CHAPTER 248 Principles of Radiation Therapy
Basic Principles of Radiation Therapy
Cellular Effects of Radiation
Radiation therapy uses ionizing radiation, which means that the radiation’s energy is sufficient to remove an electron from the outer shell of an atom (more than 124 electron volts).1 In clinical therapy, photon radiation is most commonly generated by linear accelerators or gamma-ray sources that produce a range of ionizing photon energies. When a high-energy photon interacts with an atom, the atom becomes unstable and is more apt to react with surrounding atoms. With this simple model of ionizing radiation as background, and remembering that in many ways standard radiation sources or beams act like visible light, we can explain the effects of radiation on cells.
Evidence supporting that DNA is the most critical cellular target of radiation includes (1) genetic damage and specific chromosomal abnormalities after irradiation are correlated with cell death, (2) defects in cell DNA repair mechanisms increase radiosensitivity, and (3) selective irradiation of the cell nucleus as opposed to cytoplasm has mimicked the overall effect of radiation on cells.1 This basic effect of irradiation on DNA and the cell’s subsequent repair underlie the observed biologic effects of conventional fractionated radiotherapy treatment.
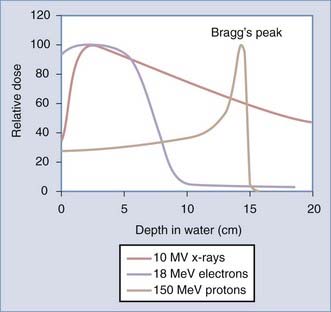
There is an active interest in increasing application of particle therapy, specifically protons. As opposed to behaving like a light wave, protons deposit their energy at a specified depth over a more tightly defined area, or Bragg’s peak. The pattern of energy distribution of particle therapy (electrons and protons) compared with photon therapy (x-ray) is shown in Figure 248-1. Particles are charged and have physical properties that cause direct damage to the DNA (rather than through reactive water molecules). Their clinical advantage over photon therapy is in less dose to normal tissues, which may be especially important in children who are at risk for radiation-induced malignancy. Electron therapy is readily available on all linear accelerators and has been used clinically for decades; however, this modality is rarely used in central nervous system (CNS) malignancies. Proton therapy requires a multimillion dollar machine called a cyclotron and thus remains less commonly available and of uncertain, albeit promising, importance in years to come.2,3
Therapeutic Ratio for Radiation
Biologic Selectivity
Normal tissue generally repairs DNA damage more efficiently than neoplastic tissue. Reasons may include metabolic differences, aberrant cell cycle control mechanisms, and inherent cellular differences. Cells require time to repair DNA damage, and the normal cell response to irradiation is to delay cell cycle progression in the G2 phase.4,5 The length of G2 delay correlates with radiation resistance because the cell has more time to repair radiation damage.6
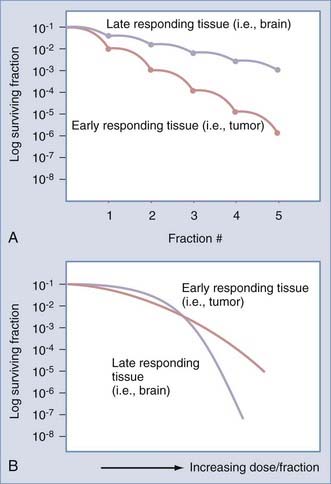
This enhanced repair capacity of normal tissue has been critically exploited by fractionated regimens with low doses of radiation (the total therapeutic dose is broken up into a series of small daily treatments). Because a high percentage of normal cells will repair damage at low doses of radiation and because neoplastic cells are less capable of efficient repair, the concept of fractionation of radiation is supported. Figure 248-2A demonstrates a schematic rendering of the effect of multiple fractions of 2 Gy on both normal tissues and tumor; the more fractions, the greater the therapeutic advantage. These principles were extended to hyperfractionated approaches (two or three small doses per day), although with limited success.7–10
Another clinical observation is that more rapidly dividing normal tissues such as skin and oral mucosa are more acutely sensitive to the effects of irradiation than are late-responding or slowly dividing and nondividing tissues such as nerve tissue.1,11,12 In contrast, late-responding tissues are more sensitive to large doses of radiation. Note that in Figure 248-2B, a large single dose of radiation is capable of greater injury to late-responding normal tissue than to the tumor.11,13 Therefore, to take advantage of the inherent differential cellular capacity to repair sublethal doses of irradiation, multiple small doses of radiation have been recommended. This basic principle supports the biologic advantage of fractionation to selectively protect late-responding nerve tissues.
Technical Selectivity
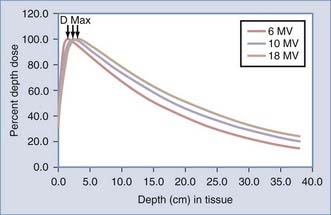
Therapeutic advantage may also be achieved by depositing more radiation dose in the tumor target than in the normal tissue. A single-photon radiation beam entering a patient begins with a region of lower dose, termed the build-up region,14 and then progressively increases until it reaches the depth of maximal dose (Dmax) that is characteristic of the radiation beam energy (Fig. 248-3). This build-up region spares the skin from the highest doses of radiation. After the radiation beam reaches Dmax, the dose decreases with depth as it is attenuated (see Fig. 248-3). Much like shining a light into a dark field, the light becomes less bright the farther you move away from its origin as it becomes attenuated by air.
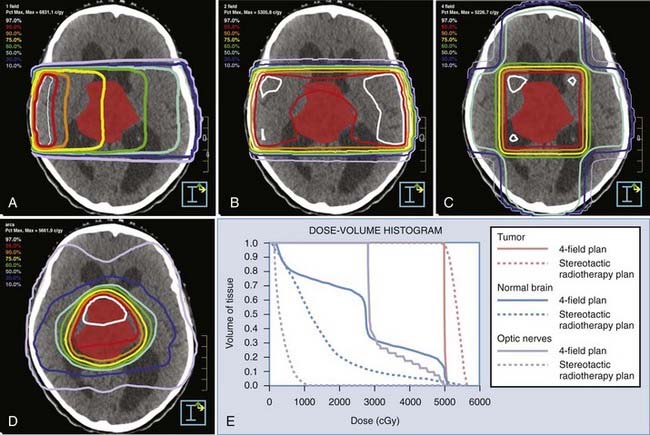
To achieve technical selectivity, several beams of radiation can be added together from several directions (Fig. 248-4). A single beam of radiation entering a patient is shown in Figure 248-4A. In this figure, a group of same dose, or isodose lines, is labeled as a percentage of maximal dose. The percentage of maximum decreases with depth owing to attenuation. When we put a tumor at the intersection of several beams of radiation oriented at divergent angles, the isodose lines are as shown in Figure 248-4B to D. Note that the tumor receives the highest dose, and areas outside the tumor now receive progressively less dose. As multiple beams are added together, the isodose lines become densely concentrated at higher percentages of maximum, as shown in Figure 248-4D. In considering these physical characteristics of radiation beams, these examples illustrate the capacity of multiple radiation beams to deliver a higher percentage of the radiation dose to the tumor, with progressively smaller areas of normal tissue receiving significant doses.
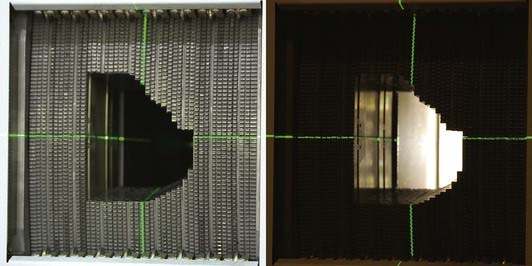
In addition to the additive effects of multiple converging beams of radiation on the target, each beam may be selectively filtered in the plane of its delivery. The intensity of light could then be altered at different areas in a given field of radiation. If we could control this factor as a radiation beam was delivered, we could then further shape the radiation beam. This is accomplished practically by a series of moving leaves that are computer controlled and move across a radiation field during treatment. This leaf-moving device is called a multileaf collimator (Fig. 248-5) and is used both to shape static beams and to modulate the intensity of each beam, hence the term IMRT.
Clinical Application of Radiation Therapy
Application of the biologic characteristics and physical characteristics to the clinical discipline of radiotherapy leads us to recognize several parameters of treatment that define both the therapeutic effect on the tumor and the likelihood of normal tissue complications. One important parameter is dose. Dose must be described as total dose as well as dose delivered at each treatment (per fraction). Multiple small doses of radiation generally yield greater biologic advantage,11,13 as illustrated in Figure 248-2A. The small doses are most commonly given once a day and are termed standard fractionation. A second method of delivery is with two or more smaller radiation doses each day, separated by time (4 to 6 hours) to allow for normal tissue repair; this is called hyperfractionation. The total dose and the pattern of treatment delivery (daily or twice daily fractionation) are both critical in determining the effect of radiation treatment on both tumor and normal tissue.11,13,15 More recently, the ability to give a single or a limited number of fractions (hypofractionation) has been studied intensively. In these regimens, the total dose and time over which they are given remains critical in defining the effects.
Another parameter that is important in describing radiation treatment is time.11,13 This has been confirmed clinically in that radiation treatment schemes with gaps or splits in treatment have been less successful than those in which a continuous course has been given.15,16 Furthermore, treatment regimens delivering the same dose over a shorter period have been more effective in treating tumors than those delivered over a longer period.15 Delivering the same dose over a shorter time is termed accelerated fractionation. Hence, treatment descriptions should note the elapsed number of days (time—both treatment and nontreatment days and including gaps in treatment) over which the treatment course was completed.
Another critical parameter for radiation treatment effect is the volume of tissue treated.17 A small area of the brain will tolerate a much larger dose without serious toxicity than will the entire brain. Furthermore, the particular volume that is treated may determine the toxicity; different areas of the nervous system have diverse tolerances, and functional neuroanatomy dictates the observed effect of a toxicity.18
Basic Paradigms of Radiation Treatment Delivery
Conventional Radiotherapy
Historically, radiotherapy planning relied on a combination of physical examination, knowledge of the disease (spread patterns), and two-dimensional image sets as defined by plain film radiographs.19,20 This technique is still used for simple treatment plans such as whole-brain radiotherapy. Treatment planning sessions are called simulations and are used to position the patient in reference to the treatment machine for appropriate beam entrance and exit. Immobilization devices such as masks (Fig. 248-6) are constructed to obtain reproducibility of the patient for daily treatment setup. Patient position is marked by the intersection of room lasers. These room lasers intersect at the center point of the axis of rotation for the treatment machine. This center point is called the isocenter for the machine. To position a patient, the intersection of these lasers with the patients’ physical anatomy makes three points (one on each side and one at the intersection from the ceiling) that are marked or tattooed (permanent marks). As such, the approximate position at the simulation is recorded permanently on the patient’s body or the mask. Plain-film simulation radiographs are obtained by using a diagnostic x-ray machine that mimics the geometry of the treatment machine. Radiographs are taken at the recorded patient position for each treatment portal or beam. These radiographs show the patient’s bony anatomy, some soft tissues, and otherwise contrasted and marked (barium or metallic indicators) areas. A film is taken for each of the beams that will be used for the treatment. The films also indicate the isocenter point. The radiation oncologist can then map the patient’s tumor from CT or magnetic resonance imaging (MRI) to the planning plain films in reference to bony anatomy. For diseases in which a spread pattern is known, the anatomy at risk may be treated on the basis of the bony landmarks alone (e.g., in tumors that seed the cerebrospinal fluid, the craniospinal axis is often treated based on the known bony anatomy defining the craniospinal contents). A treatment plan is then designed based on this mapped or otherwise identified treatment target so that the prescribed dose covers the target while minimizing the dose to nearby normal structures when possible.
Virtual Simulation and Three-Dimensional Conformal Radiotherapy
A newer way to plan and deliver radiotherapy is called virtual simulation.21–24 Rather than using the two-dimensional plain-film simulation radiograph as a treatment planning template, virtual simulation directly uses three-dimensional imaging studies such as CT and MRI for treatment planning. The first step in the virtual simulation process is to construct an immobilization device with the patient in the treatment position designated by the radiation oncologist (see Fig. 248-6). On the immobilization device or on the patient, a set of room laser points are identified, and fiducials are placed at these intersection points. A detailed imaging study is obtained with the patient in the immobilization device, and the fiducials are identified on these images. This three-dimensional imaging study can be used to construct a virtual patient in a computer that serves as the treatment planning template. Diagnostic MRI and CT images can be imported and registered with the simulation images so that tumor extent can be more precisely identified. The radiation oncologist then delineates the tumor volume, target volume, and critical structures directly on this virtual patient, and treatment portals are iteratively optimized to cover the target volume and avoid the critical structures near the target.
The ability to iteratively alter the beam arrangement is also a major advance over conventional simulation. Virtual simulation allows trials of multiple beam entrances and exits without requiring patient participation or new radiographs for each potential beam. Furthermore, treatment with oblique angles is facilitated through the use of virtual simulation because no mapping based on a radiograph is needed. The CT image set may be used to reconstruct a plain film radiograph (digitally reconstructed radiograph [DRR]) to confirm the treatment portal and arrangement for treatment quality assurance. As the use of oblique fields has become more feasible, the use of non-coplanar field arrangements has become achievable. This intensive approach to treatment planning and delivery provides a powerful tool to tightly conform the dose distribution to the target volume. This technique is termed three-dimensional conformal therapy and is desirable because the elimination of normal tissue from the treatment field may allow escalation to a higher dose of radiation, thus enhancing tumor control.25,26
Stereotactic Radiosurgery
Although virtual simulation provides a significant improvement over conventional radiotherapy, it is limited by the accuracy of its fiducial system, which is not very robust. Both conventional simulation and virtual simulation rely on room lasers and immobilization devices for registration of the patient to the treatment planning template and to the treatment machine for treatment delivery. The most accurate application of virtual simulation is a technique known as stereotactic radiosurgery. Stereotaxis is the most important part of this treatment paradigm, in that it allows precise co-identification of the virtual patient in the computer with the actual patient in world space. This is accomplished by attaching a rigid stereotactic head ring to the patient, using this ring as a frame of reference throughout the process. A system of three-dimensional fiducials is attached to the head ring during CT acquisition, providing accurate spatial identification of each pixel within the CT image set. In essence, each pixel becomes a mathematical coordinate in reference to the head ring. After the treatment coordinates (pixels) have been identified in the treatment plan, they can be directly transferred to a mechanical system that provides accurate patient localization relative to the treatment unit. To use this paradigm optimally, mechanical error in the treatment delivery device and imaging inaccuracy defined by pixel size and slice thickness must be minimized. The mechanical accuracy using the University of Florida system is 0.2 mm ± 0.1 mm,27,28 and images are obtained with 0.6-mm pixel size and 1-mm slice thickness.28 This paradigm for radiation treatment delivery has become a standard treatment option for numerous benign and malignant CNS pathologies. Because such overall accuracy is achieved, extremely conformal dose distributions are developed, and minimal normal brain receives a significant dose of radiation (see Fig. 248-4D and E). Therefore, in practical application, the requirement to spare normal tissue using biologic characteristics is minimized. Radiosurgery has abandoned the typical fractionation patterns of conventional radiotherapy and has been applied as single treatment doses designed to have maximal affect on the tumor, sparing the normal tissue by means of physical deposition of significant doses into only the tumor or lesion. This paradigm is a unique hybrid of radiation therapy and neurosurgery such that the approach to treatment is somewhere between both disciplines. In more recent years, frameless stereotactic radiosurgery systems have been developed and used successfully in the single-fraction setting.29–32 Thus, the patient is spared the morbidity of rigid frame placement.
Stereotactic Radiotherapy
Although stereotaxis allows the most accurate application of virtual simulation, the rigid head ring and ablative approach to treatment have limited the procedure to single-fraction treatments. Despite the utility of single-fraction stereotactic radiosurgery, many circumstances prohibit delivery of large single doses of radiation. For example, lesions near the optic nerve or those larger than 3.5 to 4 cm are inappropriate for radiosurgery. In the case of the optic apparatus, this location is extremely sensitive to single doses higher than about 8 Gy. This dose, unfortunately, is subtherapeutic for treating most brain tumors. Larger lesions are also limited by the dose they may receive because higher doses become too toxic to the surrounding normal brain, despite the exquisite accuracy and conformality. Furthermore, the desirability of single-fraction treatment for some lesions has been debated from a radiobiologic perspective.13,33 Therefore, the stereotactic paradigm has been extended to fractionated treatment regimens.34–37 Numerous systems have been reported, with daily delivery repeatability between 0.6 and 4.5 mm.31,32,37–47 These fractionated stereotactic systems have proved feasible although significantly less accurate than an optimized radiosurgical system. All these systems have used a modified head ring or mask device to combine patient immobilization with daily patient localization. Other systems have been designed that attempt to separate the functions of immobilization and localization.31,48,49 These systems, although more complex, tend to be more reproducible than repeat fixation mask systems. For example, the University of Florida stereotactic group has developed a system that relies on the optic tracking of infrared light-emitting diodes attached to a custom bite plate system. This system allows real-time patient tracking along six degrees of freedom and provides submillimetric precision in reproducibility of daily patient positioning.19,31,32
Bova FJ, Buatti JM, Friedman WA, et al. The University of Florida frameless high-precision stereotactic radiotherapy system. Int J Radiat Oncol Biol Phys. 1997;38:875.
Buatti JM, Bova FJ, Friedman WA, et al. Preliminary experience with frameless stereotactic radiotherapy. Int J Radiat Oncol Biol Phys. 1998;42:591.
Buatti JM, Friedman WA, Meeks SL, et al. The radiobiology of radiosurgery and stereotactic radiotherapy. Med Dosim. 1998;23:201.
Buatti JM, Meeks SL, Marcus RBJr, et al. Radiotherapy for pediatric brain tumors. Semin Pediatr Neurol. 1997;4:304.
Fowler JF. Brief summary of radiobiological principles in fractionated radiotherapy. Semin Radiat Oncol. 1992;2:16.
Fowler JF. The linear-quadratic formula and progress in fractionated radiotherapy. Br J Radiol. 1989;62:679.
Goitein M, Abrams M, Rowell D, et al. Multi-dimensional treatment planning: II. Beam’s eye-view, back projection, and projection through CT sections. Int J Radiat Oncol Biol Phys. 1983;9:789.
Hall EJ, Brenner DJ. The radiobiology of radiosurgery: rationale for different treatment regimes for AVMs and malignancies. Int J Radiat Oncol Biol Phys. 1993;25:381.
Hall EJ. The physics and chemistry of radiation absorption. In Ryan JD, Cox K, Papadopoulos D, editors: Radiobiology for the Radiologist, 4th ed, Philadelphia: Lippincott, 1994.
Khan FM. Treatment Planning II: Patient Data, Corrections, and Setup. Baltimore: Williams & Wilkins; 1994. 260-314
Leibel SA, Ling CC, Kutcher GJ, et al. The biological basis for conformal three-dimensional radiation therapy. Int J Radiat Oncol Biol Phys. 1991;21:805.
Levin WP, Kooy H, Loeffler JS, et al. Proton beam therapy. Br J Cancer. 2005;93:849.
Mandell LR, Kadota R, Freeman C, et al. There is no role for hyperfractionated radiotherapy in the management of children with newly diagnosed diffuse intrinsic brainstem tumors: results of a Pediatric Oncology Group phase III trial comparing conventional vs. hyperfractionated radiotherapy. Int J Radiat Oncol Biol Phys. 1999;43:959.
Murray KJ, Scott C, Greenberg HM, et al. A randomized phase III study of accelerated hyperfractionation versus standard in patients with unresected brain metastases: a report of the Radiation Therapy Oncology Group (RTOG) 9104. Int J Radiat Oncol Biol Phys. 1997;39:571.
Prados MD, Wara WM, Sneed PK, et al. Phase III trial of accelerated hyperfractionation with or without difluoromethylornithine (DFMO) versus standard fractionated radiotherapy with or without DFMO for newly diagnosed patients with glioblastoma multiforme. Int J Radiat Oncol Biol Phys. 2001;49:71.
Rao RD, Thome SD, O’Fallon J, et al. Safety of thrice-daily hyperfractionated radiation and BCNU for high-grade gliomas. Int J Radiat Oncol Biol Phys. 2002;53:376.
Sherouse GW, Bourland JD, Reynolds K, et al. Virtual simulation in the clinical setting: some practical considerations. Int J Radiat Oncol Biol Phys. 1990;19:1059.
Solberg TD, Selch MT, Smathers JB, et al. Fractionated stereotactic radiotherapy: rationale and methods. Med Dosim. 1998;23:209.
Thames HD, Bentzen SM, Turesson I, et al. Fractionation parameters for human tissues and tumors. Int J Radiat Biol. 1989;56:701.
Withers HR, Taylor JMG, Maciejewski B. Treatment volume and tissue tolerance. Int J Radiat Oncol Biol Phys. 1988:751.
Yamada M, Puck TT. Action of radiation on mammalian cells. IV. Reversible mitotic lag in the S3 HeLa cell produced by low doses of x-rays. Proc Natl Acad Sci U S A. 1961;47:1181.
Yeung D, Palta J, Fontanesi J, et al. Systematic analysis of errors in target localization and treatment delivery in stereotactic radiosurgery (SRS). Int J Radiat Oncol Biol Phys. 1994;28:493.
1 Hall EJ. The physics and chemistry of radiation absorption. In Ryan JD, Cox K, Papadopoulos D, editors: Radiobiology for the Radiologist, 4th ed, Philadelphia: Lippincott, 1994.
2 Kirsch DG, Tarbell NJ. New technologies in radiation therapy for pediatric brain tumors: the rationale for proton radiation therapy. Pediatr Blood Cancer. 2004;42:461.
3 Levin WP, Kooy H, Loeffler JS, et al. Proton beam therapy. Br J Cancer. 2005;93:849.
4 Little JB, Hahn GM. Life-cycle dependence of repair of potentially-lethal radiation damage. Int J Radiat Biol Relat Stud Phys Chem Med. 1973;23:401.
5 Yamada M, Puck TT. Action of radiation on mammalian cells. IV. Reversible mitotic lag in the S3 HeLa cell produced by low doses of x-rays. Proc Natl Acad Sci U S A. 1961;47:1181.
6 Muschel RJ, Zhang HB, Iliakis G, et al. Cyclin B expression in HeLa cells during the G2 block induced by ionizing radiation. Cancer Res. 1991;51:5113.
7 Mandell LR, Kadota R, Freeman C, et al. There is no role for hyperfractionated radiotherapy in the management of children with newly diagnosed diffuse intrinsic brainstem tumors: results of a Pediatric Oncology Group phase III trial comparing conventional vs. hyperfractionated radiotherapy. Int J Radiat Oncol Biol Phys. 1999;43:959.
8 Murray KJ, Scott C, Greenberg HM, et al. A randomized phase III study of accelerated hyperfractionation versus standard in patients with unresected brain metastases: a report of the Radiation Therapy Oncology Group (RTOG) 9104. Int J Radiat Oncol Biol Phys. 1997;39:571.
9 Prados MD, Wara WM, Sneed PK, et al. Phase III trial of accelerated hyperfractionation with or without difluoromethylornithine (DFMO) versus standard fractionated radiotherapy with or without DFMO for newly diagnosed patients with glioblastoma multiforme. Int J Radiat Oncol Biol Phys. 2001;49:71.
10 Rao RD, Thome SD, O’Fallon J, et al. Safety of thrice-daily hyperfractionated radiation and BCNU for high-grade gliomas. Int J Radiat Oncol Biol Phys. 2002;53:376.
11 Fowler JF. Brief summary of radiobiological principles in fractionated radiotherapy. Semin Radiat Oncol. 1992;2:16.
12 Thames HD, Bentzen SM, Turesson I, et al. Fractionation parameters for human tissues and tumors. Int J Radiat Biol. 1989;56:701.
13 Hall EJ, Brenner DJ. The radiobiology of radiosurgery: rationale for different treatment regimes for AVMs and malignancies. Int J Radiat Oncol Biol Phys. 1993;25:381.
14 Khan FM. Treatment Planning II: Patient Data, Corrections, and Setup. Baltimore: Williams & Wilkins; 1994. 260-314
15 Fowler JF. The linear-quadratic formula and progress in fractionated radiotherapy. Br J Radiol. 1989;62:679.
16 Parsons JT. Variations in Radiotherapy Fractionation: Overview. Presented at Head and Neck Cancer, Volume III: Proceedings of the 3rd International Conference on Head and Neck Cancer, San Francisco, 26-30 July 1992. Amsterdam: New York, 1993.
17 Withers HR, Taylor JMG, Maciejewski B. Treatment volume and tissue tolerance. Int J Radiat Oncol Biol Phys. 1988:751.
18 Armstrong C, Ruffer J, Corn B, et al. Biphasic patterns of memory deficits following moderate-dose partial-brain irradiation: neuropsychologic outcome and proposed mechanisms. J Clin Oncol. 1995;13:2263.
19 Buatti JM, Bova FJ, Friedman WA, et al. Preliminary experience with frameless stereotactic radiotherapy. Int J Radiat Oncol Biol Phys. 1998;42:591.
20 Khan FM. The Physics of Radiation Therapy. Baltimore: Williams & Wilkins; 1994.
21 Buatti JM, Meeks SL, Marcus RBJr, et al. Radiotherapy for pediatric brain tumors. Semin Pediatr Neurol. 1997;4:304.
22 Goitein M, Abrams M, Rowell D, et al. Multi-dimensional treatment planning. II. Beam’s eye-view, back projection, and projection through CT sections. Int J Radiat Oncol Biol Phys. 1983;9:789.
23 Sherouse GW, Bourland JD, Reynolds K, et al. Virtual simulation in the clinical setting: some practical considerations. Int J Radiat Oncol Biol Phys. 1990;19:1059.
24 Sherouse GW, Mosher CE, Novins K, et al. Virtual Simulation: concept and implementation. Presented at the The Use of Computers in Radiation Therapy. Proceedings of the Ninth International Conference, Scheveningen, The Netherlands, June 22-25, 1987.
25 Leibel SA, Ling CC, Kutcher GJ, et al. The biological basis for conformal three-dimensional radiation therapy. Int J Radiat Oncol Biol Phys. 1991;21:805.
26 Perez CA, Purdy JA, Harms W, et al. Three-dimensional treatment planning and conformal radiation therapy: preliminary evaluation. Radiother Oncol. 1995;36:32.
27 Friedman WA, Bova FJ. The University of Florida radiosurgery system. Surg Neurol. 1989;32:334.
28 Meeks SL, Bova FJ, Friedman WA, et al. LINAC scalpel radiosurgery at the University of Florida. Med Dosim. 1998;23:177.
29 Kamath R, Ryken TC, Meeks SL, et al. Initial clinical experience with frameless radiosurgery for patients with intracranial metastases. Int J Radiat Oncol Biol Phys. 2005;61:1467.
30 Breneman JC, Steinmetz R, Smith A, et al. Frameless image-guided intracranial stereotactic radiosurgery: clinical outcomes for brain metastases. Int J Radiat Oncol Biol Phys. 2009;74:702.
31 Bova FJ, Buatti JM, Friedman WA, et al. The University of Florida frameless high-precision stereotactic radiotherapy system. Int J Radiat Oncol Biol Phys. 1997;38:875.
32 Bova FJ, Meeks SL, Friedman WA, et al. Optic-guided stereotactic radiotherapy. Med Dosim. 1998;23:221.
33 Buatti JM, Friedman WA, Meeks SL, et al. The radiobiology of radiosurgery and stereotactic radiotherapy. Med Dosim. 1998;23:201.
34 Dunbar SF, Tarbell NJ, Kooy HM, et al. Stereotactic radiotherapy for pediatric and adult brain tumors: preliminary report. Int J Radiat Oncol Biol Phys. 1994;30:531.
35 Gill SS, Thomas DG, Warrington AP, et al. Relocatable frame for stereotactic external beam radiotherapy. Int J Radiat Oncol Biol Phys. 1991;20:599.
36 Kooy HM, Dunbar SF, Tarbell NJ, et al. Adaptation and verification of the relocatable Gill-Thomas-Cosman frame in stereotactic radiotherapy. Int J Radiat Oncol Biol Phys. 1994;30:685.
37 Solberg TD, Selch MT, Smathers JB, et al. Fractionated stereotactic radiotherapy: rationale and methods. Med Dosim. 1998;23:209.
38 Graham JD, Warrington AP, Gill SS, et al. A non-invasive, relocatable stereotactic frame for fractionated radiotherapy and multiple imaging. Radiother Oncol. 1991;21:60.
39 Hariz MI, Henriksson R, Lofroth PO, et al. A non-invasive method for fractionated stereotactic irradiation of brain tumors with linear accelerator. Radiother Oncol. 1990;17:57.
40 Kortmann RD, Becker G, Perelmouter J, et al. Geometric accuracy of field alignment in fractionated stereotactic conformal radiotherapy of brain tumors. Int J Radiat Oncol Biol Phys. 1999;43:921.
41 Lyman JT, Phillips MH, Frankel KA, et al. Stereotactic frame for neuroradiology and charged particle Bragg peak radiosurgery of intracranial disorders. Int J Radiat Oncol Biol Phys. 1989;16:1615.
42 Rosenthal SJ, Gall KP, Jackson M, et al. A precision cranial immobilization system for conformal stereotactic fractionated radiation therapy. Int J Radiat Oncol Biol Phys. 1995;33:1239.
43 Schwade JG, Houdek PV, Landy HJ, et al. Small-field stereotactic external-beam radiation therapy of intracranial lesions: fractionated treatment with a fixed-halo immobilization device. Radiology. 1990;176:563.
44 Scott TW, Beach JL, Mendiondo OA. A precision repeat localization head frame for fractionated stereotactic radiotherapy. Med Dosim. 1997;22:5.
45 Theodorou K, Kappas C, Tsokas C. A new non-invasive and relocatable immobilization frame for fractionated stereotactic radiotherapy. Radiother Oncol. 1998;47:313.
46 Willner J, Flentje M, Bratengeier K. CT simulation in stereotactic brain radiotherapy—analysis of isocenter reproducibility with mask fixation. Radiother Oncol. 1997;45:83.
47 Yeung D, Palta J, Fontanesi J, et al. Systematic analysis of errors in target localization and treatment delivery in stereotactic radiosurgery (SRS). Int J Radiat Oncol Biol Phys. 1994;28:493.
48 Menke M, Hirschfeld F, Mack T, et al. Photogrammetric accuracy measurements of head holder systems used for fractionated radiotherapy. Int J Radiat Oncol Biol Phys. 1994;29:1147.
49 Milliken BD, Rubin SJ, Hamilton RJ, et al. Performance of a video-image-subtraction-based patient positioning system. Int J Radiat Oncol Biol Phys. 1997;38:855.

 of a gray and is equivalent to the older unit of dose, the rad. For particle therapy, there is a recognized potential increase in the relative biologic effect of a given absorbed dose due to the denseness of ionization. Because of this, the concept of multiplying the absorbed dose by this relative biologic effect has been used and is defined as cobalt gray equivalent (CGE)—indicating the biologic effect in grays anticipated by the particle therapy dose. For protons, the generally accepted dose multiplied by a relative biologic effect of 1.1 yields the CGE. In other words, for a given absorbed dose, there would be a 10% greater biologic effect on normal tissues and tumor with protons compared with photons.
of a gray and is equivalent to the older unit of dose, the rad. For particle therapy, there is a recognized potential increase in the relative biologic effect of a given absorbed dose due to the denseness of ionization. Because of this, the concept of multiplying the absorbed dose by this relative biologic effect has been used and is defined as cobalt gray equivalent (CGE)—indicating the biologic effect in grays anticipated by the particle therapy dose. For protons, the generally accepted dose multiplied by a relative biologic effect of 1.1 yields the CGE. In other words, for a given absorbed dose, there would be a 10% greater biologic effect on normal tissues and tumor with protons compared with photons.






