Chapter 44 Principles of Pain Management
Anatomy and Physiology of the Pain Pathways
Axons of DRG neurons send the primary nociceptive afferents through the dorsal roots to the most superficial layers of the dorsal horns (Rexed laminae I and II) and to some of the deep laminae (Rexed V). The Aδ fibers conveying input from HTMs and MTs terminate primarily in laminae I and V; C fibers mainly terminate in lamina II. Neurotransmitters related to pain conduction include excitatory amino acids and neuropeptides, particularly substance P (Geracioti et al., 2006). The second-order neurons in the dorsal horn include cells that respond only to noxious stimuli (nociceptive specific neurons) and others (wide dynamic range [WDR] neurons) that respond to both nociceptive and non-nociceptive sensory stimuli.
Central Modulation of Nociception
Descending inhibitory systems appear to have three functionally interrelated neurotransmitter mechanisms: the opioid, the noradrenergic, and the serotonergic systems. Opioid precursors and their respective peptides (β-endorphin, methionine [met]-enkephalin, leucine [leu-] enkephalin, and dynorphin) are present in the amygdala, hypothalamus, PAG, raphe magnus, and the dorsal horn. Noradrenergic neurons project from the locus caeruleus and other noradrenergic cell groups in the medulla and pons. These projections are found in the dorsolateral funiculus. Stimulation of these areas produces analgesia, as does the administration (direct or intrathecal) of α2-receptor agonists such as clonidine (Khodayar et al., 2006). Many serotonergic neurons are found in the raphe magnus. These neurons send projections to the spinal cord via the dorsolateral funiculus. Administration of serotonin to the spinal cord produces analgesia, and pharmacological blockade or lesion of the raphe magnus can reduce the effects of morphine. The antinociceptive effects of antidepressants such as tricyclics and newer serotonin-norepinephrine reuptake inhibitors such as duloxetine and milnacipran are believed to reduce pain by increasing serotonin and norepinephrine concentrations in descending inhibitory pain pathways.
Opioid Receptors
The δ receptor has similar central and peripheral distribution as the µ receptors. Studies have shown that δ-opioid agonists can provide relief of inflammatory pain and malignant bone pain. Meanwhile, peripherally restricted κ-opioid agonists have been developed to target κ-opioid receptors located on visceral and somatic afferent nerves for relief of inflammatory, visceral, and neuropathic chronic pain. The potential analgesic effects, combined with a possible lower abuse rate and fewer side effects than µ-receptor agonists, makes δ- and peripherally restricted κ-opioid receptor agonists promising targets for treating pain (Vanderah, 2010).
Central Sensitization
Central sensitization plays a major role in the development of neuropathic pain syndromes. First, postsynaptic depolarization in the spinal cord in response to afferent stimulation can induce removal of magnesium blockade in N-methyl-d-aspartate (NMDA) receptors such that glutamate now induces a depolarization upon receptor binding. This process is short lasting and is called wind up (Katz and Rothenberg, 2005). It is responsible for the temporal summation of inputs. The second set of changes is related to phosphorylation of the NMDA receptor, which is a key process for longer-lasting changes in the excitability of the dorsal horn neurons that produce central sensitization. This posttranslational modification of the NMDA receptors results in dramatic changes in excitability due to removal of the voltage-dependent magnesium block in the absence of cell depolarization and also to changes in channel kinetics, such as channel opening time. NMDA receptor activation allows calcium influx into the cell, which further augments signal transduction within the dorsal horn neurons by activating a number of intracellular signal transduction kinases. As a result, relatively brief C-fiber inputs initiate very rapid changes in membrane excitability. This manifests both as a progressive increase in excitability during the course of the stimulus (wind up) and as post-stimulus changes that may last for several hours (central sensitization).
Clinically, the real meaning of peripheral and central sensitization is the enhanced and prolonged pain perception to minor stimulations, or sometimes without peripheral stimulation. Once peripheral and central sensitizations are involved, the pain is usually more difficult to treat. It is now believed that peripheral and central sensitization may be involved in a wide variety of chronic pain conditions such as reflex sympathetic dystrophy, tension headache, carpal tunnel syndrome, pain after spinal cord injury (Carlton et al., 2009), and even in pain conditions previously thought to be mainly nociceptive in nature such as fibromyalgia, epicondylalgia, and osteoarthritis (Gwilym et al., 2009). The challenge to the clinician is that when trying to make a diagnosis for a pain patient, we should not only try to localize the pain source—as most clinicians always do—we should also factor in the role of peripheral and central sensitization and what the best treatment strategy will be in each case.
Common Pain Syndromes
Trigeminal Neuralgia
The diagnosis of TN is based primarily on a history of characteristic paroxysmal pain attacks. The White and Sweet criteria are still commonly used worldwide (Box 44.1). In the majority of TN patients, the clinical examination, imaging studies, and laboratory tests are unremarkable (classic TN). In a smaller group, TN is secondary to other disease processes affecting the trigeminal system (symptomatic TN). Because a significant percentage of patients have symptomatic TN resulting from other disease processes, diagnostic MRI studies should be part of the initial evaluation of any patient with TN symptoms. Special attention should be paid to MS plaques, tumor, and subtle vascular anomalies that may be the source of root compression. Recent studies found that high-resolution three-dimensional (3D) MRI reconstruction and magnetic resonance cisternography may provide alternative tools to better identify the presence of neurovascular compression and even measure the volume of neurovascular compression at the cerebellopontine angle and predict the prognosis after initial treatment (Tanaka et al., 2009).
Carbamazepine is the first choice for treatment of TN; both controlled and uncontrolled studies confirm its clinical efficacy. Carbamazepine monotherapy provides initial symptom control in as many as 80% of TN patients. Of those initially responding to the drug, approximately 75% will continue to have long-term control of pain attacks. Controlled studies demonstrate that baclofen and lamotrigine are superior to placebo for treatments of TN. In the experience of many clinicians, baclofen is just as effective as carbamazepine and often better tolerated. A recent study found that oxcarbazepine may be effective for those who were unresponsive to the treatment of carbamazepine (Gomez-Arguelles et al., 2008). Pregabalin may also be potentially effective. If a patient is not satisfied with single medication therapy, adding another oral medication may offer additional benefits. Intravenous (IV) lidocaine or phenytoin could be effective for some severe refractory cases of TN. However, these treatments carry additional risks and require close cardiovascular monitoring. Opioid analgesics have not been proven effective for TN and should be avoided.
Posterior fossa exploration and microvascular decompression (MVD) is assumed to directly treat the cause of TN. However, this is a complex and invasive therapy with a possibility of death. With the availability of other less-invasive procedures, MVD is infrequently used and is only reserved for younger and healthier patients. Several studies demonstrated trigeminal radiofrequency rhizotomy successfully controls symptoms in over 85% of TN cases. The technique is minimally invasive. To heat the gasserian ganglion, a radiofrequency needle is inserted through the foramen ovale under the guidance of fluoroscopy. The procedure can be finished in less than 30 minutes in experienced hands. A few patients experience sensory loss and dysesthesia (analgesia dolorosa) in the distribution of the damaged trigeminal fibers with this procedure. Stereotactic radiosurgery (SRS) employs computerized stereotaxic methods to concentrate ionizing radiation on the trigeminal root entry zone. Several studies have demonstrated the high clinical efficacy and relative safety of this new technique. It is currently recommended as a first-line noninvasive surgical technique in many pain centers, especially for frail or elderly patients (Zahra et al., 2009).
Cervicogenic Headache
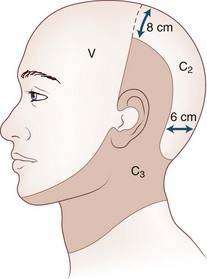
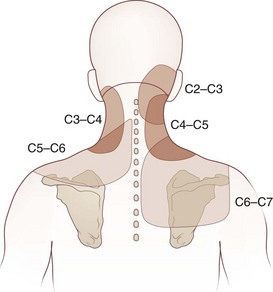
Cervicogenic headache refers to head pain originating from pathology in the neck. It is believed that pain from the C2-C3 nerve dermatome can radiate to the head and face (Fig. 44.1). An earlier study found that pain from the C2-C3 and C3-C4 cervical facet joints also can radiate to the occipital area (Fig. 44.2). The term cervicogenic headache was first introduced by Sjaastad and colleagues in 1983. However, the concept of cervicogenic headache is controversial and not well accepted by the majority of neurologists. The International Headache Society (2004) published its first diagnostic criteria in 1998 and revised it 2004. Patients with cervicogenic headache often have histories of head and neck trauma. Pain may be unilateral or bilateral. Pain is frequently localized to the occipital area, but it may also be referred to the frontal, temporal, or orbital regions. Headaches may be triggered by neck movement or sustained neck postures. This headache is constant with episodic throbbing attacks like a migraine. Patients may have other symptoms mimicking a migraine, such as nausea, vomiting, photophobia, phonophobia, and blurred vision. Owing to significant overlap of the symptoms of cervicogenic headache and migraine without aura, cervicogenic headache is often misdiagnosed as migraine. Clinicians should always consider cervicogenic headache in the differential diagnoses when evaluating a headache patient. History of head/neck injury and detailed examination of the occipital and upper cervical area should be part of the evaluation for headache. Patients with cervicogenic headache may have tenderness over the greater or lesser occipital nerve, cervical facet joints, and muscles in the upper or middle cervical region. Cervicogenic headache does not respond well to migraine medications. Treatment should be focused on removal of the pain source from the occipital-cervical junction. Initial therapy is directed to physical therapy modalities and NSAIDs. Interventional treatment such as greater occipital nerve block, cervical facet joint block, deep cervical plexus block, and botulinum toxin injections may provide effective pain relief (Zhou et al., 2010).
Complex Regional Pain Syndrome
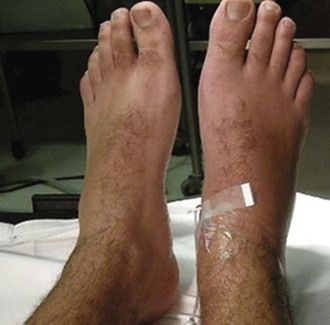
The mean age of CRPS patients ranges from 36 to 46 years, with women predominating (60%-81%). It is caused typically by an injury such as a fracture (16%-46%), strain or sprain (10%-29%), post surgery (3%-24%), and contusion or crush injury (8%-18%). Clinical features of CRPS often include pain, edema, autonomic dysfunction such as change in temperature or color in the involved limbs, motor dysfunction, and psychological abnormalities such as depression (Fig. 44.3). Schwartzman and Maleki reported the pattern of spreading of CRPS in three stages. In the early stage, CRPS often involves only one limb with pain, minor edema, and increased skin temperature. CRPS may spread from one limb to the others. In the later stage, CRPS could involve the full body and the four extremities with severe pain, edema, cold and cyanotic limbs, joint contracture, and atrophy of muscles and bones.
Tricyclic antidepressants, antiepileptics, and narcotics such as methadone are commonly used empirically for CRPS, even though clinical controlled studies have not proven their efficacy. A recent review article summarized the evidence derived from randomized controlled trials pertaining to the treatment of CRPS. The review reported clinical improvement with dimethyl sulfoxide, steroids, epidural clonidine, and intrathecal baclofen. Only bisphosphonates appear to offer clear benefits for patients with CRPS (Tran de et al., 2010). NMDA receptor modulation is a major interest of current research. It has been reported that subanesthetic infusions of ketamine might offer a promising therapeutic option in the treatment of appropriately selected patients with intractable CRPS (Schwartzman et al., 2009). A recent preliminary study reported that IV immunoglobulin treatment could potentially decrease pain in CRPS patients (Goebel et al., 2010). However, more studies are needed to further establish the safety and efficacy of these novel approaches.
Minimally invasive techniques have been used extensively for the treatment of CRPS. Techniques include sympathetic block, intravenous regional block (IVRB), somatic nerve block, epidural drug administration, intrathecal drug delivery, and neurostimulation. Stellate ganglion blocks in early-stage CRPS may significantly decrease pain and hasten clinical recovery. It may also prevent the recurrence of CRPS after reoperation of the affected extremity. In a double-blind study, IVRB with bretylium provided significantly longer analgesia than lidocaine. Good pain relief is reported with the use of epidural delivery of clonidine and ketamine and also with intrathecal baclofen and morphine. An early study with 2-year follow-up reported that spinal cord stimulation (SCS) results in a long-term pain reduction and improvement in health-related quality of life. However, a more recent randomized study with 5-year follow-up found no extra benefit in terms of pain relief for those with a combination of SCS and physical therapy, compared to those with physical therapy alone (Kemler et al., 2008). The author shares the same experience and opinion with the cited report. It seems that most RSD patients feel better immediately after the SCS implantation. However, the SCS itself may have difficulty stopping the spread of RSD, and once RSD spreads out of the area initially covered by the SCS, the pain is no longer “under control.”
Post-Stroke Pain Syndrome
Treatment of central post-stroke pain remains a challenge. Tricyclic antidepressants are still a choice of treatment. Gabapentin and lamotrigine have been used to treat central post-stroke pain syndrome in open-labeled studies. Selective posterior rhizotomy has been reported to decrease painful spasticity in the lower limbs of hemiplegic patients after a stroke. It has been reported that chronic motor cortex stimulation therapy provides pain relief for some post-stroke patients (Brown and Pilitsis, 2006). Stereotactic radiosurgery of the pituitary and deep brain stimulation (DBS) have been used to treat PSP syndrome with some success (Pickering et al., 2009).
Spinal Cord Injury and Pain
Pain after SCI originates from different sources including neuropathic, musculoskeletal, and visceral pain. Neuropathic pain after SCI is further divided into central and segmental pain. Central neuropathic pain often begins within weeks or months after injury. It is generally described as a burning, sharp, or shooting pain. Patients feel pain at or below the level of injury in areas where there is partial or complete loss of sensation to touch. Central pain is believed to be due to differentiation caused by SCI. Astrocytic activation in the spinal cord, up-regulation of chemokines, hyperexcitability of wide–dynamic range neurons in the spinal dorsal horn rostral to the lesion, and loss of γ-aminobutyric acid (GABA)ergic interneurons in laminae I-III of the spinal cord dorsal horn (Meisner et al., 2010) have been suggested to cause the neuropathic pain that follows SCI. Segmental pain often occurs around the border of injury and usually develops within the first few months after an injury. Allodynia and hyperalgesia are common. Nerve root entrapment could lead to severe segmental pain. Patients may describe stabbing or sharp pain or a band of burning pain at the level of injury. Syringomyelia with a cyst ascending from the level of the SCI may occasionally cause central pain.
Pain management after SCI is difficult. Pharmacological and rehabilitative procedures are effective in only about 38% of patients. However, the initial workup should target identifying the pain source. Different kinds of pain may respond differently to treatments. For neuropathic pain, medications such as gabapentin, amitriptyline, and nortriptyline may ease the pain in some patients. Intravenous lidocaine may provide temporary pain relief. Intrathecal baclofen therapy may reduce chronic musculoskeletal pain associated with spasticity and improve the patient’s quality of life. Intrathecal morphine and clonidine offer limited help to relieve the pain. DBS has been reported to be effective in some cases, but there is insufficient evidence to validate its routine use. Limited evidence exists for use of motor cortex stimulation (Previnaire et al., 2009). SCS lacks long-term efficacy for the relief of spasticity and pain in SCI and is believed not to be cost-effective. Dorsal root entry zone lesions and dorsal rhizotomy have also been used with limited success. Appropriate management of bowel or bladder dysfunction may help ease visceral pain. If an ascending syrinx is present, surgical drainage may be effective in relieving the pain.
Pain in Multiple Sclerosis
Pain is a common symptom in multiple sclerosis (MS). The prevalence of pain in this disease is higher than what was initially expected; some studies estimate it to be up to 86% (Bermejo et al., 2010), depending on the sample and specific questions used to assess the incidence and severity of pain. Osterberg et al. studied pain syndromes in 429 patients with definite MS, and 58% reported pain during the course of their disease; 100 (28%) had central pain, including 18 patients (5%) with trigeminal neuralgia. The majority of patients (87%) with central pain had symptoms located in the legs, while 31% were in the arms. Pain was mostly bilateral (76%) and constant. Aching, burning, and pricking were common qualities. Other reported pain syndromes in MS include the Lhermitte sign, dysesthetic pain, back pain, headache, and painful tonic spasms. Chronic pain in MS was found to have no significant relationship to gender, age of onset, disease duration, or disease course. Chronic pain can have a significant negative impact on functions in persons with MS, such as the ability to engage in household work and psychological functioning. Chronic pain is significantly related to anxiety and depression in females. In the long-term care facility, residents with MS are more physically disabled and experience more frequent pain and a higher prevalence of pressure ulcers and depression than residents without MS.
1. For pain directly related to MS, such as trigeminal neuralgia, carbamazepine is the first choice. Lamotrigine, gabapentin, oxcarbazepine, and other anticonvulsants may also be used. Painful “burning” dysesthesia may be treated with tricyclic antidepressants or carbamazepine. Further options include gabapentin or lamotrigine.
2. Pain related to spasticity may improve with adequate physiotherapy. Drug treatment includes antispastic agents like oral baclofen or tizanidine. In severe cases, intrathecal baclofen and botulinum toxin injections merit consideration.
3. Pain due to subcutaneous injections of β-interferons or glatiramer acetate may be reduced by optimizing the injection technique and by local cooling. Systemic side effects of interferons (e.g., myalgias) could be reduced by paracetamol or ibuprofen.
Even though cannabis is not legally used in the United States to treat pain, European studies indicate that cannabis-based medicines are effective in reducing pain and sleep disturbance in patients with MS-related central neuropathic pain and are mostly well tolerated (Rog et al., 2005; Thaera et al., 2009). Oral ketamine, an NMDA receptor antagonist, has also been reported to be effective in the treatment of pain and allodynia associated with MS.
Phantom Limb Pain and Stump Pain
Changes along the neuroaxis may contribute to the experience of phantom-limb pain. Spinal mechanisms are characterized by increased excitability of the dorsal horn neurons, reduction of inhibitory processes, and structural changes at the central nerve endings of the primary sensory neurons, interneurons, and the projection neurons. Supraspinal changes related to phantom-limb pain involve the brainstem, thalamus, and cortex. Reorganization of the somatosensory cortex of the human cerebral cortex in amputees has been supported by findings from several imaging studies. People with arm or hand amputations show a shift of the mouth into the hand representation in the primary somatosensory cortex (Woodhouse, 2005). Studies in human amputees have shown that reorganizational changes also occur at the thalamic level and are closely related to the perception of phantom limbs and phantom-limb pain. Neuroma in the stump may be more responsible for stump pain than phantom-limb pain. However, abnormal input originated from a neuroma in the residual limb may increase the amount of central reorganization, enhancing the chance of phantom-limb pain. Psychological factors play a role in the modulation of phantom-limb pain; the pain may be exacerbated by stress. Patients who lack coping strategies, fear the worst, or receive less social support tend to report more phantom-limb pain.
Pharmacological Management of Chronic Pain
Nonsteroidal Antiinflammatory Drugs
NSAIDs are powerful inhibitors of prostaglandin synthesis through their effect on cyclooxygenase (COX). Prostaglandins are not thought to be important pain mediators, but they do cause hyperalgesia by sensitizing peripheral nociceptors to the effects of various mediators of pain and inflammation such as somatostatin, bradykinin, and histamine. Thus, NSAIDs are used primarily to treat pain that results from inflammation and hyperalgesia. Table 44.1 lists commonly used NSAIDs.
Table 44.1 Commonly Used Oral Nonsteroidal Antiinflammatory Drugs
| Generic Name | Trade Name | Adult Dosage |
|---|---|---|
| Acetaminophen | Tylenol | 500-1000 mg q 4 h |
| Acetylsalicylic acid | Aspirin | 325-650 mg q 4 h |
| Celecoxib | Celebrex | 200 mg q 12 h |
| Choline magnesium trisalicylate | Trilisate | 500-750 mg q 8-12 h |
| Diclofenac sodium | Voltaren | 25-75 mg q 8-12 h |
| Diflunisal | Dolobid | 250-500 mg q 8-12 h |
| Etodolic acid | Lodine | 200-400 mg q 6 h |
| Fenoprofen calcium | Nalfon | 200 mg q 4-6 h |
| Flurbiprofen | Ansaid | 100 mg q 8-12 h |
| Ibuprofen | Motrin | 400-800 mg q 6-8 h |
| Indomethacin | Indocin | 25-50 mg q 8-12 h |
| Ketoprofen | Orudis | 25-75 mg q 6-8 h |
| Ketorolac | Toradol | 10 mg q 6-8 h |
| Meclofenamate sodium | Meclomen | 50 mg q 4-6 h |
| Naproxen | Naprosyn | 275-500 mg q 8-12 h |
| Phenylbutazone | Butazolidin | 100 mg q 6-8 h |
| Piroxicam | Feldene | 10-20 mg once daily |
| Salsalate | Disalcid | 500 mg q 4 h |
| Sulindac | Clinoril | 150-200 mg q 12 h |
| Tolmetin | Tolectin | 200-600 mg q 8 h |
Common side effects of NSAIDs include GI toxicity, stomach ulcers, and gastric bleeding. Renal dysfunction can occur with prolonged and excessive use of NSAIDs. Particularly at risk from excessive use of NSAIDs are elderly patients with renal dysfunction, congestive heart failure, ascites, or hypovolemia. Other adverse effects of NSAIDs include hepatic dysfunction or necrosis, asthma, vasomotor rhinitis, angioneurotic edema, urticaria, laryngeal edema, or even cardiovascular collapse. Because of the wide availability of acetaminophen and its potential toxicity (especially liver toxicity), in 2009 the U.S. Food and Drug Administration (FDA) proposed a decrease in the maximum daily dose of acetaminophen from 4000 mg to 3250 mg, reducing the maximum individual dose from 1000 to 650 mg. They relegated 500 mg tablets to prescription status and mandated new labeling on acetaminophen packaging (Krenzelok, 2009). Acetaminophen is a potential cyclooxygenase 2 (COX-2)–selective inhibitor. It may also increase cardiovascular risks.
Cardiovascular risks of NSAIDs, especially COX-2 inhibitors, have become a major focus of attention in recent years. Suggestions that the use of COX-2 inhibitors may decrease prostacyclin (PGI2) levels, with relatively unopposed platelet thromboxane A2 generation that may lead to increased thrombotic risk, have cautioned against the use of such agents. Rofecoxib (Vioxx) was withdrawn from the market in September 2004 owing to increased cardiovascular risks. A recent study found that the hazard ratio (95% confidence interval) for death was 1.70, 1.75, 1.31, 2.08, 1.22, and 1.28 for rofecoxib, celecoxib, ibuprofen, diclofenac, naproxen, and other NSAIDs, respectively (Gislason et al., 2009). Even though limited long-term data on cardiovascular risk associated with nonselective NSAIDs have been available, and some contradictory warnings and recommendations have been published recently by the American Heart Association, FDA, and independent experts (Gluszko and Bielinska, 2009), the general suggestion is that both NSAIDs and selective COX-2 inhibitors should be avoided or used with extreme caution if a patient has a high cardiovascular risks and a history of heart failure.
Antidepressants
Tricyclic antidepressants are probably the most commonly used adjunct analgesics in the management of chronic pain (Dworkin et al., 2010) (Table 44.2). The tertiary amines (amitriptyline, imipramine, doxepin, and clomipramine) and the secondary amines (nortriptyline and desipramine) both have analgesic properties. Amitriptyline is the prototype antidepressant used in this context. Clinical efficacy of tricyclics for neuropathic pain has been demonstrated by numerous well-controlled double-blind clinical studies for both neuropathic and somatic pain. Clinicians have to be familiar with the possible side effects of amitriptyline, especially in elderly patients. These adverse effects include sedation, dry mouth, constipation, urinary retention, glaucoma, orthostatic hypotension, and cardiac arrhythmias. Patients should be warned about the side effects before they start the medication. Amitriptyline should be avoided in patients with a history of heart disease (conduction disorders, arrhythmias, or heart failure) and closed-angle glaucoma. Amitriptyline should be started at a relatively low dose (10 mg) at bedtime and slowly titrated up as tolerated. Most patients report improved sleep after taking amitriptyline. The onset of pain relief may precede the anticipated onset of antidepressant effects. In general, pain relief may be expected in 7 to 14 days. The dosage required for pain management is usually lower than for depression; 75 to 100 mg at bedtime is often effective. If the patient cannot tolerate this dose or is not a good candidate for amitriptyline, other tricyclics such as nortriptyline or desipramine may be considered. These secondary amines generally have fewer anticholinergic effects and are therefore better tolerated than tertiary amines. However, their clinical efficacy is not as well established as that for amitriptyline.
Table 44.2 Tricyclic Antidepressants Commonly Used for Pain Management
| Generic Name | Trade Name | Adult Dosage Range (mg/day) |
|---|---|---|
| Amitriptyline | Elavil | 10-100 |
| Clomipramine | Anafranil | 25-200 |
| Desipramine | Norpramin | 10-200 |
| Doxepin | Sinequan | 10-200 |
| Imipramine | Tofranil | 10-200 |
| Nortriptyline | Pamelor | 10-150 |
The main advantage of the selective serotonin reuptake inhibitors (SSRIs) is the favorable side-effect profile. However, SSRIs are clearly less effective than tricyclic antidepressants. The NNT (number needed to treat to reach 50% pain relief) is 6.7 versus 2.4 (Coluzzi and Mattia, 2005). It seems that selective serotonin/noradrenaline reuptake inhibitors (SNRI) are relatively more effective for pain management than most of the SSRIs. Venlafaxine is an SNRI for which randomized controlled trials showed good pain relief effect for painful polyneuropathy and neuropathic pain following treatment of breast cancer. Duloxetine has also been demonstrated to have significant analgesic effects in diabetic polyneuropathy and fibromyalgia. Milnacipran is another SNRI; randomized double-blind placebo-controlled studies found that milnacipran is effective in controlling pain and improving global status, fatigue, and physical and mental function in patients with fibromyalgia (Arnold et al., 2010). Nausea, hyperhidrosis, and headache are the most common adverse events.
Anticonvulsants
Valproic acid has been proven to be effective in reducing the frequency of migraine attacks (Vikelis and Rapoport, 2010). Some studies found that valproates may provide significant pain relief in patients with post herpetic neuralgia and diabetic neuropathy. However, negative results have also been reported. Common side effects include tremor, ankle swelling, sedation, and GI discomfort. Weight gain and hair loss may be a major cosmetic concern, especially for younger patients. Valproate should not be used for children younger than 2 years of age because of hepatotoxicity. Generally, valproate is not the first-line choice for neuropathic pain.
Pregabalin is a GABA analog with similar structure and mechanism of action as gabapentin. It has antiepileptic, analgesic, and anxiolytic activity. Pregabalin has been approved by the FDA for the management of neuropathic pain associated with diabetic neuropathy, postherpetic neuralgia, and fibromyalgia (Straube et al., 2010). Food does not significantly affect the extent of absorption. Pregabalin is not protein bound and exhibits a plasma half-life of about 6 hours. Hepatic metabolism is negligible, and most of the oral dose (95%) appears unchanged in the urine. At a dose of 300 mg/day, about 45% of diabetic neuropathy patients had 50% pain relief. This means that pregabalin has an NNT of 2.2 for diabetic neuropathy. Pregabalin seems to be more effective than gabapentin and other anticonvulsants for neuropathic pain. Common side effects of pregabalin include dizziness, sedation, dry mouth, and peripheral edema.
Oxcarbazepine is a ketoderivative of carbamazepine, with better tolerability. It can block sodium-dependent action potentials. The medication does not induce hepatic enzymes and has fewer drug-drug interactions than carbamazepine. Multiple open studies have suggested that oxcarbazepine may be effective for the treatment of neuropathic pain. However, a double-blind controlled study did not find significant difference between oxcarbazepine and placebo for the treatment of pain due to diabetic neuropathy (Grosskopf et al., 2006).
Lamotrigine is an antiepileptic drug that stabilizes neural membranes by blocking the activation of voltage-sensitive sodium channels and inhibiting the presynaptic release of glutamate. Multiple open studies have supported the use of lamotrigine in neuropathic pain. However, controlled studies found no efficacy of lamotrigine for the treatment of neuropathic pain (Breuer et al., 2007; Rao et al., 2008). Lamotrigine is ineffective for prevention of migraine.
Topiramate has proven its efficacy and safety in the prophylactic treatment of episodic migraine in a number of randomized controlled clinical trials (Naegel and Obermann, 2010). Even though open studies and case reports continue to support the use of topiramate in the treatment of various kinds of neuropathic pain, controlled studies failed to reveal any benefit of topiramate for the treatment of neuropathic pain. The mechanisms of action include blockade of sodium channels, enhancement of GABA inhibition, and attenuation of kainate-induced responses at glutamate receptors. The starting dose is usually small (e.g., 25 mg twice a day for an adult). It may be incrementally increased weekly by 50 mg up to 200 mg/day. Topiramate may induce memory loss, word-finding difficulties, disorientation, and sedation. The other common adverse affects are renal calculi, tremors, dizziness, ataxia, headaches, fatigue, and GI upset. Topiramate may also induce significant weight loss. This medication may be more helpful in obese pain patients.
Systemic Local Anesthetics
Systemic administration of local anesthetics has been used to treat neuropathic pain syndrome. Clinical trials have provided some evidence that lidocaine and mexiletine are superior to placebo for neuropathic pain (Carroll et al., 2008). Intravenous lidocaine is used for the treatment of neuropathic pain as a second-line therapy. If a patient has a positive response to IV lidocaine therapy, a trial of oral mexiletine may be considered. However, mexiletine has a relatively high rate of adverse effects such as nausea, vomiting, tremor, dizziness, unsteadiness, and paresthesias. Given the limited number of supportive studies, mexiletine and other oral local anesthetics should only be used as second-line agents for neuropathic pain that has failed to respond to anticonvulsants or antidepressants.
Topical Analgesics
Double-blind placebo-controlled studies have confirmed the efficacy of the 5% lidocaine patch for the treatment of postherpetic neuralgia (Lin et al., 2008) and for those patients with trigger points in myofascial pain syndrome (Affaitati et el., 2009). However, the lidocaine patch may not be effective in treating pain due to traumatic rib fractures (Ingalls et al., 2010). Minimal systemic absorption occurs. The patch is usually applied 12 hours per day, with minimal systemic side effects. Topical lidocaine ointment in various concentrations (up to a compounded formulation of 10%) may offer a cost-effective alternative.
Capsaicin is the spicy ingredient in chili pepper. It can deplete substance P from the terminals of afferent C fibers, potentially leading to decreased pain perception. Capsaicin creams are effective in reducing postsurgical pain in cancer patients. When applied topically, it may initially release substance P and cause severe burning pain. Pain related to the use of capsaicin gradually decreases over a few days if the cream is applied regularly. A lower-concentration cream (0.025%) or the application of a topical local anesthetic may help some patients decrease the initial burning pain and tolerate the medication better. A recent study found that topical capsaicin might effectively decrease pain in patients with chronic migraine (Papoiu and Yosipovitch, 2010). It is important to warn patients not to get any trace of the cream on mucous membranes, since this causes severe pain.
N-Methyl-D-Aspartate Receptor Blockers
NMDA receptors are involved in the development of central sensitization associated with chronic refractory pain syndromes. NMDA antagonists may modulate CNS function, offering a novel approach to treating chronic neuropathic pain. Intravenous anesthetic doses of ketamine may induce serious side effects such as vivid hallucinations and psychosis. However, double-blind placebo-controlled studies have confirmed that low-dose IV ketamine may provide significant pain relief for CRPS type 1 without significant psychomimetic side effects (Sigtermans et al., 2009). Methadone has the property of both µ-opioid receptor agonist and NMDA antagonist. Evidence indicates that methadone has similar analgesic efficacy to morphine, but adverse effects due to prolonged half-life—particularly respiratory depression, cardiac arrhythmia, and sudden death—make it critical for providers to be familiar with methadone’s pharmacological properties before considering methadone as an analgesic therapy for chronic pain. Amantadine is a noncompetitive NMDA antagonist. Dextromethorphan, the d-isomer of the codeine analog, levorphanol, is a weak, noncompetitive NMDA receptor antagonist. Memantine is an NMDA antagonist used for the treatment of Alzheimer disease. All three of these medications possess some analgesic properties. Current data are too scant or too weak, however, to recommend clinical use of any of these drugs for chronic pain management.
Opioid Analgesics
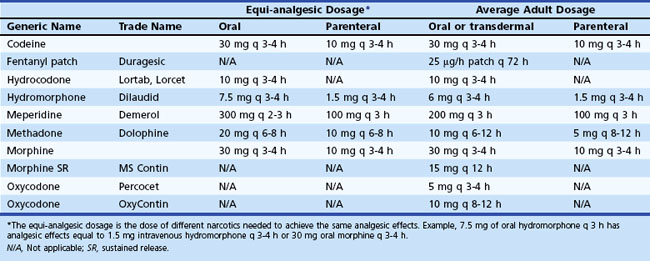
Opioids are classified according to the activity on the opioid receptors as full agonists, partial agonists, or mixed agonists-antagonists. Commonly used full agonists include hydrocodone, codeine, morphine, oxycodone, hydromorphone, methadone, and fentanyl. Buprenorphine is a partial agonist. It has lower intrinsic efficacy compared to other full opioid agonists and displays a ceiling effect to analgesia. Mixed agonist-antagonists include pentazocine, butorphanol tartrate, dezocine, and nalbuphine hydrochloride. These mediations block opioid analgesia at one type of receptor (µ) while simultaneously activating other opioid receptors (κ). Mixed agonist-antagonists should not be used together with full agonists, because they may cause withdrawal syndrome and increased pain. Table 44.3 lists commonly used narcotics and their equi-analgesic dosage.
Equi-analgesic dosage means the dose of different narcotics needed to reach the same analgesic effects. The middle two columns of Table 44.3, for example, indicate that 7.5 mg of oral hydromorphone every 3 hours may have analgesic effects equal to 1.5 mg of IV hydromorphone every 3 to 4 hours or 30 mg of oral morphine every 3 to 4 hours.
Physicians need to be familiar with side effects of opioids before prescribing these medications. Common side effects of opioids include constipation, sedation, nausea, vomiting, and respiratory depression due to overdoses. Occasionally, opioids may cause myoclonus, seizures, hallucinations, confusion, sexual dysfunction, sleep disturbances, and pruritus. Constipation is a common problem associated with opioid administration. Tolerance to the constipating effects of opioids hardly ever occurs during chronic therapy. Some patients are too embarrassed to tell the physician about constipation problems, so physicians should always ask patients about this. Mild constipation can usually managed by an increase in fiber consumption and the use of mild laxatives such as milk of magnesia. Severe constipation may be treated with a stimulating cathartic drug (e.g., bisacodyl, standardized senna concentrate, MiraLax, and similar drugs). Tapentadol is a novel centrally acting analgesic with two modes of action, µ-opioid agonist and norepinephrine reuptake inhibition. It was approved by the FDA for treatment of acute pain in the year 2008. Multiple double-blind controlled studies found tapentadol’s analgesic effects similar to morphine and oxycodone. However, tapentadol has fewer GI side effects such as nausea and vomiting (Daniels et al., 2009; Smit et al., 2010). Owing to its dual mechanism of action and better GI tolerability, there is potential for off-label use in chronic pain.
Diminishing opioid analgesic efficacy and increased pain during the course of opioid therapy is quite common. It is traditionally considered a result of opioid tolerance but could also be the result of opioid-induced hyperalgesia (OIH), which occurs when prolonged administration of opioids results in a paradoxical increase in atypical pain that appears to be unrelated to the original nociceptive stimulus. The mechanism of OIH is still unclear. However, opioid receptor desensitization, up-regulation of spinal dynorphin, and enhanced activity of excitatory transmitters such as NMDA are believed to be involved the pathogenesis of OIH (Silverman, 2009). Clinically, it is difficult to distinguish opioid tolerance and OIH. However, the issue of opioid-induced pain sensitivity should also be considered when an adjustment of opioid doses is being contemplated because opioid treatment is failing to provide the expected analgesic effects and/or there is an unexplainable pain exacerbation following a period of effective opioid treatment. Quantitative sensory testing of pain may offer the most appropriate way of diagnosing hyperalgesia. With OIH, an increased opioid dose is not always the answer. Office-based detoxification, reduction of opioid dose, opioid rotation, and the use of specific NMDA receptor antagonists are all viable treatment options for OIH.
Interventional Pain Management
Interventional pain management techniques have grown rapidly since 1990 and have become a major tool in treating acute and chronic pain. The American Society of Interventional Pain Physicians has developed evidence-based guidelines for improving compliance and the quality of care. Numerous reports have been published to investigate the long-term efficacy of interventional pain management techniques and have provided critical evidence indicating that these techniques may be useful (Manchikanti et al., 2009).
Traditionally, neurosurgeons have utilized surgical techniques to destroy part(s) of the peripheral and central nerve systems to interrupt conduction of painful information into the CNS. These techniques include resection of peripheral nerves, dorsal root ganglia, the dorsal root entry zone, the spinal thalamic tract, entire spinal cord, nuclei of the thalamus, and the sensory cortex, as well as the pituitary gland. Although these techniques may provide temporary pain relief, the pain may quickly become even worse than presurgical levels because of subsequent deafferent pain that is more difficult to treat than most somatic pain. As a result, surgical resection techniques are not commonly used any more. Instead, modern interventional pain management techniques emphasize the importance of accurate delivery of medications such as corticosteroids or local anesthetics to suppress inflammation and block conduction of painful information, respectively. Selective destruction of nerve tissue with heat generated by radiofrequency energy or freezing the nerve tissue with liquid nitrogen (cryotherapy) has largely replaced surgical resections. Nerve stimulation techniques have also evolved concomitant to neuroscientific developments in our understanding of the mechanisms of pain. Table 44.4 lists commonly used interventional pain management techniques and their indications.
Table 44.4 Commonly Used Interventional Pain Management Techniques and Indications
| Name of Procedure | Indication |
|---|---|
| Celiac plexus block | Pancreatic cancer |
| Diskography | Diagnosis of anatomical localization of diskogenic pain |
| Epidural corticosteroid injection | Lumbar or cervical radiculopathy |
| Facet joint block | Lumbar or cervical facet joint syndrome |
| Facet joint rhizotomy | Lumbar or cervical facet joint syndrome |
| Gasserian ganglion block | Trigeminal neuralgia |
| Greater occipital nerve block | Greater occipital neuralgia |
| Intravenous regional block | Complex regional pain syndromes |
| Lumbar sympathetic block | Complex regional pain syndromes of the legs |
| Percutaneous disk decompression | Lumbar or cervical disk herniation |
| Sacroiliac joint injection | Sacroiliac joint pain |
| Sphenopalatine ganglion block | Headache and facial pain |
| Spinal cord stimulator | CRPS, PVD, low back pain, angina |
| Stellate ganglion block | CRPS of arm, neck, and head; headache |
| Suprascapular nerve block | Shoulder pain |
| Vertebroplasty | Vertebral fracture |
| Motor cortex stimulation | Neuropathic pain |
| Deep brain stimulation | Neuropathic pain |
CRPS, Complex regional pain syndrome; PVD, peripheral vascular disease.
Greater Occipital Nerve Block
Greater occipital nerve block is the easiest interventional procedure for neurologists to perform in the office. For the procedure, one can palpate the posterior occipital protuberance, move 1.5 to 2 cm laterally, feel for the occipital artery pulsation and groove, then inject 2 to 3 mL of 0.5% bupivacaine with 20 mg of triamcinolone down to the bone and fan out (Fig. 44.4). According to this author’s data, for patients with occipital neuralgia after whiplash injuries, a greater occipital nerve block may provide immediate headache relief in 90% of patients and last for an average of 28 days. More rigorous clinical trials are needed to confirm the clinical efficacy of occipital nerve block for occipital neuralgia and cervicogenic headache (Ashkenazi et al., 2010). More research and education are warranted to increase clinician awareness of the existence of occipital neuralgia and cervicogenic headache, inasmuch as most neurologists seem more interested and well trained in examining the 12 pairs of cranial nerves than the greater occipital nerves.
Sphenopalatine Ganglion Block for Headache and Facial Pain
The sphenopalatine ganglion is a small triangular structure located in the pterygopalatine fossa, posterior to the middle turbinate and inferior to the maxillary nerve. It is covered by a thin layer (about 1 to 5 mm) of connective tissue and mucous membrane. Anesthetization of the sphenopalatine ganglion can be accomplished via the transnasal approach. The patient is placed supine on the treatment table with the nose pointed at the ceiling. A cotton applicator soaked with 2% to 4% lidocaine is inserted into the nose on the side of headache. To avoid mechanical discomfort, the cotton applicator should not be inserted deeply into the upper posterior wall of the nasopharynx. A slow drip of 2 to 4 mL of lidocaine over a 2- to 4-minute period into the nose through the cotton applicator often achieves the goal of a sphenopalatine ganglion block, with the local anesthetic flowing down to the back of nasopharynx by gravity. Sphenopalatine ganglion blocks have been reported to be effective in the relief of a wide variety of pain conditions of the head including acute migraine attacks, cluster headache, atypical facial pain, head and facial RSD, and postdural puncture headache (Cohen et al., 2009). Intranasal sphenopalatine ganglion block is safe and easy to perform in the clinic and may be helpful for neurologists without special training in interventional pain management techniques to treat an acute headache attack. Other methods for sphenopalatine ganglion block such as a lateral approach with fluoroscopic guidance or endoscopic sphenopalatine ganglion block have also been used. However, special training and equipment are needed.
Gasserian Ganglion Lesions for Trigeminal Neuralgia
The first choice for treatment of trigeminal neuralgia is carbamazepine. It can be used with other medication such as baclofen. Gasserian ganglion lesions are indicated when patients fail other medication treatments. These procedures include radiofrequency thermocoagulation, balloon compression, and glycerolysis. Radiofrequency thermocoagulation is the most commonly used procedure. This procedure is often performed by neurosurgeons, interventional pain specialists, or interventional radiologists with special training. The treatment requires inserting a radiofrequency needle through the face and foramen ovale into the base of the skull under the guidance of fluoroscopy, CT, or CT fluoroscopy. After the needle reaches the gasserian ganglion, radiofrequency energy is applied to induce thermocoagulation; 87% to 91% of patients experience immediate pain relief. In a 5-year follow-up, 50% of patients still had good pain relief. Common side effects include corneal anesthesia, masticator weakness, and anesthesia dolorosa. Recently, stereotactic radiosurgery for trigeminal neuralgia has been used more widely because of its noninvasive nature. Significant pain relief was achieved in 73% at 1 year, 65% at 2 years, and 41% at 5 years follow-up (Kondziolka et al., 2010). However, this procedure may be more costly than other procedures already mentioned.
Epidural Corticosteroid Injection
Pain specialists have used epidural corticosteroid injection (ESI) for decades to treat back and neck pain. The procedure is further divided into cervical, thoracic, and lumbar ESI (LESI), with the purpose of treating pain originating from different spinal regions. By 1995, there were at least 12 so-called double-blind placebo-controlled studies investigating the clinical efficacy of LESI for LBP. Of these studies, only six yielded positive results, while the other studies did not support the use of LESI for LBP. Actually, several of these studies exhibited the critical flaw of treating “low back pain” as a single entity. It is now realized that LBP is a clinical syndrome that may be caused by a variety of pathologies in the lumbar spine and adjacent organs. It is not reasonable to treat LBP with ESI, regardless of the cause. More recent well-designed placebo-controlled studies have provided clinical evidence that LESI decreases lumbar radicular pain caused by lumbar disk herniation (Roberts et al., 2009). The pain-relieving effect of LESI may last up to 3 months. Corticosteroids appear to speed the rate of recovery and return of function, allowing patients to reduce medication levels and increase activity while waiting for the natural improvement expected in most spinal disorders. Recent studies also support the use of LESI for pain relief in patients with spinal stenosis (Lee et al., 2010).
Past the age of 60, more than 90% of the normal population has a variety of degenerative spine changes including disk herniation, spinal stenosis, and foraminal stenosis. The majority of persons with these changes, however, do not have pain. It is now believed that the pain in patients with disk herniation and associated radiculopathy is not purely due to mechanical compression but is more likely due to chemical inflammation. A recent study provided convincing evidence for the role of inflammatory mediators in the pathogenesis of lumbar radicular pain and LBP in patients with lumbar degenerative diseases. In the study, the immunoreactivity of an array of cytokines was measured in lavage samples and compared with clinical response to the therapeutic injection. Ten subjects underwent repeated epidural lavage sampling 3 months after the steroid injection. It was found that interferon gamma (IFN-γ) was the most consistently detected cytokine. IFN-γ immunoreactivity was also highly correlated with reduction of pain 3 months after the epidural steroid injection. In subjects reporting significant pain relief (>50%) from the injection, mean IFN-γ immunoreactivity was significantly greater compared with patients experiencing no significant relief. The IFN-γ immunoreactivity in repeated lavage samples decreased to trace residual concentrations in patients who reported pain relief from the steroid injection. These results suggest that IFN-γ may be part of a biochemical cascade triggering pain in lumbar radicular pain (Scuderi et al., 2009). Other chemical substances such as phospholipase A2, which is responsible for the liberation of arachidonic acid from cell membranes and starting the cascade of formation of inflammatory mediators such as prostaglandin E (PGE), is also believed to play a major role in pathogenesis of LBP. Epidural corticosteroid injection has been proven to suppress the functional activity of inflammatory mediators such IFN-γ and phospholipase A2 (Scuderi et al., 2009) to decrease inflammation in the epidural space and surrounding nerve roots. With the support of evidence from both basic science and clinical studies, it is current common practice to offer patients with lumbar radicular pain due to disk herniation a trial of LESI before considering a surgical treatment for lumbar disk herniation. The procedure often prevents back surgeries. As long as pain is relieved and the patient is free of neurological deficits, a herniated disk should be left alone without further treatment.
Lumbar Facet Joint Block
The lumbar facet joint block procedure is indicated for lumbar facet joint pain syndrome. Lumbar facet joint syndrome may be found in up to 35% of patients with LBP. Clinically, this syndrome may mimic lumbar radiculopathy (sciatica). Patients may complain of LBP, often on one side, with pain radiating down the back or front of the thigh. Clinical examination may reveal tenderness on either or both sides of the lumbar spine over the lumbar facet joints. Lumbar spine extension and lateral rotation to the painful side may increase LBP because this maneuver increases pressure on the lumbar facet joints. The straight leg raising test is often negative. Traditionally, pain specialists have performed intrajoint corticosteroid injections, but over the last decade, this procedure has largely been replaced by a diagnostic medial branch (nerve innervating the lumbar facet joints) block with a small amount of local anesthetic. If the patient has significant pain relief (more than 50%) after two consecutive diagnostic medial branch blocks, facet joint rhizotomy with radiofrequency destruction of the medial branch will be performed to denervate the lumbar facet joints. A recent systematic literature review found moderate evidence to support the clinical efficacy and use of radiofrequency rhizotomy for lumbar facet joint syndrome (Datta et al., 2009).
Percutaneous Disk Decompression
Over the last decade, two new percutaneous disk decompression techniques have been reported. Introduced in 2000, DISC Nucleoplasty utilizes a unique plasma technology called Coblation to remove tissue from the center of the disk. During the procedure the DISC Nucleoplasty SpineWand is inserted into the center of the disk, where a series of channels are created to remove tissue from the nucleus. Disc DeKompressor was introduced in 2003. This procedure uses a 1.5-mm percutaneous lumbar diskectomy probe to aspirate the disk material. It is minimally invasive with less risk for nerve root damage. This technique is indicated for patients with contained disk herniation and lumbar radiculopathy. Observational studies suggest both Nucleoplasty and Disc DeKompressor may be potentially effective, minimally invasive treatments for patients with symptomatic contained disks. However, prospective randomized controlled trials are needed to confirm their clinical efficacy and to determine ideal patient selection for these procedures (Gerges et al., 2010).
Motor Cortex Stimulation
Motor cortex stimulation (MCS) has been used for the treatment of central and neuropathic pain syndromes since 1991. It has been used to treat medically unresponsive central and neuropathic pain including that due to thalamic, putaminal, and lateral medullary infarction, traumatic trigeminal neuropathy (not idiopathic trigeminal neuralgia), facial postherpetic neuralgia, brachial plexopathy, neuropathic pain after an SCI, phantom-limb pain, and CRPS. MCS has shown particular promise in the treatment of intractable neuropathic facial pain and central pain syndromes such as thalamic pain syndrome (Levy et al., 2010).
Spinal Cord Stimulation
SCS is indicated for failed back surgery syndrome, CRPS, and unremitting pain due to peripheral vascular disease. Multiple studies have found that SCS may also improve pain due to refractory angina and improve circulation in the coronary arteries. Some authors have reported treatment success with SCS for severe peripheral neuropathy, postherpetic neuralgia, chronic knee pain following total knee replacement, central pain in MS, and painful spasms of atypical stiff limb syndrome (Ughratdar et al., 2010). The value of SCS for amputation stump pain, phantom-limb pain, and SCI is yet to be established. Patients seeking SCS treatments usually have failed all other conservative treatments such as medication, physical therapy, and nerve blocks with anesthetics and/or corticosteroids. SCS is not indicated for severe depression and contraindicated for patients with a cardiac pacemaker or defibrillators.
Intrathecal Drug Delivery Systems
Commonly used medications for pain management include morphine, hydromorphone, bupivacaine, clonidine, and ziconotide, a novel peptide that functions as a calcium channel blocker. Ziconotide was approved by the FDA in 2004 for treating intractable severe chronic pain, but its serious side effects have called the clinical use of this medication into question (Ziconotide, 2008). Baclofen is a GABAB agonist. It has been used through an intrathecal delivery system for the treatment of severe spasticity and may also decrease the pain related to spasticity. Even though intrathecal opioid treatment was initially approved by the FDA for the treatment of patients with malignant pain, over the last decade, intrathecal opioids have been used extensively for nonmalignant pain such as failed back surgery syndrome. A retrospective cohort study with 3-year follow-up found a favorable outcome for intrathecal opioids. Some patients are able to eliminate oral opioids, although some increase in intrathecal opioid dosing may be required (Atli et al., 2010).
Affaitati G., Fabrizio A., Savini A., et al. A randomized, controlled study comparing a lidocaine patch, a placebo patch, and anesthetic injection for treatment of trigger points in patients with myofascial pain syndrome: evaluation of pain and somatic pain thresholds. Clin Ther. 2009;31(4):705-720.
Arnold L.M., Gendreau R.M., Palmer R.H., et al. Efficacy and safety of milnacipran 100 mg/day in patients with fibromyalgia: results of a randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2010;62:2745-2756.
Ashkenazi A., Blumenfeld A., Napchan U., et al. Peripheral nerve blocks and trigger point injections in headache management–a systematic review and suggestions for future research. Headache. 2010;50:943-952.
Atli A., Theodore B.R., Turk D.C., et al. Intrathecal opioid therapy for chronic nonmalignant pain: a retrospective cohort study with 3-year follow-up. Pain Med. 2010;11:1010-1016.
Bermejo P.E., Oreja-Guevara C., ez-Tejedor E. [Pain in multiple sclerosis: prevalence, mechanisms, types and treatment]. Rev Neurol. 2010;50(2):101-108.
Breuer B., Pappagallo M., Knotkova H., et al. A randomized, double-blind, placebo-controlled, two-period, crossover, pilot trial of lamotrigine in patients with central pain due to multiple sclerosis. Clin Ther. 2007;29(9):2022-2030.
Brown J.A., Pilitsis J.G. Motor cortex stimulation. Pain Med. 2006 May;7(Suppl 1):S140-S145.
Carlton S.M., Du J., Tan H.Y., et al. Peripheral and central sensitization in remote spinal cord regions contribute to central neuropathic pain after spinal cord injury. Pain. 2009;147(1-3):265-276.
Carroll I.R., Kaplan K.M., Mackey S.C. Mexiletine therapy for chronic pain: survival analysis identifies factors predicting clinical success. J Pain Symptom Manage. 2008;35(3):321-326.
Cohen S., Sakr A., Katyal S., et al. Sphenopalatine ganglion block for postdural puncture headache. Anaesthesia. 2009;64(5):574-575.
Coluzzi F., Mattia C. Mechanism-based treatment in chronic neuropathic pain: the role of antidepressants. Curr Pharm Des. 2005;11(23):2945-2960.
Daniels S.E., Upmalis D., Okamoto A., et al. A randomized, double-blind, phase III study comparing multiple doses of tapentadol IR, oxycodone IR, and placebo for postoperative (bunionectomy) pain. Curr Med Res Opin. 2009;25:765-776.
Datta S., Lee M., Falco F.J., et al. Systematic assessment of diagnostic accuracy and therapeutic utility of lumbar facet joint interventions. Pain Physician. 2009;12(2):437-460.
Dworkin R.H., O’Connor A.B., Audette J., et al. Recommendations for the pharmacological management of neuropathic pain: an overview and literature update. Mayo Clin Proc. 2010;85(3 Suppl):S3-14.
Geracioti T.D.Jr., Carpenter L.L., Owens M.J., et al. Elevated cerebrospinal fluid substance p concentrations in posttraumatic stress disorder and major depression. Am J Psychiatry. 2006;163(4):637-643.
Gerges F.J., Lipsitz S.R., Nedeljkovic S.S. A systematic review on the effectiveness of the Nucleoplasty procedure for discogenic pain. Pain Physician. 2010;13(2):117-132.
Gislason G.H., Rasmussen J.N., Abildstrom S.Z., et al. Increased mortality and cardiovascular morbidity associated with use of nonsteroidal anti-inflammatory drugs in chronic heart failure. Arch Intern Med. 2009;169(2):141-149.
Gluszko P., Bielinska A. Non-steroidal anti-inflammatory drugs and the risk of cardiovascular diseases: are we going to see the revival of cyclooxygenase-2 selective inhibitors? Pol Arch Med Wewn. 2009;119(4):231-235.
Goebel A., Baranowski A., Maurer K., et al. Intravenous immunoglobulin treatment of the complex regional pain syndrome: a randomized trial. Ann Intern Med. 2010;152(3):152-158.
Gomez-Arguelles J.M., Dorado R., Sepulveda J.M., et al. Oxcarbazepine monotherapy in carbamazepine-unresponsive trigeminal neuralgia. J Clin Neurosci. 2008;15(5):516-519.
Grosskopf J., Mazzola J., Wan Y., et al. A randomized, placebo-controlled study of oxcarbazepine in painful diabetic neuropathy. Acta Neurol Scand. 2006;114(3):177-180.
Gwilym S.E., Keltner J.R., Warnaby C.E., et al. Psychophysical and functional imaging evidence supporting the presence of central sensitization in a cohort of osteoarthritis patients. Arthritis Rheum. 2009;61(9):1226-1234.
Ingalls N.K., Horton Z.A., Bettendorf M., et al. Randomized, double-blind, placebo-controlled trial using lidocaine patch 5% in traumatic rib fractures. J Am Coll Surg. 2010;210(2):205-209.
Katz W.A., Rothenberg R. Section 3: The nature of pain: pathophysiology. J Clin Rheumatol. 2005;11(2 Suppl):S11-S15.
Kemler M.A., de Vet H.C., Barendse G.A., et al. Effect of spinal cord stimulation for chronic complex regional pain syndrome type I: five-year final follow-up of patients in a randomized controlled trial. J Neurosurg. 2008;108(2):292-298.
Khodayar M.J., Shafaghi B., Naderi N., et al. Antinociceptive effect of spinally administered cannabinergic and 2-adrenoceptor drugs on the formalin test in rat: possible interactions. J Psychopharmacol. 2006;20(1):67-74.
Kondziolka D., Zorro O., Lobato-Polo J., et al. Gamma Knife stereotactic radiosurgery for idiopathic trigeminal neuralgia. J Neurosurg. 2010;112(4):758-765.
Krenzelok E.P. The FDA Acetaminophen Advisory Committee Meeting—what is the future of acetaminophen in the United States? The perspective of a committee member. Clin Toxicol (Phila). 2009;47(8):784-789.
Lee J.W., Myung J.S., Park K.W., et al. Fluoroscopically guided caudal epidural steroid injection for management of degenerative lumbar spinal stenosis: short-term and long-term results. Skeletal Radiol. 2010;39(7):691-699.
Levy R., Deer T.R., Henderson J. Intracranial neurostimulation for pain control: a review. Pain Physician. 2010;13(2):157-165.
Lin P.L., Fan S.Z., Huang C.H., et al. Analgesic effect of lidocaine patch 5% in the treatment of acute herpes zoster: a double-blind and vehicle-controlled study. Reg Anesth Pain Med. 2008;33(4):320-325.
Manchikanti L., Derby R., Wolfer L., et al. Evidence-based medicine, systematic reviews, and guidelines in interventional pain management: Part 7: systematic reviews and meta-analyses of diagnostic accuracy studies. Pain Physician. 2009;12(6):929-963.
Meisner J.G., Marsh A.D., Marsh D.R. Loss of GABAergic interneurons in laminae I-III of the spinal cord dorsal horn contributes to reduced GABAergic tone and neuropathic pain after spinal cord injury. J Neurotrauma. 2010;27:729-737.
Naegel S., Obermann M. Topiramate in the prevention and treatment of migraine: efficacy, safety and patient preference. Neuropsychiatr Dis Treat. 2010;6:17-28.
Papoiu A.D., Yosipovitch G. Topical capsaicin. The fire of a ‘hot’ medicine is reignited. Expert Opin Pharmacother. 2010;11(8):1359-1371.
Pickering A.E., Thornton S.R., Love-Jones S.J., et al. Analgesia in conjunction with normalisation of thermal sensation following deep brain stimulation for central post-stroke pain. Pain. 2009;147(1-3):299-304.
Previnaire J.G., Nguyen J.P., Perrouin-Verbe B., et al. Chronic neuropathic pain in spinal cord injury: efficiency of deep brain and motor cortex stimulation therapies for neuropathic pain in spinal cord injury patients. Ann Phys Rehabil Med. 2009;52(2):188-193.
Rao R.D., Flynn P.J., Sloan J.A., et al. Efficacy of lamotrigine in the management of chemotherapy-induced peripheral neuropathy: a phase 3 randomized, double-blind, placebo-controlled trial, N01C3. Cancer. 2008;112(12):2802-2808.
Roberts S.T., Willick S.E., Rho M.E., et al. Efficacy of lumbosacral transforaminal epidural steroid injections: a systematic review. PM R. 2009;1(7):657-668.
Rog D.J., Nurmikko T.J., Friede T., et al. Randomized, controlled trial of cannabis-based medicine in central pain in multiple sclerosis. Neurology. 2005;65(6):812-819.
Schwartzman R.J., Alexander G.M., Grothusen J.R., et al. Outpatient intravenous ketamine for the treatment of complex regional pain syndrome: a double-blind placebo controlled study. Pain. 2009;147(1-3):107-115.
Scuderi G.J., Cuellar J.M., Cuellar V.G., et al. Epidural interferon gamma-immunoreactivity: a biomarker for lumbar nerve root irritation. Spine (Phila Pa 1976). 2009;34(21):2311-2317.
Sigtermans M.J., van Hilten J.J., Bauer M.C., et al. Ketamine produces effective and long-term pain relief in patients with complex regional pain syndrome type 1. Pain. 2009;145(3):304-311.
Silverman S.M. Opioid induced hyperalgesia: clinical implications for the pain practitioner. Pain Physician. 2009;12(3):679-684.
Smit J.W., Oh C., Rengelshausen J., et al. Effects of acetaminophen, naproxen, and acetylsalicylic acid on tapentadol pharmacokinetics: results of two randomized, open-label, crossover, drug-drug interaction studies. Pharmacotherapy. 2010;30(1):25-34.
Straube S., Derry S., Moore R.A., et al. Pregabalin in fibromyalgia: meta-analysis of efficacy and safety from company clinical trial reports. Rheumatology (Oxford). 2010;49(4):706-715.
Tanaka T., Sakamoto E., Shiiba S., et al. Relationship between the curative effects of carbamazepine administration and the neurovascular compression volume of the trigeminal nerve measured using magnetic resonance cisternography. Clin J Pain. 2009;25(9):752-759.
Thaera G.M., Wellik K.E., Carter J.L., et al. Do cannabinoids reduce multiple sclerosis-related spasticity? Neurologist. 2009;15(6):369-371.
The International Headache Society. The international classification of headache disorders: 2nd edition. Cephalalgia. 2004;24(Suppl 1):9-160.
Tran de Q.H., Duong S., Bertini P., et al. Treatment of complex regional pain syndrome: a review of the evidence. Can J Anaesth. 2010;57(2):149-166.
Ughratdar I., Sivakumar G., Basu S. Spinal cord stimulation to abort painful spasms of atypical stiff limb syndrome. Stereotact Funct Neurosurg. 2010;88(3):183-186.
Vanderah T.W. Delta and kappa opioid receptors as suitable drug targets for pain. Clin J Pain. 2010;26(Suppl 10):S10-S15.
Vikelis M., Rapoport A.M. Role of antiepileptic drugs as preventive agents for migraine. CNS Drugs. 2010;24(1):21-33.
Woodhouse A. Phantom limb sensation. Clin Exp Pharmacol Physiol. 2005;32(1-2):132-134.
Zahra H., Teh B.S., Paulino A.C., et al. Stereotactic radiosurgery for trigeminal neuralgia utilizing the BrainLAB Novalis system. Technol Cancer Res Treat. 2009;8(6):407-412.
Zhou L., Hud-Shakoor Z., Hennessey C., et al. Upper cervical facet joint and spinal rami blocks for the treatment of cervicogenic headache. Headache. 2010;50:657-663.
2008 Ziconotide: new drug. Limited analgesic efficacy, too many adverse effects. Prescrire Int. 2008;17(97):179-182.