Chapter 46 Principles of Neurosurgery
The basic principles of neurosurgery include but are not limited to:
 Establishing an accurate diagnosis
Establishing an accurate diagnosis
 Exercising proper patient selection for surgery
Exercising proper patient selection for surgery
Although these principles have remained constant over the past century, the practice of neurosurgery has evolved tremendously since the days of Cushing, Dandy, and Penfield.* This dramatic change has occurred as a result of a convergence of advancing technology combined with the exponential growth of neuroscience knowledge and discoveries (Apuzzo, 1996; Kaibara et al., 2000).
No one chapter can even begin to cover the gamut of neurosurgical practice in any meaningful way. This chapter is aimed at the practicing clinical neurologist who interacts with neurosurgeon colleagues. Knowing if, when, and which neurosurgical subspecialists to refer patients to can improve outcome as well as avoid unnecessary utilization of healthcare resources. In the best of both worlds, the neurologist can play a key role in the diagnostic and perioperative management plus the long-term follow-up of patients. This paradigm already exists and works well in many brain tumor programs where the neuro-oncologist is an integral member of the surgical decision-making and postoperative management team (Yasargil et al., 2004).
Establishing the Diagnosis
Inherent to surgical intervention, the vast majority of neurosurgical consultations involve diagnostic neuroimaging. In most cases, referral to a neurosurgeon occurs as a consequence of abnormal imaging results, already creating a unique patient-physician relationship. The neurosurgeon is often confronted with the situation of deciding whether the patient’s symptoms or signs, if any, correlate with the neuroimaging findings. This is in stark contrast to neurological practice, in which certain diagnoses such as headache and seizures may not receive diagnostic imaging initially or even at all. Although the finding of a neuroimaging abnormality is not a prerequisite for neurosurgical intervention (such as movement disorder surgery), neuroimaging is required nevertheless (Britz et al., 1995; Kent et al., 1994; and Nijeholt et al., 1998).
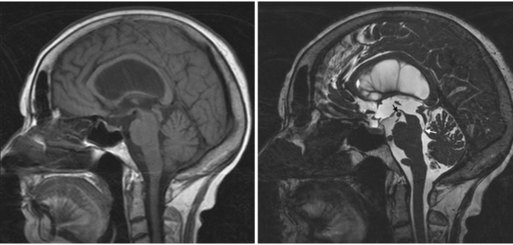
It is important to understand the difference between standard diagnostic imaging and the high-resolution neuroimaging required for neurosurgical decision making. For example, routine diagnostic magnetic resonance imaging (MRI) studies of the brain are performed with relatively thick 5-mm slices, with 2.5-mm skip between slices. Although generally adequate to detect lesions 4 mm in size or larger, partial volume averaging can obscure fine anatomical detail such as the relationship of arteries, veins, and cranial nerves to the lesion. For example, although the diagnosis of hydrocephalus may be obvious on routine diagnostic imaging, establishing the etiology of the ventriculomegaly may require high-resolution constructive interference in steady state (CISS) imaging (Fig. 46.1). In this case, a diagnosis of occult aqueductal stenosis would dramatically change the treatment, with endoscopic third ventriculostomy being the preferred treatment rather than a ventriculoperitoneal shunt.
With routine “diagnostic” imaging, the number of different MRI sequences is often limited by the concept of the study being a screening exam rather than one honing in on a certain differential diagnosis. For example, atypical arachnoid cysts can be differentiated from epidermoid cysts if repeat imaging includes a diffusion-weighted imaging (DWI) study (Tsuruda et al., 1990). In this case, an arachnoid cyst may not require referral to a neurosurgeon, whereas the epidermoid cyst should. Consultation with a neuroradiologist, if needed, could lead to proper imaging studies being performed prior to possible neurosurgical consultation.
In other cases, routine diagnostic imaging may be adequate for establishing a diagnosis but inadequate for neurosurgical decision making. In some cases, additional imaging modalities may be required, such as computed tomography (CT) imaging of bony anatomy to supplement abnormal soft-tissue findings on MRI. In other cases, special high-resolution or unique imaging sequences may be required (Barnett et al., 1993; Curtin and Hirsch, 1992). Although beyond the scope of this chapter, suffice it to state that a brief discussion with the neurosurgeon—prior to referral—may in some cases lead to essential imaging studies being obtained prior to the consultation. This may make for a much more productive and useful consultation, obviating the need to have the patient return for an otherwise unnecessary follow-up visit owing to the lack of key imaging studies at the first visit.
Patient Selection for Surgery
Patient selection for surgery is under the purview of the neurosurgeon in most cases, although for many disorders, the neurologist plays an integral and key role. As noted previously, the increasing sophistication and complexity of surgical intervention mandates intimate collaboration between neurologists, neurosurgeons, and other subspecialists. Knowing when to refer to multidisciplinary specialty centers is obviously a function of patient diagnosis and proximity to such centers. In general, community neurosurgeons are well versed in treating a wide range of neurosurgical disorders, although increasingly, many are limiting their practices to mainly treating spinal disorders. Increasing evidence suggests that for specific diagnoses, surgical outcomes are a function of surgical volume load and experience. For example, dedicated brain tumor neurosurgeons performing over 50 brain tumor operations per year generally have better patient outcomes than those only occasionally performing these surgeries (Cowan et al., 2002; and Cowan et al., 2003; D’Agostino et al., 2009; and Shahlaie et al., 2010). Similar findings have been reported for cerebral aneurysm treatment, epilepsy, movement disorders, and pediatric disorders. Most academic centers and some large group private practices have dedicated subspecialty neurosurgeons (as well as the necessary ancillary team) to comprehensively and optimally manage these patients.
Patient selection for surgery has become increasingly complex, and new treatment modalities and alternatives have arisen. Just as surgical volume load and experience varies among neurosurgeons, it may be important that the referring physician understand other important factors in patient selection for surgery. An example is vestibular schwannoma (acoustic neuroma) management. As already noted, surgical experience may play a major role with regard to patient outcome. It is important to recognize, however, that fewer patients are choosing surgery for small acoustic neuromas, and instead are undergoing stereotactic radiosurgery or radiotherapy treatment (Andrews et al., 2001; Flickinger et al., 2001; Lunsford et al., 2005; and Meijer et al., 2000). Referring to a neurosurgeon whose practice is located in an area without focal beam radiation capabilities may inadvertently lead to an emphasis on surgical alternatives. A similar situation exists with intracranial aneurysms, in which endovascular coiling has increasingly become the treatment of choice for selected patients. Typically, high-volume neurovascular surgeons are closely affiliated with (or perform themselves) endovascular treatment, and therefore this issue is a moot point (D’Agostino et al., 2009).
In some cases, the neurologist is the primary determinant of patient selection for surgical intervention. The management of movement disorders is an obvious example. In only a minority of patients is surgical intervention such as deep brain stimulation appropriate Olanow and Koller, 1998). Referral to a movement disorder neurologist first may be appropriate instead of initial referral to a functional neurosurgeon. Other examples include carotid endarterectomy (versus stenting) and cerebrovascular bypass procedures.
To some extent, patient selection for surgery begins prior to neurosurgical evaluation. There are no hard-and-fast guidelines as to what types of neurological diagnoses should be referred for neurosurgical consultation, but in most cases, the decision to refer for neurosurgical consultation is obvious. Symptomatic patients with obvious abnormalities seen on imaging, such as a large cerebral aneurysm, require neurosurgical consultation. There are other cases, however, that may not require neurosurgical consultation and can be managed well and appropriately by the neurologist. Although it may seem obvious and trite, it is important to understand that a neurosurgical practice is a surgical practice. With the increasing use of neuroimaging in our society, so-called incidentalomas are being noted by radiologists (Fainstein et al., 2004; Molitch, 2008; and Molitch, 2009). In some cases, this has led to reflexive referrals to neurosurgeons based solely on the radiology report, without the personal review of the imaging study. Referral of these nonoperative lesions to the neurosurgeon first raises the expectation of the patient that surgical intervention is advised and/or pending, and second takes up a significant amount of the neurosurgeon’s clinic time explaining why the lesion does not require surgery. Examples follow.
Arachnoid Cyst
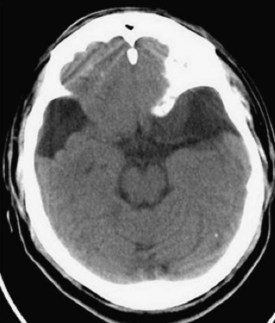
The vast majority of arachnoid cysts are small and asymptomatic and consequently, nonsurgical. A typical location is the anterior middle fossa where the anterior temporal lobe is displaced posteriorly (Fig. 46.2). Small arachnoid cysts that do not produce significant mass effect (midline shift, brainstem compression) are rarely a cause of headaches (Pierre-Kahn et al., 1990; Pradilla and Jallo, 2007). If it is equivocal whether the lesion is large enough to warrant surgical intervention, a quick film review by the neurosurgeon is often all that is needed to adequately triage the patient.
Tiny Intracranial Aneurysms
With the increasing availability and use of MRI, unruptured intracranial aneurysms less than 7 mm in size are being incidentally discovered during diagnostic imaging. Current international guidelines support repeat imaging of these lesions to ascertain whether they are enlarging or not (Asari and Ohmoto, 1993; Juvela, 2002; Mocco et al., 2004; and Wiebers et al., 2003). Because generally they do not require surgical intervention, referral to a neurosurgeon can be premature. A brief telephone conversation with the neurosurgical colleague may be all that is needed, conserving time and cost for both the patient and surgeon.
Incidental Small Cavernous Malformations
Like small aneurysms, these lesions are rarely surgical and can be followed with neurosurgical consultation (Gross et al., 2009; Robinson et al., 1991). Most neurosurgeons will gladly review imaging studies in order to differentiate which patients should be referred for consultation.
Intervertebral Disk Bulges and Annular Tears
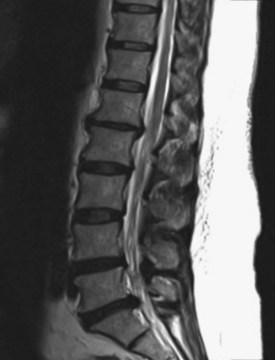
Here, the correlation of neuroimaging findings and neurological symptoms and signs is key (Fujiwara et al., 1999) (Fig. 46.3). Clinically insignificant disk bulges are so common in our aging society that a neurosurgical spine specialist could easily be inundated with nonsurgical consultations, stealing time from a practice tailored to helping appropriately referred patients (Stabler et al., 1996; Stabler et al., 1997). Admittedly, inappropriate patient referral to neurosurgeons for asymptomatic disk bulges is infrequent by neurologists, given their training and understanding of neural anatomy.
In some cases, certain diagnoses may not require immediate neurosurgical referral. A prototypical example is pseudotumor cerebri (idiopathic intracranial hypertension). It is generally accepted that surgical intervention is one of the last treatment options for this condition (Acheson, 2006; Kelman et al., 1992; Rosenberg et al., 1993). Although many, if not most, neurologists primarily manage this condition, some initiate a referral to neurosurgery for consideration for a cerebrospinal fluid (CSF) shunt procedure. Again, this sometimes raises patient expectations and leads to frustration and delay of (medical) treatment. As diuretics and weight loss are the primary treatment modalities, neurosurgical consultation may never be necessary. However, with rapid visual deterioration, optic nerve fenestration or CSF diversion may be indicated. Also, consideration of intracranial venous occlusive disease should be made for non-obese pseudotumor patients (Mokri et al., 1993; Owler et al., 2003; Owler et al., 2005). A telephone conversation with the neurosurgical colleague could direct the completion of a contrast MR venogram or CT venogram to rule out venous sinus abnormalities.
Long-Standing Overt Ventriculomegaly in Adults
It is common teaching that minimally or asymptomatic patients with overt hydrocephalus simply be monitored, and treatment considered only when the patient becomes significantly symptomatic (Fukuhara and Luciano, 2001; Kelly, 1991). Often these patients have undiagnosed aqueductal stenosis. The basis of this conservative “watch-and-wait” approach presumably arose as a consequence of the historically high complication rate associated with CSF shunt procedures in these patients (Pudenz and Foltz, 1991). This hesitance to consider early neurosurgical intervention is not unique to neurologists and underscores the importance of proper referral considerations. Our understanding and state-of-the-art management of adult hydrocephalus has significantly advanced over the past 2 decades. A neurosurgeon with particular interest in adult hydrocephalus will know that this is not a benign disorder, and that the natural history is inevitable symptomatology, nearly always prior to age 60, and that unfortunately, once a patient becomes symptomatic, it may be too late to effectively intervene. Moreover, the surgical techniques and CSF shunt technology has advanced significantly as well (Dusick et al., 2008; Feng et al., 2004). Many older-generation practicing neurosurgeons were not trained to perform endoscopic third ventriculostomies, which may be the preferred treatment, and therefore default directly to a CSF shunting procedure. Likewise, the use of outdated shunt valve technology may expose the patient to unwarranted risks of postoperative complications. The key point, which will be repeated in this chapter, is that the practice of neurosurgery has become increasingly subspecialized for certain diagnoses. For patients living in rural or even certain urban communities, longer travel to see a subspecialist may offer a better outcome.
Intractable Epilepsy, Trigeminal Neuralgia, and Hemifacial Spasm
These diagnoses have in common the clinical scenario of conditions typically treated primarily pharmacologically, and then consideration given to neurosurgical intervention only with “failure” of medical management (Sidebottom and Maxwell, 1995; Zakrzewska and Thomas, 1993). What constitutes “medically intractable” is clearly the issue at hand, and a detailed description is beyond the scope of this chapter. However, evidence suggests that prolonged (inadequate) medical management may lower the chances of improvement with surgery, and therefore earlier neurosurgical consultation should be considered (Li et al., 2004; Tyler-Kabara et al., 2002). Continued medical education with up-to-date knowledge of current guidelines is recommended.
Preoperative Surgical Planning
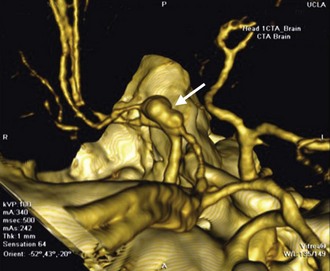
Vascular lesions are often best characterized by digital subtraction angiography, in some cases augmented by 3D angiography techniques. This modality provides functional and temporal information of the vasculature and is most helpful to characterize arteriovenous malformations (AVMs), complex aneurysms, and various tumors. Magnetic resonance angiography (MRA), however, is rarely helpful to characterize AVMs and aneurysms and therefore is a waste of medical resources if ordered. In some cases, CT angiography (CTA) is adequate for presurgical planning for straightforward intracerebral aneurysms (Papke et al., 2007) (Fig. 46.4).
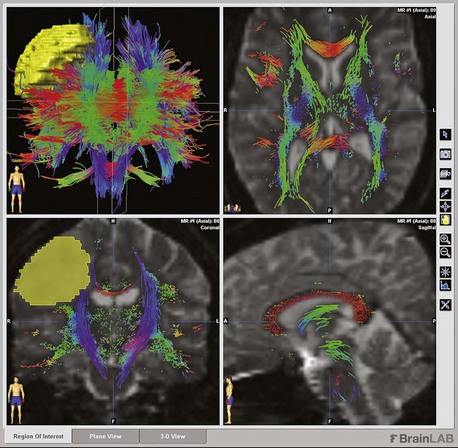
Brain tumor surgical planning can be quite complex. The location of the tumor is often the defining variable. Again, technology has significantly advanced the surgical treatment of brain tumors. Surgical planning for skull base tumors such as meningiomas and vestibular schwannomas may involve consideration of several different surgical corridors, each with inherent advantages and risks. Deep-seated intraaxial tumors that require transcortical approaches ideally should be performed with intraoperative stereotactic neuronavigation that incorporates functional imaging (fMRI), diffusion tensor imaging, white-matter tractography, and sometimes MR spectroscopy (MRS). Fiber tractography (Fig. 46.5) can help avoid damage to axonal structures like the optic radiations and arcuate fasciculus, whereas MRS can aid in biopsy site choice (Dowling et al., 2001; Kamada et al., 2005; Kamada et al., 2007). These imaging modalities are not universally available and require experienced technicians and complex postprocessing. For the neurologist, awareness of these considerations and possibilities should play a role in deciding where to refer patients. High-volume neurosurgical subspecialists who routinely use this technology should be sought for appropriate cases.
Epilepsy surgery requires a different set of diagnostic and surgical decision-making tools. When the location of the epileptogenic focus is not clear or may be attributable to numerous lesions, studies to help determine the culprit lesion are necessary. Cortical surface electrode electroencephalography (EEG), magnetoencephalography (MEG), or depth electrodes may be needed to identify the source of seizures; such modalities are generally only available at dedicated epilepsy centers (Collura et al., 1990; Matsumoto, 1990).
Spinal pathology adds additional diagnostic challenges to differentiating causes of pain and weakness. Dynamic studies such as flexion-extension spinal films can determine instability or ligamentous injury (Elsig and Kaech, 2006). Epidural or facet joint steroid injections can be both diagnostic and therapeutic in the treatment of back pain and radicular pain, directing surgical planning (Cuckler et al., 1985; Koes et al., 1995). Electromyography (EMG) and nerve conduction studies can help pinpoint the source(s) of peripheral nerve pathology (Levin et al., 1996; Tullberg et al., 1993).
Surgical Technique
Neurosurgery entails several key considerations. The type of anesthesia is tailored to the patient’s pathology and surgical plan. For example, patients with cervical stenosis and myelopathy cannot tolerate direct laryngoscopy and cervical manipulation because of the risk of cervical spinal cord injury. Therefore, awake fiberoptic intubation is performed to avoid cervical hyperextension and allow the patient to control their cervical musculature (Fuchs et al., 1999). Sometimes it is the neurologist, who has the time to comprehensively assess patients preoperatively, who diagnoses conditions such as spinal stenosis, even if it is not the primary condition requiring surgical intervention. Another example is the patient who is dependent on optimal cerebral perfusion pressure, such as in carotid stenosis or compressive cervical myelopathy, and does not tolerate the significant hypotension that is otherwise forgivable with general anesthesia. To combat this, invasive arterial blood pressure monitors are placed prior to induction, and mean arterial pressure (MAP) is kept normotensive throughout the surgery (Epstein, 1988).
Intraoperative neuromonitoring is routinely performed in a variety of neurosurgical operations. Modalities include EEG to monitor for vascular compromise, somatosensory evoked potential (SSEP) and motor evoked potential (MEP) monitoring for cortex through spinal cord viability, brainstem auditory evoked response (BAER) monitoring for acoustic nerve and brainstem injury, and direct nerve stimulation with EMG for cranial nerve and peripheral nerve monitoring. If a change from baseline is encountered in any of these monitoring modalities, surgical or anesthetic adjustments are performed (Minahan, 2002). Examples include repositioning an aneurysm clip if there is a decrease in SSEPs, EEGs that raise concerns of cortical ischemia, tailoring the extent of spinal cord tumor resection if there is a decrease in SSEP and MEP signals, or adjusting spinal pedicle screw trajectories if there is EMG conduction through the neural foramina (Dehdashti et al., 2006). Medical interventions can include increasing MAP for patients exhibiting ischemia, or inducing cortical burst suppression on EEG as a neuroprotective measure (Lavine et al., 1997).
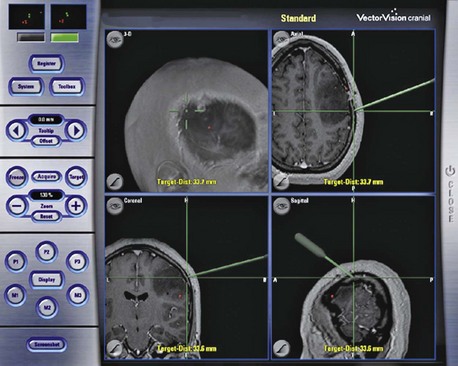
One major advancement of modern neurosurgery is intraoperative stereotactic neuronavigation. The technology entails registering (co-aligning) the patient’s head to a preoperative 3D high-resolution image (MRI or CT). The location of the tip of a pointer is then depicted on the imaging monitor (Fig. 46.6). This technology has proven helpful in determining the extent of tumor resection, especially if intraoperative CT or MRI is performed (Knauth et al., 1999). Stereotactic biopsy of lesions has become increasingly accurate (Moriarty et al., 2000). On the other hand, neuronavigation can help the surgeon avoid eloquent cortical regions such as the Broca area, Wernicke region, the corona radiata of the primary motor and sensory tracts, and the arcuate fasciculus. Concomitant fMRI and diffusion tensor imaging (DTI) with postprocessing is required to implement this technology (Wu et al., 2007). Lastly, this technology allows smaller incisions and craniotomies compared to those of the past.
Along with utilizing complex technology to avoid neurological complications is the more straightforward concept of passive brain retraction and venous drainage. Much intraoperative morbidity can be prevented by patient positioning. For intracranial surgeries, the head position can be adjusted to let gravity retract the brain from the lesion (Rolighed Larsen et al., 2002). The use of the falx cerebri as a natural retractor is helpful for interhemispheric lesions (Stone et al., 1990). Although these technical nuances may not be relevant to a neurologist’s daily practice, again it underscores the responsibility of the referring physician to guide patients to specialists who are highly experienced and use modern techniques that minimize operative morbidity.
In some highly specialized neurosurgical procedures, the neurologist plays an integral intraoperative role. One example is awake craniotomies for cortical mapping of eloquent language areas (Costello and Cormack, 2004). Another is epilepsy lesionectomy procedures in which the interpretation of intraoperative corticography is required.
Postoperative Management
The postoperative period can be critical to surgical success. In many larger hospitals, the neurointensive care unit incorporates a neurologist as the neurointensivist. This hospitalist provides minute-to-minute care that cannot be provided by a neurosurgeon while in the operating room. In general, prevention of complications is ideal. Early neurological examination establishes a postoperative neurological baseline. Improvement or decline from preoperative examination should be noted. Early identification of neurological deficits can help diagnose a hematoma, ischemia, or edema that at times can be reversible. Therefore, thorough, frequent, and sometimes continuous neuromonitoring is essential for quick identification and intervention of surgical complications (Bhatia and Gupta, 2007). Many modern intensive care units offer continuous EEG monitoring, again highlighting the natural marriage of neurointensive care with neurology (Guerit, 1999; Guerit et al., 2009).
Postoperative seizure prophylaxis is a controversial topic but is typically initiated intraoperatively and maintained up to 1 week following surgery using established antiepileptic (AED) agents (Horwitz, 1989). Although this has not been proven to decrease the incidence of postoperative seizures or epilepsy, AED use is justified to prevent catastrophic seizures that may lead to increased morbidity and mortality (Glantz et al., 2000). The neurologist can play a key role in postoperative management in this regard, helping determine the best AED agent and duration of treatment.
Another key role neurologists should play is in the long-term management and follow-up of patients with neurosurgical problems. This includes recovery from neurotrauma, intracerebral hemorrhage, hydrocephalus, brain tumor, and epilepsy surgery. Management of chronic neurological conditions is intrinsic to neurological training, and the neurologist is in many ways ideally poised to offer this care. The management of certain neuromodulatory devices such as deep brain stimulators and vagal nerve stimulators is ideally performed by trained neurologists who have a detailed understanding of the patient’s neurological status and can titrate stimulator settings much like medications (Okun et al., 2005). Although all neurosurgeons desire to be updated on the long-term sequelae of their intervention, neurosurgical practice is tailored to providing the best perioperative management of patients. It is important to understand that a minor percentage of a neurosurgeon’s practice involves outpatient encounters, and therefore this precious clinic time should be largely dedicated to evaluating and treating surgical conditions.
Acheson J.F. Idiopathic intracranial hypertension and visual function. Br Med Bull. 2006;79-80:233-244.
Andrews D.W., Suarez O., Goldman H.W., et al. Stereotactic radiosurgery and fractionated stereotactic radiotherapy for the treatment of acoustic schwannomas: comparative observations of 125 patients treated at one institution. Int J Radiat Oncol Biol Phys. 2001;50(5):1265-1278.
Apuzzo M.L. The Richard C. Schneider Lecture. New dimensions of neurosurgery in the realm of high technology: possibilities, practicalities, realities. Neurosurgery. 1996;38(4):625-637. discussion 37-39
Asari S., Ohmoto T. Natural history and risk factors of unruptured cerebral aneurysms. Clin Neurol Neurosurg. 1993;95(3):205-214.
Barnett G.H., Kormos D.W., Steiner C.P., et al. Use of a frameless, armless stereotactic wand for brain tumor localization with two-dimensional and three-dimensional neuroimaging. Neurosurgery. 1993;33(4):674-678.
Bhatia A., Gupta A.K. Neuromonitoring in the intensive care unit. I. Intracranial pressure and cerebral blood flow monitoring. Intensive Care Med. 2007;33(7):1263-1271.
Britz G.W., Haynor D.R., Kuntz C., et al. Carpal tunnel syndrome: correlation of magnetic resonance imaging, clinical, electrodiagnostic, and intraoperative findings. Neurosurgery. 1995;37(6):1097-1103.
Collura T.F., Luders H., Burgess R.C. EEG mapping for surgery of epilepsy. Brain Topogr. 1990;3(1):65-77.
Costello T.G., Cormack J.R. Anaesthesia for awake craniotomy: a modern approach. J Clin Neurosci. 2004;11(1):16-19.
Cowan J.A.Jr., Dimick J.B., Leveque J.C., et al. The impact of provider volume on mortality after intracranial tumor resection. Neurosurgery. 2003;52(1):48-53. discussion -4
Cowan J.A.Jr., Dimick J.B., Thompson B.G., et al. Surgeon volume as an indicator of outcomes after carotid endarterectomy: an effect independent of specialty practice and hospital volume. J Am Coll Surg. 2002;195(6):814-821.
Cuckler J.M., Bernini P.A., Wiesel S.W., et al. The use of epidural steroids in the treatment of lumbar radicular pain. A prospective, randomized, double-blind study. J Bone Joint Surg. 1985;67(1):63-66.
Curtin H.D., Hirsch W.L.Jr. Imaging of acoustic neuromas. Otolaryngol Clin North Am. 1992;25:553-607.
D’Agostino S.J., Harrigan M.R., Chalela J.A., et al. Clinical experience with Matrix2 360 degrees coils in the treatment of 100 intracranial aneurysms. Surg Neurol. 2009;72(1):41-47.
Davey L.M. Harvey Cushing: the New Haven years. Neurosurgery. 1999;45(5):1002-1009.
Dehdashti A.R., Pralong E., Debatisse D., et al. Multilobar electrocorticography monitoring during intracranial aneurysm surgery. Neurocrit Care. 2006;4(3):215-222.
Dowling C., Bollen A.W., Noworolski S.M., et al. Preoperative proton MR spectroscopic imaging of brain tumors: correlation with histopathologic analysis of resection specimens. AJNR Am J Neurolradiol. 2001;22(4):604-612.
Dusick J.R., McArthur D.L., Bergsneider M. Success and complication rates of endoscopic third ventriculostomy for adult hydrocephalus: a series of 108 patients. Surg Neurol. 2008;69(1):5-15.
Elsig J.P., Kaech D.L. Imaging-based planning for spine surgery. Minim Invasive Ther Allied Technol. 2006;15(5):260-266.
Epstein J.A. The surgical management of cervical spinal stenosis, spondylosis, and myeloradiculopathy by means of the posterior approach. Spine. 1988;13(7):864-869.
Fahlbusch R., Buchfelder M., Schrell U. [Neurosurgical therapy of neuroendocrinologic disorders]. Der Internist. 1985;26(5):293-301.
Fainstein Day P., Guitelman M., et al. Retrospective multicentric study of pituitary incidentalomas. Pituitary. 2004;7(3):145-148.
Feng H., Huang G., Liao X., et al. Endoscopic third ventriculostomy in the management of obstructive hydrocephalus: an outcome analysis. J Neurosurg. 2004;100(4):626-633.
Flickinger J.C., Kondziolka D., Niranjan A., et al. Results of acoustic neuroma radiosurgery: an analysis of 5 years’ experience using current methods. J Neurosurg. 2001;94(1):1-6.
Fuchs G., Schwarz G., Baumgartner A., et al. Fiberoptic intubation in 327 neurosurgical patients with lesions of the cervical spine. J Neurosurg Anesthesiol. 1999;11(1):11-16.
Fujiwara A., Tamai K., Yamato M., et al. The relationship between facet joint osteoarthritis and disc degeneration of the lumbar spine: an MRI study. Eur Spine J. 1999;8(5):396-401.
Fukuhara T., Luciano M.G. Clinical features of late-onset idiopathic aqueductal stenosis. Surg Neurol. 2001;55(3):132-136. discussion 6-7
Glantz M.J., Cole B.F., Forsyth P.A., et al. Practice parameter: anticonvulsant prophylaxis in patients with newly diagnosed brain tumors. Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2000;54(10):1886-1893.
Gross B.A., Batjer H.H., Awad I.A., et al. Brainstem cavernous malformations. Neurosurgery. 2009;64(5):E805-818. discussion E18
Guerit J.M. Medical technology assessment EEG and evoked potentials in the intensive care unit. Neurophysiol Clin. 1999;29(4):301-317.
Guerit J.M., Amantini A., Amodio P., et al. Consensus on the use of neurophysiological tests in the intensive care unit (ICU): electroencephalogram (EEG), evoked potentials (EP), and electroneuromyography (ENMG). Neurophysiol Clin. 2009;39(2):71-83.
Horwitz N.H. Prophylactic anticonvulsants for prevention of immediate and early postcraniotomy seizures. Surg Neurol. 1989;32(5):398.
Juvela S. Natural history of unruptured intracranial aneurysms: risks for aneurysm formation, growth, and rupture. Acta Neurochir (Wein). 2002;82:27-30.
Kaibara T., Saunders J.K., Sutherland G.R. Advances in mobile intraoperative magnetic resonance imaging. Neurosurgery. 2000;47(1):131-137. discussion 7-8
Kamada K., Todo T., Masutani Y., et al. Combined use of tractography-integrated functional neuronavigation and direct fiber stimulation. J Neurosurg. 2005;102(4):664-672.
Kamada K., Todo T., Masutani Y., et al. Visualization of the frontotemporal language fibers by tractography combined with functional magnetic resonance imaging and magnetoencephalography. J Neurosurg. 2007;106(1):90-98.
Kelly P.J. Stereotactic third ventriculostomy in patients with nontumoral adolescent/adult onset aqueductal stenosis and symptomatic hydrocephalus. J Neurosurg. 1991;75(6):865-873.
Kelman S.E., Heaps R., Wolf A., et al. Optic nerve decompression surgery improves visual function in patients with pseudotumor cerebri. Neurosurgery. 1992;30(3):391-395.
Kent D.L., Haynor D.R., Longstreth W.T.Jr., et al. The clinical efficacy of magnetic resonance imaging in neuroimaging. Ann Intern Med. 1994;120(10):856-871.
Knauth M., Wirtz C.R., Tronnier V.M., et al. Intraoperative MR imaging increases the extent of tumor resection in patients with high-grade gliomas. AJNR Am J Neuroradiol. 1999;20(9):1642-1646.
Koes B.W., Scholten R.J., Mens J.M., et al. Efficacy of epidural steroid injections for low-back pain and sciatica: a systematic review of randomized clinical trials. Pain. 1995;63(3):279-288.
Lavine S.D., Masri L.S., Levy M.L., et al. Temporary occlusion of the middle cerebral artery in intracranial aneurysm surgery: time limitation and advantage of brain protection. J Neurosurg. 1997;87(6):817-824.
Levin K.H., Maggiano H.J., Wilbourn A.J. Cervical radiculopathies: comparison of surgical and EMG localization of single-root lesions. Neurology. 1996;46(4):1022-1025.
Li S.T., Pan Q., Liu N., et al. Trigeminal neuralgia: what are the important factors for good operative outcomes with microvascular decompression. Surg Neurol. 2004;62(5):400-404. discussion 4-5
Ljunggren B. The case of General Wood. J Neurosurg. 1982;56(4):471-474.
Ljunggren B. [The pioneers of brain surgery (Harvey Cushing)]. Lakartidningen. 1982;79(14):1377-1378.
Lunsford L.D., Niranjan A., Flickinger J.C., et al. Radiosurgery of vestibular schwannomas: summary of experience in 829 cases. J Neurosurg. 2005;102(Suppl):195-199.
Matsumoto K. Neurosurgical applications of topographic mapping. Brain Topogr. 1990;3(1):209-218.
Meijer O.W., Wolbers J.G., Baayen J.C., et al. Fractionated stereotactic radiation therapy and single high-dose radiosurgery for acoustic neuroma: early results of a prospective clinical study. Int J Radiat Oncol Biol Phys. 2000;46(1):45-49.
Minahan R.E. Intraoperative neuromonitoring. Neurologist. 2002;8(4):209-226.
Mocco J., Komotar R.J., Lavine S.D., et al. The natural history of unruptured intracranial aneurysms. Neurosurg Focus. 2004;17(5):E3.
Mokri B., Jack C.R.Jr., Petty G.W. Pseudotumor syndrome associated with cerebral venous sinus occlusion and antiphospholipid antibodies. Stroke. 1993;24(3):469-472.
Molitch M.E. Nonfunctioning pituitary tumors and pituitary incidentalomas. Endocrinol Metab Clin N Am. 2008;37(1):151-171. xi
Molitch M.E. Pituitary tumours: pituitary incidentalomas. Best Pract Res. 2009;23(5):667-675.
Moriarty T.M., Quinones-Hinojosa A., Larson P.S., et al. Frameless stereotactic neurosurgery using intraoperative magnetic resonance imaging: stereotactic brain biopsy. Neurosurgery. 2000;47(5):1138-1145. discussion 45-46
Nijeholt G.J., van Walderveen M.A., Castelijns J.A., et al. Brain and spinal cord abnormalities in multiple sclerosis. Correlation between MRI parameters, clinical subtypes and symptoms. Brain. 1998;121(Pt 4):687-697.
Okun M.S., Tagliati M., Pourfar M., et al. Management of referred deep brain stimulation failures: a retrospective analysis from 2 movement disorders centers. Arch Neurol. 2005;62(8):1250-1255.
Olanow C.W., Koller W.C. An algorithm (decision tree) for the management of Parkinson’s disease: treatment guidelines. American Academy of Neurology. Neurology. 1998;50(3 Suppl 3):S1-S57.
Owler B.K., Parker G., Halmagyi G.M., et al. Pseudotumor cerebri syndrome: venous sinus obstruction and its treatment with stent placement. J Neurosurg. 2003;98(5):1045-1055.
Owler B.K., Parker G., Halmagyi G.M., et al. Cranial venous outflow obstruction and pseudotumor cerebri syndrome. Adv Tech Stand Neurosurg. 2005;30:107-174.
Papke K., Kuhl C.K., Fruth M., et al. Intracranial aneurysms: role of multidetector CT angiography in diagnosis and endovascular therapy planning. Radiology. 2007;244(2):532-540.
Penfield W. A technique for demonstrating the perivascular nerves of the pia mater and central nervous system. Am J Pathol. 1935;11(6):1007-1010.
Pierre-Kahn A., Capelle L., Brauner R., et al. Presentation and management of suprasellar arachnoid cysts. Review of 20 cases. J Neurosurg. 1990;73(3):355-359.
Pradilla G., Jallo G. Arachnoid cysts: case series and review of the literature. Neurosurg Focus. 2007;22(2):E7.
Preul M.C., Feindel W. Origins of Wilder Penfield’s surgical technique. The role of the “Cushing ritual” and influences from the European experience. J Neurosurg. 1991;75(5):812-820.
Preul M.C., Stratford J., Bertrand G., et al. Neurosurgeon as innovator: William V. Cone (1897-1959). J Neurosurg. 1993;79(4):619-631.
Pudenz R.H., Foltz E.L. Hydrocephalus: overdrainage by ventricular shunts. A review and recommendations. Surg Neurol. 1991;35(3):200-212.
Robinson J.R., Awad I.A., Little J.R. Natural history of the cavernous angioma. J Neurosurg. 1991;75(5):709-714.
Rolighed Larsen J.K., Haure P., et al. Reverse Trendelenburg position reduces intracranial pressure during craniotomy. J Neurosurg Anesthesiol. 2002;14(1):16-21.
Rosenberg M.L., Corbett J.J., Smith C., et al. Cerebrospinal fluid diversion procedures in pseudotumor cerebri. Neurology. 1993;43(6):1071-1072.
Sano K. [Harvey Williams Cushing]. No Shinkei Geka. 1981;9(1):32-35.
Shahlaie K., McLaughlin N., Kassam A.B., et al. The role of outcomes data for assessing the expertise of a pituitary surgeon. Curr Opin Endocrinol Diabetes Obes. 2010;17:369-376.
Shrivastava R.K., Segal S., Camins M.B., et al. Harvey Cushing’s meningiomas text and the historical origin of resectability criteria for the anterior one third of the superior sagittal sinus. J Neurosurg. 2003;99(4):787-791.
Sidebottom A., Maxwell S. The medical and surgical management of trigeminal neuralgia. J Clin Pharm Ther. 1995;20(1):31-35.
Stabler A., Bellan M., Weiss M., et al. MR imaging of enhancing intraosseous disk herniation (Schmorl’s nodes). AJNR Am J Neuroradiol. 1997;168(4):933-938.
Stabler A., Weiss M., Scheidler J., et al. Degenerative disk vascularization on MRI: correlation with clinical and histopathologic findings. Skeletal Radiol. 1996;25(2):119-126.
Stone J.L., Cybulski G.R., Crowell R.M., et al. The lateral position–dependant occipital approach–to pineal and medial occipitoparietal lesions. Technical note. Acta Neurochir (Wien). 1990;102(3-4):133-136.
Tsuruda J.S., Chew W.M., Moseley M.E., et al. Diffusion-weighted MR imaging of the brain: value of differentiating between extraaxial cysts and epidermoid tumors. AJNR Am J Neuroradiol. 1990;155(5):1059-1065. discussion 66-68
Tullberg T., Svanborg E., Isaccsson J., et al. A preoperative and postoperative study of the accuracy and value of electrodiagnosis in patients with lumbosacral disc herniation. Spine. 1993;18(7):837-842.
Tyler-Kabara E.C., Kassam A.B., Horowitz M.H., et al. Predictors of outcome in surgically managed patients with typical and atypical trigeminal neuralgia: comparison of results following microvascular decompression. J Neurosurg. 2002;96(3):527-531.
Wiebers D.O., Whisnant J.P., Huston J.3rd, et al. Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet. 2003;362(9378):103-110.
Wu J.S., Zhou L.F., Tang W.J., et al. Clinical evaluation and follow-up outcome of diffusion tensor imaging-based functional neuronavigation: a prospective, controlled study in patients with gliomas involving pyramidal tracts. Neurosurgery. 2007;61(5):935-948. discussion 48-49
Yasargil M.G., Kadri P.A., Yasargil D.C. Microsurgery for malignant gliomas. J Neurooncol. 2004;69(1-3):67-81.
Zakrzewska J.M., Thomas D.G. Patient’s assessment of outcome after three surgical procedures for the management of trigeminal neuralgia. Acta Neurochir (Wien). 1993;122(3-4):225-230.