CHAPTER 59 Principles of intravitreal application of drugs
Fundamental principles of intravitreal drug delivery
The challenge of treating posterior segment disease resides in the obstacles encountered while trying to achieve therapeutic drug concentrations at the level of the retina and choroid. Topically administered drug that is not lost immediately to the systemic circulation (less than 5% is absorbed into the eye) is absorbed through the cornea into the anterior chamber where it is eliminated through the trabecular meshwork. It achieves an even lower concentration in the vitreous because of the longer diffusion distance as well as the counterdirectional convection current from the ciliary body to the trabecular meshwork. Drugs delivered to the subTenon’s space can penetrate the more permeable sclera to achieve higher concentrations in the retina and choroid. However, the tight junctions of the retinal pigment epithelium (RPE) and choroidal blood flow provide barriers to accessing the retina. Systemically administered (either intravenous or oral) drugs are one avenue to circumvent some of these barriers, especially if the drug is lipophilic and is therefore able to bypass the blood–retinal barrier. The systemic side effects from high enough concentrations of drug required to attain intraocular efficacy, however, limit the utility of many systemically administered drugs1,2. Alternatively, drugs administered intravitreally can attain a high enough concentration for direct treatment of retinal conditions. Drugs delivered intravitreally are eliminated by outflow through either the anterior route, composed of the trabecular meshwork, or the posterior route, through the blood-retinal barrier, into the systemic circulation1,3.
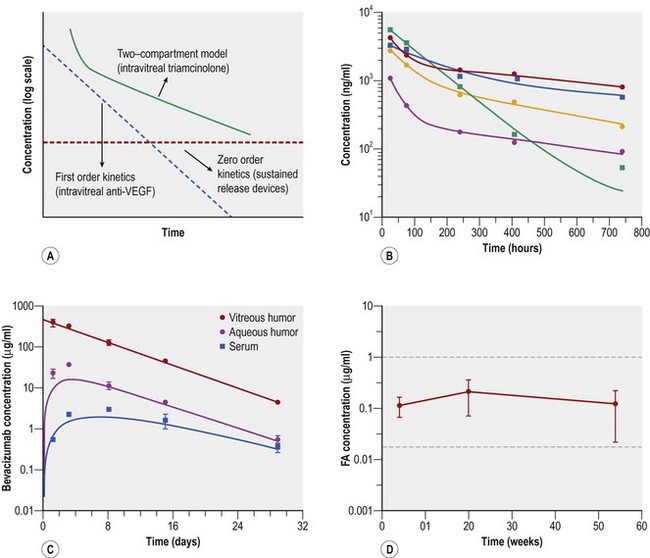
The principles to finding an ideal drug formulation for intravitreal administration require a number of qualifications. The first is that the drug should have a reasonable half-life that does not mandate frequent repeated injections and repeated risk of complications. Anti-VEGF agents require repeated injections because they have short half-lives and first order kinetics (Fig. 59.1A,C)4,5. Intravitreal steroids, such as triamcinolone, are biphasic, or follow a two-compartment model with exponential decline initially, followed by first order kinetics after 1 month (see Fig. 59.1A,B)6. In contrast, sustained-release steroid devices can demonstrate zero order kinetics (flat line shown in Figure 59.1A), releasing a constant amount of therapeutic level steroid for the lifespan of the implant (Fig. 59.1D)7. A second qualification for the ideal intravitreal drug formulation is that administration of the drug should not interfere with the transparency of the ocular media as not to interfere with vision. This comes into play with microsphere and nanosphere technology where the size of the particle can determine the level of visual obscuration after an injection. A third requisite for an intravitreally administered drug is to have the ability to be delivered at a therapeutic dose that does not cause toxicity or impede normal cellular activity3. By providing a constant lower concentration of drug over time (zero order) rather than larger spurts of drug that decrease rapidly (first order), sustained release devices are advantageous in that they provide therapeutic levels of drug without as much local or systemic toxicity.
Sustained release drug delivery platforms now available or under investigation are shown in Figure 59.2. They include devices that are suspended in the vitreous cavity by fixation to the sclera, injected into the suprachoroidal space, or into the vitreous cavity as a free-floating device, placed underneath conjunctiva, or given intrasclerally.
Intravitreal injection of drugs
Special considerations in infants
Pars plana location varies with infant development and can be located 1–1.5 mm behind the limbus in premature infants, but is 2–3.5 mm from the limbus in full-term infants. This affects the approach to needle placement during intravitreal injections8. Accordingly, the vitreous volume in infants is approximately two-thirds to three-fourths the size of adults, thus requiring adjustments in administered drug volume so as not to increase intraocular pressure too severely or cause drug toxicity to the retina.
Drugs used as intravitreal injections
Anti-bacterial
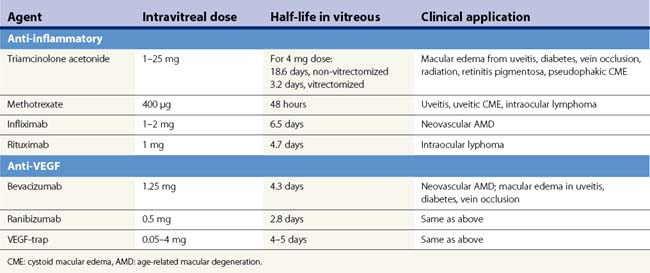
The mainstay of empiric treatment for bacterial endophthalmitis employs the use of ceftazidime, a third generation cephalosporin with increased activity against Gram-negative organisms, and vancomycin, the drug of choice for methicillin-resistant Staphylococcus aureus and other Gram-positive organisms9. Despite the fact that the fourth generation fluoroquinolones have good intravitreal penetration taken systemically, their intravitreal use has not been studied in humans although in rabbits, 625 µg of levofloxacin and 400 µg of gatifloxacin appear to be non toxic, whereas moxifloxacin was benign at doses up to 160 µg10–12. Clindamycin has been used as an intravitreal injection to treat gram positive organisms is penicillin-allergic individuals, anaerobes, and most recently has been given in conjunction with dexamethasone 400 µg intravitreally to treat posterior pole toxoplasmosis chorioretinitis without any evidence of retinal toxicity13 (Table 59.1).
Anti-fungals
Fungal endophthalmitis is most commonly treated with intravitreal amphotericin B, which has been shown to be effective against most Candida species as well as Aspergillus, Rhizopus, and Penicillium. Non-toxic doses up to 10 µg have been shown, although there have been reports of retinal necrosis when injected too close to the retina14. The liposomal formulation of amphotericin B has been shown in animal models to have less toxicity to the retina. A single case of its intravitreal application in a patient with fungal candida endophthalmitis without any evidence of retinal toxicity has been reported15. Among the newer generation triazoles (voriconazole, ravuconazole, posaconazole) that have broad anti-fungal coverage with relatively low toxicity, only voriconazole has been given intravitreally in humans. The short half-life of voriconazole results in the requirement for close observation with frequent repeat injections (Table 59.1). Sen et al. showed in their case series that five patients who had fungal endophthalmitis resistant to fluconazole and amphotericin B responded to intravitreal voriconazole16. Although the echinocandin caspofungin does not appear to reach therapeutic levels in the vitreous when given systemically, this agent shows promise as an intravitreal agent against Candida and Aspergillus. Kusbeci and colleagues demonstrated its effectiveness against C. albicans endophthalmitis in rabbits17,18.
Anti-virals
Aciclovir is an anti-viral that is effective against the herpes family of viruses. It becomes activated in virus-infected cells by a virally encoded enzyme and is therefore non-toxic to uninfected cells. Ganciclovir is a nuceloside analog of aciclovir with 10–100-fold increased activity against cytomegalovirus (CMV). Although rabbit studies show that ganciclovir given intravitreally is non-toxic at doses up to 400 µg, in humans it can be used safely at 2–5 mg even as often as every week19,20. Foscarnet is effective against herpes simplex virus, varicella zoster virus, and CMV. Intravitreal foscarnet can be given intravitreally at a dose of 2.4 mg without causing toxicity (Table 59.1). Cidofovir is a nucleoside analog that has a longer duration of action due to prolonged clearance compared with ganciclovir or foscarnet. However, it causes a high rate of uveitis (26%) and hypotony, although these complications can be prevented by prophylactically treating with probenecid and topical steroid21,22.
Steroids
Triamcinolone acetonide (TA) is a corticosteroid that was first used as an intravitreal injection by Machemer to prevent the development of proliferative vitreoretinopathy after retinal detachment repair23. It now has a variety of uses including the treatment of cystoid macular edema (CME) resulting from uveitis, diabetes, retinal vein occlusions, as well as in pseudophakic CME, radiation maculopathy, and CME related to retinitis pigmentosa (Table 59.2). The use of intravitreal triamcinolone to treat CME as a result of retinal vein occlusion was recently investigated in the SCORE (Standard Care vs. Corticosteroids in the treatment of retinal vein occlusion) studies. These studies demonstrated a significant treatment benefit in patients with central retinal vein occlusions with an odds ratio of attaining the primary outcome (≥15 letters of best corrected visual acuity improvement from baseline) of five-fold over the observation group. By 1 year, the rate of cataract formation was 33%, and 35% required IOP-lowering medication in the 4 mg treatment group (this was significantly higher than the observation group)24. No treatment benefit over standard care was demonstrated for BRVO (where standard treatment was focal or grid laser)25.
Anti-inflammatory/anti-neoplastic agents
A recent prospective interventional case series reported on the use of intravitreal methotrexate in the treatment of uveitis and uveitic CME26. They found that 400 µg in 0.1 mL of methotrexate improved visual acuity over a 6-month follow-up period in 10 of 15 intermediate uveitis, panuveitis, or uveitic CME patients. No significant toxic effects were reported26. Intravitreal methotrexate has also been used for the treatment of vitreoretinal involvement in primary CNS lymphoma27–29. Complications in one series included cataract (73%), corneal epitheliopathy (58%), maculopathy (42%), vitreous hemorrhage (8%), optic atrophy (4%), and sterile endophthalmitis (4%)30.
Infliximab is a monoclonal antibody against tumor necrosis-α (TNF) used as a systemic treatment for rheumatologic conditions and, more recently, for the treatment of non-infectious ocular inflammatory disease. It has been applied at 1–2 mg doses intravitreally towards the treatment of AMD31–33. After establishing a range of safe doses in an animal model, Theodossiadis et al. reported on three patients who received infliximab after failing to respond to intravitreal ranibizumab. All three patients had a reduction in central foveal thickness by optical coherence tomography as well as improvement in visual acuity33. The rationale for infliximab treatment in AMD is based on the growing evidence that TNF-alpha is involved in the pathogenesis of neovascular membranes in AMD34. Rituximab, a humanized murine monoclonal antibody against the CD20 B-lymphocyte antigen used in the treatment of B-cell lymphoma, is thought to be able to penetrate retinal tissue and has recently been studied for the treatment of intraocular lymphoma35–37. Kitzmann et al. reported a small series of patients who received intravitreal rituximab resulting in a reduction in lymphomatous infiltrates, although these patients received adjunctive treatments that made it difficult to ascertain the effect rituximab would have when used alone37. Another series by Ohguro et al demonstrated that rituximab given intravitreally after failed intravitreal methotrexate resulted in a reduction in subretinal and intravitreal infiltrates in two patients with intraocular lymphoma38.
Anti-VEGF agents
The anti-vascular endothelial growth factor (VEGF) agents used for the treatment of neovascular AMD have revolutionized the use of intravitreal injections for posterior segment disease. Their use is now being expanded to the treatment of CME due to diabetes, retinal vein occlusion, and uveitis. Ranibizumab is an Fab antibody fragment that binds to all isoforms of VEGF. Two large prospective, randomized controlled trials demonstrated the efficacy of ranibizumab in treating neovascular AMD compared with sham injections (MARINA), and when compared with PDT (ANCHOR), showing improvement in visual acuity in the anti-VEGF treated patients39,40. Bevacizumab (Avastin) is a full-length antibody against VEGF approved for systemic administration in the treatment of metastatic colorectal cancer. It has been used as an intravitreal injection for off-label treatment of neovascular AMD as well as for treatment of CME from retinal vein occlusion, diabetes, and uveitis. Its efficacy compared to ranibizumab (Lucentis) is currently being studied in several prospective trials, the Comparisons of Age-Related Macular Degeneration Treatment Trials (CATT) (N Engl J Med. 2011 Apr 28. [Epub ahead of print]) and the Avastin Becavizumab for CNV trial (ABC)41, although several small retrospective case series have not demonstrated a difference between the two agents42,43. Currently, Lucentis is being investigated for its efficacy in the treatment of retinal vein occlusion in the CRUISE and BRAVO trials. Six month data from these trials demonstrated a significantly larger proportion of patients having improved visual acuity and a reduction in central foveal thickness in the Lucentis vs. sham-treated groups44,45.
VEGF trap is a chimeric fusion protein composed of an Fc fragment linked to the extracellular portions of VEGF receptors 1 and 2 which binds to all forms of VEGF. It has shown promise in the treatment of neovascular AMD in Phase I studies and is under current investigation with Phase III trials (see Table 59.2)46–48.
Sustained release delivery devices
Implants
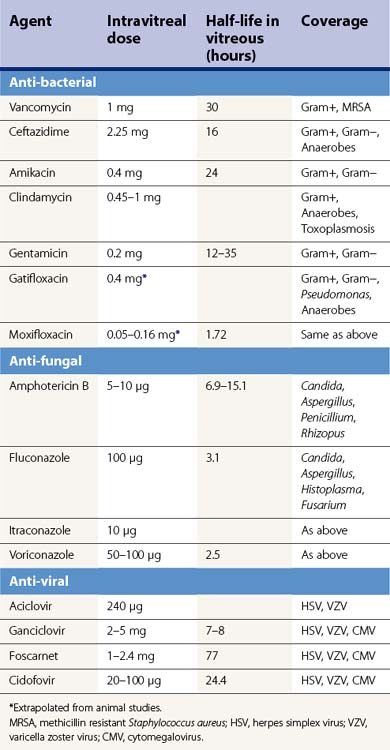
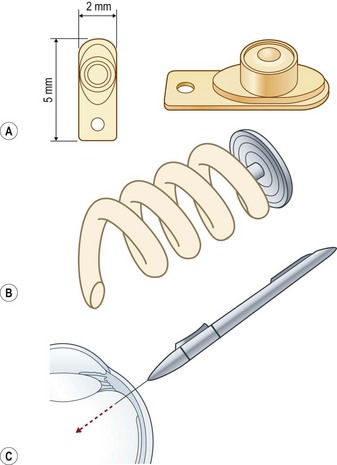
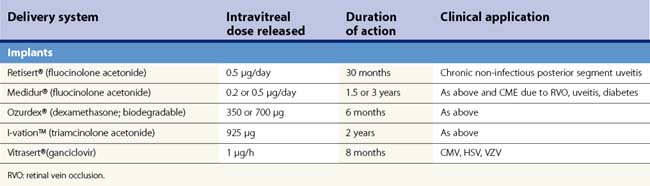
A shift from repeated intravitreal injections to sustained release intraocular delivery devices in both implantable and injectable versions has recently occurred. These delivery devices can be classified into biodegradable and non-biodegradable implants. A non-biodegradable steroid implant that has been implemented for the treatment of posterior uveitis is the fluocinolone acetonide-releasing device (Retisert®, Bausch & Lomb) that is implanted through the pars plana through a scleral opening and secured using 8-0 Prolene suture (Figs 59.2–59.3). In a 3-year clinical trial studying the efficacy of the fluocinolone acetonide intravitreal implant in the treatment of non-infectious posterior uveitis, Callanan et al. found that uveitis recurrence was reduced significantly (from 62% to 4% at 1 year), and that implanted eyes had improved visual acuity compared with non-implanted eyes (P < 0.01)49. The uveitis recurrence rate was 20% at 3 years’ post-implantation. The high rate of long term complications such as cataract (93%) and glaucoma requiring surgery (40%) limits its widespread use and has argued for combined cataract or glaucoma surgery in high risk individuals50. In a recent study reporting pooled results from three prospective randomized trials, 75% of eyes receiving the fluocinolone acetonide implant required intraocular pressure lowering therapy at some point during a 3-year course51. In the study by Callanan et al., 40% of implanted eyes required glaucoma surgery compared with 2% of non-implanted eyes49.
Other sustained release steroid-releasing implants currently under investigation include biodegradable implants (e.g. Ozurdex®, Allergan) and non-biodegradable implants (Medidur®, Alimera Sciences and I-vation™, Surmodics) (Table 59.3; Fig. 59.3). The advantage of biodegradable implants is that they can be implanted without a need for extraction once drug elution terminates. However, biodegradable implants often have non-ideal release kinetics and can have a non-controlled burst of drug release at the end of their lifespan1. Non-biodegradable implants, on the other hand, require explantation once drug release ceases (or can be left in place), but typically are longer lasting, and have closer to ideal drug-release kinetics (e.g., zero order kinetics with Retisert and I-vation, see Fig. 59.1). Ozurdex and Medidur (Illuvien implant) both have applicator systems that allow for outpatient placement through self-sealing, small gauge wounds in the office setting (see Fig. 59.3). Phase III trials evaluating the long-term efficacy and safety of Ozurdex (previously known as Posurdex) for the treatment of CME related to retinal vein occlusion have demonstrated a significantly greater percentage of patients with improved visual acuity at days 30–90 after Ozurdex injection compared with sham-injected controls52. As of June 2009, Ozurdex has been FDA approved for the treatment of retinal vein occlusions.
I-vation™ is a triamcinolone acetonide-releasing helical apparatus made of a metallic scaffold coated with a polymer derived from a combination of the non-biodegradable compounds polybutyl methacrylate and polyethylene vinyl acetate (see Figs 59.2A, 59.3B). The coating is similar to those found on drug-eluting coronary artery stents. This device can be dialed in through the pars plana through a 25-gauge sclerotomy and releases 925 µg of steroid over 2 years with near zero order kinetics (see Table 59.3)53. Drug release kinetics can be altered by varying the thickness and total surface area of the polymers in its coating. The cap portion of this helical screw remains flush against the sclera underneath the conjunctiva. I-vation has recently been investigated in a Phase I study, STRIDE (Sustained Triamcinolone Release for Inhibition of Diabetic Macular Edema), for the treatment of diabetic macular edema with early results showing a reduction in central foveal thickness after implantation54.
Medidur® is an injectable non-biodegradable intravitreal implant designed to release 0.2 or 0.5 mg of fluocinolone acetonide for a period of between 18 and 36 months (Table 59.3). It is designed for sutureless insertion in an outpatient clinic setting using a 25-gauge injector system. A Phase III trial is in process investigating the treatment of diabetic macular edema in two different doses of Medidur implanted eyes compared with sham injections53,54.
The Vitrasert® implant is a polymeric (polyvinyl acetate) non-biodegradable implant that releases 1 µg/h of ganciclovir with a duration of 8 months (Table 59.3). It was introduced in the 1990s to treat CMV retinitis in AIDS patients, but also has activity against herpes simplex virus. Studies have shown that the mean time to progression of CMV retinitis was 205 days with the ganciclovir implant which is approximately three times longer than with intravenous ganciclovir55,56.
Encapsulated cell technology
Encapsulated cell technology is a method in which viable human cell lines that secrete a specific therapeutic protein are sequestered in a porous implant that allows for diffusion of the molecule out towards target tissues, while allowing for diffusion inwards of oxygen and nutrients to maintain the health of live cells within the implant. This technology is being investigated for the treatment of retinitis pigmentosa and geographic atrophy (GA) in AMD. The NT-501, Neurotech implant consists of a semipermeable outer membrane with 15 nm pores that allows for growth factors and oxygen to reach viable human retinal pigment epithelial cells that have been engineered to secrete ciliary neurotrophic factor (CNTF). This RPE cell line is maintained on a polyethylene terephthalate yarn scaffold inside the implant. The NT-501 implant is placed into the eye through a 2 mm incision through the pars plana. CNTF is a cytokine that binds to receptors found on Muller glial cells, rods, and cone photoreceptors57. It has been demonstrated to retard photoreceptor degeneration in animal models of retinitis pigmentosa58. Preliminary results from a Phase II clinical trial for the treatment of patients with GA demonstrated increased retinal thickness, which is thought to correspond to an increase in photoreceptor size, and resulting in stabilization of visual acuity. For retinitis pigmentosa, phase I study results have been published reporting three of seven implanted eyes that could be tracked by conventional reading charts, with an improvement in acuity of 10–15 letters59. There were no serious complications in the 10 eyes that were implanted.
Microspheres
The use of biodegradable polymers such as polylactide and poly lactic co-glycolic acid (PLGA) to suspend drugs into microparticles (1–1000 µm) or nanoparticles (1–1000 nm) resulting in controlled release of drugs describes the concept of microspheres1. They provide sustained drug release on an order of weeks to months. Their advantage is that drug is released in a controlled fashion, minimizing the ‘burst’ effect that biodegradable implants have at the end of their lifespan. They are injected into the vitreous cavity and thus the disadvantages are synonymous with the complication rates associated with any other intravitreal injections, although drugs delivered in this way would need to be injected much less frequently. An additional complication is that nanoparticles may cause temporary clouding of the media, although microspheres larger than 2 µm circumvent this problem because they sink to the bottom of the vitreous cavity by gravity. This technology has been used to incorporate the pegylated anti-VEGF peptide, pegaptanib, into a vehicle that, when applied using a trans-scleral technique, released drug for up to 20 days, resulting in inhibition of VEGF-induced cell proliferation of human umbilical vein endothelial cells60. Cardillo et al. demonstrated in their case series that a microsphere preparation of triamcinolone acetonide was effective in reducing foveal thickness and improving visual acuity in patients with diabetic macular edema when compared with the conventional preparation of triamcinolone61. Microspheres appear to have a shorter half-life in vitrectomized eyes.
Liposomes
Liposomes are lipid vesicles 25–10 000 nm in diameter that can be used to encapsulate both hydrophilic and lipophilic drugs. They can be phagocytosed by cells such as retinal pigment epithelial cells thus allowing for targeted intracellular drug delivery. This technology has been utilized to create less toxic formulations of amphotericin B and gentamicin in animal models, although their efficacy in human intraocular disease has not yet been demonstrated62,63.
Future drug delivery techniques
Iontophoresis is a non-surgical technique that utilizes an applicator to deliver a weak electrical current to the sclera to drive ionically charged drug molecules across the sclera into the choroid, retina, and vitreous64,65. This technique shows future promise for the delivery of sustained release formulations with the ability to modulate dosage by altering the strength of current utilized. This technique has shown good tolerability profiles thus far66. Finally, microcannulation of drug delivery devices into the suprachoroidal space is a promising new technique that can potentially deliver drug to the macula, optic nerve, and posterior pole67.
Complications
Complications associated with intravitreal injections include pain, subconjunctival hemorrhage, transient elevation of intraocular pressure, infectious endophthalmitis, uveitis, and sterile endophthalmitis. The rate of infectious endophthalmitis following intravitreal injections has been reported to be 0.04–0.16% per injection and appears to be minimized using a standardized sterilization protocol including the use of povodine–iodine and eyelid specula68,69. Sterile endophthalmitis was reported in 1–2% of patients receiving non-preservative free triamcinolone intravitreally and can occur in patients receiving Avastin rarely as well69,70.
1 Del Amo EM, Urtti A. Current and future ophthalmic drug delivery systems. A shift to the posterior segment. Drug Discov Today. 2008;13(3–4):135-143.
2 Gaudana R, Jwala J, Boddu SH, et al. Recent perspectives in ocular drug delivery. Pharm Res. 2009;26(5):1197-1216.
3 Maurice D. Review: practical issues in intravitreal drug delivery. J Ocul Pharmacol Ther. 2001;17(4):393-401.
4 Bakri SJ, Snyder MR, Reid JM, et al. Pharmacokinetics of intravitreal ranibizumab (Lucentis). Ophthalmology. 2007;114(12):2179-2182.
5 Bakri SJ, Snyder MR, Reid JM, et al. Pharmacokinetics of intravitreal bevacizumab (Avastin). Ophthalmology. 2007;114(5):855-859.
6 Beer PM, Bakri SJ, Singh RJ, et al. Intraocular concentration and pharmacokinetics of triamcinolone acetonide after a single intravitreal injection. Ophthalmology. 2003;110(4):681-686.
7 Jaffe GJ, Yang CH, Guo H, et al. Safety and pharmacokinetics of an intraocular fluocinolone acetonide sustained delivery device. Invest Ophthalmol Vis Sci. 2000;41(11):3569-3575.
8 Aiello AL, Tran VT, Rao NA. Postnatal development of the ciliary body and pars plana. A morphometric study in childhood. Arch Ophthalmol. 1992;110(6):802-805.
9 Hegazy HM, Kivilcim M, Peyman GA, et al. Evaluation of toxicity of intravitreal ceftazidime, vancomycin, and ganciclovir in a silicone oil-filled eye. Retina. 1999;19(6):553-557.
10 Aydin E, Kazi AA, Peyman GA, et al. Intravitreal toxicity of moxifloxacin. Retina. 2006;26(2):187-190.
11 Gao H, Pennesi ME, Qiao X, et al. Intravitreal moxifloxacin: retinal safety study with electroretinography and histopathology in animal models. Invest Ophthalmol Vis Sci. 2006;47(4):1606-1611.
12 Kazi AA, Jermak CM, Peyman GA, et al. Intravitreal toxicity of levofloxacin and gatifloxacin. Ophthalmic Surg Lasers Imaging. 2006;37(3):224-229.
13 Soheilian M, Ramezani A, Azimzadeh S, et al. Randomized Trial of Intravitreal Clindamycin and Dexamethasone versus Pyrimethamine, Sulfadiazine, and Prednisolone in Treatment of Ocular Toxoplasmosis. Ophthalmology. 2011;118:134-141.
14 Gupta A, Srinivasan R, Kaliaperumal S, et al. Post-traumatic fungal endophthalmitis – a prospective study. Eye. 2008;22(1):13-17.
15 Koozekanani DD, Weinberg DV, Dubis AM, et al. Hemorrhagic Retinoschisis in Shaken Baby Syndrome Imaged with Spectral Domain Optical Coherence Tomography. Ophthalmic Surg Lasers Imaging. 2010 Mar 9:1-3.
16 Sen P, Gopal L, Sen PR. Intravitreal voriconazole for drug-resistant fungal endophthalmitis: case series. Retina. 2006;26(8):935-939.
17 Goldblum D, Fausch K, Frueh BE, et al. Ocular penetration of caspofungin in a rabbit uveitis model. Graefes Arch Clin Exp Ophthalmol. 2007;245(6):825-833.
18 Kusbeci T, Avci B, Cetinkaya Z, et al. The effects of caspofungin and voriconazole in experimental Candida endophthalmitis. Curr Eye Res. 2007;32(1):57-64.
19 Cochereau-Massin I, Lehoang P, Lautier-Frau M, et al. Efficacy and tolerance of intravitreal ganciclovir in cytomegalovirus retinitis in acquired immune deficiency syndrome. Ophthalmology. 1991;98(9):1348-1353. discussion 53–5
20 Heinemann MH. Long-term intravitreal ganciclovir therapy for cytomegalovirus retinopathy. Arch Ophthalmol. 1989;107(12):1767-1772.
21 Chavez-de la Paz E, Arevalo JF, Kirsch LS, et al. Anterior nongranulomatous uveitis after intravitreal HPMPC (cidofovir) for the treatment of cytomegalovirus retinitis. Analysis and prevention. Ophthalmology. 1997;104(3):539-544.
22 Rahhal FM, Arevalo JF, Munguia D, et al. Intravitreal cidofovir for the maintenance treatment of cytomegalovirus retinitis. Ophthalmology. 1996;103(7):1078-1083.
23 Machemer R, Sugita G, Tano Y. Treatment of intraocular proliferations with intravitreal steroids. Trans Am Ophthalmol Soc. 1979;77:171-180.
24 Scott IU, Ip MS, VanVeldhuisen PC, et al. A randomized trial comparing the efficacy and safety of intravitreal triamcinolone with standard care to treat vision loss associated with macular edema secondary to branch retinal vein occlusion: the Standard Care vs. Corticosteroid for Retinal Vein Occlusion (SCORE) study report 6. Arch Ophthalmol. 2009;127(9):1115-1128.
25 Ip MS, Scott IU, VanVeldhuisen PC, et al. A randomized trial comparing the efficacy and safety of intravitreal triamcinolone with observation to treat vision loss associated with macular edema secondary to central retinal vein occlusion: the Standard Care vs. Corticosteroid for Retinal Vein Occlusion (SCORE) study report 5. Arch Ophthalmol. 2009;127(9):1101-1114.
26 Taylor SR, Habot-Wilner Z, Pacheco P, et al. Intraocular methotrexate in the treatment of uveitis and uveitic cystoid macular edema. Ophthalmology. 2009;116(4):797-801.
27 Frenkel S, Hendler K, Siegal T, et al. Intravitreal methotrexate for treating vitreoretinal lymphoma: 10 years of experience. Br J Ophthalmol. 2008;92(3):383-388.
28 Hardwig PW, Pulido JS, Erie JC, et al. Intraocular methotrexate in ocular diseases other than primary central nervous system lymphoma. Am J Ophthalmol. 2006;142(5):883-885.
29 Velez G, Yuan P, Sung C, et al. Pharmacokinetics and toxicity of intravitreal chemotherapy for primary intraocular lymphoma. Arch Ophthalmol. 2001;119(10):1518-1524.
30 Smith JR, Rosenbaum JT, Wilson DJ, et al. Role of intravitreal methotrexate in the management of primary central nervous system lymphoma with ocular involvement. Ophthalmology. 2002;109(9):1709-1716.
31 Giansanti F, Ramazzotti M, Giuntoli M, et al. Intravitreal infliximab clearance in a rabbit model: different sampling methods and assay techniques. Invest Ophthalmol Vis Sci. 2009;50(11):5328-5335.
32 Regatieri CV, Dreyfuss JL, Melo GB, et al. Dual role of intravitreous infliximab in experimental choroidal neovascularization. Effect on the expression of sulfated glycosaminoglycans. Invest Ophthalmol Vis Sci. 2009;50(11):5487-5494.
33 Theodossiadis PG, Liarakos VS, Sfikakis PP, et al. Intravitreal administration of the anti-tumor necrosis factor agent infliximab for neovascular age-related macular degeneration. Am J Ophthalmol. 2009;147(5):825-830. 30 e1
34 Oh H, Takagi H, Takagi C, et al. The potential angiogenic role of macrophages in the formation of choroidal neovascular membranes. Invest Ophthalmol Vis Sci. 1999;40(9):1891-1898.
35 Itty S, Pulido JS. Rituximab for intraocular lymphoma. Retina. 2009;29(2):129-132.
36 Kim H, Csaky KG, Chan CC, et al. The pharmacokinetics of rituximab following an intravitreal injection. Exp Eye Res. 2006;82(5):760-766.
37 Kitzmann AS, Pulido JS, Mohney BG, et al. Intraocular use of rituximab. Eye. 2007;21(12):1524-1527.
38 Ohguro N, Hashida N, Tano Y. Effect of intravitreous rituximab injections in patients with recurrent ocular lesions associated with central nervous system lymphoma. Arch Ophthalmol. 2008;126(7):1002-1003.
39 Brown DM, Kaiser PK, Michels M, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1432-1444.
40 Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1419-1431.
41 Brown DM, Regillo CD. Anti-VEGF agents in the treatment of neovascular age-related macular degeneration: applying clinical trial results to the treatment of everyday patients. Am J Ophthalmol. 2007;144(4):627-637.
42 Gamulescu MA, Radeck V, Lustinger B, et al. Bevacizumab versus ranibizumab in the treatment of exudative age-related macular degeneration. Int Ophthalmol. 2010;30(3):261-266.
43 Landa G, Amde W, Doshi V, et al. Comparative study of intravitreal bevacizumab (Avastin) versus ranibizumab (Lucentis) in the treatment of neovascular age-related macular degeneration. Ophthalmologica. 2009;223(6):370-375.
44 Campochiaro PA, Heier JS, Feiner L, et al. Ranibizumab for macular edema following branch retinal vein occlusion: six-month primary end point results of a phase III study. Ophthalmology. 2010;117(6):1102-1112. e1
45 Brown DM, Campochiaro PA, Singh RP, et al. Ranibizumab for macular edema following central retinal vein occlusion: six-month primary end point results of a phase III study. Ophthalmology. 2010;117(6):1124-1133. e1
46 Nguyen QD, Shah SM, Browning DJ, et al. A phase I study of intravitreal vascular endothelial growth factor trap-eye in patients with neovascular age-related macular degeneration. Ophthalmology. 2009;116(11):2141-2148. e1
47 Stewart MW, Rosenfeld PJ. Predicted biological activity of intravitreal VEGF Trap. Br J Ophthalmol. 2008;92(5):667-668.
48 Nguyen QD, Shah SM, Browning DJ, et al. A phase I study of intravitreal vascular endothelial growth factor trap-eye in patients with neovascular age-related macular degeneration. Ophthalmology. 116(11), 2009. 2141–8.e1
49 Callanan DG, Jaffe GJ, Martin DF, et al. Treatment of posterior uveitis with a fluocinolone acetonide implant: three-year clinical trial results. Arch Ophthalmol. 2008;126(9):1191-1201.
50 Malone PE, Herndon LW, Muir KW, Jaffe GJ. Combined fluocinolone acetonide intravitreal insertion and glaucoma drainage device placement for chronic uveitis and glaucoma. Am J Ophthalmol. 2010;149(5):800-806.e1.
51 Bollinger KE, Smith SD. Prevalence and management of elevated intraocular pressure after placement of an intravitreal sustained-release steroid implant. Curr Opin Ophthalmol. 2009;20(2):99-103.
52 Haller JA, Bandello F, Belfort RJr, et al. Randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with macular edema due to retinal vein occlusion. Ophthalmology. 2010;117(6):1134-1146. e3
53 Lee SS, Robinson MR. Novel drug delivery systems for retinal diseases. A review. Ophthalmic Res. 2009;41(3):124-135.
54 Yasukawa T, Ogura Y. Medical devices for the treatment of eye diseases. Handb Exp Pharmacol. 2010;197:469-489.
55 Martin DF, Parks DJ, Mellow SD, et al. Treatment of cytomegalovirus retinitis with an intraocular sustained-release ganciclovir implant. A randomized controlled clinical trial. Arch Ophthalmol. 1994;112(12):1531-1539.
56 Musch DC, Martin DF, Gordon JF, et al. Treatment of cytomegalovirus retinitis with a sustained-release ganciclovir implant. The Ganciclovir Implant Study Group. N Engl J Med. 1997;337(2):83-90.
57 Beltran WA, Zhang Q, Kijas JW, et al. Cloning, mapping, and retinal expression of the canine ciliary neurotrophic factor receptor alpha (CNTFRalpha). Invest Ophthalmol Vis Sci. 2003;44(8):3642-3649.
58 Tao W, Wen R, Goddard MB, et al. Encapsulated cell-based delivery of CNTF reduces photoreceptor degeneration in animal models of retinitis pigmentosa. Invest Ophthalmol Vis Sci. 2002;43(10):3292-3298.
59 Sieving PA, Caruso RC, Tao W, et al. Ciliary neurotrophic factor (CNTF) for human retinal degeneration: phase I trial of CNTF delivered by encapsulated cell intraocular implants. Proc Natl Acad Sci USA. 2006;103(10):3896-3901.
60 Carrasquillo KG, Ricker JA, Rigas IK, et al. Controlled delivery of the anti-VEGF aptamer EYE001 with poly(lactic-co-glycolic)acid microspheres. Invest Ophthalmol Vis Sci. 2003;44(1):290-299.
61 Cardillo JA, Souza-Filho AA, Oliveira AG. Intravitreal Bioerudivel sustained-release triamcinolone microspheres system (RETAAC). Preliminary report of its potential usefulnes for the treatment of diabetic macular edema. Arch Soc Esp Oftalmol. 2006;81(12):675-677. 9–81
62 Fishman PH, Peyman GA, Lesar T. Intravitreal liposome-encapsulated gentamicin in a rabbit model. Prolonged therapeutic levels. Invest Ophthalmol Vis Sci. 1986;27(7):1103-1106.
63 Tremblay C, Barza M, Szoka F, et al. Reduced toxicity of liposome-associated amphotericin B injected intravitreally in rabbits. Invest Ophthalmol Vis Sci. 1985;26(5):711-738.
64 Hsu J. Drug delivery methods for posterior segment disease. Curr Opin Ophthalmol. 2007;18(3):235-239.
65 Myles ME, Neumann DM, Hill JM. Recent progress in ocular drug delivery for posterior segment disease: emphasis on transscleral iontophoresis. Adv Drug Deliv Rev. 2005;57(14):2063-2079.
66 Halhal M, Renard G, Courtois Y, et al. Iontophoresis: from the lab to the bed side. Exp Eye Res. 2004;78(3):751-757.
67 Olsen TW, Feng X, Wabner K, et al. Cannulation of the suprachoroidal space: a novel drug delivery methodology to the posterior segment. Am J Ophthalmol. 2006;142(5):777-787.
68 Cavalcante LL, Cavalcante ML, Murray TG, et al. Intravitreal injection analysis at the Bascom Palmer Eye Institute: evaluation of clinical indications for the treatment and incidence rates of endophthalmitis. Clin Ophthalmol. 2010;4:519-524.
69 Bhavsar AR, Googe JMJr, Stockdale CR, et al. Risk of endophthalmitis after intravitreal drug injection when topical antibiotics are not required: the diabetic retinopathy clinical research network laser–ranibizumab–triamcinolone clinical trials. Arch Ophthalmol. 2009;127(12):1581-1583.
70 Sato T, Emi K, Ikeda T, et al. Severe intraocular inflammation after intravitreal injection of bevacizumab. Ophthalmology. 2010;117(3):512-516. 6 e1–2