greater than 50 cm2. In this technique, the remaining scalp is divided into three large flaps. Two flaps are based on the superficial temporal vessels and one flap is based on the occipitals vessel. The Orticochea technique relies heavily on scoring the galea of the three flaps to expand the size of the flap and help close the defect.21
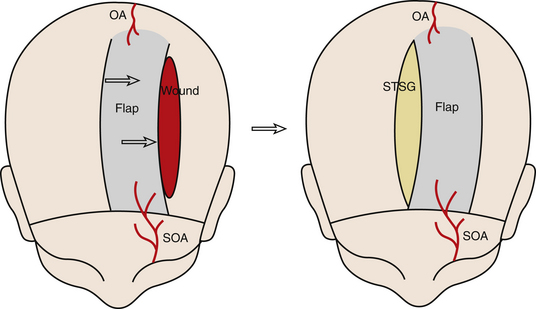
Recently, Papadopoulos et al. described the double scalping flap for coverage of a large full-thickness defect of the vertex.22 Two large posterolateral flaps are created based on the superficial temporal vessels. The flaps are then rotated anteriorly to cover the defect. A spilt-thickness skin graft is used to cover the donor site (Fig. 139-7).
In brief, good design of the above flaps entails closure of the defect without tension, incorporation of major vascular pedicles and preservation of the native hairline. Subsequent tissue expansion can be used to remove the skin grafted donor site. Wounds too large to allow local flap coverage usually require coverage with a free tissue transfer (free flap).
Free Flaps
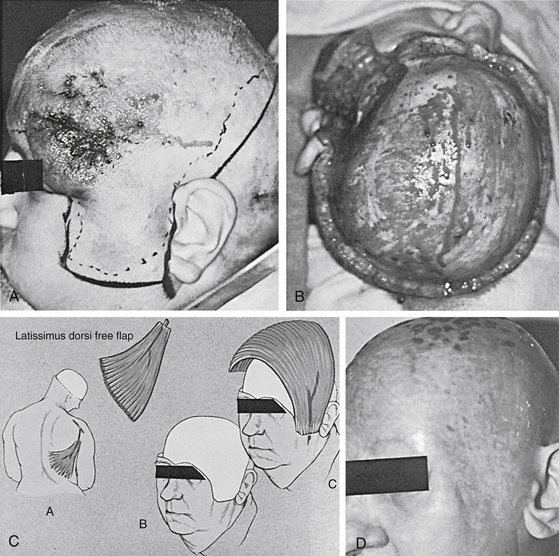
Closure of near-total scalp defects or even smaller scalp defects in the setting of previous scalp radiation requires the use of free tissue transfer. A free flap is one that is completely detached from the donor area and moved to another site on the body. After this movement, circulation to the flap is restored by the microsurgical anastomosis of flap and available recipient site vasculature. The superficial temporal artery or other branches of the external carotid system in the neck most often provide donor site vasculature for free tissue transfers used to reconstruct the scalp. Since Miller first reported use of a microsurgical technique in the scalp in 1976, many different free flaps have been used to cover large scalp defects. The latissimus dorsi muscle provides the best coverage because of its large surface area and long vascular pedicle23 (Fig. 139-8; see case report 2). Other free flaps used to cover the scalp include anterolateral thigh, radial forearm, parascapular, omentum, rectus abdominus myocutaneous flap, and even scalp from an identical twin.24–28
The addition of free flaps to the reconstructive surgeon’s armamentarium makes almost any size or type of wound reconstructable29 in a single-stage procedure. However, they have donor site morbidity and they are time consuming. Therefore, they are reserved for situations when local flaps or skin graft is not an appropriate option.
Tissue Expansion
Tissue expansion makes use of the long-observed principle that skin expands to accommodate itself to gradual stretching. In 1978, Radovan was the first to report successful clinical applications of this observation and to demonstrate prototypes of the expanders in clinical use today.9–11 The expanders that are currently in use are silicone bags with self-sealing valves placed beneath the areas to be expanded.
Tissue expansion has become a preferred technique of scalp reconstruction because it provides hair-bearing skin for reconstruction. Up to 50% of the scalp can be reconstructed with tissue expanders30 without a clinically noticeable change in the density of hair follicles in the expanded skin. Unlike many rotation flaps, the resultant advancement flap created by the expansion process does not appreciably change the orientation of the hair follicles.20
Tissue expanders are available in different sizes and shapes. In general, rectangular expanders (38%) give the most in tissue gains when compared to crescentic (32%) and round (25%) bases.31 Tissue expanders are placed adjacent to the defect in a subgaleal pocket. Saline may be injected percutaneously through a one-way valve into the expander once or twice per week to increase the volume. The expansion of tissue expanders should proceed until the expanded scalp is 20% bigger than the size of the wound to account for tissue recoil during flap advancement.32 The duration required to inflate the expander varies with each clinical situation, but it is usually between 4 and 8 weeks. At a second operation, the expander is removed, and the resultant scalp flap is used to close the defect.
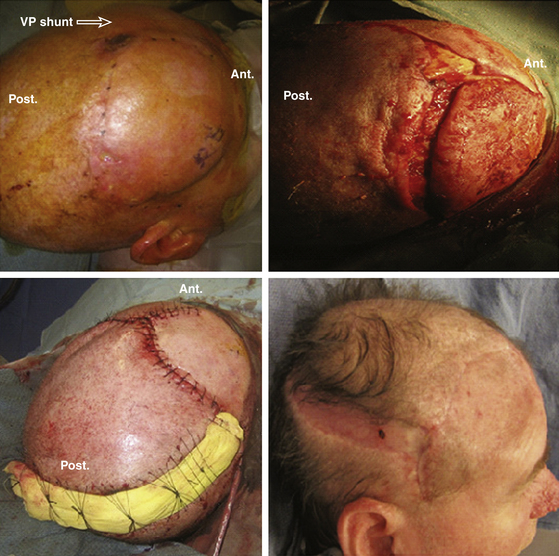
Disadvantages of tissue expansion include the need for two or more operations, the interim deformity, and usually the fact that the expander cannot be placed in the acute setting. Tissue expanders are most often placed after the wound has been closed temporarily by simpler but less definitive techniques (Fig. 139-9; see case report 3).
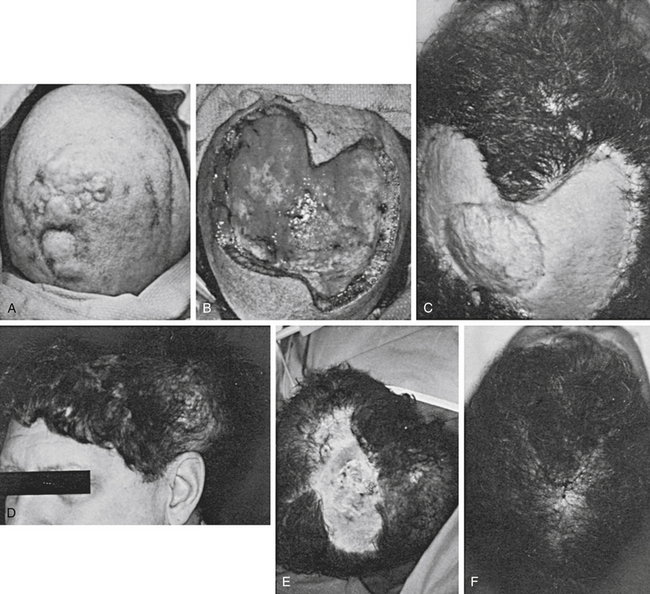
Case Report 3: Scalp Reconstruction with Skin Graft and Tissue Expansion
The lesion was excised together with a 2-cm margin of grossly normal-appearing tissue. Removal resulted in a defect down to the periosteum of 17 × 12 cm. Where the tumor was adjacent to the outer table, the outer table was excised to an area 8 cm2. The wound was immediately closed by the placement of a meshed, split-thickness skin graft of approximately 0.014-inch thickness on the patient’s periosteum and drilled-down outer table. This recipient site was dressed with Adaptic and saline-soaked gauze, which was immobilized by sewing the dressing to the intact scalp. The dressing was removed after 7 days, at which time the graft had almost completely taken. The area was observed for a recurrence for 1 year, but none occurred. During this time, small areas of skin graft breakdown occurred; these areas were managed with local dressing care. Approximately 1.5 years after radical removal of the tumor, two large tissue expanders were placed in the hair-bearing skin in the occipital and temporal areas. These expanders were gradually filled over the next 10 weeks, after which the expanders were removed and the resultant scalp flap was advanced. This action resulted in a remaining defect of approximately 8 × 10 cm. Two more tissue expanders were placed, and the expansion process was repeated. At the time of removal of the second set of expanders, the entire area of skin-grafted skull was resurfaced with hair-bearing skin.
Conclusions
Reconstruction of the scalp requires careful clinical examination to provide optimal definition of the real or anticipated defect and the status of the adjacent scalp. The size and location of a soft-tissue defect will determine the complexity of procedures required for reconstruction. Full-thickness skin coverage for the skull can usually be provided either by galeal scoring and direct advancement or by the creation of a local flap. As noted earlier, galeal scoring and advancement of wound edges usually close defects up to 3 or 4 cm in diameter. Defects larger than this can be closed by creating a rotation or bipedicle flap and by closing the secondary defect with a split-thickness skin graft on the skull periosteum. Large and complex defects usually require free tissue transfers. Tissue expanders are most often placed in the scalp secondarily to reconstruct alopecia defects with neighboring hair-bearing tissue.
Argenta L.C., Morykwas M.J. Vacuum-assisted closure: a new method for wound control and treatment. Clinical experience. Ann Plast Surg. 1997;38:563-577.
Arnold P.G., Rangarathnam C.S. Multiple-flap scalp reconstruction: Orticochea revisited. Plast Reconstr Surg. 1982;69:605-613.
Borah G.L., Hidalgo D.A., Wey P.D. Reconstruction of extensive scalp defects with rectus free flaps. Ann Plast Surg. 1995;34:281-285.
Buncke H.J., Hoffman W.Y., Alpert B.S. Microvascular transplant of two free scalp flaps between identical twins. Plast Reconstr Surg. 1982;70:605-609.
Chicarilli Z.N., Ariyan S., Cuono C.B. Single-stage repair of complex scalp and cranial defects with the free radial forearm flap. Plast Reconstr Surg. 1986;77:577-585.
Elliott L.F., Jurkiewicz T. Scalp and calvarium. In: Jurkewicz T., Mathes S., Ariyan S. Plastic Surgery: Principles and Practice. St. Louis: Mosby; 1990:419-440.
Gordon L., Buncke H.J., Alpert B.S. Free latissimus dorsi muscle flap with split-thickness skin graft cover: a report of 16 cases. Plast Reconstr Surg. 1982;70:173-178.
Ikuta Y. Microvascular free transfer of omentum. In: Vasconez L.O., Strauch B. Grabb’s Encyclopedia of Flaps. Philadelphia: Lippincott-Raven; 1998:42-44.
Koss N., Robson M.C., Krizek T.J. Scalping injury. Plast Reconstr Surg. 1975;55:439-444.
Leedy J.E., Janis J.E., Rohrich R.J. Reconstruction of acquired scalp defects: an algorithmic approach. Plast Reconstr Surg. 2005;116:54e-72e.
Lutz B.S. Aesthetic and functional advantages of the anterolateral thigh flap in reconstruction of tumor-related scalp defects. Microsurgery. 2002;22:258-264.
Manders E.K., Graham W.P.III, Schenden M.J., Davis T.S. Skin expansion to eliminate large scalp defects. Ann Plast Surg. 1984;12:305-312.
Marathe US, Sniezek JC. Use of Vacuum-Assisted Closure Device in Enhancing Closure of a Massive Skull Defect. Paper presented at Western Section Meeting of the Triological Society, January 31, 2003.
Molnar J.A., DeFranzo A.J., Marks M.W. Single-stage approach to skin grafting the exposed skull. Plast Reconstr Surg. 2000;105:174-177.
Nair S., Giannakoupoulous G. Surgical management of radiated scalp in patients with recurrent glioma. Neurosurgery. 1994;34(1):103-107.
Nordstrom R. “Stretch-back” in scalp reductions for male pattern baldness. Plast Reconstr Surg. 1984;73:422-426.
Nordstrom R.E.A., Devine J.W. Scalp stretching with a tissue expander for closure of scalp defects. Plast Reconstr Surg. 1985;75:578-581.
Orticochea M. New three-flap scalp reconstruction technique. Br J Plast Surg. 1971;24:184-188.
Papadopoulos O., Karypidis D., Moustaki M., et al. Double scalping flap: a versatile technique in scalp reconstruction. J Craniofac Surg. 2009;20:1484-1491.
Radovan C. Tissue expansion in soft-tissue reconstruction. Plast Reconstr Surg. 1984;74:482-492.
Schmidt D., Adelmann G. The course of the occipital artery—an anatomical investigation for biopsy in suspected vasculitis. Eur J Med Res. 2001;6:235-241.
Schwenn O.K., Wustenberg E.G., Konerding M.A., Hattenbach L.O. Experimental percutaneous cannulation of the supraorbital arteries: implication for future therapy. Invest Ophthalmol Vis Sci. 2005;46:1557-1560.
Urken M.K., Catanlano P.J., Sen C. Free tissue transfer for skull base reconstruction: an analysis of complications and a classification system for defining skull base defects. Arch Otolaryngol Head Neck Surg. 1993;119:1318-1325.
van Rappard J.H., Molenaar J., van Doorn K., et al. Surface-area increase in tissue expansion. Plast Reconstr Surg. 1988;82:833-839.
Zide B. Surgical Anatomy around the Orbit: the System of Zones. Philadelphia: Lippincott; 2005.
1. Koss N., Robson M.C., Krizek T.J. Scalping injury. Plast Reconstr Surg. 1975;55:439-444.
2. Strayer L.M. Augustin Belloste and the treatment of avulsion of the scalp. N Engl J Med. 1939;220:901.
3. Netolitzky J. Zur Kasuistik der Hauttransplantation. Wien Med Wochenshr. 1871;21:820.
4. Robinson E.F. Total avulsion of the scalp. Surg Gynecol Obstet. 1908;7:663.
5. Kazanjian V.H. Repair of Partial Losses of the Scalp. Plast Reconstr Surg. 1953;12:325-334.
6. Orticochea M. Four-flap scalp reconstruction technique. Br J Plast Surg. 1967;20:159.
7. Orticochea M. New three-flap scalp reconstruction technique. Br J Plast Surg. 1971;24:184-188.
8. Miller G.D., Anstee E.J., Snell J.A. Successful replantation of an avulsed scalp by microvascular anastomoses. Plast Reconstr Surg. 1976;58:133-136.
9. Radovan C. Breast reconstruction after mastectomy using the temporary expander. Plast Reconstr Surg. 1982;69:195-208.
10. Radovan C. Tissue expansion in soft-tissue reconstruction. Plast Reconstr Surg. 1984;74:482-492.
11. Nordstrom R.E.A., Devine J.W. Scalp stretching with a tissue expander for closure of scalp defects. Plast Reconstr Surg. 1985;75:578-581.
12. Zide B. Surgical Anatomy around the Orbit: the System of Zones. Philadelphia: Lippincott; 2005.
13. Schwenn O.K., Wustenberg E.G., Konerding M.A., Hattenbach L.O. Experimental percutaneous cannulation of the supraorbital arteries: implication for future therapy. Invest Ophthalmol Vis Sci. 2005;46:1557-1560.
14. Schmidt D., Adelmann G. The course of the occipital artery—an anatomical investigation for biopsy in suspected vasculitis. Eur J Med Res. 2001;6:235-241.
15. Nair S., Giannakoupoulous G. Surgical management of radiated scalp in patients with recurrent glioma. Neurosurgery. 1994;34(1):103-107.
16. Nordstrom R. “Stretch-back” in scalp reductions for male pattern baldness. Plast Reconstr Surg. 1984;73:422-426.
17. Argenta L.C., Morykwas M.J. Vacuum-assisted closure: a new method for wound control and treatment. Clinical experience. Ann Plast Surg. 1997;38:563-577.
18. Molnar J.A., DeFranzo A.J., Marks M.W. Single-stage approach to skin grafting the exposed skull. Plast Reconstr Surg. 2000;105:174-177.
19. Marathe US, Sniezek JC. Use of Vacuum-Assisted Closure Device in Enhancing Closure of a Massive Skull Defect. Paper presented at Western Section Meeting of the Triological Society, January 31, 2003.
20. Leedy J.E., Janis J.E., Rohrich R.J. Reconstruction of acquired scalp defects: an algorithmic approach. Plast Reconstr Surg. 2005;116:54e-72e.
21. Arnold P.G., Rangarathnam C.S. Multiple-flap scalp reconstruction: Orticochea revisited. Plast Reconstr Surg. 1982;69:605-613.
22. Papadopoulos O., Karypidis D., Moustaki M., et al. Double scalping flap: a versatile technique in scalp reconstruction. J Craniofac Surg. 2009;20:1484-1491.
23. Gordon L., Buncke H.J., Alpert B.S. Free latissimus dorsi muscle flap with split-thickness skin graft cover: a report of 16 cases. Plast Reconstr Surg. 1982;70:173-178.
24. Borah G.L., Hidalgo D.A., Wey P.D. Reconstruction of extensive scalp defects with rectus free flaps. Ann Plast Surg. 1995;34:281-285.
25. Ikuta Y. Microvascular free transfer of omentum. In: Vasconez L.O., Strauch B. Grabb’s Encyclopedia of Flaps. Philadelphia: Lippincott-Raven; 1998:42-44.
26. Chicarilli Z.N., Ariyan S., Cuono C.B. Single-stage repair of complex scalp and cranial defects with the free radial forearm flap. Plast Reconstr Surg. 1986;77:577-585.
27. Lutz B.S. Aesthetic and functional advantages of the anterolateral thigh flap in reconstruction of tumor-related scalp defects. Microsurgery. 2002;22:258-264.
28. Buncke H.J., Hoffman W.Y., Alpert B.S., et al. Microvascular transplant of two free scalp flaps between identical twins. Plast Reconstr Surg. 1982;70:605-609.
29. Urken M.K., Catanlano P.J., Sen C. Free tissue transfer for skull base reconstruction: an analysis of complications and a classification system for defining skull base defects. Arch Otolaryngol Head Neck Surg. 1993;119:1318-1325.
30. Manders E.K., Graham W.P.III, Schenden M.J., Davis T.S. Skin expansion to eliminate large scalp defects. Ann Plast Surg. 1984;12:305-312.
31. van Rappard J.H., Molenaar J., van Doorn K., et al. Surface-area increase in tissue expansion. Plast Reconstr Surg. 1988;82:833-839.
32. Elliott L.F., Jurkiewicz T. Scalp and calvarium. In: Jurkewicz T., Mathes S., Ariyan S. Plastic Surgery: principles and Practice. St. Louis: Mosby; 1990:419-440.