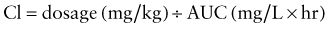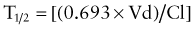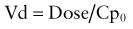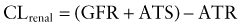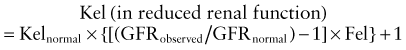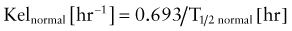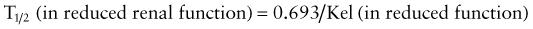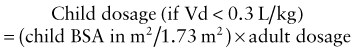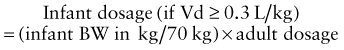Chapter 57 Principles of Drug Therapy
Pharmacogenetics is the study of how variant forms of human genes contribute to interindividual variability in drug response. The finding that drug responses can be influenced by the patient’s genetic profile has offered great hope for realizing individualized pharmacotherapy when the relationship between genotype and phenotype (disease and/or drug response) predicts drug response (Chapter 56). In the developing child, it is apparent ontogeny has the potential of modulating drug response through altering pharmacokinetics and pharmacodynamics.
General Principles of Pharmacokinetics and Pharmacodynamics
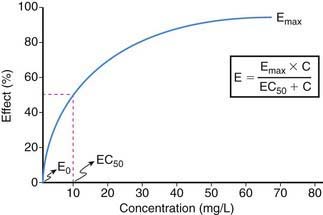
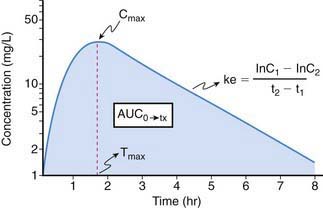
Drug effect is produced only when an exposure (amount and duration) occurs that is sufficient to produce a drug-receptor interaction capable of modulating the cellular milieu and inducing a physiologic response. Thus, exposure-response relationships for a given drug represent an interface between pharmacokinetics and pharmacodynamics that can be simply conceptualized by consideration of two profiles: plasma concentration vs. effect (Fig. 57-1) and plasma concentration vs. time (Fig. 57-2).
The relationship between drug concentration and effect for most drugs is not linear (see Fig. 57-1). At a drug concentration of 0, the effect from the drug is generally 0 or not perceptible (E0). Following administration of drug and/or escalation of dosage, the concentration increases as does the effect, first in an apparently linear fashion (at low drug concentrations) and then with a nonlinear increase in effect to an asymptotic point in the relationship where a maximal effect (Emax) is attained that does not perceptibly change with further increases in drug concentration. The point in the concentration-effect relationship where the observed effect represents 50% of the Emax is defined as EC50, a common pharmacodynamics term used to compare concentration-effect relationships between patients (or research subjects) and between drugs that may be in a given drug class. In practice, Emax can be derived either from visual interpolation of the concentration-effect profile or via mathematical curve fitting of the relationship.
Because it is rarely possible to measure drug concentrations at or near the receptor, it is necessary to use a surrogate measurement to assess exposure-response relationships. In most instances, this surrogate is represented by the plasma drug concentration vs. time curve. For drugs whose pharmacokinetic properties are best described by first-order (as opposed to zero- or mixed-order) processes, a semilogarithmic plot of plasma drug concentration vs. time data for an agent given by an extravascular route of administration (e.g., intramuscular, subcutaneous, intracisternal, oral, transmucosal, transdermal, rectal) produces a pattern depicted in Figure 57-2. The ascending portion of this curve represents a time during which the liberation of a drug from its formulation, dissolution of the drug in a biologic fluid (e.g., gastric or intestinal fluid, interstitial fluid; a prerequisite for absorption) and absorption of a drug are rate-limiting relative to its elimination. After the time (Tmax) where maximal plasma concentrations (Cmax) are observed, the plasma concentration decreases as metabolism and elimination become rate limiting, the terminal portion of this segment of the plasma concentration vs. time curve being representative of drug elimination from the body. Finally, the area under the plasma concentration vs. time curve (AUC), a concentration- and time-dependent parameter reflecting the degree of systemic exposure from a given drug dosage, can be determined by integrating the plasma concentration data over time.
Definitions of Terms
Area under the curve (AUC) is, conceptually, a measure of both the extent of drug absorbed and its persistence in the body. Thus, it is both concentration- and time-dependent. Mathematically, AUC represents the integral of blood drug levels over time from 0 to either a predetermined postdose time point (AUC0→tx) or extrapolated to infinity (AUC0→∞), which is calculated by using the apparent terminal elimination rate constant (similarly calculated from the observed plasma concentration vs. time plot; see Fig. 57-2).
where AUC can either represent the AUC0→∞ for single dose administration or the AUC from time zero to the end of the dosing interval at steady state (i.e., AUCss0→τ). Calculation of Clren requires a complete, quantitative collection of urine (usually over 24 hr) to determine the amount of drug excreted unchanged (Ae). Clnr is generally determined as the difference between Cl and Clren. For drug administration by any extravascular route, the calculation of Cl yields an “apparent” value (e.g., Cl/F) which must be corrected for bioavailability.
Elimination half-life (T1/2) of a drug represents the time for postabsorption blood or plasma concentrations to be reduced by 50%. The apparent elimination rate constant (ke) can be reliably estimated from two drug concentrations obtained on the elimination phase of the concentration vs. time curve (see Fig. 57-2). The T1/2 is then determined as the natural logarithm of 2 divided by the elimination rate constant (T1/2 = 0.693/ke). T1/2 reflects the elimination of parent drug by all of the routes involved in its clearance. Although T1/2 is often considered a surrogate indicator of drug clearance, this should be done cautiously because it depends upon the clearance and the apparent volume of distribution (Vd), as illustrated by the following equation:
where Cp0 represents the highest attainable plasma concentration following administration of a single dose. As a proportionality constant, Vd is a determinant of plasma drug concentrations attained following administration of a given drug dose. For drugs that do not distribute extensively or associate with great affinity to proteins and tissue, the Vd may dimensionally correspond to a physiologic or anatomic body space: Vd < 0.1 L/kg ≅ intravascular space, 0.1-0.3 L/kg ≅ extracellular space, 0.6-0.7 L/kg ≅ total body water space. Disease and development can influence the Vd, and thus the achievable concentration, for a given drug. Following extravascular drug administration, the apparent Vd is affected by the extent of drug absorbed (Vd/F). In instances in which a drug has incomplete absorption, plasma concentration can underestimate the true value of the Vd.
The Impact of Ontogeny on Drug Disposition
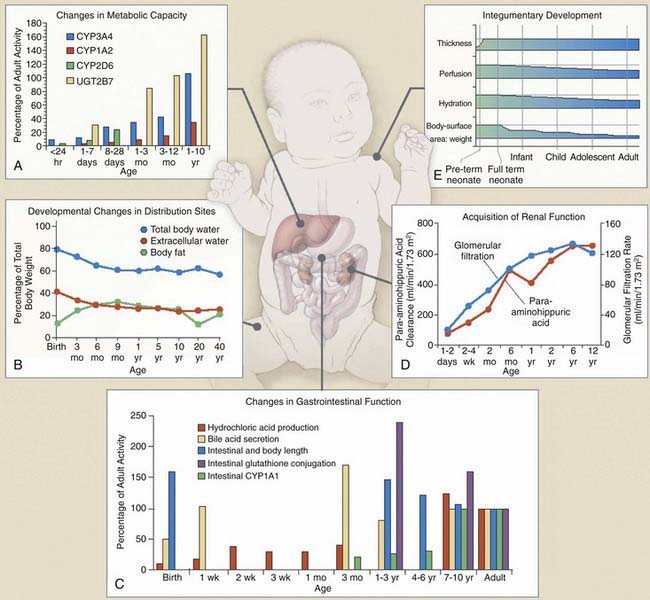
Dramatic pharmacokinetic, pharmacodynamic, and psychosocial changes occur as preterm infants mature toward term, as infants mature through the first few years of life, and as children reach puberty and adolescence (Fig. 57-3). To meet the needs of these different pediatric groups, different formulations are needed for drug delivery that can influence drug absorption and disposition, and different psychosocial issues influence compliance, timing of drug administration, and reactions to drug use. These additional factors must be considered in conjunction with known pharmacokinetic and pharmacodynamic influences of age when developing an optimal patient-specific drug therapy strategy.
Drug Absorption
Peroral Absorption
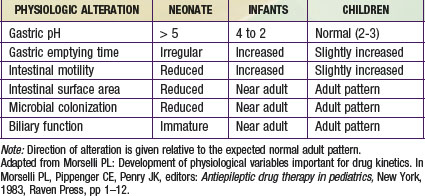
The most important factors that influence drug absorption from the GI tract are related to the physiology of the stomach, intestine, and biliary tract (see Fig. 57-3C and Table 57-1). The rate and extent of peroral absorption of drugs depends primarily on the pH-dependent passive diffusion and motility of the stomach and intestinal tract because both of these factors influence transit time of the drug. Gastric pH changes significantly throughout development, and the highest (alkaline) values occur during the neonatal period. In the fully mature neonate, the gastric pH ranges from 6 to 8 at birth and drops to 2 to 3 within a few hours of birth. However, after the first 24 hr of life, the gastric pH increases due do the immaturity of the parietal cells. As the parietal cells mature, the gastric acid secretory capacity increases (pH decreases) over the first few months of life to reach adult levels by 3-7 yr of age. As a result, the peroral bioavailability of acid-labile drugs, such as penicillin or ampicillin, is increased. In contrast, the absorption of weak organic acids (e.g., phenobarbital and phenytoin) is relatively decreased, a condition that can necessitate administering larger doses in the very young to achieve therapeutic plasma levels.
Extravascular Drug Absorption
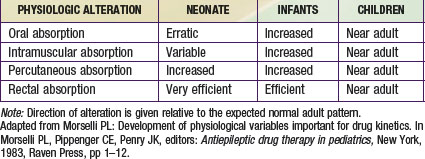
Intravenous drug administration is assumed to be the most dependable and accurate route for drug delivery, with a bioavailability of 100%. Absorption of drugs from tissues and organs (e.g., intramuscular, transdermal, and rectal) can also be affected by development (Table 57-2). Intramuscular blood flow changes with age, which can result in variable and unpredictable absorption. Reduced muscular blood flow in the first few days of life, the relative inefficiency of muscular contractions (useful in dispersing an IM drug dose), and an increased fraction of water per unit of muscle mass can delay the rate and/or extent of drugs given intramuscularly to the neonate. Muscular blood flow increases into infancy, and consequently the bioavailability of drugs given by the IM route is comparable to that seen in children and adolescents.
In contrast, mucosal permeability (rectal and buccal) in the neonate is increased and thus can result in enhanced absorption by this route. Transdermal drug absorption in the neonate and very young infant is increased due to the thinner and more hydrated stratum corneum (see Fig. 57-3E). In addition, the ratio of body surface area to body weight is greater in infants and children than in adults. Collectively, these developmental differences can predispose the child to increased exposure and risk for toxicity for drugs or chemicals placed on the skin (e.g., silver sulfadiazine, topical corticosteroids, benzocaine, diphenhydramine), and such toxicity is more likely during the first 8 to 12 mo of life.
Drug Distribution
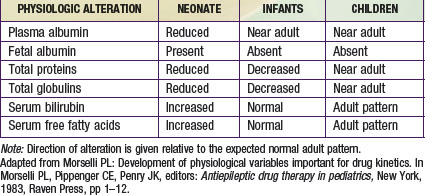
Newborns have a much higher proportion of body mass in the form of water (approximately 75% TBW) than older infants and children (see Fig. 57-3B). Also, the percentage of ECW decreases from the newborn stage (∼45%) into adulthood (∼20-30%). In fact, the increase of TBW in the neonate is attributable to ECW. The reduction in TBW is rapid in the first year of life, with adult values (55%) achieved by approximately 12 years of age. In contrast, the percentage of intracellular water (ICW) as a function of body mass remains stable from the first months of life through adulthood. The impact of developmental changes in body water spaces is exemplified by drugs such as the aminoglycoside antibiotics, compounds that distribute predominantly throughout the extracellular fluid space and have a higher Vd (0.4-0.7 L/kg) in neonates and infants than in adults (0.2-0.3 L/kg).
Albumin, total proteins, and total globulins (e.g., α1-acid glycoprotein) are the most important circulating proteins responsible for drug binding in plasma. The absolute concentration of these proteins is influenced by age, nutrition, and disease (Table 57-3). The concentrations of most all circulating plasma proteins are reduced in the neonate and young infant (about 80% of adult) and reach adult values by 1 year of age. A similar pattern of maturation is observed with α1-acid glycoprotein (an acute phase reactant capable of binding basic drugs) where neonatal plasma concentrations are approximately 3 times lower than in maternal plasma and attain adult values by approximately 1 year of age.
Drug Metabolism
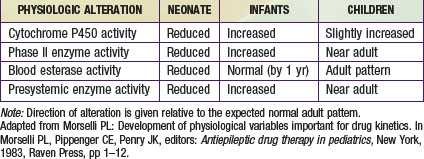
The primary organ responsible for drug metabolism is the liver, although the kidneys, intestines, lungs, adrenals, blood (phosphatases, esterases), and skin can also biotransform certain compounds. Drug metabolism occurs primarily in the endoplasmic reticulum of cells via two general classes of enzymatic processes: phase I, or nonsynthetic, and phase II, or synthetic, reactions. Phase I reactions include oxidation, reduction, hydrolysis, and hydroxylation reactions, and phase II reactions primarily involve conjugation with an endogenous ligand (e.g., glycine, glucuronide, glutathione, or sulfate). As illustrated by Figure 57-3A, many drug-metabolizing enzymes demonstrate an ontogenic profile, with generally low activity present at birth and maturation over a period of months to years (Table 57-4).
Although there are many enzymes that are capable of catalyzing the biotransformation of drugs and xenobiotics, the quantitatively most important are represented by the cytochrome P450 (CYP450) supergene family, which has at least 16 primary enzymes. The specific CYP450 isoforms responsible for the majority of human drug metabolism are represented by CYP1A2, CYP2C9, CYP2C19, CYP2D6, CYP2E1, and CYP3A4 (Chapter 56). These enzymes represent the products of genes that in some instances are polymorphically expressed, with allelic variants producing enzymes generally resulting in either no or reduced catalytic activity (a notable exception being the *17 allele of CYP2C19, which conveys increased activity). At birth, the concentration of drug-oxidizing enzymes in fetal liver (corrected for liver weight) appears similar to that in adult liver. However, the activity of these oxidizing enzyme systems is reduced, which results in slow clearance (and prolonged elimination) of many drugs that are substrates for them, including phenytoin, caffeine, diazepam, and many others. After birth, the hepatic CYP450s appear to mature at different rates. Within hours after birth, CYP2E1 activity increases rapidly, and CYP2D6 is detectable soon thereafter. CYP2C (CYP2C9 and CYP2C19) and CYP3A4 are present within the first month of life, a few months before CYP1A2. CYP3A4 activity in young infants can exceed that observed in adults as reflected by the clearance of drugs that are substrates for this enzyme, such as cyclosporine and tacrolimus.
With regard to the impact of development on drug metabolism, most therapeutic drugs are polyfunctional substrates for a host of enzymes and/or transporters. The isoform-specific ontogenic profile (see Fig. 57-3) must be considered in the context of deducing how development can affect the metabolic portion of drug clearance. True developmental dependence of drug clearance (CL) must also consider the role of pharmacogenetic constitution on the activity of enzymes and transporters (Chapter 56) and the impact of ontogeny on the nonmetabolic routes (e.g., renal drug excretion, salivary and biliary drug excretion, pulmonary drug excretion) that contribute to the overall drug clearance (Total CL = CLhepatic + CLrenal + CLnonrenal).
Renal Drug Elimination
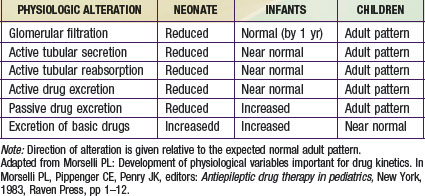
The kidney is the primary organ responsible for the excretion of drugs and their metabolites. The development of renal function begins during early fetal development and is complete by early childhood (see Fig. 57-3D; Table 57-5). Total renal drug clearance (CLrenal) can be conceptualized by considering the following equation:
where glomerular filtration (GFR), active tubular secretion (ATS), and active tubular reabsorption (ATR) of drugs can contribute to overall clearance. As is true for hepatic drug metabolism, only free (unbound) drug and/or metabolite can be filtered by a normal glomerulus and/or either secreted or reabsorbed via a renal tubular transport protein.
Renal clearance is limited in the newborn owing to anatomic and functional immaturity of the nephron unit. In term and preterm neonates, GFR averages 2-4 mL/min/1.73 m2 at birth. During the first few days of life, a drop in renal vascular resistance occurs, which results in a net increase in renal blood flow and a redistribution of intrarenal blood flow from a predominantly medullary to a cortical distribution. All of these changes are associated with a commensurate increase in GFR. In term neonates, GFR increases rapidly over the first few months of life and approaches adult values by 10-12 mo of life (see Fig. 57-3D). The rate of GFR acquisition is blunted in preterm neonates, consequent to continued nephrogenesis, which occurs in the early postnatal period. In children 2 to 5 yr of age, GFR can exceed adult values, especially during periods of increased metabolic demand (e.g., during a fever).
where the Fel represents the fraction of the drug excreted unchanged in an adult with normal renal function, GFRobserved is the value calculated (from creatinine clearance or an age-appropriate estimation equation) for the patient (in mL/min/1.73 m2), GFRnormal is the average value considered for a healthy adult (120 mL/min/1.73 m2), and Kelnormal is estimated from the average elimination T1/2 for a drug taken from the medical literature using the following equation:
Impact of Ontogeny on Pharmacodynamics
As noted earlier (see Fig. 57-1), drug action is concentration dependent, and onset and offset are generally associated with appearance and disappearance, respectively, of the drug at the receptor(s) in an amount that is sufficient to initiate the cascade of biologic effects that terminate in drug action. The minimum effective concentration of a drug is that observed with the immediate onset of effect, and the duration of action is predicated upon the maintenance of drug concentrations at the receptor within a range that is associated with the desirable pharmacologic action(s).
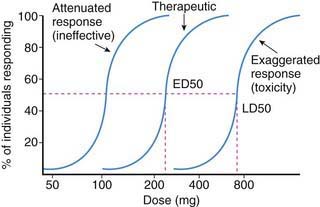
Age-related pharmacokinetic variation resulting in altered drug disposition can result in less or more drug being available at the receptor(s) consequent to whether drug clearance is decreased or increased relative to values in adults. The resulting alteration in the dose-concentration profile can result in an attenuated (ineffective) or exaggerated (toxicity) response in children, which is especially relevant for drugs with a narrow therapeutic index (Fig. 57-4). Thus, in some circumstances, apparent developmental differences in drug response and effect may be simply explained on a pharmacokinetic basis.
Additional Considerations in Pediatric Therapeutics
Monitoring Drug Levels
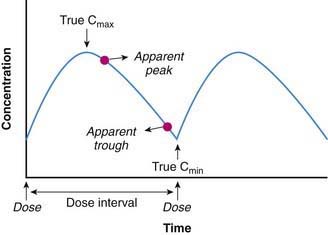
Common to both of these approaches is the need to accurately assess plasma drug concentrations in a given patient. Figure 57-5 represents a hypothetical general steady-state plasma concentration vs. time profile for a drug given by an extravascular route. It is provided to illustrate the following general principles to be recognized and followed when plasma drug level monitoring is used in patients as a tool to individualize drug treatment:
This last point is illustrated by Figure 57-5, which denotes the “true” peak (Cmax) and trough (Cmin) plasma concentrations in relation to apparent values, a situation that often occurs when “peak” and “trough” blood levels are ordered and nursing or phlebotomy procedures allow some period of leeway as to when they can be obtained. When such a discrepancy is noted and the exact timing of the samples relative to dose administration is known, corrections can be made to ensure that pharmacokinetic parameters estimated from the data are accurate. If a discrepancy is not noted, the parameters can be incorrectly estimated and the dosing regimen can be incorrectly calculated, thereby compromising the safety and/or efficacy of drug treatment.
Drug Formulation and Administration
Other Routes of Administration
In pediatric patients, percutaneous drug administration is generally reserved for agents intended to produce a local effect within the dermis (see “Drug Absorption”). Development has an impact on the barrier of the skin that, if not recognized and controlled for with proper drug administration techniques, can produce situations in which systemic toxicity can result. Similar therapeutic challenges occur when transmucosal routes (e.g., buccal, sublingual, rectal) are used for administering drug. Specifically, unpredictable systemic bioavailability can complicate treatment when the rate and/or extent of drug absorption varies. As a consequence, transmucosal drug administration to pediatric patients is now only used when the patient’s condition does not permit drug administration by the oral or the parenteral routes.
Drug-Drug Interactions
Pharmacokinetic and/or pharmacodynamic properties of drugs may be altered when 2 or more drugs are administered. Although interactions largely occur at the level of drug metabolism, they can occur at the level of drug absorption (e.g., inhibition of intestinal CYP3A4 activity by grapefruit juice or St. John’s wort and consequent reduction in presystemic clearance of CYP3A4 substrates), distribution (e.g., displacement of warfarin plasma protein binding by ibuprofen with consequent increased hemorrhagic risk), or elimination (e.g., inhibition of active tubular secretion of β-lactam antibiotics by probenecid). Drug-drug interactions can occur at the level of the receptor (via competitive antagonism); many of these are intentional and produce therapeutic benefit in pediatric patients (e.g., antihistamine reversal of histamine effects, naloxone reversal of opiate adverse effects) (Tables 57-6 and 57-7).
Table 57-6 PARTIAL LISTING OF DRUG INTERACTIONS OF POTENTIAL IMPORTANCE IN PEDIATRIC PRACTICE
| INTERACTING AGENT | ADVERSE EFFECT* |
|---|---|
| ACETAMINOPHEN | |
| Alcohol | Hepatotoxicity |
| Oral anticoagulants | ↑ Anticoagulation |
| Probenecid | ↑ Acetaminophen toxicity |
| Zidovudine | Granulocytopenia |
| ACYCLOVIR | |
| Narcotics | ↑ Narcotic toxicity? |
| Zidovudine | Lethargy |
| ALCOHOL | |
| Antidepressants (tricyclic) | ↑ Toxicity |
| Barbiturates | ↑ CNS depression (acute) |
| Benzodiazepines | ↑ CNS depression |
| Cephalosporins (not all) | Disulfiram effect |
| Chloral hydrate | ↑ CNS depression |
| Doxycycline | ↓ Antibiotic effect |
| Isoniazid | ↑ Hepatotoxicity |
| Metronidazole | Disulfiram effect |
| Phenothiazines | Impaired coordination |
| Phenytoin | ↑ Phenytoin toxicity |
| ALLOPURINOL | |
| Aluminum hydroxide | ↓ Allopurinol absorption |
| Ampicillin | Rash |
| Anticoagulants (oral) | ↑ Anticoagulant effect |
| Azathioprine | ↑ Azathioprine toxicity |
| Captopril | ↑ Cutaneous hypersensitivity |
| Cyclophosphamide | ↑ Cyclophosphamide toxicity |
| Theophylline | ↑ Theophylline toxicity |
| Thiazide diuretics | ↑ Allopurinol toxicity |
| AMINOGLYCOSIDE ANTIBIOTICS | |
| Amphotericin B | ↑ Nephrotoxicity |
| Bumetanide | ↑ Ototoxicity |
| Cisplatin | ↑ Nephrotoxicity |
| Cyclosporine | ↑ Nephrotoxicity |
| Furosemide | ↑ Nephrotoxicity and ototoxicity |
| Magnesium | ↑ Neuromuscular blockade |
| Neuromuscular blocking agents | ↑ Blockade |
| Vancomycin | ↑ Nephrotoxicity? |
| ANTACIDS | |
| β-Adrenergic blockers | ↓ Absorption |
| Captopril | ↓ Absorption |
| Cimetidine | ↓ Absorption |
| Corticosteroids | ↓ Absorption |
| Digoxin | ↓ Absorption |
| Iron | ↓ Absorption |
| Isoniazid | ↓ Absorption |
| Ketoconazole | ↓ Absorption |
| Nonsteroidal anti-inflammatory agents | ↓ Absorption |
| Phenytoin | ↓ Absorption |
| Salicylates | ↓ Absorption |
| Tetracycline | ↓ Absorption |
| Theophylline | ↑ Toxicity |
| ASPIRIN | |
| Anticoagulants (oral) | ↑ Bleeding |
| Captopril | ↓ Antihypertensive effect |
| BARBITURATES | |
| Anticoagulants (oral) | ↓ Anticoagulation |
| β-Adrenergic blockers | ↓ β Blockade |
| Carbamazepine | ↑ Production of carbamazepine epoxide |
| Chloramphenicol | ↑ Barbiturate toxicity |
| Contraceptives (oral) | ↓ Contraception |
| Corticosteroids | ↓ Steroid effect |
| Influenza vaccine (viral) | ↑ Barbiturate toxicity |
| Rifampin | ↓ Barbiturate effect |
| Theophylline | ↓ Theophylline effect |
| Valproate | ↑ Barbiturate toxicity |
| BLEOMYCIN | |
| Oxygen | ↑ Pulmonary toxicity |
| CAPTOPRIL | |
| Allopurinol | ↑ Cutaneous hypersensitivity |
| Aspirin | ↓ Antihypertensive effect |
| Cimetidine | Neuropathy |
| Nonsteroidal anti-inflammatory agents | ↓ Antihypertensive effect |
| Potassium | Hyperkalemia |
| Spironolactone | Hyperkalemia |
| CARBAMAZEPINE | |
| Anticoagulants (oral) | ↓ Anticoagulation |
| Antidepressants (tricyclic) | ↑ Toxicity (both drugs) |
| Cimetidine | ↑ Carbamazepine toxicity |
| Contraceptives (oral) | ↓ Contraception |
| Corticosteroids | ↓ Steroid effect |
| Cyclosporine | ↓ Cyclosporine effect |
| Erythromycins | ↑ Carbamazepine toxicity |
| Influenza vaccine (viral) | ↑ Carbamazepine toxicity |
| Isoniazid | ↑ Toxicity (both drugs) |
| Phenytoin | ↓ Carbamazepine effect |
| Theophylline | ↓ Theophylline effect |
| Valproate | ↓ Valproate effect |
| CIMETIDINE | |
| Alcohol | ↑ Alcohol effect |
| Antacids | ↓ Cimetidine effect |
| Anticoagulants (oral) | ↑ Anticoagulation |
| Antidepressants (tricyclic) | ↑ Antidepressant toxicity |
| Benzodiazepines | ↑ Benzodiazepine toxicity |
| β-Adrenergic blocking agents | ↑ β-Blockade toxicity |
| Captopril | Neuropathy |
| Carbamazepine | ↑ Carbamazepine toxicity |
| Digoxin | ↑ Digoxin toxicity |
| Ketoconazole | ↓ Ketoconazole absorption |
| Metoclopramide | ↓ Cimetidine effect |
| Phenytoin | ↑ Phenytoin toxicity |
| Theophylline | ↑ Theophylline toxicity |
| CONTRACEPTIVES (ORAL) | |
| Anticoagulants (oral) | ↓ Anticoagulation |
| Antidepressants (tricyclic) | ↑ Antidepressant toxicity |
| Barbiturates | ↓ Contraception |
| Carbamazepine | ↓ Contraception |
| Griseofulvin | ↓ Contraception |
| Penicillins (ampicillin, oxacillin) | ↓ Contraception? |
| Phenytoin | ↓ Contraception |
| Rifampin | ↓ Contraception |
| Theophylline | ↑ Theophylline toxicity |
| CYCLOSPORINE | |
| Alkylating agents | ↑ Nephrotoxicity |
| Aminoglycosides | ↑ Nephrotoxicity |
| Amphotericin B | ↑ Nephrotoxicity |
| Carbamazepine | ↓ Cyclosporine effect |
| Erythromycins | ↑ Cyclosporine toxicity |
| Furosemide | Gout |
| Ketoconazole | ↑ Nephrotoxicity |
| Metoclopramide | ↑ Cyclosporine toxicity |
| Nafcillin | ↓ Cyclosporine effect |
| Phenytoin | ↓ Cyclosporine effect |
| Rifampin | ↓ Cyclosporine effect |
| DIGOXIN | |
| Antacids | ↓ Absorption |
| Anticholinergics | ↑ Digoxin toxicity |
| Cholestyramine | ↓ Absorption |
| Cimetidine | ↑ Digoxin toxicity |
| Diuretics (hypokalemia) | ↑ Digoxin toxicity |
| Phenytoin | ↓ Digoxin effect |
| Quinidine | ↑ Digoxin toxicity |
| Verapamil | ↑ Digoxin toxicity |
| ERYTHROMYCINS | |
| Anticoagulants (oral) | ↑ Anticoagulation |
| Astemizole (Hismanal) | ↑ Astemizole toxicity: arrhythmias |
| Carbamazepine | ↑ Carbamazepine toxicity |
| Cyclosporine | ↑ Cyclosporine toxicity |
| Phenytoin | ↓ Phenytoin effect |
| Terfenadine (Seldane) | ↑ Terfenadine toxicity: arrhythmias |
| Theophylline | ↑ Theophylline toxicity |
| FLUOROQUINOLONES | |
| Antacids | ↓ Antibiotic effect |
| Theophylline | ↑ Theophylline toxicity |
| GRISEOFULVIN | |
| Anticoagulants (oral) | ↓ Anticoagulants |
| Contraceptive (oral) | ↓ Contraceptive |
| ISONIAZID | |
| Alcohol | Hepatitis |
| Antacids | ↓ Isoniazid absorption |
| Carbamazepine | ↑ Toxicity (both) |
| Ketoconazole | ↓ Ketoconazole effect |
| Phenytoin | ↑ Phenytoin toxicity |
| Rifampin | ↑ Hepatotoxicity |
| Valproate | ↑ Hepatic and CNS toxicity |
| KETOCONAZOLE | |
| Antacids | ↓ Absorption |
| Anticoagulants (oral) | ↑ Anticoagulation |
| Cimetidine | ↓ Ketoconazole effect |
| Cyclosporine | ↑ Nephrotoxicity |
| Isoniazid | ↓ Ketoconazole effect |
| Phenytoin | Altered metabolism of both drugs |
| Rifampin | ↑ Effects of both drugs |
| METHOTREXATE | |
| Blood transfusion | ↑ Toxicity |
| Cisplatin | ↑ Methotrexate toxicity |
| Etretinate | ↑ Hepatotoxicity |
| Nonsteroidal anti-inflammatory drugs | ↑ Methotrexate toxicity |
| Trimethoprim/sulfamethoxazole | Megaloblastic anemia |
| METOCLOPRAMIDE | |
| Carbamazepine | Neurotoxicity |
| Cimetidine | ↓ Cimetidine effect |
| Cyclosporine | ↑ Cyclosporine toxicity |
| Digoxin | ↓ Absorption |
| Narcotics | ↑ Sedation |
| NIFEDIPINE | |
| β-Adrenergic blockers | Heart failure, atrioventricular block |
| Cyclosporine | ↑ Gingival hyperplasia |
| Phenytoin | ↑ Phenytoin toxicity |
| Prazosin | Hypotension |
| Quinidine | ↓ Quinidine effect |
| PHENYTOIN | |
| Alcohol | ↑ Toxicity (acute) |
| Antacids | ↓ Phenytoin effect |
| Anticoagulants (oral) | ↓ Phenytoin toxicity, ↑↓ anticoagulation |
| Antidepressants (tricyclic) | ↑ Phenytoin toxicity |
| Carbamazepine | ↓ Carbamazepine effect |
| Chloramphenicol | ↑ Toxicity (both drugs) |
| Cimetidine | ↑ Phenytoin toxicity |
| Contraceptives (oral and implant) | ↓ Contraception |
| Corticosteroids | ↓ Corticosteroid effect |
| Cyclosporine | ↓ Cyclosporine effect |
| Digoxin | ↓ Digoxin effect |
| Dopamine | Hypotension |
| Folic acid | ↓ Phenytoin effect |
| Isoniazid | ↑ Phenytoin toxicity |
| Miconazole | ↓ Phenytoin effect |
| Neuromuscular blocking agents | ↓ Blockade |
| Nifedipine | ↑ Phenytoin toxicity |
| Quinidine | ↓ Quinidine effect |
| Rifampin | ↓ Phenytoin effect |
| Theophylline | ↓ Effects (both drugs) |
| Valproate | ↑ Phenytoin toxicity |
| QUINIDINE | |
| Amiodarone | ↑ Quinidine toxicity |
| Anticoagulants (oral) | ↑ Anticoagulation |
| Barbiturates | ↓ Quinidine effect |
| Cimetidine | ↑ Quinidine toxicity |
| Digoxin | ↑ Digoxin toxicity |
| Metoclopramide | ↓ Quinidine effect |
| Phenytoin | ↓ Quinidine effect |
| Procainamide | ↑ Procainamide toxicity |
| Rifampin | ↓ Quinidine effect |
| Verapamil | Hypotension |
| RIFAMPIN | |
| Anticoagulants (oral) | ↓ Anticoagulation |
| Barbiturates | ↓ Barbiturate effect |
| β-Adrenergic blockers | ↓ β Blockade |
| Chloramphenicol | ↓ Chloramphenicol effect |
| Contraceptives (oral) | ↓ Contraception |
| Corticosteroids | ↓ Corticosteroid effect |
| Cyclosporine | ↓ Cyclosporine effect |
| Isoniazid | ↑ Hepatotoxicity |
| Ketoconazole | ↓ Effects (both drugs) |
| Phenytoin | ↓ Phenytoin effect |
| Quinidine | ↓ Quinidine effect |
| Theophylline | ↓ Theophylline effect |
| Verapamil | ↓ Verapamil effect |
| THEOPHYLLINE | |
| Barbiturates | ↓ Theophylline effect |
| β-Adrenergic blockers | ↑ Theophylline toxicity |
| Carbamazepine | ↓ Theophylline effect |
| Cimetidine | ↑ Theophylline toxicity |
| Erythromycins | ↑ Theophylline toxicity |
| Fluoroquinolones | ↑ Theophylline toxicity |
| Influenza vaccine (viral) | ↑ Theophylline toxicity |
| Interferon | ↑ Toxicity? |
| Marijuana smoking | ↓ Theophylline effect |
| Phenytoin | ↓ Effect (both drugs) |
| Rifampin | ↓ Theophylline effect |
| Tobacco smoking | ↓ Theophylline effect |
| Troleandomycin | ↑ Theophylline toxicity |
| TRIMETHOPRIM/SULFAMETHOXAZOLE | |
| Anticoagulants (oral) | ↑ Anticoagulation |
| Antidepressants (tricyclic) | Depression |
| Mercaptopurine | ↓ Antileukemia effect |
| Methotrexate | Megaloblastic anemia |
| VALPROATE | |
| Barbiturates | ↑ Phenobarbital toxicity |
| Benzodiazepines | ↑ Diazepam toxicity |
| Carbamazepines | ↓ Valproate effect |
| Cimetidine | ↑ Valproate toxicity? |
| Ethosuximide | ↑ Ethosuximide toxicity? |
| Phenytoin | ↑ Phenytoin toxicity |
CNS, central nervous system; ?, possible effect.
* When possible, an alternative drug combination should be given. Otherwise, drug levels and signs of toxicity must be monitored.
Modified from Rizack M, Hillman C: The Medical Letter handbook of adverse drug interactions, New Rochelle, NY, 1989, The Medical Letter.
Table 57-7 PARTIAL LISTING OF ANTI-VIRALS USED TO TREAT HIV AND DRUG INTERACTIONS OF POTENTIAL IMPORTANCE IN PEDIATRIC PRACTICE*
| RITONAVIR | |
| Amiodarone† | Hypotension, bradycardia, sinus arrest |
| Colchicine† | ↑ Colchicine levels |
| Ergot alkaloids† | Nausea, vomiting, vasospastic ischemia |
| Pimozide† | Cardiotoxicity |
| Quinidine† | Cardiotoxicity |
| Rifabutin | ↑ Rifabutin levels (↓ rifabutin by 75%) |
| Voriconazole† | ↓ Voriconazole efficacy |
| SAQUINAVIR | |
| Bepridil† | Risk of arrhythmias |
| Darunavir | ↓ Exposure to darunavir |
| Eplerenone | ↑ Eplerenone levels and side effects |
| Ergot alkaloids† | Nausea, vomiting, vasospastic ischemia |
| Loperamide | ↓ Saquinavir levels (avoid if prolonged treatment) |
| Midazolam† | Excessive sedation and respiratory depression |
| Pimozide† | Cardiotoxicity |
| Quinidine† | Hypotension, bradycardia, sinus arrest |
| Rifabutin | ↓ Saquinavir and ↑ rifabutin levels |
| Rifampin† | ↓ Saquinavir and ↑ hepatotoxicity |
| Sildenafil† | Cardiovascular events |
| INDINAVIR | |
| Amiodarone† | Hypotension, bradycardia, sinus arrest |
| Ergot alkaloids† | Nausea, vomiting, vasospastic ischemia |
| Omeprazole | ↓ Indinavir exposure and efficacy |
| Pimozide† | Cardiotoxicity |
| Rifabutin/rifampin/rifapentine | ↑ Rifabutin toxicity and ↓ indinavir levels |
| Sildenafil† | ↑ Sildenafil levels (hypotension, visual changes, priapism) |
| Statins | ↑ Risk of myopathy or rhabdomyolysis |
| Triazolam† | Excessive or prolonged sedation |
| ATAZANAVIR | |
| Amiodarone | Risk of cardiotoxicity (QT prolongation, torsades de pointes) |
| Diltiazem | Prolonged PR interval |
| Ergot alkaloids* (theoretical) | Vasospasm, ischemia |
| Etravirine† | ↓ Atazanavir levels, ↑ etavirine levels |
| Fosamprenavir† | ↓ Atazanavir levels |
| H2-receptor antagonists/Proton pump inhibitors | ↓ Atazanavir levels |
| Minocycline | ↓ Atazanavir levels |
| Nevirapine | ↓ Atazanavir levels, ↑ nevirapine levels |
| Pimozide† | Cardiotoxicity |
| Rifampin† | ↓ Atazanavir levels |
| Tipranavir | ↓ Atazanavir levels |
| EFAVIRENZ | |
| Etravirine (and other non-nucleoside reverse transcriptase inhititors) | ↓ Etravirine levels |
| Amprenavir/Fosamprenavir | ↓ Amprenavir levels |
| Azole antifungals | ↓ Azole levels |
| Maraviroc | ↓ Maraviroc levels |
| Nevirapine | ↓ Efavirenz levels, ↑ adverse effects |
| Rifabutin | ↓ Rifabutin levels |
| Rifampin | ↓ Efavirenz levels |
| NEVIRAPINE | |
| Amprenavir/Fosamprenavir | ↓ Amprenavir levels |
| Atazanavir | ↑ Nevirapine, ↓ atazanavir bioavailability |
| Fluconazole | ↑ Nevirapine exposure |
| Itraconazole | ↓ Itraconazole bioavailability |
| Rifampin | ↓ Nevirapine levels |
| Voriconazole | ↑ Nevirapine levels and/or ↑ or ↓ voriconazole levels |
| STAVUDINE | |
| Didanosine | ↑ risk for pancreatitis, hepatotoxicity, and severe peripheral neuropathy |
| Doxorubicin | ↓ Stavudine efficacy |
| Hydroxyurea | Fatal pancreatitis, hepatotoxicity |
| ZIDOVUDINE | |
| Dapsone | Neutropenia |
| Ganciclovir | Anemia and neutropenia |
| Pyrazinamide | ↓ Pyrazinamide efficacy |
| Ribavirin | ↓ Zidovudine efficacy; lactic acidosis; hepatic decompensation; neutropenia; and anemia |
| TENOFOVIR | |
| Didanosine | Neuropathy, diarrhea, pancreatitis, severe lactic acidosis |
* This table is not meant to be an all-inclusive list of drug-drug interactions. Care should be taken when prescribing all medications, and the potential of interactions should be considered. The practitioner is encouraged to assess the possibility of all interactions when prescribing medications.
† Considered to be drugs that are a contraindicated drug-drug interaction. An alternate drug combination should be given. If not possible, drug levels and signs of toxicity must be monitored.
Micromedex® 2.0. Thomson Reuters. Copyright 1974-2010.
Drug-drug interactions that occur at the level of drug metabolism can be somewhat predictable based on a priori knowledge of a given drug’s biotransformation profile. Information pertaining to drug-substrate interaction can be useful in ascertaining the direction (e.g., enzyme inhibition → reduced clearance → higher plasma concentration → enhanced effect vs. enzyme induction → increased clearance → reduced plasma concentration → diminished effect) of a drug-drug interaction. This information can be found in primary and secondary literature and drug labeling, but it might not be complete or updated. The data compilation from Indiana University (http://medicine.iupui.edu/clinpharm/ddis) appears to be the most complete and useful.
One of the most daunting challenges for the clinician is to determine if a drug-drug or drug-food interaction will be clinically significant. Extensive databases of reported and/or potential (e.g., theoretical mechanism- or metabolism-based) drug interactions exist and are widely available on the Internet (e.g., http://www.medscape.com/druginfo/druginterchecker; http://www.drugs.com; http://www.umm.edu/adam/drug_checker.htm); some of these assess the potential significance of the interaction. Many computer-based information systems used by hospital and community pharmacies routinely screen a patient’s medication profile (generally restricted to prescribed drugs) against new prescriptions to evaluate the potential for drug-drug interactions.
Abdel-Rahman SM, Kearns GL. The pharmacokinetic-pharmacodynamic interface: determinants of anti-infective drug action and efficacy in pediatrics. In: Feigin RD, Cherry JD, Demmler-Harrison GJ, et al, editors. Textbook of pediatric infectious disease. ed 6. Philadelphia: Saunders/Elsevier; 2009:3156-3178.
Bartelink IH, Rademaker CM, Schobben AF, et al. Guidelines on paediatric dosing on the basis of developmental physiology and pharmacokinetic considerations. Clin Pharmacokinet. 2006;45:1077-1097.
Biomarkers Definitions Working Group. Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther. 2001;69:89-95.
Centers for Disease Control and Prevention. Revised product labels for pediatric over-the-counter cough and cold medicines. MMWR Morb Mortal Wkly Rep. 2008;57:1180.
Chen N, Aleksa K, Woodland C, et al. Ontogeny of drug elimination by the human kidney. Pediatr Nephrol. 2006;21:160-168.
Conroy S, Carroll WD. Prescribing in paediatrics. Arch Dis Child Educ Pract Ed. 2009;94:55-59.
2009 Generic drug revisited. Med Lett. 2009;51:81-82.
Hines RN. The ontogeny of drug metabolism enzymes and implications for adverse drug events. Pharmacol Ther. 2008;118:250-267.
Johnson TN, Rostami-Hodjegan A, Tucker GT. Prediction of the clearance of eleven drugs and associated variability in neonates, infants and children. Clin Pharmacokinet. 2006;45:931-956.
Johnson TN, Thomson M. Intestinal metabolism and transport of drugs in children: the effects of age and disease. J Pediatr Gastroenterol Nutr. 2008;47:3-10.
Kokki H, Kumpulainen E, Lehtonen M, et al. Cerebrospinal fluid distribution of ibuprofen after intravenous administration in children. Pediatrics. 2007;120:e1002-e1008.
Paul IM. Advances in pediatric pharmacology, therapeutics, and toxicology. Adv Pediatr. 2005;52:321-365.
Ritschel WA, Kearns GL, editors. Handbook of basic pharmacokinetics … including clinical applications, ed 7, Washington, DC: American Pharmacists Association, 2009.
Thomson SA, Tuleu C, Wong IC, et al. Minitablets: new modality to deliver medicines to preschool-aged children. Pediatrics. 2009;123:e235-238.
Yaffe SJ, Aranda JV, editors. Neonatal and pediatric pharmacology: therapeutic principles in practice, ed 3, Philadelphia: Lippincott Williams & Wilkins, 2004.
Zandieh SO, Goldman DA, Keohane CA, et al. Risk factors in preventable adverse drug events in pediatric outpatients. J Pediatr. 2008;152:225-231.