Chapter 51 Principles of Neutrophil (Granulocyte) Transfusions
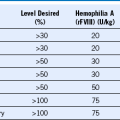
Table 51-1 Infectious Problems in Neutropenic Patients Treated With Historical Granulocyte Transfusions in 34 Studies
Collection and Transfusion of Neutrophil Concentrates
Donor stimulation using only properly timed corticosteroids (≥24 hours before leukapheresis, NOT immediately before) will increase the yield to approximately 2 × 1010 PMNs. Stimulation with G-CSF, alone or in combination with corticosteroids, will produce higher but variable PMN yields. Yields of 4 to 8 × 1010 PMNs are achieved regularly, and posttransfusion blood PMN counts frequently increase to 1 to 3 × 109/L, with PMNs detected in the recipient’s bloodstream for several hours after GTX. Currently PMN donors are optimally stimulated using 300 to 480 mcg G-CSF given subcutaneously plus 8 mg dexamethasone taken orally approximately 12 hours before beginning leukapheresis.1 Recent reports suggest that adrenal corticosteroids might cause posterior subcapsular cataracts in PMN donors. Although these reports are not in total agreement, it seems logical to include this cautionary information in donor consent forms or to stimulate PMN donors using only G-CSF—accepting lower PMN doses with the last option.2
Author’s Approach to Therapeutic Granulocyte Transfusions
Collect PMNs (4 to 8 × 1010) from allogeneic blood donors, as follows:
1. Stimulate neutrophilia by giving the donor 300 to 480 mcg of granulocyte colony-stimulating factor (G-CSF) subcutaneously 12 hours before beginning leukapheresis, with or without 8 mg dexamethasone orally 12 hours before beginning leukapheresis.
2. Process 10 L of donor blood using a continuous-flow blood separator with citrated hydroxyethyl starch solution infused throughout the collection.
1 Clarke K, Szer J, Shelton M, et al. Multiple granulocyte transfusions facilitating unrelated bone marrow transplantation in a patient with very severe aplastic anemia complicated by suspected fungal infection. Bone Marrow Transplant. 1995;16:723.
2 Hester JP, Dignani MC, Anaissie EJ, et al. Collection and transfusion of granulocyte concentrates from donors primed with granulocyte stimulating factor and response of myelosuppressed patients with established infection. J Clin Apheresis. 1995;10:188.